Abstract
Background/Objective
During adolescence, chronotype shifts towards “eveningness.” “Eveningness” is related to negative physical and mental health outcomes. Little is known about what influences the shift in chronotype beyond pubertal status. The current study examined the influence of earlier depression predicting later individual differences in adolescent chronotype, accounting for pubertal status, and the prospective prediction of later increases in depression from earlier chronotype.
Methods
Youth (age M=12.06, SD=2.35; 56.5% girls) from the community completed repeated assessments of depression, including both self-reports (14 assessments) and diagnostic interviews (8 assessments), over a 48-month period. At the 36-month time-point, participants completed chronotype and pubertal development measures. Regression and ANOVA analyses examined: (1) the influence of earlier depression levels (baseline to 36-months) upon chronotype, and (2) chronotype (at 36 months) upon later depression (48 months).
Results
Youth with higher earlier depression symptoms (β=−.347, p<.001) and history of depression diagnosis (β= −.13, p=.045) showed a greater eveningness preference controlling for pubertal status, age and gender. Further, depression diagnosis history interacted with pubertal status to predict chronotype: (F(1,243)=4.171, p=.045) such that the influence of depression on chronotype was greatest among postpubertal youth (t=3.271, p=.002). Chronotype (greater eveningness preference) predicted prospective increases in depression symptoms (β= −.16, p=.03) and onset of depressive episode (b=−.085, OR=.92, p=.03) one year later.
Conclusion
Depression, experienced earlier in life, predicts greater preference for eveningness, especially among postpubertal youth. In turn, later depression is predicted by evening preference. These findings suggest the reciprocal interplay between mood and biological rhythms, especially depression and chronotype, during adolescence.
Keywords: Youth, Sleep, Circadian Rhythm, Depression, Puberty, Morningness, Eveningness
Introduction
Prominent theories on biological rhythms and mood (Ehlers, Frank, & Kupfer, 1988; Wehr, Wirz-Justice, Goodwin, Duncan, & Gillin, 1979) highlight the critical importance of circadian patterns in the development of later depression and the likely reciprocal and longitudinal relation between dysregulated circadian patterns and depression. However, limited empirical evidence, especially using prospective longitudinal designs, exists regarding the interplay between circadian patterns and depression over time. This paucity is particularly notable during the critical developmental period of adolescence, when rates of depression increase and circadian timings change.
Children, adolescents, and adults exhibit meaningful individual differences in their daily patterns of sleep and alertness. Some demonstrate a pattern of falling asleep early, waking early, and generally feeling most alert during earlier periods of the day (i.e., “larks”), whereas others exhibit a much later timing (i.e., “owls”) (Danesi & Natale, 2002; Roenneberg, Wirz-Justice, & Merrow, 2003b; Adan et al., 2012). Most individuals fall in between the extremes. These relatively stable individual differences in overall alertness preference are referred to as chronotype. It has previously been shown that questionnaire measures of chronotype, which assess respondents on their degree of “morningness” or “eveningness” preference, correlate reasonably well with objective measures of circadian rhythm, such as the nightly onset of melatonin production (Duffy, Dijk, Hall, & Czeisler, 1999). Exhibiting a preference for eveningness has been linked with various negative outcomes, including depression (Drennan, Klauber, Kripke, & Goyette, 1991; Kitamura et al., 2010; Hidalgo et al., 2009; Merikanto et al., 2013; Chelminski, Ferraro, Petros, & Plaud, 1999; Alvaro, Roberts, & Harris, 2014; Pabst, Negriff, Dorn, Susman, Bin Huang, 2009), poor academic performance (Giannotti, Cortesi, Sebastiani, & Ottaviano, 2002; Merikanto et al., 2013), physical inactivity and higher rates of smoking, alcohol use and obesity (Roenneberg, Allebrandt, Merrow, & Vetter, 2012; Urbán, Magyaródi, & Rigó, 2011).
The timing of sleep and alertness is believed to be governed by a complex interaction between a homeostatic sleep system and a circadian system (Borbély, 1982). The homeostatic system increases the “pressure” to sleep with each waking minute (then dissipates during sleep), while the circadian system promotes patterns of alertness and sleepiness following a roughly 24-hour rhythm. This rhythm is governed by a central pacemaker, the hypothalamic suprachiasmatic nucleus (SCN), which sets the internal clock based on exposure to various zeitgebers (translated as “time-givers”). The primary zeitgeber is the daily fluctuation in light and dark, however other factors such as the timing of activity, meals, and social interactions, also contribute to adjustments to this clock and the overall rhythm (Roenneberg & Merrow, 2007). Chronotype, in theory, should reflect both the typical timing of an individual’s sleep-wake cycles as well as the chronological patterning of their daily activities and feelings of alertness.
Chronotype is not static, but rather changes throughout the lifespan. Prepubertal youth tend to hold a stronger preference towards morning, and then most youth shift toward an evening preference during adolescence, followed by a slow reversion toward a morning preference in adulthood (Kim, Dueker, Hasher, & Goldstein, 2002; Carskadon, Vieira, & Acebo, 1993; Roenneberg et al., 2004). Puberty is the most clearly identified factor underlying the shift during adolescence (Roenneberg et al., 2004; Carskadon et al., 1993), while other potential predictors have been understudied. This change in preference toward eveningness is normative, however it does not occur at the same time or to the same degree for all adolescents. Importantly, puberty is also linked with a rise in depression, such that prevalence rates rise significantly for postpubertal relative to prepubertal youth (Merikangas, He, Burstein, & Swanson, 2010; Hankin et al., 2015). Thus, depression may be an important factor affecting individual differences in changes in chronotype, though this possibility has not yet been studied.
The majority of studies have used a cross-sectional design (Hidalgo et al., 2009) or cross-sectional analysis (Antypa, Vogelzangs, Meesters, Schoevers, & Penninx, 2015) to examine the relationship between chronotype and maladaptive outcomes. This approach limits the ability to rigorously test prospective associations and temporal precedence. Few studies employed a longitudinal design, and in this small literature, chronotype has been used to predict later depression diagnoses during adolescence (Mooney, 2015). To better understand the longitudinal interplay between chronotype and depression, including both prediction of individual differences in chronotype and later forecasting of depression during adolescence, a prospective design is required. In the current study, we used a prospective multi-wave longitudinal approach with repeated measures of depression to enable a more accurate and precise assessment of depression predicting later individual differences in chronotype (Moffitt et al., 2010), and chronotype predicting subsequent increases in depression.
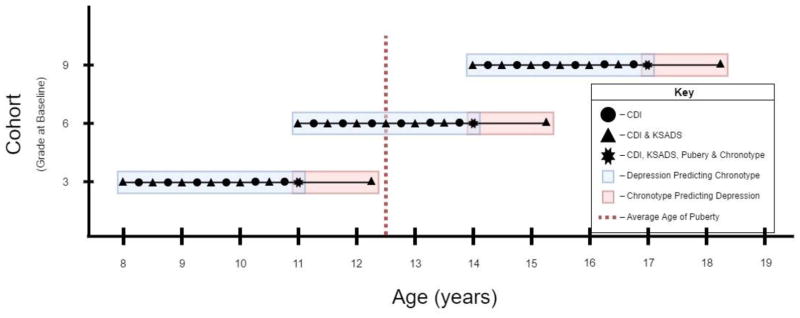
We examined two main questions. First, we investigated whether individual differences in depression (both categorical depression diagnoses and dimensional symptom levels) would predict an increased evening preference after controlling for pubertal status. Further, we analyzed whether pubertal status moderated the effect of depression upon chronotype such that the influence of earlier depression would be greatest for postpubertal youth. The shift toward evening preference appears to occur predominantly after the onset of puberty (Roenneberg et al., 2004; Carskadon et al., 1993), thus the possibility of an effect among prepubertal youth is unlikely. Second, we addressed whether a greater preference toward eveningness would predict future increases in depression (diagnoses or symptoms). To examine these questions, the current study used data from a longitudinal study of 3rd, 6th, and 9th graders from the general community who were followed for 4 years with repeated measures of depression (see Figure 1 for study design and alignment to two main study questions). Chronotype was assessed only at 36 months post-baseline, when participants were in 6th, 9th and 12th grades. An additional follow-up of depression occurred one year after chronotype was measured (48 months post-baseline). This prospective longitudinal design allows for an examination of both depression and individual differences in chronotype during adolescence, a developmental period in which chronotype is experiencing the most change and depression rates accelerate.
Figure 1.
The accelerated longitudinal design allowed for examination of our two questions developmentally. Question 1 examined depression symptoms and diagnosis measured prospectively across 3-years (blue section) to predict individual differences in chronotype. Question 2 examined the influence of chronotype upon later depression (orange section). Average age of pubertal onset (Patton & Viner, 2007) is indicated on the graph to show when the sample typically transitions.
Methods
Participants
Participants were recruited by letters mailed to families within the broader Denver area public schools with a child in 3rd, 6th or 9th grade (Hankin et al., 2015). Youth and a caretaker were then followed with repeated measures at regular intervals for 48 months (Figure 1). The original sample consisted of 360 youth who were 8–16 years old at the baseline assessment (M=12.06, SD=2.35) and one caregiver (mothers=85.5%). At the time that chronotype was assessed (36-months post-baseline), the sample with data for analysis of chronotype, depression, and pubertal status consisted of 255 youth who were 11–19 years old (M=15.03, SD=2.31). Participants were 56.5% female. For ethnicity, 11% reported being Hispanic/Latino; for race, youth indicated the following: 82% White, 5% African American, 4% Asian, 1% American Indian and 8% more than one race. Hollingshead 4 factor index for SES (Hollingshead, 1975) average was 48.65 (SD = 11.26), and 18% reported receiving free/reduced lunch. Youth who did not provide data on chronotype at 36-months did not differ from those with complete data on any baseline variable: gender (t=0.989, p=0.323), age (t=−1.29, p=0.198) or depression scores (t=−1.629, p=0.104). Of the 255 participants with complete data at 36-months, 185 completed a 1-year follow-up after chronotype assessment (4-years post-baseline). Youth who completed this follow-up at 4-years were not significantly different from those who completed the 36-month assessment in terms of gender (t=0.149, p=0.881), age (t=1.426, p=0.155), depression scores (t=−.169, p=0.866), or chronotype (t=−1.866, p=0.063). As there were no significant differences due to patterns of missing data at the different measurement waves, Full Information Maximum Likelihood (FIML) was used to address missing data and provide more accurate, nonbiased, robust statistical estimates for data analysis (Graham, 2009).
Procedure
Figure 1 illustrates study timeline and when assessments were obtained. Participants completed the Children’s Depression Inventory (CDI) and were interviewed via the Schedule for Affective Disorders and Schizophrenia in School-Aged Children (KSADS) at baseline. The CDI was given at follow-up assessments occurring every 3 months after the baseline visit for the following three years. Diagnostic data on youth experiencing an episode of depression was obtained via KSADS at regular 6-month intervals over the three-year period (Figure 1). At the 36-month follow-up, chronotype and pubertal status were assessed via child-report. Approximately 12 months later (48 months post-baseline), CDI and KSADS again were obtained. This resulted in 14 total CDI, and 8 KSADS assessments of depression, thus making for a rigorous longitudinal study with reliable, accurate assessment of depression over time.
Parents provided informed consent, and youth provided assent. The Institutional Review Board at the University of Denver approved all procedures. Youth and parents were reimbursed for participation (Hankin et al., 2015).
Measures
Depression symptoms were assessed with the Children’s Depression Inventory (CDI, Kovacs, 1992). The CDI is a widely-used questionnaire to measure self-reported depression symptoms in children and adolescents. Youth were asked to respond to one of three statements for each item (e.g., 0-I am sad once in a while to 2-I am sad all the time), with higher scores indicating greater levels of depression symptoms and a theoretical minimum of 0 and a maximum of 54. The CDI is reliable and valid (Klein, Dougherty, & Olino, 2005). Internal consistency was good (α = .79 to .90 across all time points). The CDI assessments were averaged across 13 timepoints (T1 to 36-month post-baseline) to create an overall score of depression severity over 3 years, used to predict individual differences in chronotype (at 36 month post-baseline). The 48-month CDI assessment was the sum of the items for the single timepoint being predicted by chronotype (at 36 month post-baseline).
Depression diagnoses across the study were assessed via the Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997. The K-SADS-PL is a widely used semi-structured interview used to assess past and present diagnoses of depression disorder among youths according to Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; 2000). Youth and a parent were interviewed individually and separately. Best estimate practices, which have been shown to be a reliable and valid approach for integrating data (Klein et al., 2005), were used to combine reports between parent and child in order to arrive at youth-diagnoses of depressive episodes. Interrater reliability for the KSADS was good (κ = .91). All K-SADS-PL data were dichotomized to indicate the presence/absence of an episode of depression over 7 timepoints (baseline to 36-month) to create an overall depression variable representing prospective onset of depressive episode over the 3 years. At the 48 month timepoint, KSADS data was dichotomized to indicate the presence/absence of an episode of depression for the single assessment representing the epoch elapsed from 36 to 48 months.
Chronotype was measured with the Morningness/Eveningness Scale in Children (MESC, Carskadon et al., 1993). The MESC assessed chronotype with items about youth preference towards morning or evening through ratings of the preferred timing of events and how they may perform if activities were scheduled at specific times. Children were posed a scenario and they have to identify the statement that best fits them and are scored on a 1 – 4 or 5 scale (e.g., “Gym class is set for 7:00 in the morning. How do you think you’ll do? Answer choices: “My best!”, “Okay”, “Worse than usual”, “Awful!”). Higher scores indicate a greater preference for morning with a theoretical minimum of 10 and maximum of 43. Previous research has suggested cutoffs of ≤23 for evening-type, and ≥30 for morning-type (Díaz-Morales & Sorroche, 2008). The MESC has good reliability and validity (Carskadon, Mancuso, & Rosekind, 1989; Giannotti et al., 2002). Internal consistency in this study was acceptable (α = 0.79). The current study assessed chronotype once at the 36-month timepoint when youth were in 6th, 9th, and 12th grades.
Pubertal status was measured with the Pubertal Development Scale (PDS, Petersen, Crockett, Richards, & Boxer, 1988). The PDS is a commonly used questionnaire to determine pubertal status within youth. Children are asked about various aspects of physical development and told to pick the answer that best describes them (e.g., Would you say your height: Answer choices: “Has not begun to increase yet”, “Has just started to increase”, “Has been increasing for a while”, “Seems to have reached its maximum”) with some items pertaining to specific sexes (i.e., breast growth, menses, facial hair). We followed standard PDS scoring to create prepubertal and postpubertal groups separately for girls and boys. Reliability and validity of the PDS is high (Petersen et al., 1988; Shirtcliff, Dahl, & Pollak, 2009). Internal consistency in this study was acceptable for both boys (α = 0.80) and girls (α = 0.84). The current study assessed pubertal status of the participants at the 36-months follow-up when youth were in 6th, 9th, and 12th grades.
Data Analysis Plan
To assess question 1, we used linear regression analyses, implemented in R (Team, 2014), to examine the prospective relationship between earlier depression and chronotype while controlling for gender, age and pubertal status. We then investigated the interaction between depression and pubertal status in prospectively predicting chronotype by conducting a linear regression with CDI-based dimensional depression symptoms and a 2×2 ANOVA for KSADS depression diagnoses, with gender and age as covariates. For question 2, separate regression analyses were used to examine chronotype predicting later CDI (linear regression) and depression diagnosis (logistic regression) after controlling for prior depression to enable prospective prediction of depression at 48 months as a function of earlier chronotype (at 36months).
Results
Preliminary analyses
Table 1 reports descriptive statistics for demographic information, chronotype and depression measurements for the overall sample, by gender and grade. The mean for chronotype in this sample (M=26.87, SD=5.38) fell within established norms (Carskadon et al., 1993) and was consistent with an intermediate-type. The majority of youth were identified as an intermediate-type (approximately 79%), with 11% being considered evening-type. T-tests showed no significant gender difference for chronotype. Consistent with past work, postpubertal participants showed significantly greater preference towards evening (t=3.54, d=0.46, p<.001). There was a significant gender difference for the CDI, with girls reporting higher depression symptoms (M=4.50) compared to boys (M=3.49; t=2.3, d=.30 p=.022). Over the entire duration of the study, 39.7% of participants experienced at least one episode of depression with girls displaying a marginally significant higher rate of depression diagnoses (X2(1)=3.034, p=.082). Correlations are reported in Table 2. Chronotype was moderately correlated with puberty, age, and depression (measured as a mean score from baseline to 36 months, and as a separate follow-up at 48-months), such that eveningness was associated with postpubertal, older participants and higher levels of depression.
Table 1.
Means, Standard Deviations and t-scores
| Gender | Pubertal Status | Chronotype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Variable | Male (n=111) | Female (n=144) |
t- score |
df | p- value |
Prepubertal (n=100) |
Postpubertal (n=148) |
t- score |
df | p- value |
Morning Type |
Intermediate Type |
Evening Type |
||||
|
|
|
||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||||
|
| |||||||||||||||||
| Chronotype | 27.42 | 5.11 | 26.45 | 5.57 | −1.43 | 253 | 0.153 | 28.13 | 5.13 | 25.74 | 5.28 | 3.54 | 246 | <.001 | - | - | - |
| Age | 14.89 | 2.28 | 15.14 | 2.33 | 0.85 | 253 | 0.398 | 13.74 | 1.89 | 15.96 | 2.14 | −8.38 | 246 | <.001 | 14.12 | 15.2 | 15.54 |
| Average CDI across 36mo | 3.49 | 2.79 | 4.5 | 3.92 | 2.3 | 253 | 0.022 | 3.33 | 2.6 | 4.61 | 3.98 | −2.84 | 246 | 0.005 | 2.87 | 3.83 | 6.58 |
| CDI at 48mo | 3.4 | 4.12 | 4.57 | 6.53 | 1.41 | 183 | 0.003 | 3.34 | 4.09 | 4.59 | 6.3 | −1.49 | 178 | 0.001 | 3.26 | 3.3 | 6.66 |
Chronotype (low score – Evening Preference)
Age at 36-month timepoint
CDI (Children’s Depression Inventory; low score – low depression symptoms)
Table 2.
Correlation Table
| Chronotype at 36mo | Puberty at 36mo | History of Depression across 36mo | Average CDI across 36mo | Depressive Episode at 48mo | CDI at 48mo | Gender | Age at 36mo | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Chronotype at 36mo | 1 | −0.22** | −0.20** | −0.36** | −0.24** | −0.36** | 0.09 | −0.23** |
|
|
||||||||
| Puberty at 36mo | 1 | 0.20** | 0.17** | 0.20** | 0.11 | −0.61** | 0.47** | |
|
|
||||||||
| History of Depression across 36mo | 1 | 0.47** | 0.44** | 0.38** | .20 | 0.29** | ||
|
|
||||||||
| Average CDI across 36mo | 1 | 0.32** | 0.66** | −0.11 | 0.24** | |||
|
|
||||||||
| Depressive Episode at 48mo | 1 | 0.36** | −0.09 | 0.28** | ||||
|
|
||||||||
| CDI at 48mo | 1 | −0.1 | 0.07 | |||||
|
|
||||||||
| Gender | 1 | −0.05 | ||||||
|
|
||||||||
| Age at 36mo | 1 | |||||||
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Chronotype (low score – Evening Preference)
Pubertal Status (0 – Prepubertal; 1 – Postpubertal)
History of Depression (0 – No history of depression; 1 – History of depression)
CDI (Children’s Depression Inventory; low score – low depression symptoms
Gender (0 – Female; 1 – Male)
Predicting Later Chronotype from Earlier Depression
Depression symptoms exhibited a significant effect upon chronotype such that individuals with higher CDI scores showed a greater preference towards evening (β=−.347, p<.001, SE=.08), when controlling for age, gender and pubertal status. Next, we conducted a linear regression analysis with MESC scores as the dependent variable, earlier depression diagnosis as predictor, with pubertal status, age and gender as covariates. We found a significant effect of depression diagnosis upon chronotype such that those who had experienced an episode of depression over the 3 years exhibited a greater evening preference (β=−.13, p=.045, SE=.71). Upon including depression (CDI or diagnosis) in the regression, the influence of pubertal status is no longer significant. These regression analyses show that earlier depression (both symptoms and diagnosis) predicts chronotype even after controlling for pubertal status, age and gender.
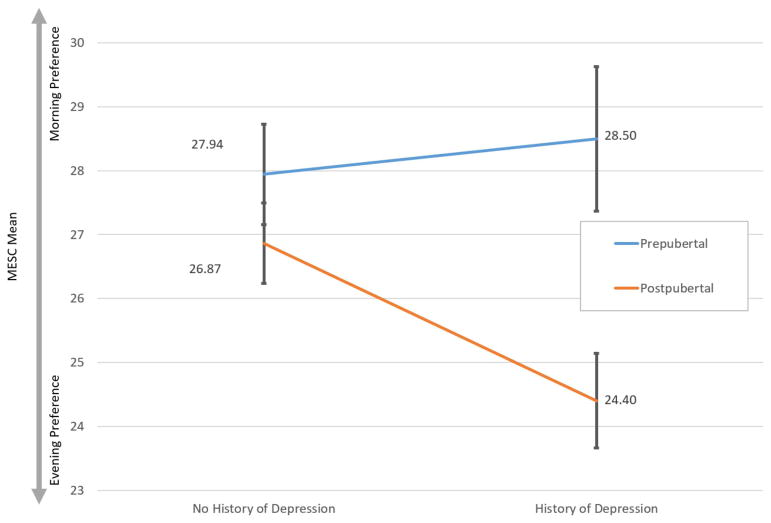
We further investigated whether depression and pubertal status interacted to predict individual differences in chronotype. ANOVA revealed a significant interaction between depression diagnosis and pubertal status (F(1,243)=4.171, p=.045) such that the effect of depression upon chronotype was moderated by pubertal status (Figure 2). Follow-up analyses showed that prepubescent youth show similar chronotype preferences regardless of history of depression. However, postpubertal youth with a history of depression exhibit significantly greater evening preference compared to postpubertal adolescents with no depression history (t=3.271, p=.002) (Figure 3). The regression for CDI scores interacting with pubertal status was not significant (β= −0.039, p=.85, SE=.215). Regression coefficients are reported in Table 3.
Figure 2.
The effect of depression upon chronotype is moderated by pubertal status such that postpubertal youth with a history of depression show a greater preference towards eveningness. MESC – Morningness/Eveningness Scale in Children
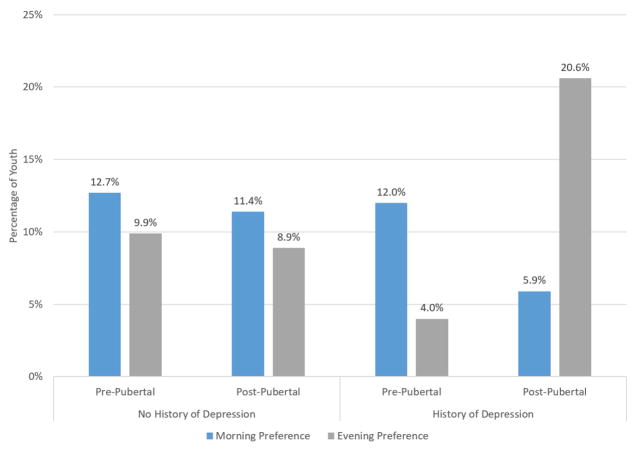
Figure 3.
The percentage of youth categorized as having an evening preference increases two-fold when considering both history of depression and post-pubertal status. Intermediate type omitted from visualization to allow for proper scaling between morning and evening preferences.
Table 3.
Regression Coefficients
| Variable | Average CDI Predicting Chronotype | History of Depression Predicting Chronotype | CDI/Puberty Interaction | History of Depression/Puberty Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B | SE B | β | B | SE B | β | B | SE B | β | Mean Square | Df | F | |
| Puberty at 36mo | −1.72 | 0.994 | −0.159 | −1.956 | 1.042 | −0.178 | −1.518 | 1.277 | −0.14 | 153.03 | 1 | 5.73* |
| Gender | −0.721 | 0.866 | −0.067 | −0.778 | 0.913 | −0.072 | −0.727 | 0.868 | −0.068 | 28.17 | 1 | 1.059 |
| Age at 36mo | −0.145 | 0.173 | −0.063 | −0.234 | 0.181 | −0.1 | −0.151 | 0.175 | −0.065 | 42.71 | 1 | 1.606 |
| Averaged CDI across 36mo | −0.454 | 0.0861 | −0.327** | - | - | - | −0.414 | 0.179 | −0.298* | - | - | - |
| History of Depression Across 36mo | - | - | - | −1.448 | 0.718 | −0.131* | - | - | - | 42.09 | 1 | 1.56 |
| CDI/Puberty Interaction | - | - | - | - | - | - | −0.054 | 0.215 | −0.039 | - | - | - |
| History of Depression/Puberty Interaction | - | - | - | - | - | - | - | - | - | 104.83 | 1 | 3.93* |
p<.05,
p<.01
Predicting Prospective Depression from Earlier Chronotype
Our second question concerned whether eveningness would predict prospective changes in depression. First, we conducted a logistic regression analysis, with pubertal status and history of depression diagnosis through the 36 months of the study as covariates, to predict onset of clinical depression one year later (at 48 month KSADS evaluation) based on individual differences in chronotype (assessed at the 36-month follow-up). Chronotype significantly predicted future onset of depressive episode (b = −.085, SE = .04, OR = .92, Wald = 4.28, p = .03) even after controlling for prior clinical depression, such that individuals with an evening preference were more likely to have a future depressive episode. Next, we conducted a linear regression analysis with CDI scores (48-month follow-up) as the dependent variable and individual differences in chronotype as predictor with pubertal status as covariate. Chronotype (a more evening preference) predicted significant prospective elevations of depression symptoms one year later (β = −.16, t = −2.10, p = .03), even after controlling for earlier symptoms of depression and pubertal status. Overall, these results show that earlier chronotype predicts later depression prospectively assessed one-year later, such that adolescents with a more evening preference were significantly more likely to receive a depression diagnosis episode and exhibit dimensionally higher depressive symptoms.
Exploratory Analyses
The above results show that average dimensional depression scores (CDI averaged over the 3 years) predicted individual differences in chronotype. Still, with our intensive repeated measures design with 13 CDI measures, it is possible that growth trajectories in depressive symptoms predict later individual differences in chronotype. To address this exploratory analysis, we first conducted latent growth curve modeling to ascertain the best fitting longitudinal growth model of CDI scores measured repeatedly across the 3 years. After establishing depression growth, we conducted analyses that related individual differences in chronotype with both intercept (mean levels) and slope (rate of longitudinal growth) of CDI. Significant associations between chronotype and CDI score intercept would show relations for average levels of depressive symptoms, whereas relations between chronotype and depression slope would suggest that trajectories of earlier depressive symptoms are associated with later individual differences in chronotype. Growth curve analyses were conducted using Structural Equation Modeling (SEM), implemented in R, and we specified linear and quadratic models. Standard SEM model fit indices were used to determine which growth model most accurately represented the data. We fit these models to the CDI data obtained from waves 3–33 months to obtain a longitudinal growth model that did not contain any overlapping timepoints with other predictor variables of interest (e.g., chronotype at 36 months).
The linear growth model provided the best fit to the data (CFI=.934, RMSEA=0.073)1 . There was significant variance in both intercept (16.06, p < .001) and linear slope (0.016, p < .001). SEM analyses that regressed individual differences in chronotype at 36 months onto the latent intercept and slope showed that only the intercept was significantly related to chronotype, such that youth with higher mean CDI scores showed a preference for eveningness (β = −.357; p<.001). These findings confirm our initial approach and further show that earlier average levels of depression significantly predict later individual differences in chronotype. Longitudinal trajectories of depressive symptoms did not relate to chronotype after controlling for average depression levels.
Discussion
Youth chronotype, as an indicator of biological rhythms, is important as it has links to mental and physical health problems (Urbán et al., 2011; Fleig & Randler, 2009; Johnson, Chilcoat, & Breslau, 2000; R. Roberts, Lewinsohn, & Seeley, 1995; Aronen, Paavonen, Fjallberg, Soinninen, & Torronen, 2000; Gaultney, Terrell, & Gingras, 2005; Kahn, Van de Merckt, Rebuffat, & Mozin, 1989; Owens, Spirito, & McGuinn, 2000; Gregory, 2002). But little is known about reciprocal effects between depression and chronotype development during the critical developmental period spanning childhood to later adolescence. Results from this longitudinal study showed that high levels of depression across 3-years, including both dimensional symptoms as well as a history of depression diagnosis, predicted a greater preference towards eveningness above and beyond the effect of pubertal status. Moreover, postpubertal youth with a history of depression diagnosis showed a greater shift towards evening preference when compared to prepubertal youth without a history of depression. Finally, youth exhibiting a more evening preference were significantly more likely to become depressed (both diagnosis and dimensional symptom scores) one year later, even after controlling for prior depression.
The National Sleep Foundation (2015) recommends that adolescents sleep between 8 and 10 hours, but only 15% report reaching 8.5 hours on weekdays. Among this age group, inadequate nocturnal sleep has been associated with decreased self-esteem, academic functioning and overall mental health (Fredriksen, Rhodes, Reddy, & Way, 2004; R. Roberts, Roberts, & Duong, 2009). This chronic sleep deficiency is in part due to increased academic demands and early school start times (Carskadon, Wolfson, & Acebo, 1998). Adolescents who hold an evening preference tend to go to bed later (Randler, Bilger, & Díaz-Morales, 2009) and are often forced to wake up early to attend school, and as a result they are even less likely to get the recommended 8 to 10 hours of nightly sleep, and thus experience excessive daytime fatigue (Taillard, Philip, & Bioulac, 1999). An evening phase preference often forces a mismatch between sleep and wake cycles to daily demands, such as school, and may place youth at heightened risk for negative outcomes, including poor academic functioning and depressive states (Vollmer, Schaal, Hummel, & Randler, 2011).
Specific results from this longitudinal study extend knowledge on the developmental interplay between chronotype and the development of depression. Consistent with past work, youth reporting a preference for eveningness were significantly more likely to exhibit later depression (Drennan et al., 1991; Kitamura et al., 2010; Hidalgo et al., 2009; Merikanto et al., 2013; Chelminski et al., 1999; Alvaro et al., 2014; Pabst et al., 2009). However, previous work has been done using cross-sectional methodology, whereas the current study used a longitudinal design that assessed depression again one year after measuring chronotype and after controlling for past depression history. Moreover, a history of earlier depression predicted individual differences in adolescents’ chronotype, such that youth who had experienced a history of depression were more likely to exhibit an evening preference. Congruent with previous work, we found the majority of youth fell within the intermediate range for chronotype preference. Importantly, though, the percentage of youth with an evening preference doubled when comparing post-pubertal adolescents with a history of depression relative to all the other groups (evening preference from ~10% to 20%) (Figure 3). This shows that the development and progression of the shift in chronotype during adolescence may be more complex than previously understood. Depression history may play an influential role in a larger shifting system of youths’ chronotype.
Taken together, these findings are consistent with an interdependent, reciprocal process between youths’ chronotype and depression, in which higher rates of depression influence individual differences in chronotype, resulting in an overall elevation of depression symptoms and the endurance of the cycle. While presently the mechanisms underlying the chronotype shift are unknown, one possibility suggested by these results is that depression-related emotions and behaviors may play a role in altering circadian and sleep processes. Because the circadian rhythm becomes entrained to the external light/dark schedule through timing of particular environmental cues (e.g., exposure to light patterns, timing of meals, changing temperature, social interactions), resulting in a preference towards morning or evening (Roenneberg, Daan, & Merrow, 2003a), elevated levels of depression may result in the alteration of such cues. It has been shown that exposure to light is the strongest regulator of circadian rhythms (Wirz-Justice, 2006), and the timing of such exposure may be altered while experiencing depression. For example, individuals experiencing depression symptoms, such as experiencing low levels of energy, will spend more time indoors with less exposure to natural light. In a study of 100 children and adolescents, Armitage et al. (2004) monitored activity and light exposure levels for five consecutive days and found that depressed adolescents obtained less light exposure, spent less time in bright light, and had lower activity levels than healthy controls. Such lack of exposure can delay circadian rhythms and promote an evening chronotype (Wirz-Justice, 2006).
Further research has examined the influence of non-photic cues such as the timing of particular activities (Mistlberger & Skene, 2004) and has suggested that it is difficult to disentangle such cues from the influence of light due to the sensitivity to light in humans. It may be the case that such cues play a role in altering/regulating the exposure to light (through masking) rather than acting as strict zeitgebers themselves (Mistlberger & Skene, 2004). Keeping this in mind, it may be the case that depressive symptoms, such as social withdrawal and changes in appetite and eating, will influence the timing of light exposure (e.g., refusing to go outside to eat with colleagues and isolating oneself inside)(Stetler, Dickerson, & Miller, 2004), ultimately altering the overall rhythm (towards an evening preference), resulting in further delay, disruption and overall decrease of sleep periods (Szuba, Yager, Guze, Allen, & Baxter, 1992). Another potential mechanism in which depression may alter chronotype pertains to core body temperature. Individuals experiencing a depressive episode have demonstrated an increase in the amount of variability of their circadian temperature phase when compared to controls (Rausch et al., 2003). Declining core body temperature has been shown to be associated with sleep initiation (Zulley, Wever, Aschoff, 1981; Campbell and Broughton, 1994), thus variability in circadian patterns of temperature could play a role in disrupting or delaying sleep initiation and altering chronotype. Further research examining these complex relationships is clearly needed.
This study has various strengths and weaknesses. Regarding strengths, the age of the current sample aligns with the development of the shift in chronotype (Danesi & Natale, 2002; Randler et al., 2009); along with an increase in experiencing depression (Hankin et al., 1998). We also used repeated measurement of depression symptoms and diagnoses over an extended period of time throughout adolescence to establish reliable and valid depression levels, including both symptoms and diagnosis. A limitation is that chronotype was only measured at a single time-point, so we could not investigate prospective change in chronotype. Future studies should implement repeated measurements of chronotype so that overall change in chronotype and its relationship to changing depressive symptoms can be examined.
Our findings suggest a cyclical interplay between chronotype and depression and that it may be important to not only monitor post-pubertal youth for depressive symptoms, but also to inquire about the timing of their sleep and activity patterns. Adolescents with strong eveningness tendencies may be at heightened risk for the emergence of depressive symptoms, and should be monitored carefully. Helping youth establish regular sleep-wake patterns that are compatible with their academic and social commitments may confer benefits across numerous domains.
Acknowledgments
Funding Source: All phases of this study and writing were supported by NIMH grants: R01-MH 077195, 1R01MH105501, 1R21MH102210 and 1K23MH108640
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Contributors’ Statements:
Dustin Haraden: Mr. Haraden conceptualized the study, designed the analyses, drafted the manuscript, and approved the final manuscript as submitted.
Benjamin Mullin: Dr. Mullin aided in the conceptualization of the results and the overall impact, critically reviewed the manuscript, and approved the final manuscript as submitted.
Benjamin Hankin: Dr. Hankin conceptualized the study, designed the larger data collection study and coordinated and supervised data collection, designed the analyses, critically reviewed the manuscript, and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.) Washington, DC: 2000. [Google Scholar]
- 2.Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian Typology: A Comprehensive Review. Chronobiology International. 2012;29(9):1153. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- 3.Alvaro PK, Roberts RM, Harris JK. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Medicine. 2014;15(8):934. doi: 10.1016/j.sleep.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BWJH. Chronotype associations with depression and anxiety disorders in a large cohort study. Depression and Anxiety. 2015;33(1):75. doi: 10.1002/da.22422. [DOI] [PubMed] [Google Scholar]
- 5.Armitage R, Hoffmann R, Emslie G, Rintelman J, Moore J, Lewis K. Rest-activity cycles in childhood and adolescent depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(6):761–769. doi: 10.1097/01.chi.0000122731.72597.4e. [DOI] [PubMed] [Google Scholar]
- 6.Aronen E, Paavonen EJ, Fjallberg M, Soinninen M, Torronen J. Sleep and psychiatric symptoms in school-age children. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;6(4):289. doi: 10.1046/j.1467-0658.2000.00093-19.x. [DOI] [PubMed] [Google Scholar]
- 7.Borbély AA. A two process model of sleep regulation. Human Neurobiology. 1982 [PubMed] [Google Scholar]
- 8.Campbell SS, Broughton RJ. Rapid decline in body temperature before sleep: fluffing the physiological pillow? Chronobiology international. 1994;11(2):126–131. doi: 10.3109/07420529409055899. [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA, Mancuso J, Rosekind MR. Impact of part-time employment on adolescent sleep patterns. Sleep Res 1989 [Google Scholar]
- 10.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993 doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 11.Carskadon MA, Wolfson AR, Acebo C. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. SLEEP-NEW …. 1998 doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 12.Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness–morningness” dimension in ‘depressive’ college students. Journal of Affective Disorders. 1999;52(1–3):19. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 13.Danesi E, Natale V. Gender and Circadian Typology. Biological Rhythm Research. 2002;33(3):261. doi: 10.1076/brhm.33.3.261.8261. [DOI] [Google Scholar]
- 14.Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disorders. 1991;23(2):93. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- 15.Duffy JF, Dijk D-J, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. Journal of Investigative Medicine. 1999;47(3) [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Morales JF, Sorroche MG. Morningness-Eveningness in Adolescents. The Spanish Journal of Psychology. 2008;11(01):201. doi: 10.1017/s1138741600004248. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms: a unified approach to understanding the etiology of depression. Archives of General Psychiatry. 1988;45(10):948. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- 18.Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eating Behaviors. 2009;10(2):115. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: Tracking the Effects of Adolescent Sleep Loss During the Middle School Years. Child Development. 2004;75(1):84. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- 20.Gaultney JF, Terrell DF, Gingras JL. Parent-Reported Periodic Limb Movement, Sleep Disordered Breathing, Bedtime Resistance Behaviors, and ADHD. Behavioral Sleep Medicine. 2005;3(1):32. doi: 10.1207/s15402010bsm0301_5. [DOI] [PubMed] [Google Scholar]
- 21.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 22.Graham JW. Missing Data Analysis: Making It Work in the Real World. Annual Review of Psychology. 2009;60(1):549. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 23.Gregory GG. A Two-State Stochastic Model of REM Sleep Architecture in the Rat. Journal of Neurophysiology. 2002;88(5):2589. doi: 10.1152/jn.00861.2001. [DOI] [PubMed] [Google Scholar]
- 24.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107(1):128. doi: 10.1037/0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 25.Hankin BL, Young JF, Abela JRZ, Smolen A, Jenness JL, Gulley LD, … Oppenheimer CW. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. Journal of Abnormal Psychology. 2015;124(4):803. doi: 10.1037/abn0000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves MLF. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry and Clinical Neurosciences. 2009;63(3):283. doi: 10.1111/j.1440-1819.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead AB. Unpublished manuscript. Yale University; New Haven, CT: 1975. Four factor index of social status. [Google Scholar]
- 28.Johnson EO, Chilcoat HD, Breslau N. Trouble sleeping and anxiety/depression in childhood. Psychiatry Research. 2000;94(2):93. doi: 10.1016/s0165-1781(00)00145-1. [DOI] [PubMed] [Google Scholar]
- 29.Kahn A, Van de Merckt C, Rebuffat E, Mozin MJ, Sottiaux M, Blum D, Hennart P. Sleep problems in healthy preadolescents. Pediatrics. 1989 [PubMed] [Google Scholar]
- 30.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Dueker GL, Hasher L, Goldstein D. Children’s time of day preference: age, gender and ethnic differences. Personality and Individual Differences. 2002;33(7):1083. doi: 10.1016/s0191-8869(01)00214-8. [DOI] [Google Scholar]
- 32.Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, … Mishima K. Evening Preference Is Related To The Incidence Of Depressive States Independent Of Sleep-Wake Conditions. Chronobiology International. 2010;27(9–10):1797. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- 33.Klein DN, Dougherty LR, Olino TM. Toward Guidelines for Evidence-Based Assessment of Depression in Children and Adolescents. Journal of Clinical Child & Adolescent Psychology. 2005;34(3):412. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs M. Children’s depression inventory: Manual. Multi-Health Systems 1992 [Google Scholar]
- 35.Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2010 doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merikanto I, Lahti T, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, … Partonen T. Evening Types Are Prone To Depression. Chronobiology International. 2013;30(5):719. doi: 10.3109/07420528.2013.784770. [DOI] [PubMed] [Google Scholar]
- 37.Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biological Reviews. 2004;79(3):533. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- 38.Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, Poulton R. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine. 2010;40(06):899. doi: 10.1017/s0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mooney A. Morningness-eveningness and adolescent mental health: a prospective longitudinal study. 2015 (Doctoral dissertation, The University of Melbourne Australia) [Google Scholar]
- 40.Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental & Behavioral Pediatrics. 2000;21(1):27–34. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Pabst SR, Negriff S, Dorn LD, Susman EJ, Bin Huang. Depression and Anxiety in Adolescent Females: The Impact of Sleep Preference and Body Mass Index. Journal of Adolescent Health. 2009;44(6):554. doi: 10.1016/j.jadohealth.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patton GC, Viner R. Pubertal transitions in health. The Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- 43.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117. doi: 10.1007/bf01537962. [DOI] [PubMed] [Google Scholar]
- 44.Randler C, Bilger S, Díaz-Morales JF. Associations among Sleep, Chronotype, Parental Monitoring, and Pubertal Development among German Adolescents. The Journal of Psychology. 2009;143(5):509. doi: 10.3200/JRL.143.5.509-520. [DOI] [PubMed] [Google Scholar]
- 45.Rausch JL, Johnson ME, Corley KM, Hobby HM, Shendarkar N, Fei Y, … Leibach FH. Depressed Patients Have Higher Body Temperature: 5-HT Transporter Long Promoter Region Effects. Neuropsychobiology. 2003;47(3):120. doi: 10.1159/000070579. [DOI] [PubMed] [Google Scholar]
- 46.Roberts R, Lewinsohn P, Seeley J. Symptoms of DSM-III-R Major Depression in Adolescence: Evidence from an Epidemiological Survey. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34(12):1608. doi: 10.1097/00004583-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Roberts R, Roberts CR, Duong HT. Sleepless in adolescence: Prospective data on sleep deprivation, health and functioning. Journal of Adolescence. 2009;32(5):1045. doi: 10.1016/j.adolescence.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roenneberg T, Merrow M. Entrainment of the Human Circadian Clock. Cold Spring Harbor Symposia on Quantitative Biology. 2007;72(1):293. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- 49.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social Jetlag and Obesity. Current Biology. 2012;22(10):939. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 50.Roenneberg T, Daan S, Merrow M. The art of entrainment. Journal of Biological …. 2003a doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- 51.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Current Biology. 2004;14(24):R1038. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 52.Roenneberg T, Wirz-Justice A, Merrow M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. Journal of Biological Rhythms. 2003b;18(1):80. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 53.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal Development: Correspondence Between Hormonal and Physical Development. Child Development. 2009;80(2):327. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology. 2004;29(10):1250. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Szuba MP, Yager A, Guze BH, Allen EM, Baxter LR. Disruption of social circadian rhythms in major depression: A preliminary report. Psychiatry Research. 1992;42(3):221. doi: 10.1016/0165-1781(92)90114-i. [DOI] [PubMed] [Google Scholar]
- 56.Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. Journal of Sleep Research. 1999;8(4):291. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 57.Team, R. 2014 R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. There is no corresponding record for this reference, 2014. [DOI] [Google Scholar]
- 58.Urbán R, Magyaródi T, Rigó A. Morningness-Eveningness, Chronotypes and Health-Impairing Behaviors in Adolescents. Chronobiology International. 2011;28(3):238. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vollmer C, Schaal S, Hummel E, Randler C. Association Among School-related, Parental and Self-related Problems and Morningness-Eveningness in Adolescents. Stress and Health. 2011;27(5):413. doi: 10.1002/smi.1393. [DOI] [Google Scholar]
- 60.Wehr T, Wirz-Justice A, Goodwin F, Duncan W, Gillin J. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- 61.Wirz-Justice A. Biological rhythm disturbances in mood disorders. International Clinical Psychopharmacology. 2006;21(Supplement 1):S11. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 62.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflügers Archiv European Journal of Physiology. 1981;(4):314–318. doi: 10.1007/BF00581514. 0031-6768/81/0391/0314. [DOI] [PubMed] [Google Scholar]