Abstract
Introduction
Parkinson’s disease (PD) is characterized by bradykinesia, rigidity, postural instability and tremor. Several pathologic processes can produce this syndrome, but neurodegeneration accompanied by neuronal inclusions composed of α-synuclein (Lewy bodies) is considered the typical pathologic correlate of PD.
Methods
The neuropathologic features of PD are reviewed based upon personal experience and review of the literature. Molecular pathology of PD is summarized from cell biological and animal studies.
Results
The pathologic feature that correlates with signs and symptoms of PD is neuronal loss in the substantia nigra with dopaminergic denervation of the striatum. Neuronal degeneration in the substantia nigra preferentially affects the ventrolateral cell group that projects to posterolateral putamen and is accompanied by formation of Lewy bodies composed of aggregated α-synuclein. Some patients with PD are found at autopsy to have other pathologic processes, such as multiple system atrophy, progressive supranuclear palsy and cerebrovascular disease (vascular Parkinsonism). The peripheral autonomic nervous system is also affected. The triggering event in PD is unknown, but recent studies suggest a role for loss of nuclear membrane integrity. Once α-synuclein aggregates forms, evidence supports cell-to-cell propagation.
Conclusion
PD is a multisystem synucleinopathy caused by poorly characterized genetic and environmental factors that produces degeneration in selectively vulnerable neuronal populations.
Keywords: Parkinson disease, neuropathology, α-synuclein, Lewy body
Introduction
Parkinson’s disease (PD) is a progressive neurological disorder characterized by bradykinesia, tremor, rigidity and postural instability. There are a number of disorders that can have some or all of these clinical features, and the clinical syndrome is referred to as “Parkinsonism.” Disorders in which Parkinsonism is a prominent feature are referred to as “Parkinsonian disorders.” Parkinsonian disorders include degenerative vascular, traumatic and toxic etiology (Table 1). In autopsy series the most common neurodegenerative causes of Parkinsonism are α-synucleinopathies (Lewy body disease (LBD) and multiple system atrophy (MSA)) and tauopathies (progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD)). These disorders are defined based upon the predominant protein that accumulates within degenerating neurons and glia. Some of these are considered atypical Parkinsonian disorders because they have other clinical features, such as autonomic dysfunction in MSA, vertical gaze palsy in PSP, and higher order cortical deficits (e.g., apraxia) in CBD. While relatively uncommon, cerebrovascular disease can sometimes be associated with Parkinsonism (“vascular PD” (VaP)), usually a lower body Parkinsonism [1].
Table 1.
Parkinsonian disorders
| Classification | Examples |
|---|---|
| Degenerative parkinsonism | |
| α-Synuclein | Parkinson’s disease, multiple system atrophy |
| Tau | Progressive supranuclear palsy; corticobasal degeneration; Guam Parkinson dementia complex; chronic traumatic encephalopathy |
| TDP-43 | Perry syndrome; frontotemporal lobar degeneration (FTLD-TDP) |
| Nonspecific | Genetic PD (PINK1, PRKN, POLG1, some forms of LRRK2) |
| Non-degenerative parkinsonism | |
| Vascular | Vascular parkinsonism |
| Toxic | MPTP; manganese poisoning |
| Drug-induced | Antipsychotic medications |
| Infectious | Influenza virus (post-encephalitic parkinsonism) |
Parkinsonian disorders classified by major etiologic factor
Common features of disorders presenting as PD
Degenerative parkinsonian disorders can be inherited or sporadic, but they all have selective loss of dopaminergic neurons in the substantia nigra that project to the basal ganglia. Within the substantia nigra, the ventrolateral cell groups (i.e. A9 or nigrostriatal pathway) are most vulnerable, while dorsal and medial cell groups (i.e. A10 or mesolimbic pathway) are more resistant [2]. The biological basis for selective vulnerability of dopaminergic neurons may reside in pacemaker-like properties of these cells, leading to frequent intracellular calcium transients [3]. Calcium buffering may be relatively deficient in A9 neurons compared with A10 neurons [4], leading to cellular stress and eventual disruption of cellular homeostasis. Cell death is associated with disruption of nuclear membrane integrity and release of proaggregant nuclear factors [5], such as histones [6], that may trigger α-synuclein aggregation. Once aggregation begins, it may subsequently spread to other cells by direct or indirect means. Propagation of abnormal forms of α-synuclein can be modeled in cellular [7] and animal systems [8], and it is the most popular hypothesis to explain the progressive involvement of select neuronal systems in PD [9].
Clinicopathologic findings and frequency of disorders presenting as PD
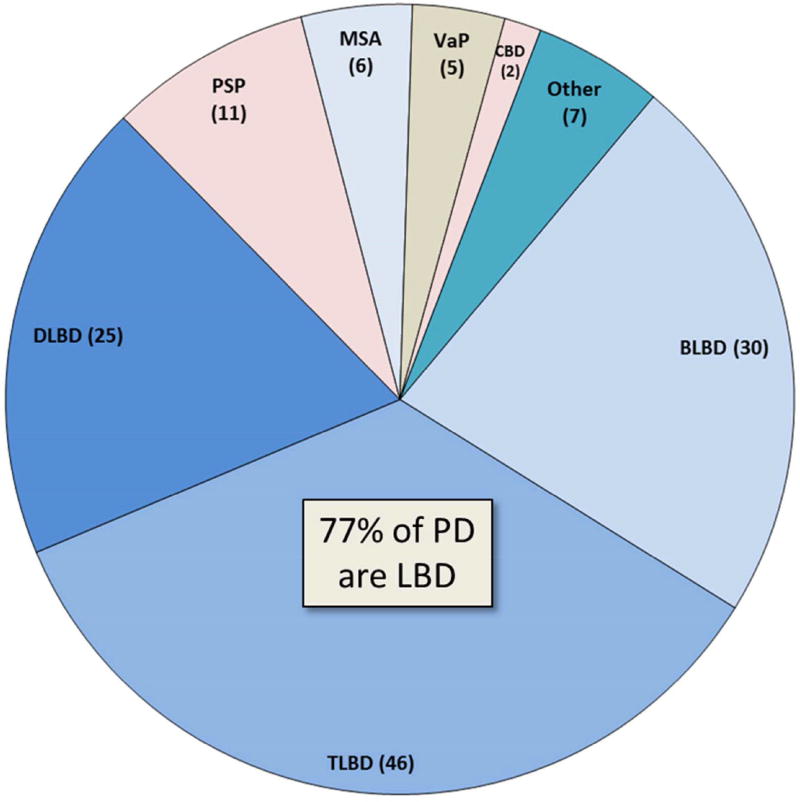
In the Mayo Clinic brain bank of 132 patients with antemortem diagnosis of PD, without dementia, the most common pathologic finding was LBD (77%) (Figure 1) and 81% had α-synucleinopathy (LBD + MSA). Only 10% had tauopathy (PSP + CBD); 4% had VaP. PSP is most often clearly differentiated from PD, and the diagnostic accuracy of PSP in the Mayo Clinic brain bank, which houses over 1200 pathologically confirmed PSP brains, is over 80% [10]. While PSP has neuronal loss and degeneration in the substantia nigra as in PD, it is not associated with α-synuclein pathology, but rather tau pathology, with globose neurofibrillary tangles rather than Lewy bodies in affected nigral neurons. The additional involvement of basal ganglia, thalamus, subthalamic nucleus, as well as caudal brainstem and cerebellum in PSP leads to a clinical syndrome clearly different from PD in most cases. The exception is so-called PSP-P [11], in which patients at least early in the course have features of PD, including response to dopamine replacement therapy and asymmetrical Parkinsonism. In PSP-P tau pathology tends to be milder than in typical PSP, and it is associated with disproportionate brainstem compared to diencephalic pathology.
Figure 1. Frequency of neuropathologic disorders with antemortem clinical diagnosis of PD.
The pie chart shows the frequency of pathologic disorders that present with Parkinsonism without dementia in the Mayo Clinic brain bank (n=132). The most common pathology is that of LBD (brainstem predominant (BLBD), transitional (TLBD) or diffuse (DLBD)). The next most frequent disorder is progressive supranuclear palsy (PSP), followed by multiple system atrophy (MSA) and vascular Parkinsonism (VaP). Corticobasal degeneration rarely presents as PD. Other rare causes of PD include nonspecific substantia nigra degeneration associated with basal ganglia pathology or with frontotemporal degeneration (with or without motor neurons disease).
Although MSA is an α-synucleinopathy like PD, it is pathologically distinct – the major pathology is in oligodendroglia rather than neurons. Neuronal inclusions occur, but they do not resemble Lewy bodies, and their distribution is quite different from Lewy bodies in PD [12]. Neuronal inclusions in MSA tend to be most frequent in the putamen, pontine nuclei and inferior olivary nucleus. Inclusions can be detected in the substantia nigra and locus ceruleus, but even here they rarely resemble Lewy bodies. In contrast to PD, where the basal ganglia are morphologically unremarkable (even when there are significant numbers of Lewy neurites), the posterolateral putamen in MSA has macroscopic atrophy and discoloration, and there is severe neuronal loss and gliosis with excessive iron accumulation, especially in MSA-P. These putaminal changes can be detected on antemortem neuroimaging, if it is performed as part of the diagnostic evaluation. MSA-P is the form of MSA presenting primarily as a Parkinsonian disorder, as opposed to cerebellar ataxia (MSA-C). The diagnostic accuracy of pathologically confirmed MSA in the Mayo Clinic brain bank is less than that for PSP (about 70%). Cases with autopsy confirmed MSA are most often misdiagnosed clinically as either PSP (47%) or atypical PD (34%). The most frequent disorder clinically misdiagnosed as MSA, but not associated with MSA pathology at autopsy is PD or dementia with Lewy bodies with significant autonomic dysfunction [13]. As clinicians becoming increasingly aware of the multisystem nature of α-synuclein pathology in PD, including frequent, if not invariable, involvement of both central and peripheral components of the autonomic nervous system, it becomes increasingly important to differentiate PD from MSA by criteria other than only autonomic dysfunction.
Some rare disorders can resemble PD include those with nonspecific substantia nigra degeneration. In the brain bank one such patient carried a LRRK2 mutation. The patient had nonspecific substantia nigra degeneration, without Lewy bodies or tau pathology [14]. Most LRRK2 patients have Lewy body pathology, with a lower frequency showing predominantly tau pathology [15].
Substantia nigra degeneration is often observed in frontotemporal degeneration with or without motor neuron disease, and Parkinsonism is not uncommon in frontotemporal degeneration[16], but because of significant involvement of brain regions outside of the extrapyramidal system, it is rarely misdiagnosed as PD.
Rare familial forms of Parkinsonism can have substantia nigra degeneration with TDP-43 pathology, rather that α-synuclein or tau pathology. An example of this is Perry syndrome, which is caused by mutations in DCTN1 [17]. DCTN1 encodes the dynactin subunit p150Glued and is a motor protein involved in axonal transport. These patients present with Parkinsonism, depression and central hypoventilation and have pallidonigral TDP-43 proteinopathy as the underlying pathologic process.
An exceptional case of familial Parkinsonism associated with only nonspecific substantia nigra degeneration was found to have mutation in polymerase gamma−1 (POLG1) [18], a nuclear gene that encodes mitochondrial DNA polymerase gamma, which is necessary for mitochondrial function, replication and repair. Mitochondrial metabolism is linked to other familial and sporadic forms of PD, most notably familial PD associated with mutations in PINK1 and PRKN. Autopsy studies of these disorders also fail to show consistent association with Lewy related pathology [19].
Lewy bodies and Lewy-related pathology in PD
Lewy bodies are intraneuronal inclusions composed of aggregates of α-synuclein. Only a subset of mature inclusions is visible with routine histologic methods. Immunohistochemistry for α-synuclein reveals not only the classical Lewy body in vulnerable neurons of the substantia nigra, raphe nuclei, mesopontine tegmentum, locus ceruleus, basal nucleus of Meynert and dorsal motor nucleus of the vagus, but also pale staining inclusions in less vulnerable neuronal populations, such as those in the amygdala and neocortex. These pale staining and poorly circumscribed lesions are referred to as pre-inclusions or cortical type Lewy bodies. Another major location of aberrant α-synuclein in PD is within cell processes (mostly axonal), so-called Lewy neurites. Abnormal α-synuclein within neurites represents the largest burden of pathology in a given brain region and in some brain regions (e.g., basal ganglia), it is the major form of abnormal α-synuclein, with few on no Lewy bodies detected. While most of the α-synuclein immunoreactive pathology in PD is within neurons, most cases have at least a few oligodendroglial inclusions in the midbrain and basal ganglia [20]. Numerous oligodendroglial cytoplasmic inclusions (GCI) are the pathologic hallmark of MSA [21], and glial inclusions seem to be increased in early onset PD related to genetic mutations in the gene for α-synuclein [22].
The presence of α-synuclein in cytoplasmic inclusions represents aberrant cytologic localization, since α-synuclein is normally a natively unfolded protein enriched in presynaptic terminals where it plays a role in synaptic vesicle release[23]. Aberrant α-synuclein in PD has not only an abnormal conformation (“amyloid-like”) that promotes is aggregation, but it also has pathologic post-translational modifications, including phosphorylation, truncation and oxidative damage [24].
Distribution of pathology in PD
It has been known for many years that Lewy-related pathology in PD extends beyond the substantia nigra, but Braak and co-workers were the first to put this process into a coherent staging scheme [9]. In this scheme, neuronal pathology occurs early in the dorsal motor nucleus of the vagus in the medulla and the anterior olfactory nucleus in the olfactory bulb, followed by locus ceruleus neurons in the pons and then dopaminergic neurons in the substantia nigra. In later stages, pathology extends to the basal forebrain, amygdala and the medial temporal lobe structures, with convexity cortical areas affected in the last stages.
The Braak PD staging scheme has been confirmed in several studies, although there may be individual cases that fail to fit the scheme [25, 26]. For example, there may be involvement in the substantia nigra without obvious involvement of dorsal motor nucleus of the vagus [27]. Subsequent iterations of the Braak scheme proposed that autonomic neurons in peripheral; autonomic ganglia and central autonomic neurons of the spinal cord may be affected before the dorsal motor neurons of the vagus [28]. The basal ganglia is affected in PD (although not initially addressed by Braak), but significant Lewy-related pathology in the basal ganglia is relatively late in the disease course [29].
As is clear from the previous discussion of pathologic findings in clinically diagnosed PD (without dementia), many patients have widespread (diffuse) or at least limbic (transitional) Lewy related pathology at autopsy. The latter cases would be consistent with Braak PD stage 6[9]. In this small autopsy series, brainstem predominant LBD was detected in less than one-third of PD cases with Lewy-related pathology. While BLBD cases would be predicted to have low likelihood of the dementia with Lewy bodies (DLB) syndrome (dementia with Parkinsonism, hallucinations, dream enactment behavior and fluctuations [30]), most of those with TLBD or DLBD would be predicted to have intermediate- to high-likelihood of the DLB. Additional studies are warranted to determine the factors that are needed to modify the clinical syndrome in order to produce clinically overt neurocognitive impairment, but one obvious difference from patients who come to autopsy with the DLB is the degree of concomitant Alzheimer type pathology – it was quite low in this PD without dementia cohort (Table 2). The median Braak neurofibrillary tangle stage was only II (out of VI), and the median Thal amyloid phase was only 2 (out of 5). Concomitant Alzheimer type pathology would be predicted to be greater in those with clinically significant cognitive deficits. A limitation of this brain bank survey is that most of the cases (80%) were not evaluated at Mayo Clinic, but rather referred from outside resources for neuropathologic study and research at the Mayo Clinic brain bank.
Table 2.
Neuropathologic characteristics of PD with Lewy-related pathology (N=101)
| LBD type | Age at death |
Sex (M:F) | Braak NFT stage (0 – VI) |
Thal amyloid phase (0 – 5) |
Brain weight (grams) |
|---|---|---|---|---|---|
| BLBD | 78 (71, 84) | 24:6 | II (I, III)* | 0 (0, 1)* | 1190 ± 32 |
| TLBD | 78 (74, 85) | 35:11 | II (II, III) | 1 (0, 1)* | 1200 ± 26 |
| DLBD | 79 (68, 84) | 18:7 | III (II, IV) | 3 (0. 4) | 1210 ± 31 |
Demographic and pathologic features of PD with Lewy-related pathology; Alzheimer type pathology summary measures for NFT (Braak stage) and amyloid plaques (Thal phase);
Abbreviations: LBD = Lewy body disease; BLBD = brainstem predominant Lewy body disease; TLBD = transitional/limbic LBD; DLBD = diffuse/neocortical LBD; M =- male; F=female, NFT = neurofibrillary tangle. data is median (25th percentile, 75th percentile);
indicate p<0.05 compared to DLBD for Thal phase and Braak stage with ANOVA on Ranks.
Concluding remarks
Neuropathology is an important adjunct to clinical neurology. Obtaining autopsies on patients followed in the clinic, especially those enrolled in natural history studies, biomarker studies or clinical treatment trials is important, but a resource that is underutilized by many movement disorder specialists. Support for movement disorders brain banks is not a high priority of funding agencies, yet there is much to be learned from postmortem studies that can improve clinical diagnosis and uncover changes that can be used to understand fundamental disease mechanisms.
Acknowledgments
Supported by NINDS P50-NS072187, the Mangurian Foundation Lewy Body Dementia Program, CurePSP/Tau Consortium Brain Bank, and The Robert E. Jacoby Professorship
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 3.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J Biol Chem. 2013;288:10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 5.Jiang P, Gan M, Yen SH, Moussaud S, McLean PJ, Dickson DW. Proaggregant nuclear factor(s) trigger rapid formation of alpha-synuclein aggregates in apoptotic neurons. Acta Neuropathol. 2016;132:77–91. doi: 10.1007/s00401-016-1542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang P, Gan M, Yen SH, McLean PJ, Dickson DW. Histones facilitate alpha-synuclein aggregation during neuronal apoptosis. Acta Neuropathol. 2017;133:547–558. doi: 10.1007/s00401-016-1660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 10.Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–1026. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- 11.Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson's syndrome. Brain. 2007;130:1566–1576. doi: 10.1093/brain/awm104. [DOI] [PubMed] [Google Scholar]
- 12.Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–309. doi: 10.1093/brain/awv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85:404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Kalia LV, Lang AE, Hazrati LN, Fujioka S, Wszolek ZK, Dickson DW, Ross OA, Van Deerlin VM, Trojanowski JQ, Hurtig HI, Alcalay RN, Marder KS, Clark LN, Gaig C, Tolosa E, Ruiz-Martinez J, Marti-Masso JF, Ferrer I, Lopez de Munain A, Goldman SM, Schule B, Langston JW, Aasly JO, Giordana MT, Bonifati V, Puschmann A, Canesi M, Pezzoli G, Maues De Paula A, Hasegawa K, Duyckaerts C, Brice A, Stoessl AJ, Marras C. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015;72:100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24:375–398. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konno T, Ross OA, Teive HAG, Slawek J, Dickson DW, Wszolek ZK. DCTN1-related neurodegeneration: Perry syndrome and beyond. Parkinsonism Relat Disord. 2017 doi: 10.1016/j.parkreldis.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta SH, Dickson DW, Morgan JC, Singleton AB, Majounie E, Sethi KD. Juvenile onset Parkinsonism with "pure nigral" degeneration and POLG1 mutation. Parkinsonism Relat Disord. 2016;30:83–85. doi: 10.1016/j.parkreldis.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takanashi M, Li Y, Hattori N. Absence of Lewy pathology associated with PINK1 homozygous mutation. Neurology. 2016;86:2212–2213. doi: 10.1212/WNL.0000000000002744. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta Neuropathol (Berl) 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- 21.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 22.Fujishiro H, Imamura AY, Lin WL, Uchikado H, Mark MH, Golbe LI, Markopoulou K, Wszolek ZK, Dickson DW. Diversity of pathological features other than Lewy bodies in familial Parkinson's disease due to SNCA mutations. Am J Neurodegener Dis. 2013;2:266–275. [PMC free article] [PubMed] [Google Scholar]
- 23.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson DW. Alpha-synuclein and the Lewy body disorders. Curr Opin Neurol. 2001;14:423–432. doi: 10.1097/00019052-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 26.Parkkinen L, Soininen H, Alafuzoff I. Regional distribution of alpha-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:363–367. doi: 10.1093/jnen/62.4.363. [DOI] [PubMed] [Google Scholar]
- 27.Jellinger KA. Alpha-synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution--a pilot study. Acta Neuropathol (Berl) 2003;106:191–201. doi: 10.1007/s00401-003-0725-y. [DOI] [PubMed] [Google Scholar]
- 28.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboi Y, Dickson DW. Dementia with Lewy bodies and Parkinson's disease with dementia: are they different? Parkinsonism Relat Disord. 2005;11(Suppl 1):S47–S51. doi: 10.1016/j.parkreldis.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 30.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O'Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]