Abstract
Swine are a key reservoir host for influenza A viruses (IAVs), with the potential to cause global pandemics in humans. Gaps in surveillance in many of the world's largest swine populations impede our understanding of how novel viruses emerge and expand their spatial range in pigs. Although US swine are intensively sampled, little is known about IAV diversity in Canada's population of ~12 million pigs. By sequencing 168 viruses from multiple regions of Canada, our study reveals that IAV diversity has been underestimated in Canadian pigs for many years. Critically, a new H1 clade has emerged in Canada (H1α-3), with a two-amino acid deletion at H1 positions 146–147, that experienced rapid growth in Manitoba's swine herds during 2014–2015. H1α-3 viruses also exhibit a higher capacity to invade US swine herds, resulting in multiple recent introductions of the virus into the US Heartland following large-scale movements of pigs in this direction. From the Heartland, H1α-3 viruses have disseminated onward to both the east and west coasts of the United States, and may become established in Appalachia. These findings demonstrate how long-distance trading of live pigs facilitates the spread of IAVs, increasing viral genetic diversity and complicating pathogen control. The proliferation of novel H1α-3 viruses also highlights the need for expanded surveillance in a Canadian swine population that has long been overlooked, and may have implications for vaccine design.
Keywords: Influenza A virus, zoonotic, phylogeography, viral emergence, reassortment, swine
Abbreviations
BEAST, Bayesian evolutionary analysis sampling trees; BSSVS, Bayesian stochastic search variable selection; CswH1, classical H1 viruses; GTR, general time-reversible; HPD, highest posterior density; IAV, influenza A virus; IAV-S, swine influenza A virus; MCC, maximum clade credibility; MCMC, Markov chain Monte Carlo; ML, maximum likelihood; pdmH1, pandemic H1 viruses; tMRCA, time to the most recent common ancestor; TRswH3, triple reassortant H3N2 viruses; UCLN, uncorrelated lognormal.
Introduction
Influenza A viruses (IAV-S) have circulated in Canada’s large swine population for many decades [1], presenting a threat to human and animal health. Canada has over 12 million swine and is the world’s fifth largest exporter of pork. Canada’s swine production is concentrated in three provinces: Quebec (~4 million pigs), Ontario (~3 million) and Manitoba (~3 million) (Fig. S1, available in the online Supplementary Material). On average, ~7 million pigs are transported each year from Canada to the United States prior to end-stage production, particularly to the swine-dense ‘corn belt’ regions, including the Heartland and Midwest (Fig. S1), resulting in regular viral traffic across the US–Canadian border [2]. North American IAV-S lineages, including classical H1 viruses (CswH1) and triple-reassortant H3N2 viruses (TRswH3), are present in Canadian pigs [3, 4]. Since 2000, several novel influenza viruses have been identified in Canadian pigs, including viruses of human and avian origin [5–8]. Novel human-like H1 viruses were identified in Ontario pigs in 2003–2004, shortly before the emergence of similar human-like H1N2 viruses in US swine (H1δ−1) [8] (described as the 1B.2.1 lineage in a recently developed nomenclature system [9]). In 2009, a human-like H3N2 virus was detected in Saskatchewan (A/swine/Saskatchewan/02903/2009/H3N2), which is most closely related to the seasonal H3N2 viruses that circulated in humans during the early 2000s [10]. The first documented human-to-swine transmission (‘reverse zoonosis’) of the 2009 H1N1 pandemic virus (pdmH1N1, 1A.3.3.2) was identified in swine in Alberta in May 2009 [11]. Additional reverse zoonosis events of pdmH1N1 viruses have occurred in Canadian swine since 2009 [12]. However, the full diversity of IAV-S in Canada remains unknown, as Canada's swine herds are under-sampled compared to the intensive surveillance of IAV-S in US swine [13–16].
The evolution of IAV-S in North American swine is driven by multiple processes: (a) reverse zoonotic transmission from humans, (b) reassortment, (c) long-distance movement of animals ('swine flows') and (d) antigenic drift. Although the antigenic evolution of the haemagglutinin (HA) protein is not considered to be as rapid in swine as in human hosts [17], multiple drift variants of the CswH1 lineage have been identified in US swine since the 1980s: H1α (1A.1), followed by H1β (1A.2), the currently dominant H1γ viruses (1A.3.3.3) and the recently described H1γ-2 viruses (1A.3.2) [18]. Traditionally, in North America the entire pig production cycle from breeding to market was managed in ‘farrow-to-finish’ operations. Today, major phases of production (farrow-to-wean, wean-to-feeder and feeder-to-market) are often split across different sites. This multisite production model requires the frequent transport of pigs, sometimes for hundreds or thousands of miles. For example, pigs raised in the Appalachia and Southeast regions of the US are often transported long-distance to the fertile agricultural regions located in the Heartland and Corn Belt regions, including Iowa and Indiana, for fattening prior to end-stage production. Although such strategies are beneficial to abattoirs and pork processing plants, and may reduce production costs, large-scale pig movements facilitate the long-distance spread of IAV-S between regions [19].
To investigate the genetic diversity of IAV-S in Canada, and to further understand the ecology and evolution of IAV-S in North America, we sequenced the complete genomes of 168 viruses collected in Canadian swine during 2012–2015 (GenBank accession numbers ALZ42991– ALZ46694, listed in Table S1). We discovered that asymmetrical trade between Canada and the United States has produced important differences in the evolution of IAV-S in these countries, including the widespread circulation of highly divergent H1α viruses in Canadian swine, which have recently returned to the United States and have the potential to become established in US herds.
Results
Genetic diversity of H1 viruses in the United States and Canada
Differences were observed in the genetic diversity of H1 viruses in the United States and Canada. In Canada, H1α (1A.1) viruses were the most frequently identified H1 viruses. In Manitoba, where sampling was more intensive, more than three-quarters (62/81) of the H1 viruses identified were H1α (Fig. 1). In contrast, H1α viruses died out long ago in US herds, and have only been identified sporadically following new viral introductions from Canada (see below). Notably, the two dominant H1 viruses in the United States, H1γ (1A.3.3.3) and H1δ-1 (1B.2.2), were not observed in any Canadian province during 2009–2016 (Fig. 1), even when all available H1 sequences were included in the phylogeny (Fig. S2). H1β (1A.2) and H1δ-2 (1B.2.1) were also not identified in any part of Canada, despite frequent detection of the latter in the Appalachia region (Fig. 1), where the virus is thought to have originated [19]. In contrast to H1, H3 viruses are found in both the United States and Canada, with evidence of frequent cross-border transmission. Of the six H3 lineages (IV-A to IV-F) that have recently been described in US swine [16], two were identified in Canada: H3-IV(B) and H3-IV(C). H3-IV(C) viruses were introduced from the Midwest into Ontario, and H3-IV(B) viruses were introduced into Manitoba on at least two occasions from the Heartland (Fig. S3). Why H3 viruses do not exhibit the same strong spatial structuring as H1 viruses remains unclear, but may relate to lower competition experienced by a single H3-IV lineage that has been dominant for many years in North American swine. Recently, viruses with new human-origin H3 and N2 antigens have emerged in US swine, but have not yet reached Canada, and may alter H3 dynamics in North America in years to come [20]. The pdmH1N1 viruses (1A.3.3.2) were also observed in both the US and Canada, but primarily as a result of independent transmission events from humans to pigs in both countries, with only limited evidence of dissemination from Ontario to the Midwest (Fig. S3). Human-origin pandemic H1 viruses comprised a high proportion of the H1 viruses identified in multiple Canadian provinces (Fig. 1), but primarily from provinces where sampling is very low and sensitive to bias. Some clades of viruses with the non-reassorted pandemic genotype exhibited sustained transmission over multiple years and in multiple Canadian provinces (Fig. S3), potentially in contrast to the United States, where the pandemic HA tends to be lost during reassortment events [21].
Fig. 1.

Spatial distribution of H1 viruses in US and Canadian swine. In each Canadian province and US region (similar to those defined in Fig. S1), the proportion of H1 viruses from each major lineage is presented. The classical H1 lineages include H1α (shaded blue), H1β (red), H1γ (orange) and H1γ-2 (green). Human seasonal virus-origin lineages include H1δ-1 (teal) and H1δ-2 (purple). Human pandemic-origin viruses (pdm) are shaded light blue.
A time-scaled maximum clade credibility (MCC) tree indicates that the H1α viruses identified in Canada are highly divergent from the H1γ, H1γ-2 and H1β viruses that presently circulate in US swine, with a common ancestor dating back to the 1980s (Figs 2 and S4). Although H1α viruses closely related to US viruses were isolated in Canada as early as 1981 [e.g. A/swine/Ontario/2/1981(H1N1)], the 1981 introduction did not transmit onward in Canada, and present-day H1α viruses evolved from subsequent introductions of H1α viruses into Canada (Fig. 2). The population of Canadian H1α viruses comprises at least three distinct clades (H1α-1, H1α-2 and H1α-3, Table 1), which may represent three independent viral introductions from the United States to Canada during the 1990s (Fig. 2). Resolving when and how many times H1α viruses entered Canada is complicated by the low availability of samples prior to 2000, when these introductions are estimated to have occurred. It is not possible to distinguish whether the isolate A/St Hyacinthe/106/1991(H1N1) from Quebec represents a progenitor of the H1α-1 population or a separate introduction that did not transmit. A single virus from Alberta [A/swine/Alberta/SD0042/2014(H1N2)] that is basal to the H1α-3 clade (Figs 2 and S4) could represent a fourth viral introduction into Canada, but again additional samples are needed.
Fig. 2.
Evolutionary relationships between classical H1 viruses. Time-scaled MCC tree inferred for H1 sequences from 185 classical swine IAVs collected in US and Canadian swine during 1973–2017, including all available H1α sequences from the United States and Canada. The colour of each branch indicates the most probable location state, consistent with the shading used in Fig. 1. Background non-H1α viruses collected from swine in the US are shaded grey, for clarity. Posterior probabilities are provided for key nodes. Canada’s three H1α lineages and the US lineages H1β, H1γ, or H1γ-2 are labelled. The 1981 Ontario viruses (e.g. A/swine/Ontario/2/1981(H1N1)) are indicated. Amino acid substitutions are indicated above the branches where they occurred. Asterisks identify the 10 introductions of H1α-3 viruses into US swine herds; the hash (#) indicates the three introductions of H1α-1 viruses into the USA. Arrows indicate the introduction of H1α-3 viruses (introduction #4, Table 2) into the US Heartland and Appalachia regions. A similar tree with tip labels is provided in Fig. S4.
Table 1. Characteristics of Canada’s three H1α lineages.
| H1α-1 | H1α-2 | H1α-3 | |
|---|---|---|---|
| tMRCA (95 % HPD) | 1997.59–1999.63 | 2001.13–2003.88 | 2011.09–2012.27 |
| Genetic signatures | na | na | ∆146–147 (HA) |
| Canadian provinces | AB, MB, ON, QC, SK | MB | MB |
| US introductions | n=3 (IL, MN) | n=0 | n=10 (IA, IL, IN, MN, NC, NE) |
| Collection dates | 2003–2014 | 2006–2014 | 2013–2017 |
| Subtypes | H1N1 | H1N1, H1N2 | Predominantly H1N2 |
| No. US introductions | n=3 | n=0 | n=10 |
| % genetic similarity (H1γ) | 86.6 % | 86.0 % | 85.3 % |
| % genetic similarity (H1α-1) | – | 86.5 % | 87.1 % |
The relatively low availability of sequence data available for Canada's H1α viruses, compared to US herds, gives the illusion that the three clades are closely related on the phylogeny. However, the H1α-1, H1α-2 and H1α-3 clades have actually diverged substantially from one another. The genetic similarity between H1α-1 and H1α-3 (86.8 %) is lower than the genetic similarity between the US H1γ (1A.3.3.3) and H1β (1A.2) viruses (89.1 %), which are known to provide little antigenic cross-protection [22]. The genetic similarity between H1α-1 and H1α-2 viruses (86.5 %), and between H1α-1 and H1α-3 viruses (87.1 %), is comparable to the genetic similarity between H1α-1 and H1γ viruses (86.6 %) (Table 1). Several amino acid changes differentiate the Canadian H1α lineages (Figs 2 and S5). Notably, H1α-3 viruses are defined by a two-amino acid deletion in the HA (∆146–147). US swine lineages H1δ-1 and H1δ-2 have a similar single-amino acid deletion in the HA (∆147), which first emerged in human seasonal H1N1 viruses in 1995 (e.g., A/Beijing/262/1995/H1N1) (Fig. S6). The ∆147 deletion also emerged independently in a small number of H1 viruses isolated from swine in Germany.
Genetic diversity of the NA segment
Of the 200 viruses in Canada for which whole-genome sequence data were available, the vast majority (168/200, 84 %) had an NA segment from the N2-2002 lineage (Table S1). All Canadian H1α-1 viruses retained the classical N1, similar to US H1γ and H1β viruses, as did the majority (75 %) of Canadian H1α-2 viruses. N1 viruses were identified in five different Canadian provinces during 2009–2014 (Alberta, Manitoba, Quebec and Saskatchewan). The Canadian N1 lineage has circulated in Canada since the early 2000s, and it is possible that N1 viruses are more prevalent in less intensively sampled regions of Canada. In contrast to the H1α-1 and H1α-2 lineages, almost all Canadian H1α-3 viruses in Manitoba had the N2-2002 segment, which was acquired via multiple reassortment events with H3N2 viruses co-circulating in Manitoba (Fig. S3) and has largely replaced the N1 in Manitoba. Out of 150 total viruses with whole-genome sequences from Manitoba, collected 2009–2016, only two (1.3 %) had the classical N1 segment (Table S1).
Genetic diversity of internal gene segments
Triple-reassortant internal genes (TRIGs) were identified at high rates in Canada, similar to the dominance of this internal gene constellation in US swine. The most frequently identified genotype in Canada during 2009–2015 was the H3N2 subtype with TRIGs (n=59 viruses, genotype H3-T, Fig. 3a and Table S1). However, the repeated introduction of human pandemic H1N1 viruses since 2009 has increased the diversity of IAV-S internal genes in Canadian swine, similar to US swine. The internal genes of pandemic H1N1 viruses introduced from humans since 2009 have reassorted extensively with H3 and H1α viruses in Canada, generating a multitude of new reassortant genotypes (Fig. 3a). Many of the genotypes differed by only one, or sometimes two segments, and this network of relationships is visualized in Fig. S7. The two reassortant genotypes that were detected the most frequently were H3N2 viruses with pandemic PA and MP segments (n=37 viruses, genotype H3-O), and H1N2 viruses (H1α) with pandemic PA and MP segments (n=50 viruses, genotype H1c-N) (Fig. 3a). In Manitoba, the proportion of viruses with the reassortant H3-O and H1c-N genotypes increased rapidly during 2012–2015, coinciding with a decline in the proportion of viruses with non-reassorted H3-T genomes (Fig. 3b). The proliferation of pandemic PA and MP segments in Manitoba swine is similar to the rise of the pandemic MP in US herds (and to a lesser extent, the pandemic PA segment) (Fig. 3c). However, the precise arrangement of segments differs in pigs in the US and Canada, and H1c-N and H3-O genotypes are not identified frequently in US swine herds (Fig. S8), where genotypes with different combinations of pandemic PA, NP and MP segments have been identified [16, 23]. Viruses with a single pandemic MP segment were the most frequently identified H3N2 genotype in US herds during 2009–2016 [23], and were associated with H3N2v infections of humans in the United States, but were not detected in swine in Canada. Manitoba viruses with the H1c-N and H3-O genotypes are closely related and interspersed on trees inferred for segments other than HA (Fig. S3), indicating that most of the genome has been conserved during transmission, while the H1α and H3 antigens have been repeatedly exchanged via reassortment.
Fig. 3.
Whole-genome diversity of IAV-S in Canadian swine. (a) Twenty genotypes were identified among the 200 IAV-S collected from swine in Canada during 2009–2016. Genotypes H3-I and H3-O are similar to genotypes 10 and 7, reported in [16], and genotypes 22 and 11 in Rajao et al. [23]. The number of viruses identified in Canada with each genotype is provided in the final column. A phylogeny presenting the evolutionary relationships of the H1c genotypes identified in Canada and the United States is provided in Fig. S14. (b) The proportion of viruses collected in Manitoba with either the H3-T genotype or H3-O or H1c-N genotype during 2012–2015. (c) The proportion of viruses in the US and Manitoba with each of the pandemic segments (excluding viruses with all eight pandemic segments).
Introductions of Canadian H1α-3 viruses into the United States
Of the three H1α lineages in Canada, H1α-1 viruses have circulated the longest and have been identified in the largest number of Canadian provinces (Table 1). However, relatively few introductions of H1α-1 viruses from Canada into the United States were evident on the phylogeny (n=3), and none since 2009 (Fig. 2). Importantly, no introduction of H1α-1 viruses sustained transmission in US herds. In contrast, Manitoba's new H1α-3 viruses have been introduced into the United States at least 10 times since 2013, with evidence of spatial dissemination and early establishment in US swine herds (Fig. S9 and Table 2). As H1α-3 viruses spread across the United States, they have retained the ∆146–147 HA deletion, as well as the H1N2 subtype. The first introduction of H1α-3 viruses into the United States occurred in 2013, represented by the single isolate A/swine/Minnesota/A01394082/2013(H1N2) (introduction #1, Table 2). Subsequently, four introductions of H1α-3 viruses into the US were estimated to have occurred in 2014, another four in 2015, and then a tenth introduction in 2016. A reconstruction of the demographic history of H1α-3 viruses using a Bayesian Skygrid plot indicates that the eight viral introductions that occurred during 2014–2015 coincide with a period of rapid growth of the H1α-3 virus population (Fig. 4a).
Table 2. Ten discrete introductions of H1α-3 viruses from Manitoba, Canada, into the United States from 2013 to 2017.
| Intro | Collection dates (MM/YYYY) | US states | No. viruses | Subtype | tMRCA (95 % HPD) | Representative virus |
|---|---|---|---|---|---|---|
| 1 | 10/2013 | MN | n=1 | H1N2 | n/a | MN/A01394082/2013/H1N2 |
| 2 | 10/2014 – 03/2016 (18 mos.) | MN | n=4 | H1N2 | 2014.6 (2014.2–2014.8) | MN/A01566110/2014/H1N2 |
| 3 | 01/2015 – 3/2017 (26 mos.) | CA, IA, IL, IN, KS, MN | n=7 | H1N2 | 2014.9 (2014.7–2015.0) | IL/A01775416/2015/H1N2 |
| 4 | 02/2015 – 03/2017 (25 mos.) | CA, IA, MN, NC | n=22 | H1N2 | 2014.9 (2014.8–2015.1) | NC/A01668802/H1N2 |
| 5 | 03/2015 – 08/2016 (18 mos.) | IA, NE | n=3 | H1N2 | 2015.0 (2014.6–2015.1) | NE/A01841831/2015/H1N2 |
| 6 | 04/2015 – 03/2016 (12 mos.) | IA | n=4 | H1N2 | 2014.7 (2014.5–2015.0) | IA/A02077467/2015/H1N2 |
| 7 | 07/2015 | IA | n=1 | H1N2 | n/a | IA/A02076932/H1N2 |
| 8 | 10/2015 – 12/2016 (14 mos.) | IA | n=6 | H1N2 | 2015.5 (2015.3–2015.7) | IA/A01729216/2016/H1N2 |
| 9 | 03/2016 – 11/2016 (9 mos.) | MN | n=4 | H1N2 | 2015.5 (2015.1–2015.8) | MN/A01942902/2016/H1N2 |
| 10 | 07/2016-10/2016 (4 mos.) | IA | n=2 | H1N2 | 2016.3 (2016.0–2016.5) | IA/A01778107/2016/H1N2 |
Fig. 4.
Population growth and spatial dispersal of H1α-3 viruses. (a) The spatial dispersal of H1α-3 viruses from Manitoba into the United States was inferred from the MCC tree and visualized using SpreaD3 [44]. Black lines project the MCC tree up to four time points, and the radii of the red circles are proportional to the number of lineages maintained per location. Westward movements are depicted by lines with an upward curvature, while eastward movements are depicted by lines with a downward curvature. A dotted line indicates that viral transmission probably did not proceed directly between two locations, and probably involved unsampled intermediary locations, given empirical knowledge of US pig movements. All connections represent highly supported transmission events with Bayes factor >10 000. (b) A Bayesian Skygrid plot for H1α-3 viruses in North America depicts the growth of the mean effective population size (Ne) over time (blue line) with 95 % highest posterior density interval (blue shading). Red bars indicate the number of H1α-3 viruses in the dataset per quarter. Vertical green lines represent the estimated timing of 10 H1α-3 virus introductions from Canada into the United States (based on tMRCAs, Table 2).
To date, H1α-3 viruses have been identified in eight US states (California, Illinois, Indiana, Iowa, Kansas, Minnesota, Nebraska and North Carolina) encompassing four US regions (Appalachia, Heartland, Midwest and Pacific) (Table 2). All introductions of H1α-3 viruses into the US were first identified in the Heartland region, following the large-scale movements of pigs from Manitoba into states such as Minnesota and Iowa. The vast majority (8/10) of H1α-3 virus introductions from Manitoba were confined to the Heartland region, with no evidence of spread to other regions. However, for two viral introductions (#3 and #4, Table 2), the Heartland served as a source of H1α-3 viruses identified in the Midwest, Appalachia and Pacific regions (Figs 4b and S10). Following initial identification in the Heartland in January 2015, the virus associated with introduction #3 disseminated onward to the Midwest region by mid-2016, and to the Pacific region by December 2016 [A/swine/California/A01671936/2016(H1N2)]. Introduction #4 also was identified first in the Heartland in early 2015 [A/swine/Iowa/A01840670/2015(H1N2)], with subsequent dissemination to the Pacific region by December 2015 [A/California/A01459157/2015(H1N2)] and the Appalachia region by March 2016. H1α-3 viruses associated with introduction #4 have continued to circulate in the Appalachia region as of March 2017, and have the potential to become established. In fact, H1α-3 viruses transmitted sufficiently in Appalachia to transmit back to the Heartland, following large-scale movements of pigs in a direction that has been described previously [19].
Sources of viral diversity in the US Heartland and Midwest
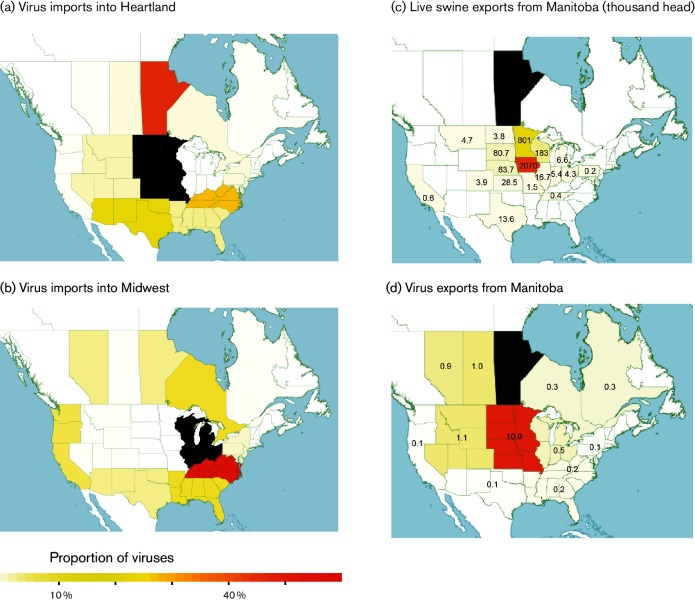
To further understand the spatial evolution of IAV-S diversity in North America, we quantified gene flow from multiple regions into two major destinations of North American swine flows: the Heartland and Midwest regions. The Bayesian phylogeographic approach summarized patterns across trees inferred separately for each segment and lineage (Fig. S11). We found that Manitoba is a major source of IAV-S in the US Heartland (Fig. 5a), but not in the Midwest, which imports IAV-S predominantly from Appalachia, the Southeast and Ontario (Fig. 5b). These patterns of viral gene flow align with geography, and also correspond with the direction of pig movements, as Manitoba exports swine primarily to the Heartland. Manitoba exported almost three million live pigs to the Heartland states of Iowa and Minnesota in 2015, and these two states account for ~87 % of Manitoba's total US live swine exports (Fig. 5c). The finding that Ontario is an important source of IAV-S in the Midwest is consistent with Ontario's highest density of swine farms in the southeast near Toronto and Detroit (Fig. S12). Transporting pigs from Ontario to Michigan requires 3 h of driving, compared with over 20 h to navigate around the natural barriers of the Great Lakes and Canadian Shield to reach Manitoba or the US Heartland.
Fig. 5.
Movements of pigs and viruses in North America. Each region is shaded according to the proportion of total ‘Markov jump’ counts from that particular region (source) into the (a) Heartland or (b) Midwest (destination): red, high proportion of jumps, important source of viruses; light yellow, low proportion of jumps, not an important source of viruses; black, destination. The extremely large number of jumps between the Heartland and Midwest were excluded in order to better visualize movements between more distant regions of interest. (c) US states are shaded according to the number of live swine imported from Manitoba in 2015 (per 1000 head): white, no imported swine; orange, over 1 million imported swine. (d) Each US state is shaded according to the number of importations of IAV-S from Manitoba, measured by Markov jump counts, summed across all phylogenies.
Discussion
By sequencing the complete genomes of IAV-S collected from multiple provinces in Canada, this study addresses long-standing gaps in our knowledge of IAV-S diversity and evolution in Canada, one of the world's largest swine producers. Although the United States and Canada share one of the most heavily trafficked border crossings for live swine, differences in their IAV-S populations have been sustained for many years, particularly for the H1 subtype. The identification of at least three genetically distinct H1α clades (H1α-1, H1α-2 and H1α-3) in Canada highlights the extent of under-studied H1 diversity that has circulated in Canada. Although all of Canada's H1α viruses were categorized as 1A.1.1 in a recently developed global nomenclature system [9], this was based on very limited data from Canada, and our study demonstrates that at least one of the three clades identified in this study (H1α-3) meets the criteria for new classification as a lineage, including evidence of recent circulation and at least 10 viruses. Canadian swine herds still remain highly undersampled, and it is possible that H1α-1 and H1α-2 viruses circulate at high enough levels to merit their own classification, but further surveillance in Canada is needed. The circulation of distinct H1α lineages in Canada has implications for vaccine design, as commercial vaccines formulated for US swine herds are unlikely to provide protection against Canada’s drifted H1α viruses. Although H1α viruses still represent only a small fraction of influenza viruses sequenced from US pigs at this time, they have spread spatially across the US at an alarming rate, and have the potential to become established and expand in Appalachia and other US regions. Although only two H1α viruses were identified in the Pacific region, these viruses actually represented two independent journeys of approximately 3000 km, highlighting the rapidity with which IAV-S can spread in North America when conditions are conducive.
A major outstanding question is what specific conditions facilitated the rapid spread of H1α-3 viruses in the US in recent years, after decades with no evidence of successful invasions of H1α-1 viruses or human-like A/swine/Saskatchewan/02903/2009(H3N2)-like viruses [10] from Canada. Although swine movements are good predictors of how viruses move between locations, these models do not consider the factors that determine whether invasions succeed in becoming established in a new location, at least sufficiently to be detected by surveillance. H1α-1 viruses may be less prevalent in Canadian pig populations destined for US export. Alternatively, differences in invasion rates may be driven by intrinsic properties of viruses, including lower clinical attack rates, greater antigenic drift, or genetic changes with phenotypes yet to be determined (e.g., the ∆146–147 deletion). Pig exports from Canada to the United States have remained fairly stable since the 1990s, and are not likely to account for temporal differences in rates of virus invasion. Surveillance biases may account for the lack of detection of H1α-1 viruses in US herds prior to 2009, but not since 2009.
In contrast to humans and wild birds, which follow symmetrical movement patterns along routes of migration, workflows and air travel, swine flows are frequently asymmetrical, producing complex patterns of IAV-S dispersal that are greatly in need of further study. Since the 2009 H1N1 pandemic, there have been global efforts to characterize the evolution of IAV-S [24–26], including regions that have been under-sampled [27–32]. Although our sequencing efforts substantially increased the number of whole-genome sequences from Canadian swine, Manitoba remains the only province in Canada with more than 50 IAV-S genome sequences available since 2010. Understanding how ongoing animal movements between multiple centres of North American swine production facilitate the continual emergence and rapid spatial dispersal of new viral lineages will require sampling with equivalent intensity in Ontario, Quebec, Saskatchewan, Alberta and undersampled regions of the United States. Understanding the complex ecology of H1 viruses in North America is not only critical for vaccine design and informing strategies for viral control, but may provide insights into viral interactions, including the vaccine-associated enhanced respiratory disease (VAERD) observed when vaccinated pigs are challenged with antigenic variants of the same H1 subtype [33].
Methods
Sample collection
IAV-S was isolated from lung tissue and nasal swabs collected as part of both active and passive surveillance projects on pigs in Alberta, Saskatchewan and Manitoba. The samples were submitted through Prairie Diagnostic Services, Inc. (PDS) and the University of Minnesota Veterinary Diagnostic Laboratory (UMVDL). Samples were initially screened using IAV-S Matrix gene real time RT-PCR protocols approved by either the Canadian Food Inspection Agency (CFIA) or the United States Department of Agriculture (USDA). PCR-positive samples were subtyped using a commercial kit (Life Technologies #4485541), and virus isolation was attempted on all samples with matrix PCR Ct <32. A total of 179 viral isolates from 2012 to 2015 were prepared for sequencing from three Canadian provinces (Alberta, Saskatchewan and Manitoba), along with seven from six highly under-represented US states (Alabama, Arkansas, Kentucky, Maryland, Montana and Utah).
RNA extraction and M-RTPCR
Extraction of viral RNA was performed using 140 µl of viral allantoic fluid in Qiagen AVL buffer using a QiaAmp Viral RNA Mini kit (Qiagen, Hilden, Germany)/ZR96 Viral RNA kit (Zymo, Irvine, CA, USA) hybrid protocol. In brief, specimen lysis was performed in Qiagen buffer AVL in a 96 deep-well plate. Lysate was transferred to a ZR-96 spin plate (Zymo), and samples were processed according to the manufacturer’s protocol. The influenza A genomic eight RNA segments were simultaneously amplified from 3 uls of purified RNA using a multisegment RT-PCR strategy [34].
IAV-S full-genome sequencing assembly and annotation
Illumina libraries were prepared using the Nextera DNA sample preparation kit (Illumina, Inc., San Diego, CA, USA) with half-reaction volumes as described previously [35]. For samples requiring extra coverage, Ion Torrent PGM (Thermo Fisher Scientific) was used, where 100 ng of pooled DNA amplicons were sheared for 7 min and Ion Torrent-compatible barcoded adapters were ligated to the sheared DNA using the Ion Xpress Plus fragment library kit (Thermo Fisher Scientific) to create 400 bp libraries. Sequence reads were sorted by barcode, trimmed and de novo assembled using CLC Bio’s clc_novo_assemble program. Curated assemblies were validated and annotated with the viral annotation software – Viral Genome ORF Reader, VIGOR 3.0 [36] – before submission to GenBank. All sequences generated as part of this study were submitted to GenBank as part of the Bioproject IDs and assigned accession numbers (Bioproject IDs PRJNA263044 and PRJNA183620; GenBank accessions available in Table S1).
Spatial distribution of H1 swine lineages in the United States and Canada
The proportion of H1 viruses from each lineage was estimated for 13 spatial locations in North America, including each of the 5 Canadian provinces from which IAV-S sequence data is publicly available [Alberta (AB), Manitoba (MB), Ontario (ON), Quebec (QC) and Saskatchewan (SK)], and eight US regions: (a) Appalachia [(Kentucky (KY); North Carolina (NC), Tennessee (TN), Virginia (VA) and West Virginia (WV)], (b) Heartland [Kansas (KS), Iowa (IA), Minnesota (MN), Missouri (MO), Nebraska (NE), North Dakota (ND) and South Dakota (SD)], (c) Mid-Atlantic [Delaware (DE), Maryland (MD), New Jersey (NJ), New York (NY) and Pennsylvania (PA)], (d) Midwest [Illinois (IL), Indiana (IN), Michigan (MI), Ohio (OH) and Wisconsin (WI)], (e) Mountain [Colorado (CO), Idaho (ID), Montanta (MT), Nevada (NV), Utah (UT) and Wyoming (WY)], (f) Pacific [California (CA), Oregon (OR) and Washington (WA)], (g) Southeast [Alabama (AL), Arkansas (AR), Florida (FL), Georgia (GA), Louisiana (LA), Mississippi (MS) and South Carolina (SC)] and (h) Southwest [Arizona (AZ), New Mexico (NM), Oklahoma (OK) and Texas (TX)]. No viruses were available from the New England region. All H1 sequences (of at least 800 nt) collected during 2009–2017 in swine in the United States and Canada were downloaded from the Influenza Virus Resource at GenBank on 23 May 2017 (n=4111). Viruses that are known to have been collected intensively as part of ongoing studies of IAV-S diversity at agricultural fairs were not included (n=270) [37], as these are not likely to be representative of commercial swine herds.
Phylogenetic analysis
The final data set included (a) 168 genomes from Canadian IAV-S, collected 2012–2015, that were successfully sequenced for this study; (b) 5 genomes from highly under-represented US states (Alabama, Arkansas, Kentucky, Maryland and Montana) that also were successfully sequenced for this study; and (c) 648 genomes from US and Canadian IAV-S, collected 2009–2015, that were available from the Influenza Virus Resource at GenBank [38]. In total, these data represented 29 US states and 5 Canadian provinces: Alabama, Alberta, Arkansas, California, Colorado, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Manitoba, Maryland, Michigan, Minnesota, Missouri, Montana, Nebraska, New York, North Carolina, North Dakota, New York, Ohio, Oklahoma, Ontario, Pennsylvania, Quebec, Saskatchewan, South Dakota, Tennessee, Texas, Virginia, Wisconsin and Wyoming.
Sequence alignments were constructed for each of the six internal gene segments (PB2, PB1, PA, NP, MP and NS) and for the H1, H3, N1 and N2 antigenic segments separately using muscle v3.8.3, [39] with manual correction in Se-Al v 2.0 (available at http://tree.bio.ed.ac.uk/software/seal/). The initial phylogenetic trees were inferred using the neighbour-joining method available in PAUP v 4.0b10 (available at http://paup.csit.fsu.edu) for each of the 10 alignments to assign each sequence to a genetic lineage. For the internal gene segments, viruses were categorized as triple-reassortant internal gene (TRIG) lineage or pdmH1N1. For the NP, MP and NS segments, the TRIG lineage is a continuation of the classical lineage, as classical NP, MP and NS segments were incorporated into triple-reassortant viruses during the reassortment [40]. Reference sequences for pdmH1N1 from humans and swine, including A/California/04/2009(H1N1), were included to assist in identification of the pdmH1N1 lineage. H1 and N1 segments were categorized as classical or pdmH1N1 [no human seasonal H1N1 virus (delta) origin viruses were identified in Canada]. All H3 sequences belonged to the same North American swine H3 lineage. The sequence alignment for each segment was further separated into each of these lineages, and trees were inferred independently for each alignment. Due to frequent reassortment, the number of sequences per lineage varied: PB2-TRIG (n=701 sequences), PB1-TRIG (n=726 sequences), PA-TRIG (n=500 sequences), NP-TRIG (n=543), MP-TRIG (n=234), NS-TRIG (n=686), PB2-pdm (n=531 sequences), PB1-pdm (n=529 sequences), PA-pdm (n=730 sequences), NP-pdm (n=622), MP-pdm (n=809), NS-pdm (n=460), H1-classical (n=182 sequences), H3 (n=356 sequences), H1-pdm (n=490), N1-classical (n=132 sequences), N1-pdm (n=462) and N2 (n=507 sequences). While the main analysis focused on viruses for which whole-genome sequences were available, additional phylogenies were inferred for the larger amount of sequence data available for the H1 (n=1651 classical H1 sequences; n=1611 delta H1 +human H1 sequences) inferred using the maximum likelihood (ML) method available in the program RAxML v 7.2.6, [41] incorporating a general time-reversible (GTR) model of nucleotide substitution with a gamma-distributed (Γ) rate variation among sites. To assess the robustness of each node, a bootstrap resampling process was performed (500 replicates), again using the ML method available in RAxML v 7.2.6.
Phylogenetic relationships were inferred for each of the data sets separately using the time-scaled Bayesian approach with MCMC available via the beast v 1.8.4 package [42] and the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). A strict molecular clock was used, with a constant population size, and a general-time reversible (GTR) model of nucleotide substitution with gamma-distributed rate variation among sites. We also repeated the analysis using a relaxed (UCLN) molecular clock, which produced similar tree topologies and tMRCAs for nodes of interest (Fig. S13). For viruses for which only the year of viral collection was available, the lack of tip date precision was accommodated by sampling uniformly across a 1-year window from 1 January to 31 December. The MCMC chain was run separately at least 3 times for each of the data sets and for at least 100 million iterations with sub-sampling every 10 000 iterations, using the BEAGLE library to improve computational performance [43]. All parameters reached convergence, as assessed visually using Tracer v 1.6, with statistical uncertainty reflected in values of the 95 % highest posterior density (HPD). At least 10 % of the chain was removed as burn-in, and runs for the same lineage and segment were combined using LogCombiner v 1.8.0 and downsampled to generate a final posterior distribution of 1000 trees that was used in the subsequent spatial analysis. For a more detailed tree of the evolution of classical H1 viruses, we also inferred a MCC tree for a data set including all available H1α sequences from Canada and closely related US viruses, and representative background sequences from older US classical H1α sequences from the 1970s, 1980s and 1990s, and more recent H1β, H1γ and H1γ−2 viruses (n=185). For visualization purposes, we also inferred an MCC tree for viruses solely from the H1α-3 lineage (n=109). The dispersal of H1α-3 viruses from Manitoba into the United States is visualized using SpreaD3 [44] at four time points. We applied a Skyride coalescent model to infer and visualize the effective population size (Ne) dynamics of this lineage.
Spatial analysis of IAV-S in Canada
The phylogeographical analysis considered the same 13 locations as the analysis of proportions of H1 lineages described above. Although a larger number of viruses were available from the Heartland and Midwest regions, these regions also have the largest swine population sizes. The location state was specified for each viral sequence, allowing the expected number of location state transitions in the ancestral history conditional on the data observed at the tree tips to be estimated using ‘Markov jump’ counts [45], which provided a quantitative measure of asymmetry in gene flow between regions. For computational efficiency the phylogeographic analysis was run using an empirical distribution of 1000 trees [46], allowing the MCMC chain to be run for 25 million iterations, sampling every 1000. A Bayesian stochastic search variable selection (BSSVS) was employed to improve the statistical efficiency for all data sets. Maximum clade credibility (MCC) trees were summarized using TreeAnnotator v 1.8.0 and the trees were visualized in FigTree v 1.4.2. Heat maps were constructed using the R package to summarize Markov jump counts inferred over the totality of phylogenies (all segments and all IAV-S lineages). The trade value (USD) for live swine trade between Canada and the United States for the years 1996–2012 was obtained from the United Nations’ Commodity Trade Statistics database (available at http://comtrade.un.org, accessed 20 March 2014) (Table S2). Swine population sizes in the US and Canada were obtained from Statistica (available at https://www.statista.com/statistics/194371/top-10-us-states-by-number-of-hogs-and-pigs/) and from a 2014 report produced by Statistics Canada, ‘The changing face of the Canadian hog industry' (available at www.statcan.gc.ca/pub/96-325-x/2014001/article/14027-eng.htm). The number of pigs exported from Manitoba to US states was obtained from a report prepared for the Department of Agribusiness and Agricultural Economics at the University of Manitoba titled ‘Manitoba pig and pork industry 2015’ (available at http://manitobapork.com/wp-content/uploads/2012/11/Manitoba-pig-and-pork-profile-2015.pdf).
Genetic distance between classical H1 viruses
To evaluate genetic divergence between the Canadian H1α viruses and other classical H1 viruses identified in US swine, we estimated the pairwise distance (p distance) using mega v 7.0.2 [47], with 1st+2nd+3rd+non coding codon positions included and all ambiguous positions removed for each sequence pair. We calculated the average percentage of genetic similarity [(1−p distance)×100] between the classical H1 lineages – H1β, H1γ, H1γ−2 and H1pdm – as well as the three bootstrap-supported clades of Canadian H1α viruses (H1α-1, H1α-2 and H1α-3). To examine whether important mutations have occurred in the evolutionary history of the classical H1 lineage, we identified lineage-specific amino acid substitutions in the four antigenic sites previously defined in the H1 HA protein (classical swine lineage) – the Sa and Sb sites that are proximal to the receptor-binding pocket, the Ca site (formed by Ca1 and Ca2 subsites) that is at the subunit interface and the Cb site within the vestigial esterase domain [48] – using A/Puerto Rico/8/34(H1N1) as a reference sequence for site numbering [49]. Additionally, we noted lineage-specific mutations occurring in other sites of the H1 protein [50, 51]. Illustrations were generated using the Hemagglutinin Structure Prediction (HASP) server [52]. To determine the role of positive selection in the evolution of the H1 protein, we estimated site-specific ratios of non-synonymous/synonymous substitution rates (dN/dS), using the Renaissance counting (RC) methodology, implemented in BEAST, which combines estimates of the number of synonymous and non-synonymous substitutions at each site with an empirical Bayes procedure to produce a posterior distribution of d N/d S ratios for all sites in the alignment [53].
Funding information
This work was funded with federal funds from the Centers of Excellence for Influenza Research and Surveillance (CEIRS), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) and Department of Health and Human Services (HHS) under contract no. HHSN272201400008C. This work was supported by the Multinational Influenza Seasonal Mortality Study (MISMS), an on-going international collaborative effort to understand influenza epidemiology and evolution, led by the Fogarty International Center, NIH. The sequencing work was supported by the NIAID/NIH Genomic Centers for Infectious Diseases (GCID) programme (U19-AI-110819). Funding for Canadian surveillance was provided by the Saskatchewan Ministry of Agriculture, Saskatchewan Agricultural Development Fund (20140241) and Alberta Agriculture and Forestry (2014R060R, 2016R0011R).
Acknowledgements
We would like to thank Wendy Wiese and Lotus Smasal at University of Minnesota Veterinary Diagnostic Laboratory for their virology work. The content is solely the responsibility of the authors and does not represent official views of the National Institutes of Health.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
References
- 1.Olsen CW. The emergence of novel swine influenza viruses in North America. Virus Res. 2002;85:199–210. doi: 10.1016/S0168-1702(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 2.Nelson MI, Viboud C, Vincent AL, Culhane MR, Detmer SE, et al. Global migration of influenza A viruses in swine. Nat Commun. 2015;6:6696. doi: 10.1038/ncomms7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen CW, Karasin AI, Carman S, Li Y, Bastien N, et al. Triple reassortant H3N2 influenza A viruses, Canada, 2005. Emerg Infect Dis. 2006;12:1132–1135. doi: 10.3201/eid1207.060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grgić H, Costa M, Friendship RM, Carman S, Nagy É, et al. Genetic characterization of H1N1 and H1N2 influenza A viruses circulating in Ontario pigs in 2012. PLoS One. 2015;10:e0127840. doi: 10.1371/journal.pone.0127840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karasin AI, Olsen CW, Anderson GA. Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J Clin Microbiol. 2000;38:2453–2456. doi: 10.1128/jcm.38.6.2453-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karasin AI, Brown IH, Carman S, Olsen CW. Isolation and characterization of H4N6 avian influenza viruses from pigs with pneumonia in Canada. J Virol. 2000;74:9322–9327. doi: 10.1128/JVI.74.19.9322-9327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karasin AI, West K, Carman S, Olsen CW. Characterization of avian H3N3 and H1N1 influenza A viruses isolated from pigs in Canada. J Clin Microbiol. 2004;42:4349–4354. doi: 10.1128/JCM.42.9.4349-4354.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karasin AI, Carman S, Olsen CW. Identification of human H1N2 and human-swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005) J Clin Microbiol. 2006;44:1123–1126. doi: 10.1128/JCM.44.3.1123-1126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson TK, Macken CA, Lewis NS, Scheuermann RH, van Reeth K, et al. A phylogeny-based global nomenclature system and automated annotation tool for H1 hemagglutinin genes from swine influenza A viruses. mSphere. 2016;1:e00275-16. doi: 10.1128/mSphere.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson MI, Wentworth DE, Culhane MR, Vincent AL, Viboud C, et al. Introductions and evolution of human-origin seasonal influenza A viruses in multinational swine populations. J Virol. 2014;88:10110–10119. doi: 10.1128/JVI.01080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howden KJ, Brockhoff EJ, Caya FD, Mcleod LJ, Lavoie M, et al. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can Vet J. 2009;50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- 12.Pasma T, Joseph T. Pandemic (H1N1) 2009 infection in swine herds, Manitoba, Canada. Emerg Infect Dis. 2010;16:706–708. doi: 10.3201/eid1604.091636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson TK, Nelson MI, Kitikoon P, Swenson SL, Korslund JA, et al. Population dynamics of cocirculating swine influenza A viruses in the United States from 2009 to 2012. Influenza Other Respir Viruses. 2013;7:42–51. doi: 10.1111/irv.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducatez MF, Hause B, Stigger-Rosser E, Darnell D, Corzo C, et al. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg Infect Dis. 2011;17:1624–1629. doi: 10.3201/1709.110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corzo CA, Culhane M, Juleen K, Stigger-Rosser E, Ducatez MF, et al. Active surveillance for influenza A virus among swine, Midwestern United States, 2009–2011. Emerg Infect Dis. 2013;19:954–960. doi: 10.3201/eid1906.121637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitikoon P, Nelson MI, Killian ML, Anderson TK, Koster L, et al. Genotype patterns of contemporary reassorted H3N2 virus in US swine. J Gen Virol. 2013;94:1236–1241. doi: 10.1099/vir.0.051839-0. [DOI] [PubMed] [Google Scholar]
- 17.Lewis NS, Russell CA, Langat P, Anderson TK, Berger K, et al. The global antigenic diversity of swine influenza A viruses. Elife. 2016;5:e12217. doi: 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson TK, Campbell BA, Nelson MI, Lewis NS, Janas-Martindale A, et al. Characterization of co-circulating swine influenza A viruses in North America and the identification of a novel H1 genetic clade with antigenic significance. Virus Res. 2015;201:24–31. doi: 10.1016/j.virusres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Nelson MI, Lemey P, Tan Y, Vincent A, Lam TT, et al. Spatial dynamics of human-origin H1 influenza A virus in North American swine. PLoS Pathog. 2011;7:e1002077. doi: 10.1371/journal.ppat.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajão DS, Gauger PC, Anderson TK, Lewis NS, Abente EJ, et al. Novel reassortant human-like H3N2 and H3N1 influenza A viruses detected in pigs are virulent and antigenically distinct from swine viruses endemic to the United States. J Virol. 2015;89:11213–11222. doi: 10.1128/JVI.01675-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson MI, Stratton J, Killian ML, Janas-Martindale A, Vincent AL. Continual reintroduction of human pandemic H1N1 influenza a viruses into swine in the United States, 2009 to 2014. J Virol. 2015;89:6218–6226. doi: 10.1128/JVI.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detmer SE, Gramer MR, King VL, Mathur S, Rapp-Gabrielson VJ. In vivo evaluation of vaccine efficacy against challenge with a contemporary field isolate from the α cluster of H1N1 swine influenza virus. Can J Vet Res. 2013;77:24–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Rajão DS, Walia RR, Campbell B, Gauger PC, Janas-Martindale A, et al. Reassortment between Swine H3N2 and 2009 pandemic H1N1 in the United States resulted in influenza a viruses with diverse genetic constellations with variable virulence in pigs. J Virol. 2017;91:e01763-16. doi: 10.1128/JVI.01763-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijaykrishna D, Smith GJ, Pybus OG, Zhu H, Bhatt S, et al. Long-term evolution and transmission dynamics of swine influenza A virus. Nature. 2011;473:519–522. doi: 10.1038/nature10004. [DOI] [PubMed] [Google Scholar]
- 25.Watson SJ, Langat P, Reid SM, Lam TT, Cotten M, et al. Molecular epidemiology and evolution of influenza viruses circulating within European swine between 2009 and 2013. J Virol. 2015;89:9920–9931. doi: 10.1128/JVI.00840-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis NS, Russell CA, Langat P, Anderson TK, Berger K, et al. The global antigenic diversity of swine influenza A viruses. Elife. 2016;5:e12217. doi: 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meseko CA, Odaibo GN, Olaleye DO. Detection and isolation of 2009 pandemic influenza A/H1N1 virus in commercial piggery, Lagos Nigeria. Vet Microbiol. 2014;168:197–201. doi: 10.1016/j.vetmic.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Reiche AS, Ramírez AL, Müller ML, Orellana D, Sosa SM, et al. Origin, distribution, and potential risk factors associated with influenza A virus in swine in two production systems in Guatemala. Influenza Other Respir Viruses. 2017;11:182–192. doi: 10.1111/irv.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cappuccio JA, Pena L, Dibarbora M, Rimondi A, Pineyro P, et al. Outbreak of swine influenza in Argentina reveals a non-contemporary human H3N2 virus highly transmissible among pigs. J Gen Virol. 2011;92:2871–2878. doi: 10.1099/vir.0.036590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson MI, Schaefer R, Gava D, Cantão ME, Ciacci-Zanella JR. Influenza A viruses of human origin in swine, Brazil. Emerg Infect Dis. 2015;21:1339–1347. doi: 10.3201/eid2108.141891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng YM, Iannello P, Smith I, Watson J, Barr IG, et al. Transmission of influenza A(H1N1) 2009 pandemic viruses in Australian swine. Influ Other Respi Viruses. 2012;6:e42. doi: 10.1111/j.1750-2659.2012.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinoco YO, Montgomery JM, Kasper MR, Nelson MI, Razuri H, et al. Transmission dynamics of pandemic influenza A(H1N1)pdm09 virus in humans and swine in backyard farms in Tumbes, Peru. Influenza Other Respir Viruses. 2016;10:47–56. doi: 10.1111/irv.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajão DS, Chen H, Perez DR, Sandbulte MR, Gauger PC, et al. Vaccine-associated enhanced respiratory disease is influenced by haemagglutinin and neuraminidase in whole inactivated influenza virus vaccines. J Gen Virol. 2016;97:1489–1499. doi: 10.1099/jgv.0.000468. [DOI] [PubMed] [Google Scholar]
- 34.Zhou B, Donnelly ME, Scholes DT, St George K, Hatta M, et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza a viruses. J Virol. 2009;83:10309–10313. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geoghegan JL, Tan Lev, Kühnert D, Halpin RA, Lin X, et al. Phylodynamics of enterovirus A71-associated hand, foot, and mouth disease in Viet Nam. J Virol. 2015;89:8871–8879. doi: 10.1128/JVI.00706-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Sundaram JP, Stockwell TB. VIGOR extended to annotate genomes for additional 12 different viruses. Nucleic Acids Res. 2012;40:W186–W192. doi: 10.1093/nar/gks528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson MI, Stucker KM, Schobel SA, Trovão NS, das SR, et al. Introduction, evolution, and dissemination of influenza A viruses in exhibition swine in the United States during 2009 to 2013. J Virol. 2016;90:10963–10971. doi: 10.1128/JVI.01457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Zaslavsky L, et al. The influenza virus resource at the National Center for Biotechnology Information. J Virol. 2008;82:596–601. doi: 10.1128/JVI.02005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, et al. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol. 1999;73:8851–8856. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 42.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suchard MA, Rambaut A. Many-core algorithms for statistical phylogenetics. Bioinformatics. 2009;25:1370–1376. doi: 10.1093/bioinformatics/btp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bielejec F, Baele G, Vrancken B, Suchard MA, Rambaut A, et al. SpreaD3: interactive visualization of spatiotemporal history and trait evolutionary processes. Mol Biol Evol. 2016;33:2167–2169. doi: 10.1093/molbev/msw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minin VN, Suchard MA. Counting labeled transitions in continuous-time Markov models of evolution. J Math Biol. 2008;56:391–412. doi: 10.1007/s00285-007-0120-8. [DOI] [PubMed] [Google Scholar]
- 46.Lemey P, Rambaut A, Bedford T, Faria N, Bielejec F, et al. Unifying viral genetics and human transportation data to predict the global transmission dynamics of human influenza H3N2. PLoS Pathog. 2014;10:e1003932. doi: 10.1371/journal.ppat.1003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, et al. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science. 2010;328:357–360. doi: 10.1126/science.1186430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 50.Dubois RM, Aguilar-Yañez JM, Mendoza-Ochoa GI, Oropeza-Almazán Y, Schultz-Cherry S, et al. The receptor-binding domain of influenza virus hemagglutinin produced in Escherichia coli folds into its native, immunogenic structure. J Virol. 2011;85:865–872. doi: 10.1128/JVI.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sriwilaijaroen N, Suzuki Y. Molecular basis of the structure and function of H1 hemagglutinin of influenza virus. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:226–249. doi: 10.2183/pjab.88.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ambroggio XI, Dommer J, Gopalan V, Dunham EJ, Taubenberger JK, et al. HASP server: a database and structural visualization platform for comparative models of influenza A hemagglutinin proteins. BMC Bioinformatics. 2013;14:197. doi: 10.1186/1471-2105-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemey P, Minin VN, Bielejec F, Kosakovsky Pond SL, Suchard MA. A counting renaissance: combining stochastic mapping and empirical Bayes to quickly detect amino acid sites under positive selection. Bioinformatics. 2012;28:3248–3256. doi: 10.1093/bioinformatics/bts580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.