Abstract
Background and objectives
Fibrinogen has been reported to be involved in kidney tubulointerstitial fibrosis and podocyte injury in mouse models. However, the relationship between urinary fibrinogen and kidney outcomes has not been clarified in patients with CKD.
Design, setting, participants, & measurements
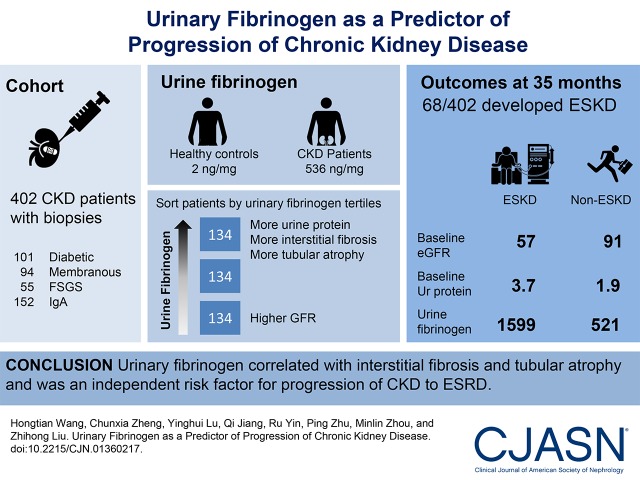
We evaluated 402 patients with CKD and kidney biopsies, including 101 with diabetic nephropathy, 94 with idiopathic membranous nephropathy, 55 with idiopathic FSGS, and 152 with IgA nephropathy. We quantified urinary fibrinogen by ELISA and tested associations with kidney histology and progression to ESRD.
Results
Median (interquartile range) urinary fibrinogen-to-creatinine ratio was 536 (191–1461) ng/mg for patients with CKD, significantly higher than 2 (2–3) ng/mg for healthy controls (P<0.001). Urinary fibrinogen was positively correlated with urine protein (r=0.64; P<0.001) and interstitial fibrosis and tubular atrophy (r=0.10; P=0.04), and it was negatively correlated with eGFR (r=−0.20; P<0.001). Over a median follow-up period of 35 months (interquartile range, 24–78 months), 68 of 402 patients (17%) developed ESRD. Higher urinary fibrinogen level was associated with increased risk of ESRD (hazard ratio, 2.12; 95% confidence interval, 1.31 to 3.26) per log10 higher urinary fibrinogen-to-creatinine ratio (P=0.003) adjusting for age, sex, BP, urine protein, disease type, eGFR, and interstitial fibrosis and tubular atrophy. For prediction of ESRD, the addition of urinary fibrinogen to eGFR, urine protein, and BP increased the area under the receiver operating curve from 0.73 to 0.76, and the Akaike information criterion improved from 333.6 to 327.0.
Conclusions
Urinary fibrinogen correlated with interstitial fibrosis and tubular atrophy and was an independent risk factor for progression of CKD to ESRD.
Keywords: chronic kidney disease; fibrinogen; predictor; progression; Glomerulonephritis, IGA; Podocytes; Glomerulonephritis, Membranous; risk factors; Diabetic Nephropathies; creatinine; blood pressure; Glomerulosclerosis, Focal Segmental; glomerular filtration rate; Confidence Intervals; Renal Insufficiency, Chronic; Blood Pressure Determination; Disease Progression; Kidney Failure, Chronic
Introduction
CKD has been a worldwide public health problem with severe burden (1,2) and a high-risk contributor to cardiovascular disease (3–5); 8%–16% of the global population is estimated to suffer from CKD with increased complications, including ESRD (4). There is a common molecular pathway for disease progression in advanced CKD after the kidney injury arrives at a threshold (6). Interstitial fibrosis and tubular atrophy, an inevitable phase of various CKD (7,8), could predict long-term kidney outcome of patients with CKD (9–13).
However, evaluation of tubulointerstitial injury is only achieved with kidney biopsy. Developing a noninvasive surrogate marker that can reflect the extent of tubulointerstitial injury and progression of CKD is urgently needed. Identification of pathophysiologically important markers also helps to discriminate those patients at high risk for progression to ESRD and then treat them timely and effectively. Other than the traditional risk factors, including age, hypertension, urine protein, and eGFR, whether other nontraditional factors could serve as potential predictors of poor kidney outcome is worthy of investigation.
Fibrinogen is a soluble 340-kD protein mainly synthesized by the liver with the central function in hemostasis (14). In our previous study, urinary fibrinogen levels were significantly elevated in patients with proteinuric kidney disease (15). Our subsequent study showed that fibrinogen could destroy actin cytoskeleton and induce apoptosis in podocytes via the Toll-like receptor 4-p38 mitogen activited protein kinase-NFκB p65 signaling pathway in vitro (16). Fibrinogen also links with inflammation (17). Additionally, fibrinogen has been shown to stimulate cultured podocytes to secrete some chemokines and cytokines in vitro (18–20). The increased deposition of fibrinogen in kidney tissues can promote tubulointerstitial fibrosis (16,20,21). In addition, plasma fibrinogen is related to the decline of eGFR in patients with CKD stages 3 and 4 (22,23).
On the basis of the above studies, we aimed to explore whether urinary fibrinogen as a noninvasive biomarker predicted long-term kidney outcomes in patients with CKD and biopsy-proven glomerular diseases.
Materials and Methods
Enrollment of Patients and Controls
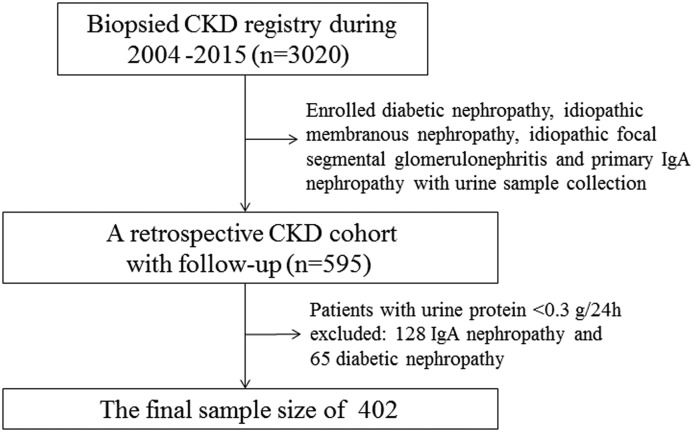
We enrolled 402 patients with CKD, including diabetic nephropathy, idiopathic membranous nephropathy, idiopathic focal segmental GN, and IgA nephropathy, with a wide range of urine protein values and eGFRs from 2004 to 2015 at the National Clinical Research Center of Kidney Disease, Jinling Hospital. Fifty healthy people were enrolled as normal controls. The flow chart of participant inclusion is shown in Figure 1. The study complied with the Declaration of Helsinki principles and was approved by the Ethics Committee of Jinling Hospital, and all of the participants gave written informed consent.
Figure 1.
Flow chart for selection of 402 patients with kidney biopsies.
Inclusion and Exclusion Criteria
The inclusion criteria were (1) urine protein >0.3 g/24 h, (2) a diagnosis proven by kidney biopsy, (3) eGFR≥15 ml/min per 1.73 m2, and (4) age 18–65 years old. The exclusion criteria were (1) secondary focal segmental GN, membranous nephropathy, or IgA nephropathy (caused by drug, inflammatory diseases, cancers, atherosclerotic lesions, liver disease, or brain ischemia); (2) viral infections; and (3) a family history of kidney disease.
Data Collection
Clinical and laboratory data were collected within 1 month of kidney biopsy, including age, sex, BP, 24-hour urinary protein, serum albumin, and eGFR calculated using the equation of the Chronic Kidney Disease Epidemiology Collaboration (24).
Definitions
The kidney end point of our study was the progression to ESRD during the follow-up period defined as eGFR<15 ml/min per 1.73 m2, treatment with chronic dialysis, or transplantation for >3 months (25). AKI was excluded from this outcome. Patients who did not reach ESRD were censored at their last follow-up visit. Interstitial fibrosis and tubular atrophy were scored as follows: zero, absent; one, <25%; two, 25%–50%; and three, >50% of the total area (25,26). Global glomerulosclerosis was defined as sclerosis area >50% of a glomerulus, and the definition of segmental glomerulosclerosis was any amount of the tuft involved in sclerosis but not involving the whole tuft or the presence of an adhesion.
Follow-Up
Follow-up assessments by medical record occurred at 1, 2, 3, 6, 9, and 12 months and then once every 6 months for patients with CKD until the patients reached ESRD or the final visit. We obtained urine samples from 402 patients at the time of biopsy.
Urine Sample Collection
The first voided urine samples were collected in sterile plastic tubes in the morning before renal biopsy and then centrifuged at 800×g for 10 minutes at 4°C to remove cell debris. The supernatants of the samples were stored at −80°C until further analysis.
Urinary Fibrinogen Assay
An ELISA was performed using the Fibrinogen Human ELISA Kit (ab108841; Abcam) according to the manufacturer’s instructions. The performance characteristics for the ELISA assay are as follows. The minimum detectable concentration is typically approximately 1.0 ng/ml. Average recovery is 96%. The coefficient of variation is 4% in intra-assay and 8.6% in interassay. The samples were measured in duplicate. The urinary creatinine concentration was used for normalization, with the urinary fibrinogen-to-creatinine ratio considered as the primary predictor variable.
Statistical Analyses
We analyzed all data using SPSS19.0 statistical software (SPSS, Chicago, IL). Variables with non-normal distribution were expressed as medians and interquartile ranges (25th and 75th percentiles) or percentages for categorical variables. The two-tailed Mann–Whitney U test or Kruskal–Wallis test was used to analyze the differences between two or more groups. Correlations between variables were estimated using the Spearman rank correlation coefficient. Urinary fibrinogen-to-creatinine ratio was log transformed for regression analysis (27).
Analysis of kidney survival was estimated with the Kaplan–Meier method. Univariable Cox regression analysis assessed potential confounding factors of urinary fibrinogen in influencing ESRD. Multivariable Cox regression analysis was used to adjust for other variables. Multiplicative interaction between log10 (fibrinogen-to-creatinine ratio) and diabetes as the cause of CKD, eGFR, and urine protein was performed in multivariable Cox models adjusted for other risk factors. Area under the receiver operating curve was calculated to determine the discrimination ability of the corresponding models (6). Likelihood ratio test, Akaike information criterion (AIC), integrated discrimination improvement, and net reclassification improvement were used to compare the different prediction models to analyze the added prognostic value of urinary fibrinogen (28). The net reclassification improvement used in the study was the category-free method. P<0.05 was considered significant.
Results
Baseline Characteristics of the Study Cohort
A total of 402 patients with CKD from Jinling Hospital were retrospectively reviewed (Figure 1). The clinical and demographic information of the participants is shown in detail in Table 1. Median (interquartile range) urinary fibrinogen-to-creatinine ratio of 536 (191–1461) ng/mg for patients with CKD was significantly higher than 2 (2–3) ng/mg for healthy controls (P<0.001).
Table 1.
Baseline characteristics of 402 patients with kidney biopsies stratified by urinary fibrinogen-to-creatinine ratio and 50 healthy control subjects
| Variables | Healthy Control Subjects, n=50 | All Patients with CKD, n=402 | Patients with CKD by Urinary Fibrinogen-to-Creatinine Ratio | ||
|---|---|---|---|---|---|
| <313 ng/mg, n=134 | 314–1246 ng/mg, n=134 | >1247 ng/mg, n=134 | |||
| Age, yr | 35 (22–45) | 41 (31–50) | 38 (31–50) | 41 (31–50) | 46 (36–52) |
| Women, % | 46 | 42 | 39 | 43 | 44 |
| Systolic BP, mmHg | 110 (100–122) | 130 (120–140) | 128 (119–140) | 127 (118–140) | 135 (125–148) |
| eGFR, ml/min per 1.73 m2 | 112 (96–128) | 82 (53–111) | 94 (59–115) | 87 (64–114) | 75 (39–104) |
| Urine protein, g/24 h | — | 2.4 (1.0–4.7) | 0.9 (0.7–1.6) | 2.6 (1.4–4.5) | 4.6 (3.3–7.6) |
| Serum albumin, g/dl | 4.3 (4.1–4.5) | 3.5 (2.6–4.1) | 4.0 (3.6–4.4) | 3.3 (2.6–3.9) | 2.9 (2.3–3.5) |
| Urinary fibrinogen to creatinine, ng/mg | 2 (2–3) | 536 (191–1461) | 131 (52–209) | 608 (458–836) | 2938 (1683–5782) |
| Disease type, n | |||||
| IgAN | — | 152 | 89 | 45 | 18 |
| IMN | — | 94 | 22 | 34 | 38 |
| DN | — | 101 | 17 | 27 | 57 |
| Focal segmental GN | — | 55 | 5 | 28 | 22 |
—, No data; IgAN, IgA nephropathy; IMN, idiopathic membranous nephropathy; DN, diabetic nephropathy.
Associations of Urinary Fibrinogen with Baseline Clinical Parameters
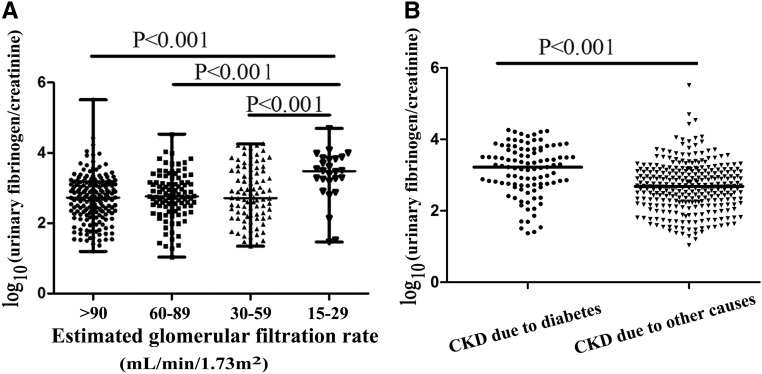
Urinary fibrinogen-to-creatinine ratio levels of patients with CKD stage 4 were higher than those of patients with CKD stages 1–3 (Figure 2A). Compared with those in patients with CKD without diabetes, urinary fibrinogen-to-creatinine ratio levels were higher in patients with CKD and diabetes (Figure 2B).
Figure 2.
Distributions of urinary fibrinogen-to-creatinine ratios by eGFR and cause of CKD among 402 patients with kidney biopsies. (A) Distributions of urinary fibrinogen-to-creatinine ratios by eGFR. (B) Distributions of urinary fibrinogen-to-creatinine ratios by cause of CKD.
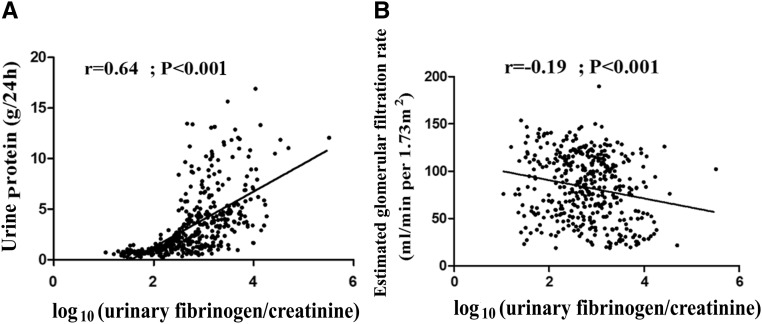
Urinary fibrinogen was significantly positively correlated with urine protein (r=0.64; P<0.001), whereas it was inversely correlated with eGFR (r=−0.20; P<0.001) (Figure 3). There were also significant correlations between urinary fibrinogen and age (r=0.18; P<0.001) and serum albumin (r=−0.45; P<0.001).
Figure 3.
Correlations of urinary fibrinogen-to-creatinine ratio with (A) urine protein and (B) eGFR among 402 patients with kidney biopsies.
Correlation of Urinary Fibrinogen with Interstitial Fibrosis and Tubular Atrophy
Because interstitial fibrosis and tubular atrophy can predict long-term kidney outcome of patients with CKD (29), we next assessed the association of urinary fibrinogen with interstitial fibrosis and tubular atrophy. There was a significant positive correlation between urinary fibrinogen and interstitial fibrosis and tubular atrophy (r=0.10; P=0.04). However, there was no significant correlation between urinary fibrinogen and glomerular damage (global sclerosis [r=0.03; P=0.56] and segmental sclerosis [r=−0.09; P=0.08]).
Urinary Fibrinogen and ESRD
During a median follow-up period of 35 (interquartile range, 24–78) months, 68 of 402 (17%) patients developed ESRD. Comparing patients with without incident ESRD with those without incident ESRD, there were significant differences with regards to eGFR, urine protein, urinary fibrinogen, disease type, global sclerosis, segmental sclerosis, and interstitial fibrosis and tubular atrophy at baseline. Urinary fibrinogen levels, baseline urine protein, and kidney pathologic lesions of ESRD group were more severe, and kidney function in the ESRD group was worse than that of non-ESRD group (Table 2).
Table 2.
Comparison of baseline characteristics of 402 patients with kidney biopsies by development of ESRD during follow-up
| Variables | Non-ESRD, n=334 | ESRD, n=68 | P Value |
|---|---|---|---|
| Age, yr | 41 (31–50) | 43(31–51) | 0.51 |
| Women, % | 44 | 34 | 0.13 |
| Follow-up, mo | 35.8 (24.5–83.1) | 42.8 (27.1–63.8) | 0.94 |
| eGFR ml/min per 1.73 m2 | 91 (61–113) | 57 (34–83) | <0.001 |
| Systolic BP, mmHg | 129 (120–140) | 140 (125–150) | 0.11 |
| Urine protein, g/24 h | 1.9 (1.0–4.4) | 3.7 (2.4–5.6) | <0.001 |
| Serum albumin, g/dl | 3.5 (2.6–4.1) | 3.6 (2.7–3.9) | 0.91 |
| Urinary fibrinogen to Cr, ng/mg | 521.1 (177.5–1451.3) | 1599.9 (654.9–4148.6) | <0.001 |
| Global sclerosis, % | 8 (0–27) | 17 (33–58) | <0.001 |
| Segmental sclerosis, % | 0 (0–7) | 3 (0–14) | 0.01 |
| IFTA | 1 (1–2) | 2 (1–2) | <0.001 |
| Disease type, n (%) | <0.001 | ||
| IgAN | 130 (39) | 22 (32) | |
| IMN | 89 (27) | 5 (7) | |
| DN | 64 (19) | 37 (54) | |
| Focal segmental GN | 51 (15) | 4 (6) |
Cr, creatinine; IFTA, interstitial fibrosis and tubular atrophy; IgAN, IgA nephropathy; IMN, idiopathic membranous nephropathy; DN, diabetic nephropathy.
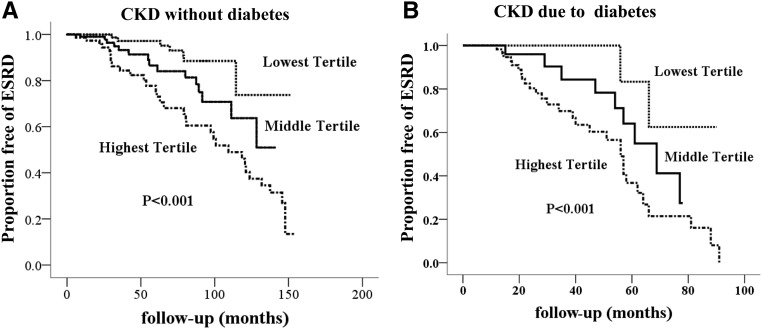
Kaplan–Meier analysis showed that patients with CKD and higher urinary fibrinogen levels at baseline had a significantly higher risk for the development of ESRD during the follow-period due to both diabetes and other causes (Figure 4). Univariable Cox regression analysis showed that urinary fibrinogen was a risk factor for kidney survival. After adjustment for traditional risk factors, such as baseline eGFR, urine protein, BP, and interstitial fibrosis and tubular atrophy, the multivariable Cox regression revealed that the association of urinary fibrinogen with new-onset ESRD remained statistically significant as shown in Table 3. There were no statistically significant interactions after adjustment for classic risk factors (urinary fibrinogen × diabetes, P=0.39; urinary fibrinogen × eGFR, P=0.07; urinary fibrinogen × urine protein, P=0.24).
Figure 4.
Survival free of ESRD according to urinary fibrinogen-to-creatinine tertile among 402 patients with kidney biopsies. (A) Patients with CKD due to causes other than diabetes (n=301). (B) Patients with CKD due to diabetes (n=101).
Table 3.
Cox regression analysis for incident ESRD according to baseline variables among 402 patients with kidney biopsies
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, yr | 1.01 (0.99 to 1.03) | 0.23 | ||
| Sex | 1.50 (0.91 to 2.48) | 0.12 | ||
| Systolic BP, >140 mmHg | 1.03 (1.02 to 1.04) | <0.001 | 1.00 (0.99 to 1.02) | 0.68 |
| eGFR, ≥15 ml/min per 1.73 m2 | 0.96 (0.95 to 0.97) | <0.001 | 0.98 (0.97 to 0.99) | <0.001 |
| Disease type | <0.001 | <0.001 | ||
| IgAN | 1.0 (Reference) | 1.0 (Reference) | ||
| IMN | 0.54 (0.13 to 0.98) | 0.001 | 0.15 (0.03 to 0.70) | 0.02 |
| DN | 3.61 (1.75 to 5.42) | 0.001 | 3.13 (1.59 to 6.18) | 0.001 |
| Focal segmental GN | 3.32 (1.26 to 10.23) | 0.02 | 2.10 (0.56 to 7.85) | 0.27 |
| Serum albumin, g/dl | 1.02 (0.99 to 1.05) | 0.16 | ||
| Log10 urine protein | 3.36 (1.83 to 6.12) | <0.001 | 2.63 (1.36 to 6.45) | 0.002 |
| Log10 urinary fibrinogen to Cr | 2.72 (1.91 to 3.87) | <0.001 | 2.12 (1.31 to 3.26) | 0.003 |
| IFTA | 3.41 (2.47 to 4.72) | <0.001 | 1.76 (1.22 to 2.52) | 0.002 |
| Global sclerosis | 1.19 (1.00 to 1.43) | 0.06 | ||
| Segmental sclerosis | 1.10 (0.55 to 2.20) | 0.80 | ||
HR, hazard ratio; 95% CI, 95% confidence interval; IgAN, IgA nephropathy; IMN, idiopathic membranous nephropathy; DN, diabetic nephropathy; Cr, creatinine; IFTA, interstitial fibrosis and tubular atrophy.
Urinary Fibrinogen and Prediction of ESRD
The areas under the receiver operating curve for prediction model 1 (including eGFR, urine protein, BP, age, and sex) and prediction model 2 (adding log10 [urinary fibrinogen-to-creatinine ratio] to model 1) for incident ESRD were 0.73 (95% confidence interval, 0.66 to 0.80) and 0.76 (95% confidence interval, 0.68 to 0.82), respectively. The model including urinary fibrinogen-to-creatinine ratio was also superior as evaluated by the likelihood test (P=0.003) and lower AIC. The integrated discrimination improvement (P<0.01) and net reclassification improvement (P=0.001) in model 1 were significantly higher compared with those in model 2 (Table 4).
Table 4.
Prediction of ESRD among 402 patients with kidney biopsies
| Variables | All Patients with CKD, n=402 | Subgroup with CKD Due to Cause Other Than Diabetes, n=301 | Subgroup with CKD Due to Diabetes, n=101 | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 1 + Log10 (Urinary Fibrinogen to Cr) | Model 1 | Model 1 + Log10 (Urinary Fibrinogen to Cr) | Model 1 | Model 1 + Log10 (Urinary Fibrinogen to Cr) | |
| AUC | 0.73 | 0.76 | 0.84 | 0.85 | 0.71 | 0.74 |
| LR test | 0.003a | 0.07a | 0.02a | |||
| AIC | 333.6 | 326.9b | 141.1 | 139.9 | 133.9 | 130.6b |
| IDI | 0.02 (95% CI, 0.01 to 0.04); P<0.01a | 0.01 (95% CI, <−0.01 to 0.01); P=0.20a | 0.05 (95% CI, <0.01 to 0.09); P=0.04a | |||
| NRI | 0.43 (95% CI, 0.17 to 0.69); P=0.001a | 0.13 (95% CI, −0.27 to 0.53); P=0.34a | 0.48 (95% CI, 0.07 to 0.88); P=0.02a | |||
Model 1 included eGFR, urine protein, and BP (adjusted for age and sex); model 2 included panel + log10 (urinary fibrinogen to Cr). Cr, creatinine; AUC, area under the curve; LR, likelihood ratio; AIC, Akaike information criterion; IDI, integrated discrimination improvement; 95% CI, confidence interval; NRI, net reclassification improvement.
P value (model 1 versus model 2).
Significant between models 1 and 2.
Kaplan–Meier analysis showed that the kidney survival of patients with CKD due to diabetes was worse than that of patients without diabetes (P<0.001). In the subgroup of patients with CKD due to diabetes, addition of urinary fibrinogen improved the area under the curve from 0.71 (model 1) to 0.74 (model 2), and AIC decreased from 133.9 (model 1) to 130.6 (model 2). The likelihood test showed that the addition of urinary fibrinogen enhanced the prediction power (P=0.02). In comparison with model 2, there were significant improvements in discrimination (P=0.04) and reclassification (P=0.02) in model 1 (Table 4).
Discussion
In this study, urinary fibrinogen was shown to be an independent risk predictor of CKD progression to ESRD among patients with biopsy-proven glomerular diseases. We found that baseline urinary fibrinogen levels in patients with CKD were elevated, negatively correlated with eGFR and serum albumin, and positively correlated with urine protein across a range of kidney disease types (diabetic nephropathy, idiopathic membranous nephropathy, idiopathic focal segmental GN, and IgA nephropathy) and CKD stages 1–4. Baseline eGFR and urine protein are established risk factors of CKD progression (6). Our previous study showed that urinary fibrinogen could reflect the disease activity for patients with focal segmental GN (16). These prior results suggested that urinary fibrinogen may be a potential predictor for kidney disease progression.
In this study, urinary fibrinogen was shown to be positively correlated with interstitial fibrosis and tubular atrophy. Tubulointerstitial injury is an established predictor of CKD progression (26,27). However, interstitial fibrosis and tubular atrophy can only be assessed directly by kidney biopsy. Previous studies by others have shown that the deposition of fibrinogen in the mouse kidney promotes kidney fibrosis by triggering resident fibroblast proliferation and activating TGF-β1/phosphorylated Smad2 signaling (21,30). Thus, measurement of urinary fibrinogen could provide a noninvasive method to identify patients at high risk of kidney functional loss, monitor kidney fibrosis, and predict disease progression in CKD.
We observed that patients with CKD and higher baseline urinary fibrinogen levels had a significant higher risk for the incidence of ESRD during the follow-period. Additionally, patients with diabetes had worse kidney prognosis than patients without diabetes. In Cox regression analysis, urinary fibrinogen was shown to be a significant independent risk factor for ESRD incidence, even after adjustment for age, sex, BP, urine protein, disease type, eGFR, and interstitial fibrosis and tubular atrophy. Also, there were no interactions of urinary fibrinogen × diabetes, urinary fibrinogen × eGFR, and urinary fibrinogen × urine protein. Therefore, urinary fibrinogen provided additional prognostic information in patients with CKD stages 1–4 independent of traditional risk factors.
We also found that the addition of urinary fibrinogen undoubtedly improved prediction accuracy of CKD progression to ESRD. The inclusion of urinary fibrinogen on the basis of urine protein, BP, and baseline eGFR improved the accuracy of prediction of an unfavorable kidney outcome with higher area under the curve and lower AIC compared with only traditional combination of urine protein, BP, and eGFR. Lower AIC indicates better discrimination (28). The precise mechanism why the addition of urinary fibrinogen model was especially notable in patients with diabetes needs to be studied further. One of the possible mechanisms was the different pathophysiologic process in diabetic and nondiabetic nephropathy.
Fibrinogen not only enhanced the predictive ability for CKD progression but also, plays an important pathophysiologic role in kidney diseases. Fibrinogen is considered as one of the ligands of Toll-like receptor 4, which is expressed in podocytes and inflammatory cells (31,32). In vitro, combination of fibrinogen with Toll-like receptor 4 in podocytes together stimulates podocytes to release Chemokine (C C motif) ligand 2, Chemokine (C C motif) ligand 7, C-X-C motif chemokine ligand 1, C-X-C motif chemokine ligand 5 and monocyte chemoattractant protein-1 (17–19). In condition of urine protein, fibrinogen appears in Bowman’s space and may combine the damaged podocytes (16,19). Our prior study showed that fibrinogen could lead to rearrangement of actin cytoskeleton and apoptosis in podocytes via activation of the Toll-like receptor 4 signaling pathway in vitro, and elevated urinary fibrinogen was closely associated with podocyte damage in adriamycin-treated mice (16). Therefore, increased urinary fibrinogen levels were associated with the glomerular injury mediated by the Toll-like receptor 4 signaling pathway.
Of course, there are a few limitations in this study. First, our findings are on the basis of Chinese patients with CKD and need to be validated in other races. Second, the measurement of urinary fibrinogen was only performed once at baseline because of limits on urine sample collection. Sequential measurements of urinary fibrinogen levels would help to further evaluate their association with incident ESRD. Third, information regarding renin-angiotensin-aldosterone system inhibitor and immunosuppression were missing. This was a retrospective study with different disease types and treatment regimens. If possible in the future, a prospective study will be designed including hemoglobin, renin-angiotensin-aldosterone system inhibitor, immunosuppression, and other patient factors to explore their potential effect on CKD prognosis.
In summary, our data provided new evidence that urinary fibrinogen is an independent risk factor for poor kidney prognosis in proteinuric patients with CKD. Its addition to classic markers, such as eGFR, BP, urine protein, and interstitial fibrosis and tubular atrophy, could improve the prediction of incident ESRD in these patients.
Disclosures
None.
Acknowledgments
All of the human samples were obtained from the Renal Biobank of the National Clinical Research Center of Kidney Diseases.
This work was funded by Major International (Regional) Joint Research Project grant 81320108007, Major Research Plan of the National Natural Science Foundation grant 91442104, National Key Research and Development Program of China grant 2016YFC0904100, and Innovation Capability Development Project of Jiangsu Province grant BM2015004.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ayodele OE, Alebiosu CO: Burden of chronic kidney disease: An international perspective. Adv Chronic Kidney Dis 17: 215–224, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS: Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martini S, Nair V, Keller BJ, Eichinger F, Hawkins JJ, Randolph A, Böger CA, Gadegbeku CA, Fox CS, Cohen CD, Kretzler M; European Renal cDNA Bank; C-PROBE Cohort; CKDGen Consortium : Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol 25: 2559–2572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Whitman IR, Feldman HI, Deo R: CKD and sudden cardiac death: Epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 23: 1929–1939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M; ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium : Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM: Focal segmental glomerular sclerosis in adults: Presentation, course, and response to treatment. Am J Kidney Dis 25: 534–542, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Xu F, Chen DM, Zeng CH, Liu ZH: The clinical course and long-term outcome of primary focal segmental glomerulosclerosis in Chinese adults. Clin Nephrol 80: 130–139, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, Wang R, Lv Z, Chen J, Tian J, Chen N, Pan X, Fu P, Hu Z, Wang L, Fan Q, Zheng H, Zhang D, Wang Y, Huo Y, Lin H, Chen S, Sun S, Wang Y, Liu Z, Liu D, Ma L, Pan T, Zhang A, Jiang X, Xing C, Sun B, Zhou Q, Tang W, Liu F, Liu Y, Liang S, Xu F, Huang Q, Shen H, Wang J, Shyr Y, Phillips S, Troyanov S, Fogo A, Liu ZH: A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult chinese patients. Am J Kidney Dis 60: 812–820, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Mise K, Hoshino J, Ueno T, Hazue R, Hasegawa J, Sekine A, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N, Fujii T, Hara S, Ohashi K, Takaichi K, Ubara Y: Prognostic value of tubulointerstitial lesions, urinary N-Acetyl-β-d-Glucosaminidase, and urinary β2-Microglobulin in patients with Type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol 11: 593–601, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An Y, Xu F, Le W, Ge Y, Zhou M, Chen H, Zeng C, Zhang H, Liu Z: Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant 30: 257–266, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Zuo K, Wu Y, Li SJ, Xu F, Zeng CH, Liu ZH: Long-term outcome and prognostic factors of idiopathic membranous nephropathy in the Chinese population. Clin Nephrol 79: 445–453, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Mosesson MW: Fibrinogen and fibrin structure and functions. J Thromb Haemost 3: 1894–1904, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zheng C, Xu F, Liu Z: Urinary fibrinogen and renal tubulointerstitial fibrinogen deposition: Discriminating between primary FSGS and minimal change disease. Biochem Biophys Res Commun 478: 1147–1152, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Zheng C, Xu X, Zhao Y, Lu Y, Liu Z: Fibrinogen links podocyte injury with Toll-like receptor 4 and is associated with disease activity in FSGS patients [published online ahead of print April 13, 2017]. Nephrology (Carlton) 10.1111/nep.13046 [DOI] [PubMed] [Google Scholar]
- 17.Davalos D, Akassoglou K: Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 34: 43–62, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, Hauser P, Pippin JW, Shankland SJ, Smith KD, Stoelcker B, Liu G, Gröne HJ, Krämer BK, Alpers CE: TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19: 704–713, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motojima M, Matsusaka T, Kon V, Ichikawa I: Fibrinogen that appears in Bowman’s space of proteinuric kidneys in vivo activates podocyte Toll-like receptors 2 and 4 in vitro. Nephron, Exp Nephrol 114: e39–e47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders HJ, Schlöndorff D: Toll-like receptors: Emerging concepts in kidney disease. Curr Opin Nephrol Hypertens 16: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sörensen I, Susnik N, Inhester T, Degen JL, Melk A, Haller H, Schmitt R: Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int 80: 1035–1044, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Goicoechea M, de Vinuesa SG, Gómez-Campderá F, Aragoncillo I, Verdalles U, Mosse A, Luño J: Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl 111: S67–S70, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis 51: 212–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Bao H, Liu Z, Liu X, Gao E, Zeng C, Zhang H, Liu Z, Hu W: Risk factors for renal survival in Chinese patients with Myeloperoxidase-ANCA-associated GN. Clin J Am Soc Nephrol 12: 417–425, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An Y, Xu F, Le W, Ge Y, Zhou M, Chen H, Zeng C, Zhang H, Liu Z: Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant 30: 257–266, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Betz BB, Jenks SJ, Cronshaw AD, Lamont DJ, Cairns C, Manning JR, Goddard J, Webb DJ, Mullins JJ, Hughes J, McLachlan S, Strachan MWJ, Price JF, Conway BR: Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int 89: 1125–1135, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Nicholson ML, McCulloch TA, Harper SJ, Wheatley TJ, Edwards CM, Feehally J, Furness PN: Early measurement of interstitial fibrosis predicts long-term renal function and graft survival in renal transplantation. Br J Surg 83: 1082–1085, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Craciun FL, Ajay AK, Hoffmann D, Saikumar J, Fabian SL, Bijol V, Humphreys BD, Vaidya VS: Pharmacological and genetic depletion of fibrinogen protects from kidney fibrosis. Am J Physiol Renal Physiol 307: F471–F484, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders HJ, Banas B, Schlöndorff D: Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM: Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol 7: 1271–1285, 2007 [DOI] [PubMed] [Google Scholar]