Abstract
Background and objectives
Epidemiologic studies suggest that higher serum phosphaturic hormone fibroblast growth factor 23 levels are associated with increase morbidity and mortality. The aim of the FGF23 Reduction Efficacy of a New Phosphate Binder in CKD Trial was to evaluate the effect of sevelamer carbonate on serum C-terminal fibroblast growth factor 23 levels in normophosphatemic patients with CKD stage 3b/4.
Design, setting, participants, & measurements
Patients with CKD, eGFR between 45 and 15 ml/min per 1.73 m2, fasting serum phosphate concentration >3.1 mg/dl, and serum C-terminal fibroblast growth factor 23 >80 relative units/ml were included in our double-blind, placebo-controlled, randomized multicenter study. All patients received 100,000 IU cholecalciferol at time of randomization. Participants received either placebo or sevelamer carbonate 4.8 g daily during a 12-week period. Biologic parameters, including serum C-terminal fibroblast growth factor 23, intact fibroblast growth factor 23, and α-klotho, were evaluated at baseline and 12 weeks after inclusion.
Results
Of 96 screened patients, 78 (mean±SD age: 63±13 years old; 70% men; mean eGFR: 27±9 ml/min per 1.73 m2) met the inclusion criteria. At baseline, mean eGFR was 27±9 ml/min per 1.73 m2, mean serum phosphate level was 3.8±0.5 mg/dl, and median (interquartile range) serum C-terminal fibroblast growth factor 23 level was 157 (120–241) relative units/ml. After 12 weeks of treatment, urinary phosphate-to-creatinine ratio fell significantly in the sevelamer group. The sevelamer and placebo groups did not differ significantly in terms of median change in serum C-terminal fibroblast growth factor 23 levels: the median (interquartile range) change was 38 (−13–114) relative units/ml in the placebo group and 37 (−1–101) relative units/ml in the sevelamer group (P=0.77). There was no significant difference in serum intact fibroblast growth factor 23, α-klotho, or phosphate levels changes between the two groups. Serum total and LDL cholesterol levels fell significantly in the sevelamer group.
Conclusions
In our double-blind, placebo-controlled, randomized study performed in normophosphatemic patients with CKD, a 12-week course of sevelamer carbonate significantly reduced phosphaturia without changing serum phosphorus but did not significantly modify serum C-terminal fibroblast growth factor 23 and intact fibroblast growth factor 23 or α-klotho levels.
Keywords: chronic kidney disease; fibroblast; mineral metabolism; KLOTHO; FGF23; phosphate binders; randomized controlled trials; sevelamer; fibroblast growth factor 23; Phosphorus; Cholesterol, LDL; creatinine; Cholecalciferol; Double-Blind Method; glomerular filtration rate; Fasting; Random Allocation; Fibroblast Growth Factors; Hypophosphatemia, Familial; Renal Insufficiency, Chronic; Phosphates; Epidemiologic Studies
Introduction
CKD is an established risk factor for cardiovascular morbidity and mortality; it results in some extent from disturbed mineral and bone metabolism, leading to arterial calcification, increased pulse wave velocity, left ventricular hypertrophy, and heart failure (1). Hyperphosphatemia is a very common complication in patients with advanced CKD (including those on dialysis), and it is associated with severe morbidity and increased mortality risk (2).
As CKD progresses, serum phosphate levels rise progressively but generally remain within the normal range until late-stage CKD (stage 4 or 5). Fibroblast growth factor 23 (FGF23) is a recently identified phosphaturic hormone. Serum FGF23 level may be a sensitive early biomarker of phosphorus metabolism disorders in patients with CKD and normal serum phosphate levels (3). Elevated FGF23 is an independent risk factor for the progression to ESRD in patients with CKD with relatively preserved residual kidney function (4). Lastly, high serum FGF23 levels are reportedly associated with vascular calcification (5), left ventricular hypertrophy (6), cardiovascular events (7,8), and mortality (9,10).
In addition to FGF23, its coreceptor α-klotho has emerged as pivotal player in calcium-phosphate homeostasis (11). There is growing interest in using α-klotho as a biomarker of kidney function, because low circulating α-klotho levels were associated with adverse kidney disease outcomes (12). Most experimental studies supported that α-klotho deficiency is associated with cardiovascular disease (13,14). In patients on dialysis, serum α-klotho was not independently associated with cardiovascular disease in one study (15), whereas other reports found an independent link between low serum α-klotho and cardiovascular risk (16,17).
In France, different phosphate binders are currently approved for the treatment of hyperphosphatemia. However, these drugs are approved only in patients on maintenance dialysis and hyperphosphatemic patients with CKD not requiring dialysis. It has been suggested that the early administration of phosphate binders in nondialyzed patients may decrease serum FGF23 levels and prevent its detrimental consequences. Studies of the effects of dietary and pharmacologic phosphate reductions on serum FGF23 levels in patients with CKD have yielded conflicting results. Several researchers have reported that the administration of various phosphate binders lowered serum FGF23 levels in hyperphosphatemic patients on maintenance dialysis and patients with CKD not requiring dialysis with normal or elevated serum phosphate levels (18–22), whereas others failed to find evidence of a significant decrease of serum FGF23 levels (23–25). However, most of these studies were single-center trials and were not placebo controlled, because they compared calcium-based phosphate binders with noncalcium phosphate binders. In addition, the effect of phosphate binders on α-klotho has not been extensively evaluated; two recent reports suggested an elevation of serum α-klotho levels in patients on hemodialysis treated with sevelamer carbonate (26,27).
The double-blind, placebo-controlled, parallel group, randomized, multicenter trial entitled FGF23 Reduction Efficacy of a New Phosphate Binder in CKD (FRENCH) was designed to evaluate the safety and efficacy of the noncalcium-based phosphate binder sevelamer carbonate in normophosphatemic patients with CKD. The study’s primary objective was to assess sevelamer carbonate’s effect, relative to placebo, on serum C-terminal FGF23 levels. The secondary objective was to evaluate the safety of sevelamer administration and its effect on other CKD–mineral bone disease markers, including α-klotho.
Materials and Methods
Study Design
The FRENCH double-blind, placebo-controlled, parallel group, randomized, multicenter trial recruited 96 patients between December of 2010 and December of 2012. After screening, 78 patients were randomized by 14 nephrology outpatient clinics in France: Amiens (Picardie), Valenciennes (Nord Pas de Calais), Rouen (Normandie), Nancy (Lorraine), Saint Etienne (Rhone Alpes), Lyon (Rhone Alpes) (two centers), Paris (Ile de France; two centers), Saint Ouen (Ile de France), Marseille (Provence Alpes Côte d'Azur [PACA]), Nice (PACA), Montpellier (Languedoc Roussillon), and Bordeaux (Aquitaine). The study’s objectives and procedures were approved by an independent investigational review board (CPP Nord Ouest II, Amiens, France) and the French drug agency. The study was registered at clinicaltrials.gov (NCT01220843) in October of 2010.
Study Population
Each patient provided written informed consent to participate in the study. The inclusion criteria were as follows: age 18 years old or over; CKD stage 3b/4 (defined as an eGFR between 15 and 45 ml/min per 1.73 m2 according to the Modification of Diet in Renal Disease Formula); fasting serum phosphate concentration >3.1 mg/dl; serum C-terminal FGF23 concentration >80 relative units (RU)/ml; no concomitant treatment with a phosphate binder; willingness to abstain from taking any of the following medications during all of the study period: antacids and phosphate binders containing aluminum, magnesium, calcium, or lanthanum and treatment for hyperparathyroidism (active vitamin D and calcimimetics) and native vitamin D; no participation in any clinical trial using an investigational product or device during the 30 days preceding the first protocol visit; and ability to read French and understand the study objectives. To note, the inclusion criterion in the initial protocol was a C-terminal FGF23 concentration >120 RU/ml. However, at the start of the inclusion process, we found that this value was too high for adequate recruitment, because there were only 18 patients recruited in 1 year. Hence, we decided to draft an amendment to the protocol and lower the cutoff of C-terminal FGF23. The amendment was approved by the investigational review board in September of 2011.
Main exclusion criteria included the use of phosphate binders, active vitamin D, or calcimimetics; kidney transplantation or parathyroidectomy; a serum phosphate level >5.6 mg/dl; a serum 25 hydroxyvitamin D level <20 ng/ml; a serum intact parathyroid hormone level <30 or >600 pg/ml; a serum total calcium level <8.4 mg/dl; predisposition to or the presence of intestinal or ileus obstruction or severe gastrointestinal motility disorder (such as severe constipation); previous major gastrointestinal surgery; alcohol or drug abuse (excluding tobacco and as judged by the investigator); arrhythmia treated with an antiarrhythmic agent; epilepsy treated with an anticonvulsant; and pregnancy or breastfeeding.
Study Procedures
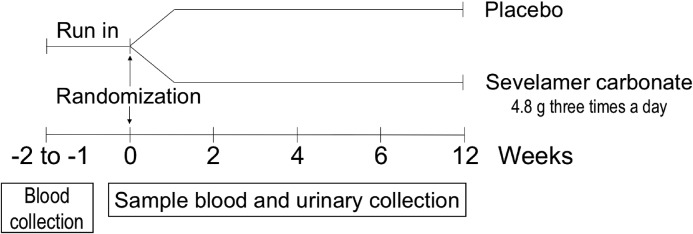
After a screening visit, participants completed a 1- to 2-week run-in period, during which baseline blood and urine samples were collected. This was followed by a 12-week treatment period with five follow-up visits (Figure 1). At randomization, all patients received 100,000 IU cholecalciferol. Patients returned to the clinic 2, 4, 6, and 12 weeks after randomization.
Figure 1.
Study design.
The study personnel and participants were blinded to the use of phosphate binder. The study participants took a dose of 4.8 g sevelamer (or placebo) three times a day for 12 weeks. If the serum phosphate level decrease below 2.5 mg/dl, the dose of treatment was divided by two. Adherence to study medication was monitored via pill counts.
Treatment Allocation and Randomization
Centralized randomization was performed by the clinical research unit at Amiens University Hospital. A data manager configured an interactive web response system (running Ennov Clinical software V6.2; Ennov SA, Paris, France) to randomize patients using a minimization algorithm. The randomization was stratified by center and took account of the initial circulating concentration of C-terminal FGF23 (FGF23≤250 RU/ml versus FGF23>250 RU/ml). A list of codes was randomly generated and then sent to the subcontractor responsible for drug labeling.
After informed consent was signed at visit 0, each patient received a unique five-digit patient number: the first two digits corresponded to the center number, and the next three began at 001 and increased with inclusion at each center. When the patient’s eligibility was confirmed at visit 1, the nephrologist investigator entered the patient into the interactive web response system, which then indicated the code of the treatment allocation of the patient. At each investigating center, the central pharmacy delivered the corresponding treatment (placebo or sevelamer carbonate) to the patient. This allocation procedure was repeated when treatment was handed over to the patient at visit 3. Only the study’s data manager and the subcontractor responsible for drug labeling had access to the listing indicating the correspondence between the unit treatment number and the type of treatment (placebo or sevelamer carbonate).
Clinical and Laboratory Evaluations
All patients underwent a clinical examination and an interview (to establish their personal medical history). Age, sex, anthropometric data, and medication use were recorded. Fasting blood samples and 24-hour urine samples were collected after inclusion in the study and at each study visit. All of the samples were assayed for C-terminal FGF23 in the same central laboratory using an ELISA (Human FGF23 C-Terminal ELISA Kit; Immutopics International, San Clemente, CA; intra-assay coefficient of variation: <2.4%; interassay coefficient of variation: <4.7%; limit of detection: 1.5 RU/ml).
Serum human intact FGF23 was measured according to the manufacturer’s instructions using an ELISA (Human FGF23 Intact ELISA Kit; Immutopics International; intra-assay coefficient of variation: <4.1%; interassay coefficient of variation: <9.1%; limit of detection: 1.5 pg/ml) along with human soluble α-klotho (Human Soluble α-Klotho Assay Kit; Immuno Biologic Laboratories Co. Ltd., Fujioka, Japan; intra-assay coefficient of variation: <3.6%, interassay coefficient of variation: <11.4%; limit of detection: 6.15 pg/ml).
Outcomes
The primary efficacy criterion was the change in the C-terminal FGF23 level from baseline (randomization) to week 12. Secondary outcomes included changes in serum mineral metabolism parameters, cholesterol, and urinary parameters.
Statistical Analyses
Sample Size Determination.
We estimated that a sample size of 80 patients would detect a 30% reduction in median C-terminal FGF23 levels in the sevelamer carbonate group compared with the placebo group after 3 months (30% reduction was obtained in a previous study [21]) with a power of 90%, a two-sided α-risk of 5%, and an anticipated dropout rate of 15% (in a Wilcoxon–Mann–Whitney test on the basis of the conservative assumption of a normal distribution of changes in serum C-terminal FGF23 levels after 12 weeks compared with baseline).
Analyses.
All randomized patients were included in an intention to treat analysis. The per protocol population comprised all randomized patients who were protocol compliant 12 weeks after randomization. Data were expressed as the mean±SD, median (interquartile range [IQR]), and range or frequency as appropriate. Patients were stratified by treatment group. Intergroup comparisons were performed with a chi-squared test (for categorical variables) and t test or the Mann–Whitney test (for continuous variables). We compared the change over time between randomization and week 12 in C-terminal FGF23 and other parameters in the two study groups and also tested for significant changes from baseline to week 12 within each study group in a repeated measures ANOVA. Response to treatment was defined as a decrease in C-terminal FGF23 levels of at least 30%. A chi-squared test was used to compare the treatment responses in the two groups. Safety (including adverse events reports) was analyzed in each study group. The threshold for statistical significance was set to P≤0.05. All statistical analyses were performed using SPSS software (version 18.0; SPSS Inc., Chicago, IL) for Windows (Microsoft Corp., Redmond, WA) and SAS software (version 9.2; SAS Institute Inc., Cary, NC).
Results
The Study Population
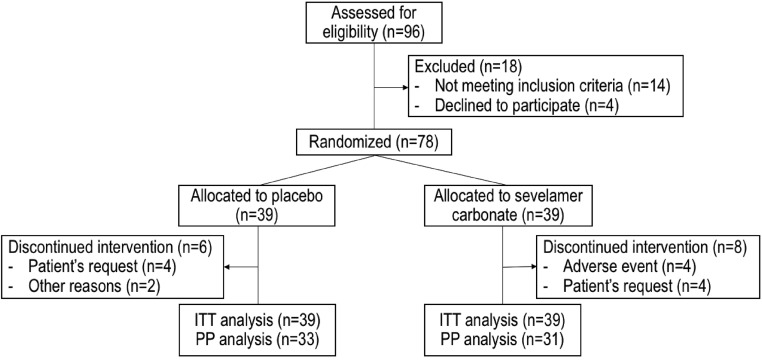
A total of 96 patients were screened; 78 met the inclusion criteria and were randomized to receive placebo (n=39) or sevelamer carbonate (n=39). The main reason for noninclusion was a serum C-terminal FGF23 level below the chosen cutoff. The intention to treat and per protocol populations were made up of 78 and 64 randomized patients, respectively (Figure 2). The mean adherence rate was 88% in the placebo group and 86% in the sevelamer group in the intention to treat population. In the sevelamer group, there was no change in the prescribed dose (4.8 g) during the study period.
Figure 2.
Study flow chart. ITT, intention to treat; PP, per protocol.
The baseline characteristics of randomized patients are summarized in Table 1; the two treatment groups did not differ significantly, with the exception of higher 25 hydroxyvitamin D levels in the sevelamer group than in the placebo group. The patient mean±SD age was 63±13 years old, 55 were men (71%), the mean eGFR was 27±9 ml/min per 1.73 m2, the mean serum phosphate level was 3.8±0.5 mg/dl, and the median serum C-terminal FGF23 level was 157 (IQR, 120–241) RU/ml.
Table 1.
Baseline characteristics of the randomized patients
| Variables | All, n=78 | Placebo, n=39 | Sevelamer, n=39 |
|---|---|---|---|
| Age, yr | 63±13 | 63±14 | 63±13 |
| Men, n (%) | 55 (71) | 28 (72) | 27 (69) |
| Body mass index, kg/m2 | 28±5 | 28±5 | 28±5 |
| GFR MDRD, ml/min per 1.73 m2 | 27±9 | 28±10 | 25±8 |
| SBP, mmHg | 142±20 | 143±20 | 141±20 |
| DBP, mmHg | 79±10 | 80±10 | 78±10 |
| Serum C-terminal FGF23, RU/ml | 157 (120–241) | 142 (120–221) | 166 (120–249) |
| Serum intact FGF23, pg/ml | 72 (47–103) | 71 (42–118) | 72 (53–109) |
| Serum α-klotho, pg/ml | 809 (674–981) | 784 (648–1060) | 830 (714–927) |
| Serum phosphorus, mg/dl | 3.8±0.5 | 3.9±0.5 | 3.8±0.6 |
| Serum calcium, mg/dl | 9.4±0.4 | 9.3±0.4 | 9.4±0.4 |
| Serum creatinine, mg/dl | 2.6±0.9 | 2.6±0.8 | 2.7±0.9 |
| Serum intact PTH, pg/ml | 97 (61–127) | 103 (71–141) | 91 (58–123) |
| Serum 25 hydroxyvitamin D, ng/ml | 31±12 | 29±11 | 34±18 |
| Serum 1,25 dihydroxyvitamin D, ng/ml | 28 (22–40) | 31 (23–39) | 26 (20–42) |
| CRP, mg/L | 3 (2–8) | 3 (2–4) | 5 (3–10) |
| Serum triglycerides, mg/dl | 137 (93–200) | 151 (97–256) | 136 (89–163) |
| Serum total cholesterol, mg/dl | 182±42 | 183±39 | 179±45 |
| Serum HDL cholesterol, mg/dl | 54±19 | 53±20 | 54±19 |
| Serum LDL cholesterol, mg/dl | 96±39 | 92±39 | 99±40 |
| Serum hemoglobin, g/dl | 12.6±1.4 | 12.5±1.3 | 12.7±1.5 |
| Urinary phosphate-to-urinary creatinine ratio, mmol/mmol | 2.0±0.6 | 2.0±0.5 | 2.0±0.7 |
| Urinary calcium-to-urinary creatinine ratio, mmol/mmol | 0.1±0.1 | 0.1±0.1 | 0.1±0.1 |
| Urinary creatinine, mmol/L | 5.8±2.2 | 5.4±1.8 | 6.2±2.5 |
Data are quoted as the mean±SD for normally distributed variables, the median (interquartile range) for non-normally distributed variables, or the number (percentage) for binary variables. GFR MDRD, GFR calculated with the Modification of Diet in Renal Disease formula; SBP, systolic BP; DBP, diastolic BP; FGF23, fibroblast growth factor 23; RU, relative units; PTH, parathyroid hormone; CRP, C-reactive protein.
Primary Efficacy Criterion
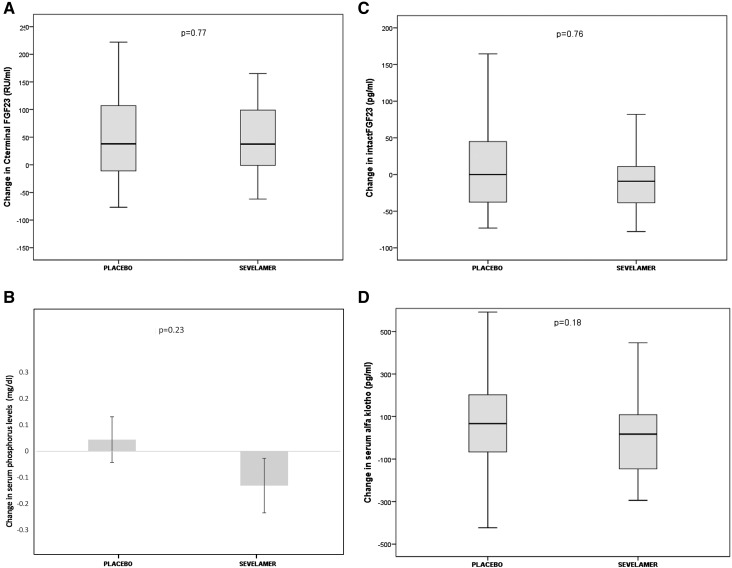
The sevelamer and placebo groups did not differ significantly in terms of the median change in C-terminal FGF23 levels (P=0.77), because this parameter did not change significantly in either group. Indeed, in the placebo group, the median change was 38 (IQR, −13–114) RU/ml given a median level of 236 (IQR, 128–273) RU/ml (Table 2) after 12 weeks of treatment. In the sevelamer group, the median change was 37 (IQR, −1–101) RU/ml given a median of 238 (IQR, 142–276) RU/ml at the end of study. The per protocol analysis gave similar results (Figure 3A). At the second visit after randomization, the C-terminal FGF23 level had increased by about 30% in both treatment arms (Table 2). There were two responders (defined as a ≥30% fall in C-terminal FGF23) in the placebo group and one in the sevelamer carbonate group. Only 20 patients (ten in each group) displayed a fall in the C-terminal FGF23 level after 12 weeks of treatment. In this subset, there were no significant correlations between the change in C-terminal FGF23 levels and the baseline C-terminal FGF23 level or the change in other biomarkers of mineral bone disease.
Table 2.
Change over time in clinical biochemistry parameters during the study period
| Variables | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 |
|---|---|---|---|---|---|
| Serum C-terminal FGF23, RU/ml | |||||
| Placebo | 142 (120–221) | 204 (119–274) | 175 (116–258) | 212 (126–253) | 236 (128–273) |
| Sevelamer | 166 (120–249) | 200 (122–306) | 212 (131–299) | 222 (132–298) | 238 (142–276) |
| Serum intact FGF23, pg/ml | |||||
| Placebo | 71 (42–118) | 104 (56–162) | 101 (79–135) | 102 (73–187) | 112 (74–204) |
| Sevelamer | 72 (53–109) | 113 (73–168) | 113 (87–180) | 110 (77–147) | 129 (78–159) |
| Serum phosphorus, mg/dl | |||||
| Placebo | 3.9±0.5 | 3.9±0.7 | 3.8±0.6 | 3.9±0.7 | 3.9±0.6 |
| Sevelamer | 3.8±0.5 | 3.8±0.7 | 3.6±0.6 | 3.7±0.7 | 3.7±0.6 |
| Serum calcium, mg/dl | |||||
| Placebo | 9.3±0.5 | 9.3±0.5 | 9.2±0.4 | 9.3±0.4 | 9.3±0.4 |
| Sevelamer | 9.4±0.4 | 9.4±0.5 | 9.4±0.5 | 9.3±0.5 | 9.3±0.6 |
| Serum intact PTH, pg/ml | |||||
| Placebo | 103 (71–141) | 93 (62–115) | 98 (72–134) | 87 (67–140) | 96 (67–123) |
| Sevelamer | 91 (58–123) | 79 (52–141) | 94 (67–147) | 87 (52–158) | 96 (68–126) |
| Serum creatinine, mg/dl | |||||
| Placebo | 2.6±0.8 | 2.6±0.8 | 2.6±0.8 | 2.6±0.8 | 2.6±0.8 |
| Sevelamer | 2.6±0.8 | 2.6±0.8 | 2.6±0.8 | 2.6±0.8 | 2.6±0.8 |
| GFR MDRD, ml/min per 1.73 m2 | |||||
| Placebo | 28±10 | 27±8 | 28±9 | 27±9 | 28±8 |
| Sevelamer | 25±8 | 26±9 | 26±8 | 27±8 | 26±9 |
Data are quoted as the mean±SD for normally distributed variables and the median (interquartile range) for non-normally distributed variables. FGF23, fibroblast growth factor 23; RU, relative units; PTH, parathyroid hormone; GFR MDRD, GFR calculated with the Modification of Diet in Renal Disease formula.
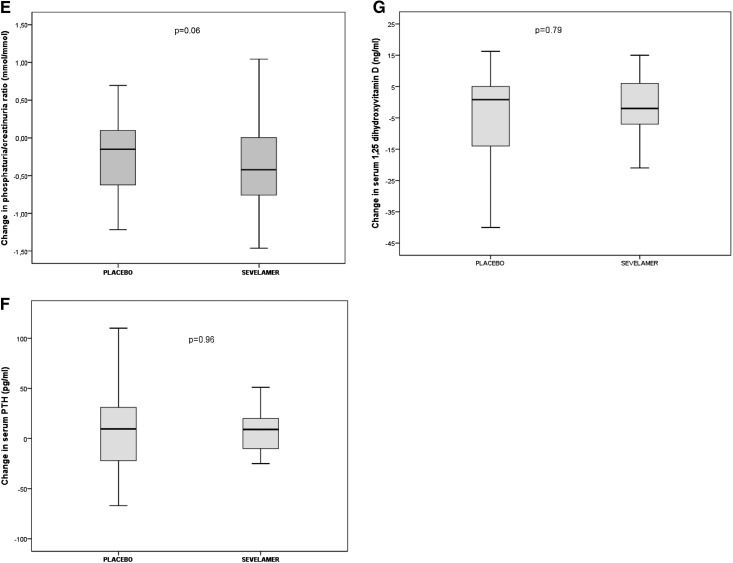
Figure 3.
No significant difference between the treatment groups for serum C-terminal fibroblast growth factor 23 (FGF23) level or in related variables. Except where noted otherwise, the band within each box marks the median, the ends of the boxes mark the first and third quartiles, and the whiskers mark the range. Change in (A) serum C-terminal fibroblast growth factor 23 level, (B) serum phosphorus (mean change; error bars indicate SD), (C) serum intact FGF23 levels, (D) serum α-klotho level and phosphorus level, (E) phosphaturia-to-creatinuria ratio level, (F) serum parathyroid hormone (PTH) level, and (G) serum 1,25 dihydroxyvitamin D.
Secondary Efficacy Criteria
The sevelamer and placebo groups did not differ significantly in terms of the change in serum phosphate levels. The serum phosphate level was stable in the placebo and sevelamer groups (Figure 3B). There was no intergroup difference in the change in intact FGF23 levels (Figure 3C) and α-klotho levels (Figure 3D). After 12 weeks of treatment, the urinary phosphate-to-creatinine ratio fell significantly between baseline and week 12 in the sevelamer group (2.03±0.64 mmol/mmol at randomization to 1.65±0.57 mmol/mmol at week 12; P=0.003), when it is stable in placebo group (Figure 3E). There was no intergroup difference in the change in serum parathyroid hormone levels (Figure 3F) and 1,25 dihydroxyvitamin D levels (Figure 3G).
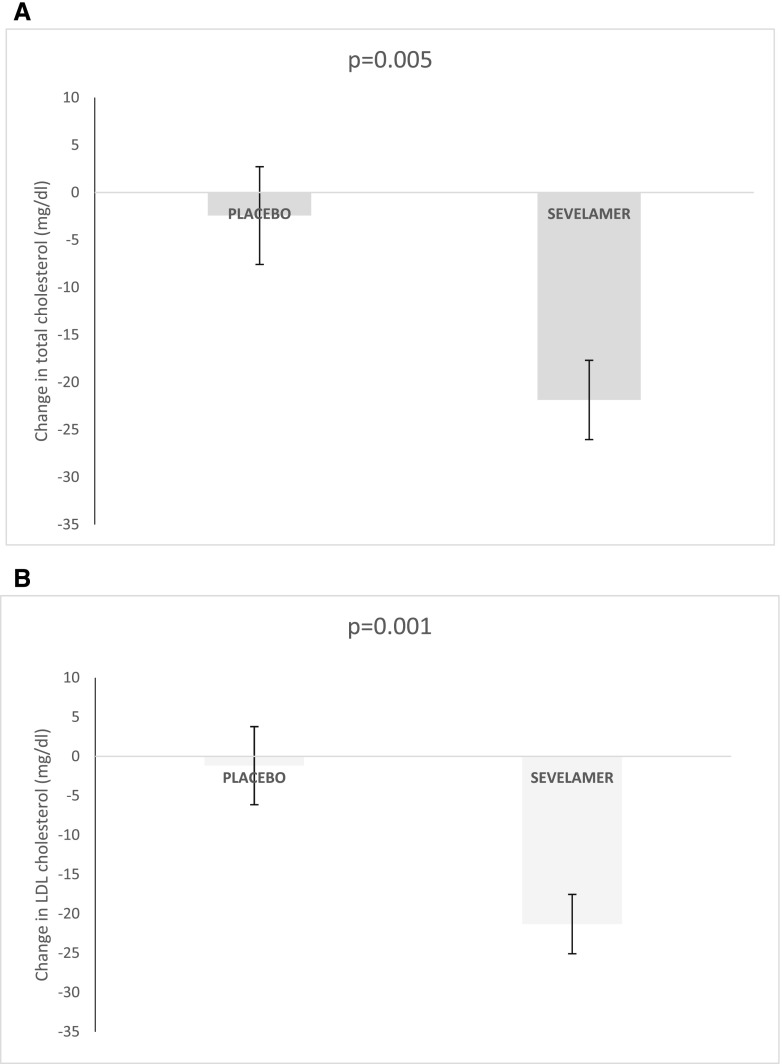
The sevelamer and placebo groups differed significantly with regard to the change of serum total cholesterol and LDL cholesterol levels at week 12 (P<0.001 and P=0.001, respectively). The mean decrease in total cholesterol was 21±25 mg/dl in the sevelamer group and 2±30 mg/dl in the placebo group (P<0.001). (Figure 4).
Figure 4.
Total and LDL cholesterol decreased more for the sevelamer group. Change in (A) serum total cholesterol level and (B) serum LDL cholesterol.
Changes in other serum (i.e., calcium, creatinine, urea, albumin, C-reactive protein, total alkaline phosphatases, and 25 hydroxyvitamin D) and urinary (calcium, urea, and creatinine) parameters were similar in the sevelamer and placebo groups.
Adverse events were more frequent in the sevelamer group (Table 3). A number of serious adverse events occurred but were not considered to be linked to the investigational product. Most of the adverse events affected the gastrointestinal system. It is noteworthy that patients who experienced gastrointestinal side effects and those who did not showed similar changes in C-terminal FGF23 levels. None of the patients displayed a serum phosphate level below 2.5 mg/dl during the study, and therefore, no dose adjustment was required.
Table 3.
Summary of adverse events in the randomized study population (Medical Dictionary for Regulatory Activities 18.1 English, classification)
| Adverse Events | Placebo | Sevelamer Carbonate |
|---|---|---|
| Serious adverse events | 0 | 11 |
| Related to investigational product | 0 | 0 |
| Cardiac disorders | 0 | 1 (9%) |
| Myocardial infarction | 0 | 1 |
| Reproductive system and breast disorders | 0 | 1 (9%) |
| Prostatitis | 0 | 1 |
| Renal and urinary disorders | 0 | 3 (27%) |
| Acute renal disease | 0 | 1 |
| Urinary tract obstruction | 0 | 1 |
| Acute urinary retention | 0 | 1 |
| Nervous system disorders | 0 | 1 (9%) |
| Headache | 0 | 1 |
| Gastrointestinal disorders | 0 | 1 (9%) |
| Acute abdominal pain | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 0 | 2 (18%) |
| Rhabdomyolysis | 0 | 1 |
| Chest pain | 0 | 1 |
| Vascular disorders | 0 | 1 (9%) |
| Hypertension | 0 | 1 |
| Injury, poisoning, and procedural complications | 0 | 1 (9%) |
| Fall | 0 | 1 |
| Nonserious adverse events | 17 | 39 |
| Surgical and medical procedure | 0 (0%) | 1 (3%) |
| Skin lesion excision | 0 | 1 |
| Skin and subcutaneous tissue disorders | 0 (0%) | 2 (5%) |
| Dyshidrosis | 0 | 1 |
| Redness | 0 | 1 |
| Renal and urinary disorder | 1 (6%) | 1 (3%) |
| Acute renal injury | 0 | 1 |
| Macroscopic hematuria | 1 | 0 |
| Nervous system disorder | 0 (0%) | 1 (3%) |
| Vertigo | 0 | 1 |
| Gastrointestinal disorders | 4 (23%) | 17 (43%) |
| Bloating | 1 | 1 |
| Gastroesophageal burning | 0 | 1 |
| Constipation | 0 | 4 |
| Diarrhea | 0 | 5 |
| Abdominal pain | 1 | 0 |
| Dysphagia | 0 | 1 |
| Nausea | 0 | 2 |
| Gastroesophageal reflux disease | 0 | 1 |
| Digestive disorder | 0 | 1 |
| Intestinal transit disorder | 1 | 0 |
| Vomiting | 1 | 1 |
| Musculoskeletal and connective tissue disorders | 3 (18%) | 2 (5%) |
| Arm pain | 1 | 0 |
| Leg pain | 1 | 0 |
| Muscular pain | 0 | 1 |
| Weakness of lower extremities | 1 | 0 |
| Polyarthralgia | 0 | 1 |
| Respiratory, thoracic, and mediastinal conditions | 0 (0%) | 2 (5%) |
| Breathlessness | 0 | 1 |
| Breathing difficult | 0 | 1 |
| Vascular disorders | 1 (6%) | 0 (0%) |
| Orthostatic hypotension | 1 | 0 |
| Infections and infestations | 2 (12%) | 2 (5%) |
| Cervical abscess | 0 | 1 |
| Dental infection | 1 | 0 |
| Urinary tract infection | 1 | 0 |
| Superinfection | 0 | 1 |
| Metabolism and nutrition disorders | 1 (6%) | 4 (10%) |
| Gout attack | 0 | 1 |
| Hyperkaliemia | 0 | 3 |
| Hypertriglyceridemia | 1 | 0 |
| General disorders and administration site conditions | 5 (29%) | 6 (15%) |
| Disease aggravation | 1 | 0 |
| Pain | 0 | 1 |
| Thoracic pain | 1 | 0 |
| Tiredness | 1 | 0 |
| Drug intolerance | 0 | 1 |
| Edema | 2 | 3 |
| Inflammatory reaction | 0 | 1 |
| Neoplasms: benign, malignant, and perinatal conditions | 0 (0%) | 1 (3%) |
| Lipoma | 0 | 1 |
Discussion
In the FRENCH double-blind, placebo-controlled, parallel group, randomized, multicenter study, we failed to show a statistically significant difference in the change in serum C-terminal and intact FGF23 levels and α-klotho comparing patients with CKD stage 3b/4 treated with placebo with those receiving sevelamer for 12 weeks. The FRENCH Trial is a robust multicenter study of the effect of sevelamer carbonate on FGF23 levels and α-klotho levels in patients with CKD stage 3b/4. In addition to the double-blind, placebo-controlled design, the study featured pill counts to provide a reliable estimate of adherence to treatment.
Targeting FGF23 in patients with early-stage CKD seems to be a valid approach: (A) FGF23 concentrations rise early in the course of disease, before increases serum phosphate levels (3), and (B) there is a strong association between elevated FGF23 and mortality (9). However, lowering FGF23 by reducing intestinal absorption of phosphate via oral phosphate binders and/or the restriction of dietary phosphate has shown inconsistent results—particularly in nondialyzed patients with CKD. Furthermore, there is marked interstudy heterogeneity in terms of methodologies, patient populations with CKD, and comparator treatments. Our results agree with the findings of a recent single-center, placebo-controlled trial, in which the change in serum C-terminal FGF23 levels was similar in the placebo and sevelamer groups (25). Comparisons of lanthanum carbonate and placebo have yielded similarly negative results in normophosphatemic, nondialyzed patients with CKD (19,24,28). Recently, Spatz et al. (23) reported that serum C-terminal FGF23 levels did not significantly decrease in hyperphosphatemic patients with advanced CKD during sevelamer carbonate treatment. However, the study by Spatz et al. (23) was not randomized and lacked a control group. Randomized studies comparing calcium-based binders with noncalcium-based binders suggest that the latter (sevelamer [21,22] and lanthanum [29]) have a greater lowering effect on serum FGF23 levels in normo- or hyperphosphatemic nondialyzed patients. A significant difference between the two arms might be related to the fact that treatment with the comparator (calcium carbonate) is often associated with an increase in FGF23 levels.
In this study, all of the patients were free from vitamin D deficiency, because they received 100,000 IU cholecalciferol at the time of randomization. The increase of FGF23 concentrations after the vitamin D repletion could prevent us from detecting an effect of sevelamer carbonate on FGF23 compared with placebo. However, this vitamin D supplementation mimed clinical practice, where the use of cholecalciferol supplementation is very frequent. Indeed, Mariani et al. (30) found 44% vitamin D supplementation among nondialysis patients with CKD and eGFR of 42.1 ml/min per 1.73 m2 in multicenter, prospective, observational cohort study of 3939 participants.
It was recently reported that a 12-week treatment with a novel, iron-based phosphate binder (ferric citrate hydrate) resulted in a significant reduction in intact FGF23 levels in hyperphosphatemic patients with advanced CKD (mean eGFR: 9±6 ml/min per 1.73 m2) (31). The combination of dietary phosphate restriction with phosphate binder treatments seems to be more effective than either approach alone (32,33), although the magnitude of the reported decrease varies from one study to another.
In contrast to the literature data from patients on hemodialysis, we failed to observe a change in serum phosphate in the sevelamer group. This is in agreement with other studies in patients with CKD stages 3–5, which failed to show a larger change in serum phosphate with the use of phosphate binders (19,21,25,28,34). Indeed, in a recent controlled trial of three commercially available phosphate binders, Block et al. (25) reported a slight reduction in serum phosphate levels (mean serum phosphorus declined from 4.2 to 3.9 mg/dl over 9 months). However, a recent study of a crossover dietary intervention (to evaluate the circadian patterns of serum phosphate in patients with CKD [32]) found that serum phosphate levels were consistently at their lowest in the morning and at their highest in the middle of afternoon. Given that most studies of phosphate binders have evaluated fasting (morning) serum phosphate concentrations, the effects of binders on serum phosphate may have been underestimated. Hence, these new data raise the question of whether morning phosphate sampling should be routinely performed in therapeutic studies of phosphate binders. Lastly, we observed a significantly decrease in urinary phosphate-to-creatinine ratio levels (in contrast to serum levels) in the sevelamer group, meaning that adherence to treatment was reliable and that sevelamer reduced intestinal phosphate absorption.
Correction of α-klotho deficiency could represent an interesting therapeutic target to prevent kidney disease progression and cardiovascular disease. Only limited data (26,27) suggested an elevation of serum α-klotho levels in patients treated with sevelamer. However, the quality and thus, accuracy of the commercially available α-klotho assays have been questioned. In this study, the sevelamer and placebo groups did not differ significantly in terms of change in serum α-klotho levels.
As described above, we found that patients randomized to the sevelamer group had significantly lower total and LDL cholesterol levels after 12 weeks of treatment (relative to patients in the placebo group). Indeed, sevelamer binds bile acids as well as phosphate in the gut, which contribute to a reduction in serum LDL cholesterol levels in subjects with CKD and subjects without CKD (35). Indeed, sevelamer is known to lower serum LDL and total cholesterol levels in predialysis patients with CKD (22,36,37).
The most common adverse events reported in the study were gastrointestinal disorders (such as constipation and diarrhea), which are frequently reported by patients taking phosphate binders. Most of the symptoms were mild but prompted patients to stop taking the study medication.
This study’s limitations included the lack of dietary monitoring and a standardized diet due to logistic constraints and the multicenter nature of the study. The value of combining dietary phosphate restriction with the administration of phosphate binders for reducing FGF23 levels has been emphasized (19). However, our protocol seems closer to the real life clinical use, in which phosphate diet restriction is challenging to achieve in the long term. Furthermore, we cannot rule out underdosing, poor compliance (despite the reduction in cholesterol levels), and decreased statistical power due to small sample size. Supplementation with cholecalciferol at inclusion induced an increase in FGF23 levels (albeit to a similar extent in the two treatments arms).
The study’s main strength is its nationwide, multicenter design, which is more representative of French population care and decreases bias due to patient care practices in a single center. One other strength was the screening for FGF23 before randomization—although a higher cutoff might have been required to see a reduction in FGF23 levels. Furthermore, the study was monitored to check adherence to treatment; this aspect was especially valuable, because study participants had to take as many as six pills per day, which could represent a risk of nonadherence.
The FRENCH double-blind, placebo-controlled, parallel group, randomized, multicenter trial evaluated the use of the noncalcium-based phosphate binder sevelamer carbonate in nondialyzed patients with CKD. Sevelamer carbonate significantly reduced phosphaturia but failed to significantly modify serum phosphate, C-terminal FGF23, intact FGF23, or α-klotho levels. Our findings and other recent data suggest that the widely used phosphate binder sevelamer carbonate does not consistently decrease levels of biomarkers of mineral disorders (including FGF23 and phosphate). Accordingly, the putative benefits of phosphate binders need to be evaluated in randomized, controlled trials with hard clinical end points (cardiovascular events and mortality, for example).
Disclosures
Sophie Liabeuf, Jean-Philippe Ryckelynck, Najeh El Esper, Romuald Mentaverri, Eric Thervet, Christian Combe, Bertrand Dussol, Philippe Vanhille, Luc Frimat, and Dominique Prié have no disclosure in relation with the present article. Dr. Fouque reports personal fees from Amgen, Fresenius Medical Care, Sanofi, Astellas, Vifor, and Fresenius Kabi. Pablo Urena Torres has received speaker’s honoraria, clinical trial studies honoraria, and advisory board honoraria from Amgen, Shire, Astellas, Hemotech, and Vifor-Fresenius. Gabriel Choukroun reports research grants from Genzyme and Sanofi and honorarium for conference from Genzyme, Amgen and Vifor pharma.
Supplementary Material
Acknowledgments
The study was sponsored by Amiens University Hospital. Sanofi Genzyme provided financial assistance, drugs, and placebo.
A list of the collaborators is available in Supplemental Appendix.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Continued Search for Therapies to Favorably Modify Phosphate and FGF23 Levels in CKD,” on pages 1911–1913.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03030317/-/DCSupplemental.
References
- 1.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GFM: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CAM, Lash JP, Hsu C-Y, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P; MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke H-D, Choukroun G, Drueke TB, Massy ZA; European Uremic Toxin (EUTox) Work Group : FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int 23: 2017–2025, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St. John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-o M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD, Deo R, Rahman M, Feldman HI, Go AS, Isakova T, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the chronic renal insufficiency cohort study. JAMA Cardiol 1: 548–556, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez OM, Wolf M, Taylor EN: Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol 6: 2871–2878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kim HR, Nam BY, Kim DW, Kang MW, Han J-H, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI, Kim CH, Oh HJ, Yoo T-H, Kang S-W, Han DS, Han SH: Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis 61: 899–909, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Buiten MS, de Bie MK, Bouma-de Krijger A, van Dam B, Dekker FW, Jukema JW, Rabelink TJ, Rotmans JI: Soluble Klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol 15: 197, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdallah E, Mosbah O, Khalifa G, Metwaly A, El-Bendary O: Assessment of the relationship between serum soluble Klotho and carotid intima-media thickness and left ventricular dysfunction in hemodialysis patients. Kidney Res Clin Pract 35: 42–49, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-González JF, Donate-Correa J, Muros de Fuentes M, Pérez-Hernández H, Martínez-Sanz R, Mora-Fernández C: Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart 100: 34–40, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Parra E, Gonzalez-Casaus ML, Galán A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A: Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant 26: 2567–2571, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Isakova T, Gutiérrez OM, Smith K, Epstein M, Keating LK, Jüppner H, Wolf M: Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 26: 584–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T; ROD21 Clinical Research Group : Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 9: 336–339, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira RB, Cancela ALE, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RMA: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C: Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: A randomized clinical trial. Am J Kidney Dis 59: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Spatz C, Roe K, Lehman E, Verma N: Effect of a non-calcium-based phosphate binder on fibroblast growth factor 23 in chronic kidney disease. Nephron Clin Pract 123: 61–66, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Ureña-Torres P, Prié D, Keddad K, Preston P, Wilde P, Wan H, Copley JB: Changes in fibroblast growth factor 23 levels in normophosphatemic patients with chronic kidney disease stage 3 treated with lanthanum carbonate: Results of the PREFECT study, a phase 2a, double blind, randomized, placebo-controlled trial. BMC Nephrol 15: 71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenglet A, Liabeuf S, El Esper N, Brisset S, Mansour J, Lemaire-Hurtel A-S, Mary A, Brazier M, Kamel S, Mentaverri R, Choukroun G, Fournier A, Massy ZA: Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study. Nephrol Dial Transplant 32: 870–879, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Lin H-H, Liou H-H, Wu M-S, Lin C-Y, Huang C-C: Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum Klotho levels in chronic haemodialysis patients. Nephrology (Carlton) 19: 672–678, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, Ross W, Windus D, Dávila-Román VG, Hruska KA: Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol 38: 158–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano S, Ojeda R, Rodríguez M, Almadén Y, Rodríguez M, Martín-Malo A, Aljama P: The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol 80: 17–22, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Mariani LH, White MT, Shults J, Anderson CAM, Feldman HI, Wolf M, Reese PP, Denburg MR, Townsend RR, Lo JC, Cappola AR, Carlow D, Gadegbeku CA, Steigerwalt S, Leonard MB; CRIC Study Investigators : Increasing use of vitamin D supplementation in the chronic renal insufficiency cohort study. J Ren Nutr 24: 186–193, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Block GA: Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9: 543–552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ix JH, Anderson CAM, Smits G, Persky MS, Block GA: Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: A crossover study. Am J Clin Nutr 100: 1392–1397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigrist M, Tang M, Beaulieu M, Espino-Hernandez G, Er L, Djurdjev O, Levin A: Responsiveness of FGF-23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): Results of a randomized trial. Nephrol Dial Transplant 28: 161–169, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, Edwards NC, Steeds RP, Ferro CJ: Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 24: 842–852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braunlin W, Zhorov E, Guo A, Apruzzese W, Xu Q, Hook P, Smisek DL, Mandeville WH, Holmes-Farley SR: Bile acid binding to sevelamer HCl. Kidney Int 62: 611–619, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Di Iorio B, Bellasi A, Russo D; INDEPENDENT Study Investigators : Mortality in kidney disease patients treated with phosphate binders: A randomized study. Clin J Am Soc Nephrol 7: 487–493, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.