Abstract
Background and objectives
Fistulas, the preferred form of hemodialysis access, are difficult to establish and maintain. We examined the effect of a multidisciplinary vascular access team, including nurses, surgeons, and radiologists, on the probability of using a fistula catheter-free, and rates of access-related procedures in incident patients receiving hemodialysis.
Design, setting, participants, & measurements
We examined vascular access outcomes in the first year of hemodialysis treatment before (2004–2005, preteam period) and after the implementation of an access team (2006–2008, early-team period; 2009–2011, late-team period) in the Calgary Health Region, Canada. We used logistic regression to study the probability of fistula creation and the probability of catheter-free fistula use, and negative binomial regression to study access-related procedure rates.
Results
We included 609 adults (mean age, 65 [±15] years; 61% men; 54% with diabetes). By the end of the first year of hemodialysis, 102 participants received a fistula in the preteam period (70%), 196 (78%) in the early-team period (odds ratios versus preteam, 1.47; 95% confidence interval, 0.92 to 2.35), and 139 (66%) in the late-team period (0.85; 0.54 to 1.35). Access team implementation did not affect the probability of catheter-free use of the fistula (odds ratio, 0.87; 95% confidence interval, 0.52 to 1.43, for the early; and 0.89; 0.52 to 1.53, for the late team versus preteam period). Participants underwent an average of 4–5 total access-related procedures during the first year of hemodialysis, with higher rates in women and in people with comorbidities. Catheter-related procedure rates were similar before and after team implementation; relative to the preteam period, fistula-related procedure rates were 40% (20%–60%) and 30% (10%–50%) higher in the early-team and late-team periods, respectively.
Conclusion
Introduction of a multidisciplinary access team did not increase the probability of catheter-free fistula use, but resulted in higher rates of fistula-related procedures.
Keywords: arteriovenous fistula, vascular access, Central Venous Catheters, hemodialysis, Aged, Odds Ratio, Logistic Models, Confidence Intervals, Comorbidity, renal dialysis, Probability, Surgeons, diabetes mellitus, Radiologists, Fistula, Canada
Introduction
Arteriovenous fistulas are promoted as the preferred form of vascular access for hemodialysis (1–3), on the basis of studies showing lower rates of vascular access complications (4,5), morbidity, and mortality (6) in people using fistulas compared with people using central venous catheters or grafts. Use of catheters is associated with the highest risk of adverse outcomes, and has been used as an indicator of suboptimal patient care (7). However, fistulas are difficult to establish and maintain. Approximately half of newly created fistulas fail to mature or require repeated access-related procedures to become suitable for regular use or to maintain patency, especially in people who are older or have comorbid conditions (8).
Given the limited ability of individual nephrologists to attend to the multiple and complex aspects of vascular access care, most kidney care programs have established multidisciplinary vascular access teams (access teams) (9–12). Access teams include a medical director, one or more dedicated vascular access coordinators, vascular surgeons, and interventional nephrologists or radiologists (13). Through enhanced multidisciplinary interaction and patient education, access teams facilitate vascular access creation and deliver ongoing vascular access care, including prevention and treatment of access complications. With pressure to increase the use of fistulas for hemodialysis access, they have historically been charged with increasing the number of people using fistulas within their programs. Although existing studies suggest that these initiatives are associated with an increased placement of fistulas (13–15), they do not report on whether the newly created fistulas function well enough to allow catheter removal—the main goal of promoting fistulas. In addition, little is known about possible unintended consequences of promoting fistulas, such as increased rates of access-related procedures.
We examined the effect of an access team on vascular access outcomes using data from a large renal program in the province of Alberta, Canada (16). We studied the probability of attaining catheter-free fistula use in people starting hemodialysis as an indicator of a successful fistula (i.e., a fistula considered suitable for long-term cannulation for hemodialysis). We also studied the rates of vascular access–related procedures as a measure of hemodialysis access–related morbidity, and as an indicator of potentially unintended consequences of promoting fistulas (17). To inform vascular access policy and practice, we studied these outcomes across patient categories defined by age, sex, and comorbidities.
Materials and Methods
Study Design, Data Sources, and Ethics Approval
We did a retrospective cohort study examining vascular access outcomes before and after the implementation of an access team in the Calgary Health Region of the Southern Alberta Renal Program (16). The Southern Alberta Renal Program provides kidney care in southern Alberta (catchment area of about 2 million people) and hemodialysis services for >800 patients. Approximately 80% of these patients live in the Calgary Health Region. We used data captured in the Southern Alberta Renal Program dialysis database (18) and the Dialysis Measurement, Analysis, and Reporting (DMAR) system (19). DMAR is an interactive web-based application that uses standardized data-coding practices to collect complete and high-quality data. Details on DMAR data elements and quality verification methods are reported in the Supplemental Material. The Conjoint Health Research Ethics Board at the University of Calgary approved this study.
Multidisciplinary Vascular Access Team
The access team was fully implemented at the beginning of 2006 and consists of a medical director (a nephrologist), dedicated nursing staff, access surgeons, and interventional radiologists (Table 1). Nursing staff and nephrologists in eight hemodialysis units assist in identifying patients on hemodialysis who are suitable for arteriovenous fistula creation. As there are no standard criteria to define eligibility for a fistula, a vascular access coordinator screens all patients who are expected to continue hemodialysis therapy long-term, and arranges multidisciplinary assessment if necessary. Patients who undergo fistula creation are seen in follow-up clinics after surgery; monthly rounds are scheduled to evaluate challenging cases. The team is also responsible for vascular access monitoring and surveillance, and for arranging referral to surgery or interventional radiology as required.
Table 1.
Multidisciplinary vascular access team structure and functions
| Composition/Activity | Multidisciplinary Vascular Access Team |
|---|---|
| Structure | Director (nephrologist); three registered nurse coordinators; three surgeons; three radiologists |
| Patient identification | Access team nurses review all new hemodialysis education referrals in CKD clinic and in all patients starting hemodialysis weekly. The nurses see all patients within 4 wk of dialysis modality education before hemodialysis start, or within 4 wk of hemodialysis start |
| Referral | Patients identified in predialysis clinics or hemodialysis facilities are referred to the team coordinators who meet with them directly and arrange vascular access clinic visit or surgery |
| Vascular access clinic structure | Two simultaneous weekly vascular access clinics: surgeon clinic (vascular access creation/complications) and the nephrologist clinic (6-wk routine postoperative assessments/complications); common waiting list for the first available vascular access surgery or diagnostic imaging procedure |
| Vascular access team activities | Physical exam (clinical monitoring) and vascular access planning for all referrals; ultrasound vein mapping when veins are not visible or arteries not palpable; physical exam and ultrasound of an existing access; guidance about needling for hemodialysis staff; catheter removal if a catheter is in place and the fistula can be used reliably; diagnostic imaging and intervention orders/booking |
| Maturation assessment | All fistulas are assessed for patency (auscultation) at 1 and 3 wk after creation, and for maturation at 6 and 8 wk (full physical examination and ultrasound). The team nurses use the rule of 6’s to assess maturation: 0.6 cm in diameter, 600 ml/min flow (assumed on the basis of thrill and bruit), and within 0.6 cm of skin surface (ultrasound exam). In case a fistula is considered not mature at 8 wk or a stenosis is suspected (arm elevation or augmentation test) an x-ray/angioplasty is arranged; a surgical procedure is considered if needed |
| Vascular access surveillance (blood flow in the access) | Performed every 8 wk by the access team using ultrasound dilution technique |
| Policy review and protocol updates | Referral for x-ray/angioplasty (i.e., venous pressure >150 mmHg with 15G needles on three consecutive runs at blood pump speed of 200 ml/min; signs of access dysfunction [difficulty needling, poor clearance, prolonged bleeding post dialysis, arm swelling, etc.] and access flow <500 ml/min; or access flow reduction ≥20%); referral for surgery if stenosis >3 cm or multiple lesions |
| Rounds | Difficult cases are discussed monthly |
| Education | Staff education and quality assessment/improvement reports |
Participants
We included all adults (18 years of age or older) who initiated hemodialysis in the Calgary Health Region of the Southern Alberta Renal Program between January 1st, 2004 and November 30th, 2011. We followed participants until November 30th, 2012. People were excluded if they had a life expectancy of <1 year due to documented metastatic cancer or other terminal illness at the time of dialysis initiation, if they started hemodialysis using an arteriovenous graft (use of grafts for hemodialysis access is uncommon in Canada, which limits generalization), or if there was a plan for peritoneal dialysis or kidney transplantation from a living kidney donor at the time of initiation of dialysis.
Exposure
To assess the effect of access team implementation, we categorized the study cohort by year of hemodialysis start. Because the team was implemented at the beginning of 2006, we defined 2004–2005 as the preteam period, 2006–2008 as the early-team period, and 2009–2011 as the late-team period. To address common limitations of before-after designs (e.g., history, instrumentation/reporting, testing and maturation threats, and Hawthorne and regression to the mean phenomena), we assessed the early and late effect of access team implementation to study the consistency of its effects over time.
Outcomes
The primary outcome was the probability of attaining catheter-free fistula use within 1 year of hemodialysis initiation. We first studied the probability of receiving a fistula creation before, or after, hemodialysis start. We defined catheter-free fistula use as use of a fistula in a patient without a central venous catheter in place, for any duration. A catheter-free fistula use period began on the day the catheter was removed for people who started hemodialysis with a catheter, and on the start date of hemodialysis if they were using a fistula exclusively when they started hemodialysis. Finally, we studied the rates of access-related procedures (total, catheter-related, and fistula-related procedures), defined as the number of individual procedures a participant received over the time at risk. We studied all outcomes during the first year of hemodialysis. A list of individual procedures captured in DMAR can be found in Supplemental Table 1.
Statistical Analyses
Descriptive Statistics.
We use standard analysis methods for qualitative and quantitative variables to describe and compare baseline characteristics across time periods. We used standard survival methods to study patient mortality and the risk of abandonment of a functional fistula across periods (5). We followed participants until the earliest of death, hemodialysis withdrawal due to recovery of kidney function, transfer to peritoneal dialysis or to another center, receipt of a kidney transplant, or the study end date (1 year after the start date of hemodialysis) if they were still at risk.
Probability of Fistula Attempt and Use.
We used logistic regression to estimate the odds ratios for fistula attempt and catheter-free use for any duration during the first year of hemodialysis. We used mixed-effects logistic regression to study the probability that a participant used the fistula catheter-free each day of his or her follow-up. We used this approach for two reasons. First, to study the proportion of follow-up time spent using a fistula catheter-free, which is not normally distributed and is best addressed with logit models. Second, this approach allowed us to account for the within-subject correlation in the data.
Procedure Rates.
After assessment for distributional assumptions and excess zeroes (20), we modeled the count outcomes at 1 year to estimate and compare access procedure rates using a negative binomial model. We estimated procedure rates in all participants and by receipt of a fistula creation attempt.
Methods for power analysis, model building, and checking are reported in the Supplemental Material.
Results
Population
Between January 1st, 2004 and November 30th, 2011, 635 people started hemodialysis in the Calgary Health Region of Southern Alberta without a plan for peritoneal dialysis or a kidney transplant from a living donor. From this cohort, we excluded 13 patients due to life expectancy of <1 year and 13 because they started hemodialysis using a graft; the remaining 609 were included in the study. Baseline characteristics were similar across the three periods (Table 2), including age and prevalence of comorbidities (diabetes, congestive heart failure, vascular disease). In more recent years, more people started hemodialysis in intensive care units, and more people received >12 months of predialysis care (Table 2). By the end of 1 year from the hemodialysis start date, 467 participants were still treated with hemodialysis, 75 people died, 11 received a kidney transplant, 39 recovered kidney function, 13 were transferred to another center, and four started peritoneal dialysis. Patient survival at 1 year was similar across study periods (Supplemental Figure 1). None of the participants received a graft during the first year of hemodialysis.
Table 2.
Characteristics of the study cohort at hemodialysis start by study period
| Characteristic | Pre–Vascular Access Team (2004–2005), n=146 | Early Vascular Access Team (2006–2008), n=252 | Late Vascular Access Team (2009–2011), n=211 |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR) | 66 (55–75) | 69 (58–77) | 63 (55–76) |
| Body mass index, median (IQR)a | 26.7 (22.9–33.1) | 26.4 (23.2–31.2) | 26.2 (22.1–30.8) |
| Men, n (%) | 86 (59) | 163 (65) | 126 (60) |
| Comorbidities, n (%) | |||
| Diabetes | 85 (58) | 133 (53) | 112 (53) |
| Vascular diseaseb | 65 (44) | 138 (55) | 99 (47) |
| Congestive heart failure | 26 (18) | 55 (22) | 49 (23) |
| Cancer | 15 (10) | 39 (16) | 30 (14) |
| Characteristics of dialysis start | |||
| Median eGFR (IQR), ml/min per 1.73 m2a | 10 (8–13) | 9 (7–12) | 9 (7–12) |
| Inpatient start, n (%) | 60 (41) | 115 (46) | 109 (52) |
| Intensive care unit start, n (%) | 3 (2) | 21 (8) | 17 (8) |
| Predialysis care received, n (%) | |||
| None | 19 (13) | 27 (11) | 35 (17) |
| <4 mo | 41 (28) | 68 (27) | 42 (20) |
| 4–<12 mo | 38 (26) | 32 (13) | 45 (21) |
| ≥12 mo | 48 (33) | 125 (50) | 89 (42) |
| Timing of first fistula attempt, before (versus after) dialysis start, n (%) | 50 (34) | 87 (35) | 65 (31) |
Over time, there was a significant increase in the proportion of people who started hemodialysis treatment in intensive care units (P=0.03) and of people who received longer predialysis care (P<0.01). IQR, interquartile range.
Missing data: body mass index was not available for 31 participants (16, 9, and 6 in the pre-, early, and late periods, respectively); eGFR was not available for 38 participants (30, 7, and 1 in the pre-, early, and late periods, respectively).
Vascular diseases include: coronary, cerebrovascular, and peripheral vascular diseases.
Arteriovenous Fistula Creation Attempts and Functional Fistula Patency
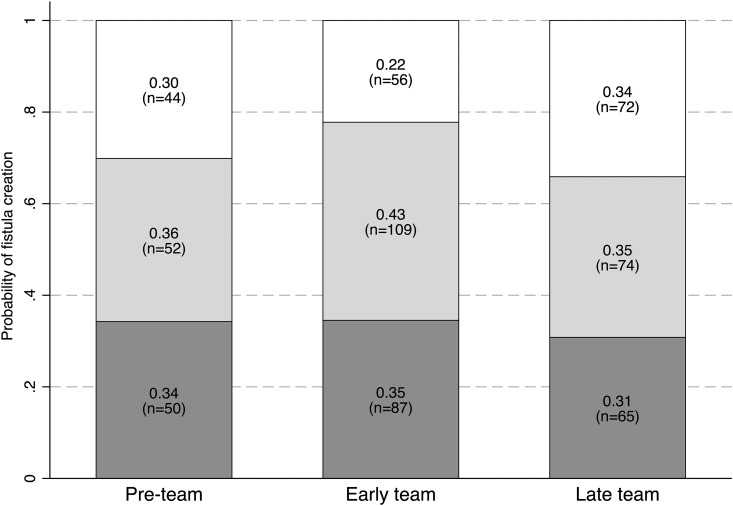
By the end of the first year of dialysis, 102 participants received a fistula creation attempt in the preteam period (70%), 196 received a fistula creation attempt in the early-team period (78%), and 139 in the late-team period (66%). Relative to the preteam period, the probabilities of receiving a fistula attempt were not different in the early- or late-team periods (Supplemental Table 1). In all three periods, about one in two fistula attempts occurred before hemodialysis start (Figure 1). After team implementation, fistula creation occurred earlier relative to the dialysis start date in people who received their fistula after dialysis start (Table 3). As compared with the preteam period, assisted functional survival probabilities of the fistula (time from the first catheter-free use to abandonment) were lower in the early-team period but similar in the preteam and late-team periods, both in crude and in adjusted analyses (Supplemental Figure 2).
Figure 1.
Probabilities of fistula attempt by the end of hemodialysis year 1 were similar across study periods. Probabilities of receiving a fistula creation attempt before (dark gray) or after (light gray) hemodialysis start, and probabilities of no attempt (white). Relative to the preteam period, the odds ratios and 95% confidence intervals for receiving a fistula attempt within the first year of hemodialysis start (versus no attempt) were 1.47 (0.92 to 2.35) in the early-team period, and 0.85 (0.54 to 1.35) for the late-team period (n=609; logistic regression model adjusted for age, sex, diabetes, and cardiovascular disease status; Supplemental Table 1).
Table 3.
Time to fistula creation, time to first fistula use, and days of catheter-free fistula use during the first year of hemodialysis
| Variable | Pre–Vascular Access Team (2004–2005) | Early Vascular Access Team (2006–2008) | Late Vascular Access Team (2009–2011) | P Value |
|---|---|---|---|---|
| Days from dialysis start to creationa | 89 (67–127) | 75 (37–119) | 58 (26–112) | 0.04 |
| Days from fistula creation to first useb | 120 (90–235) | 203 (113–377) | 189 (117–331) | <0.001 |
| Days of catheter-free usec | 96 (0–293) | 43 (0–249) | 86 (0–248) | 0.71 |
| Days of follow-up timec | 325 (13–365) | 336 (6–365) | 340 (20–365) | 0.85 |
Summary measures are medians and interquartile ranges.
Analysis restricted to those who received the fistula after hemodialysis start (n=235).
Analysis restricted to those who used their fistula (n=311).
Time from fistula creation attempt date (or dialysis start date in the case of predialysis attempts; n=437) to end of year 1 (or earlier if follow-up was for <1 yr due to death, transfer out of the program, recovery of kidney function, receipt of a kidney transplant, or transfer to peritoneal dialysis).
Catheter-Free Use of the Fistula
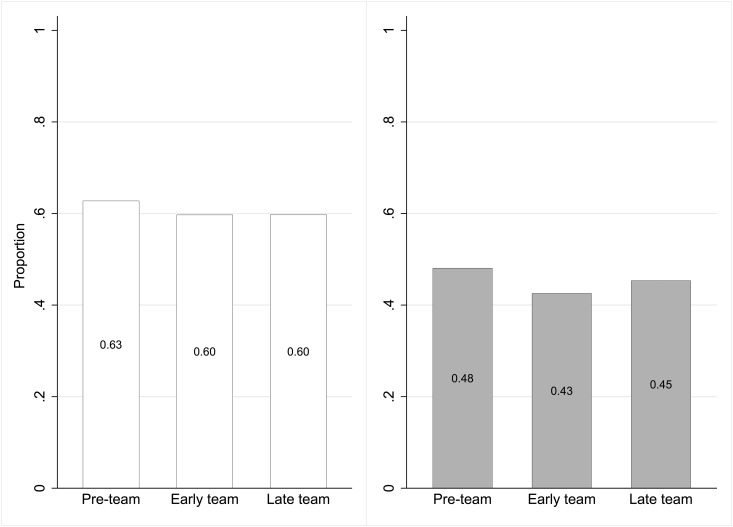
The number of days during which participants used their fistula catheter-free was similar across periods (Table 3). Among people who received a fistula creation attempt (n=437), the proportion who attained at least 1 day of catheter-free fistula use in the first year of hemodialysis was 63% in the preteam period, 60% in the early-team period, and 60% in the late-team period (Figure 2, left panel; Supplemental Table 2). The probabilities that a participant used their fistula catheter-free during each day of follow-up were also similar across periods (Figure 2, right panel; Supplemental Table 3). The median time interval between creation and first use of the fistula was significantly longer after team implementation (Table 3).
Figure 2.
Probabilities of catheter-free use of the fistula were similar across study periods. Relative to the preteam period, the odds ratios and 95% confidence intervals for catheter-free fistula use for at least 1 day (left panel) were 0.87 (0.52 to 1.43) in the early-team period and 0.89 (0.52 to 1.53) for the late-team period (n=437; logistic model adjusted for age, sex, diabetes, and cardiovascular disease status; Supplemental Table 2). The right panel represents the proportion of days of follow-up participants used their fistula catheter-free in each period. Odds ratios and 95% confidence intervals were 0.68 (0.24 to 1.96) and 0.85 (0.28 to 2.61), respectively, in the early and late period relative to the preteam period (n=437; logistic model adjusted for age, sex, diabetes, and cardiovascular disease status; Supplemental Table 3).
Access-Related Procedure Rates
During the first year of hemodialysis therapy, each study participant received an average of four access-related procedures before the implementation of the access team (Table 4). These rates increased by 20% in the early-team period, but were similar to baseline in the late-team period. Although rates of catheter-related procedures were similar across periods, fistula-related procedures did increase by 30%–40%, when creations were included, and by 50%–60%, when creations were excluded, after the implementation of the access team (Table 4). Rates of procedures in nonmaturing fistulas tended to increase over time, but the change was not statistically significant.
Table 4.
Procedure rates and incidence rate ratios in the first year of hemodialysis
| Outcomes | Pre–Vascular Access Team (2004–2005), n=146 | Early Vascular Access Team (2006–2008), n=252 | Late Vascular Access Team (2009–2011), n=211 |
|---|---|---|---|
| All participants (n=609) | |||
| All procedures | 4 (2.9 to 5.5) | 4.7 (3.4 to 6.4) | 4.3 (3.1 to 5.9) |
| Incidence rate ratios | 1 (Reference) | 1.2 (1.1 to 1.4)a | 1.1 (0.9 to 1.3) |
| Catheter-related procedures | 3.8 (2.1 to 6.7) | 3.8 (2.2 to 6.6) | 3.5 (2 to 5.9) |
| Incidence rate ratios | 1 (Reference) | 1 (0.8 to 1.3) | 0.9 (0.7 to 1.2) |
| Fistula-related procedures (including creation attempts) | 1.1 (0.8 to 1.6) | 1.5 (1 to 2.2) | 1.4 (1 to 2) |
| Incidence rate ratios | 1 (Reference) | 1.4 (1.2 to 1.6)a | 1.3 (1.1 to 1.5)a |
| Fistula-related procedures (without creation attempts) | 0.5 (0.3 to 0.8) | 0.8 (0.5 to 1.3) | 0.7 (0.4 to 1.2) |
| Incidence rate ratios | 1 (Reference) | 1.6 (1.2 to 2)a | 1.5 (1.1 to 1.9)a |
| Participants who received a fistula attempt (n=437) | n=102 | n=196 | n=139 |
| All procedures | 4.2 (3 to 5.9) | 4.5 (3.3 to 6.3) | 4.6 (3.3 to 6.4) |
| Incidence rate ratios | 1 (Reference) | 1.1 (0.9 to 1.2) | 1.1 (0.9 to 1.3) |
| Catheter-related procedures | 2.7 (1.3 to 5.3) | 2.2 (1.2 to 4.3) | 2.3 (1.2 to 4.3) |
| Incidence rate ratios | 1 (Reference) | 0.8 (0.6 to 1.1) | 0.9 (0.6 to 1.2) |
| Fistula-related procedures (including creation attempts) | 1.9 (1.4 to 2.7) | 2.4 (1.7 to 3.3) | 2.4 (1.7 to 3.3) |
| Incidence rate ratios | 1 (Reference) | 1.3 (1.1 to 1.5)a | 1.3 (1.1 to 1.5)a |
| Fistula-related procedures (without creation attempts) | 0.8 (0.5 to 1.4) | 1.2 (0.7 to 1.9) | 1.2 (0.7 to 1.9) |
| Incidence rate ratios | 1 (Reference) | 1.5 (1.1 to 1.8)a | 1.4 (1.1 to 1.9)a |
| Procedures to promote maturation | 0.2 (0.0 to 0.7) | 0.2 (0.0 to 0.8) | 0.2 (0.1 to 1.1) |
| Incidence rate ratios | 1 (Reference) | 1.3 (0.6 to 2.8) | 1.7 (0.8 to 3.7) |
| Participants who did not receive a fistula attempt (n=172) | n=44 | n=56 | n=72 |
| Catheter-related procedures | 5.1 (2 to 13) | 8.5 (3.2 to 22) | 5.5 (2.2 to 13) |
| Incidence rate ratios | 1 (Reference) | 1.7 (0.9 to 2.8) | 1.1 (0.6 to 1.8) |
Reported rates represent the number of procedures per person-years (mean and 95% confidence intervals). All negative binomial models are adjusted for age, sex, diabetes, and cardiovascular disease status. The incidence rate ratios provide an estimate of the change in procedure rate relative to the preteam period. Full models are reported in the Supplemental Material (Supplemental Tables 4–13).
Fistula-related procedure rates were significantly increased after access team implementation.
We found similar results after stratifying the analyses on the basis of whether participants received (n=437) or did not receive (n=172) a fistula creation attempt. Rates of catheter-related procedures were not significantly reduced in those who attempted fistula creation. However, the rate of fistula-related procedures (including attempts) increased, from an average of 1.9 procedures per year before team implementation to 2.4 procedures per year after team implementation (Table 4).
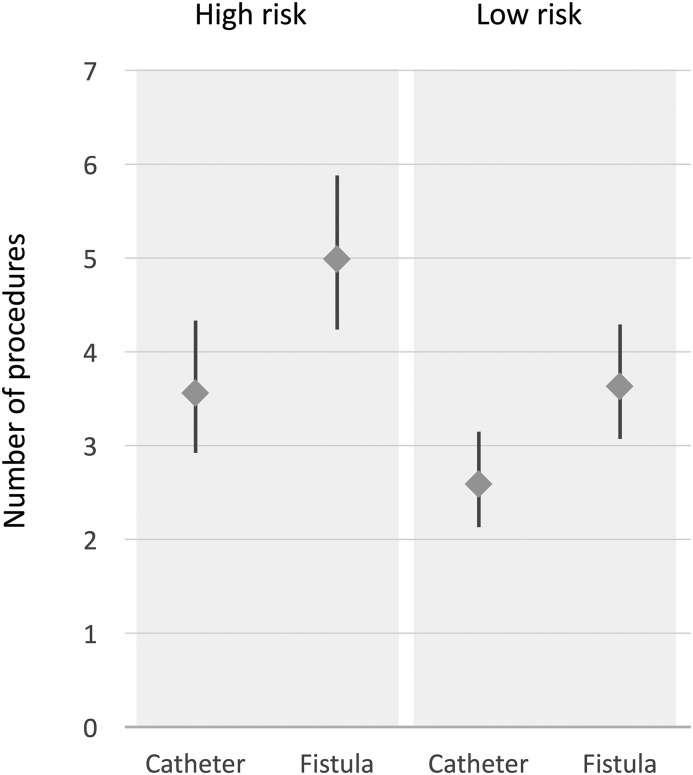
Patient characteristics, e.g., female sex, cardiovascular disease, and diabetes, were the main drivers of procedure rates regardless of study period (Supplemental Tables 4–13). For example, we estimated that a 70-year-old woman with diabetes and cardiovascular disease would receive on average five access-related procedures during the first year of hemodialysis therapy if she selected a fistula-based strategy for vascular access, compared with 3.5 procedures if she selected a catheter-based strategy (Figure 3).
Figure 3.
Predicted number of procedures during the first year of hemodialysis were higher in women and people with comorbidities. High risk and low risk refer to characteristics associated with increased incidence of access-related procedures. High risk: 70-year-old women with diabetes and cardiovascular disease; low risk: 50-year-old men without diabetes or cardiovascular disease. Catheter refers to a catheter-based access strategy (fistula creation never attempted); fistula refers to a fistula-based access strategy (the patient received a fistula creation attempt).
Other Analyses
Results were the same across categories of sex, age, or diabetes (prespecified interaction terms). Results were also the same excluding participants who were censored or died earlier than 1 year from study entry.
Discussion
In this before-after study of incident patients on hemodialysis, we found no relationship between implementation of an access team and attainment of catheter-free fistula use within 1 year of hemodialysis initiation. Although probabilities of fistula creation were high in all study periods, this outcome and successful fistula use did not change after the implementation of an access team. We did, however, find a significant increase in fistula-related procedures over time. People who started hemodialysis received, on average, 4–5 access-related procedures in the first year of treatment, regardless of the study period and the access strategy chosen. Although we found that people who received a fistula attempt underwent a significantly greater number of fistula-related procedures after the implementation of an access team, it was among women and people with diabetes and cardiovascular disease that we observed the greatest number of access-related procedures across all study periods. The clinical consequences of this burden of procedures and their effect on the health system remain to be determined.
Based largely on observational data, most guidelines recommend a “Fistula First” policy given the lower rates of morbidity and mortality associated with fistula use compared with the use of central venous catheters or grafts. US Renal Data System data show a steady increase in prevalent fistula use in the four months after hemodialysis initiation over the last decade, along with a doubling of procedure rates across all vascular access types (14). The Dialysis Outcomes and Practice Patterns Study (DOPPS) recently reported an increase in the United States in prevalent fistula use from 24% in 1997 to 68% in 2013, and a concomitant decline in catheter use from 27% in 1997 to 15% in 2013 (15). Data from prevalent patients, however, may overestimate benefits and underestimate harms of interventions due to survivorship bias. Although in the United States and Canada about 70%–80% of people start hemodialysis with catheters, detailed data on procedure rates are not available from these countries. A 2013 DOPPS survey of 72 United States sites reported that 60% of hemodialysis programs had at least one vascular access coordinator, but they did not report whether an access team was present (15). The survey also reported that the percentage of patients using a fistula or a catheter did not differ across facilities with or without at least one vascular access coordinator (15). Although there is conflicting information regarding the effectiveness of access teams on outcomes such as successful (i.e., catheter-free) fistula use, it is possible that vascular access–related procedure rates may be unnecessarily increased after the implementation of these teams. Our study does support this hypothesis.
Data on the true benefits of an access team are limited. Gathering accurate information regarding vascular access use across a patient’s dialysis career is difficult and requires high-quality data assessing time periods of independent, catheter-free fistula use and catheter use. Placement of a fistula does not imply catheter-free use and many fistulas fail to mature or require many procedures before use. Such data are not easily captured in most databases. To the best of our knowledge, this is the first study to assess the probability of catheter-free fistula use among incident patients on hemodialysis both before and after the implementation of an access team. Although fistulas were placed earlier relative to the hemodialysis start date and left to mature longer after access team implementation, we found no change in the probability of catheter-free fistula use after access team implementation. We also found, for the first time, that on average a patient on hemodialysis undergoes four to five access-related procedures during the first year of hemodialysis therapy. Although this rate of access-related procedures remained about the same in the late period after access team implementation, we found that fistula-related procedures increased. The consequences of the high burden of access-related procedures deserve further study, especially in high-risk patients defined by sex and comorbidity (21).
The result of our study may be due to bias or chance, or reflect a true lack of association between implementation of an access team and catheter-free fistula use. First, although misclassification of outcome data is unlikely, other forms of bias may have affected these results, including patient selection and unmeasured confounding. Before-after designs, however, tend to overestimate benefits of interventions by attributing to the intervention any reduced risk of poor outcomes that are more likely due to the improvement of care over time. Consistent with this hypothesis, we found a significant increase in the proportion of people receiving predialysis care over time. Second, although false negative results are possible in any study, our power calculation suggests that our study had sufficient power to detect a clinically relevant effect, if it existed. Results were consistent across sensitivity analyses that excluded people with a follow-up of <1 year. Finally, results from our study could be truly negative, suggesting that an access team may not increase catheter-free fistula use among incident patients on hemodialysis. This could be due to several factors, including an increasing number of people with worse prognoses (e.g., starting dialysis in intensive care units); enhanced patient selection, whereby fistula procedures are minimized in patients at high risk of fistula failure; or the limited ability of coordinated care to meaningfully affect these outcomes. Implementation of strategies for access monitoring and surveillance, for example, has been shown to reduce the risk of thrombosis, but not to prolong the longevity of an arteriovenous access (22). Further research is needed to identify new strategies to deliver optimal, personalized, and patient-centered vascular access care. Although access teams may not improve vascular access outcomes, they may reduce health spending by shifting care from physicians to lower-cost providers and enhance patient engagement and promote shared decision-making. These important outcome measures should be evaluated in future studies.
Our study has strengths, including the high-quality data that is captured through the database we used, allowing for accurate assessment of fistula attempts and procedure rates (23). Limitations include our study’s retrospective and single-program design that may limit generalizability. Further, patient-related factors such as patient experience, preferences, and quality of life were not measured in this study and therefore we could not assess the effect of the access team on these important outcomes. In conclusion, we found no difference in catheter-free fistula use before and after implementation of an access team, and higher rates of fistula-related procedures, suggesting that more in vascular access care may not necessarily be better. The consequences of this excess burden of procedures deserve careful examination, especially in people at risk for adverse outcomes. Further studies are needed to assess the effect of vascular access teams on vascular access and patient outcomes, including measures of quality of life, within hemodialysis programs.
Disclosures
None.
Supplementary Material
Acknowledgments
This work has been supported by grants from the Canadian Institute of Health Research awarded to P.R. and R.Q. (FRN 119366, FRN 130514). P.L. is supported by a Canadian Institute of Health Research postdoctoral fellowship.
The study sponsors had no influence on the design and set-up of the study, or write-up of the present manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03430317/-/DCSupplemental.
References
- 1.Caring for Australians with Renal Impairment : Dialysis guidelines: Vascular access, 2012. Available at http://www.cari.org.au/Dialysis/dialysis%20vascular%20access/dialysis_vascular_access.html. Accessed July 30, 2017
- 2.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF; Canadian Society of Nephrology Committee for Clinical Practice Guidelines : Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Ravani P, Gillespie BW, Quinn RR, MacRae J, Manns B, Mendelssohn D, Tonelli M, Hemmelgarn B, James M, Pannu N, Robinson BM, Zhang X, Pisoni R: Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol 24: 1668–1677, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, Manns B, Tonelli M, Strippoli GF, James MT: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn RR, Ravani P: Fistula-first and catheter-last: Fading certainties and growing doubts. Nephrol Dial Transplant 29: 727–730, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fistula first breakthrough initiative, 2015. Available at http://www.fistulafirst.org. Accessed July 30, 2017
- 10.Peters VJ, Clemons G, Augustine B: “Fistula first” as a CMS breakthrough initiative: Improving vascular access through collaboration. Nephrol Nurs J 32: 686–687, 2005 [PubMed] [Google Scholar]
- 11.Vassalotti JA, Jennings WC, Beathard GA, Neumann M, Caponi S, Fox CH, Spergel LM; Fistula First Breakthrough Initiative Community Education Committee : Fistula first breakthrough initiative: Targeting catheter last in fistula first. Semin Dial 25: 303–310, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Ontario Renal Network : Vascular access working group, 2013. Available at http://www.renalnetwork.on.ca/hcpinfo/body_and_vascular_access/vafactsheets/#.WabVaVIZPOY. Accessed July 30, 2017
- 13.Kiaii M, MacRae JM: A dedicated vascular access program can improve arteriovenous fistula rates without increasing catheters. J Vasc Access 9: 254–259, 2008 [PubMed] [Google Scholar]
- 14.Lok CE, Foley R: Vascular access morbidity and mortality: Trends of the last decade. Clin J Am Soc Nephrol 8: 1213–1219, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Pisoni RL, Zepel L, Port FK, Robinson BM: Trends in US vascular access use, patient preferences, and related practices: An update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis 65: 905–915, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, Radkevich V, Murphy B: Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J Am Soc Nephrol 16: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Lacson E Jr, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM: Balancing fistula first with catheters last. Am J Kidney Dis 50: 379–395, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA: The Southern Alberta Renal Program database: A prototype for patient management and research initiatives. Clin Invest Med 24: 164–170, 2001 [PubMed] [Google Scholar]
- 19.Oliver MJ, Verrelli M, Zacharias JM, Blake PG, Garg AX, Johnson JF, Pandeya S, Perl J, Kiss AJ, Quinn RR: Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 27: 810–816, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Weaver CG, Ravani P, Oliver MJ, Austin PC, Quinn RR: Analyzing hospitalization data: Potential limitations of Poisson regression. Nephrol Dial Transplant 30: 1244–1249, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravani P, Quinn RR, Oliver MJ, Karsanji DJ, James MT, MacRae JM, Palmer SC, Strippoli GF: Pre-emptive correction for haemodialysis arteriovenous access stenosis. Cochrane Database Syst Rev (1): CD010709, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, Mysore P, Lewin AM, Hiremath S, MacRae J, James MT, Miller L, Hemmelgarn BR, Moist LM, Garg AX, Chowdhury TT, Ravani P: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.