Abstract
Background and objectives
Young adults receiving RRT face additional challenges in life. The effect of established kidney failure on young adulthood is uncertain. We aimed to establish the psychosocial and lifestyle status of young adults receiving RRT.
Design, setting, participants, & measurements
Our study was a systematic review and meta-analysis of 16–30-year olds receiving RRT compared with the general population. We selected randomized, controlled trials; cohort studies; or cross-sectional studies without language restriction and extracted proportions of sociodemographic and lifestyle outcomes or validated psychologic health tests producing quality of life, wellbeing, and self-esteem scores. We undertook random effects meta-analysis.
Results
There were 60 studies with a total of 15,575 participants. Studies were largely single-center cross-sectional studies of those transplanted in childhood. Compared with healthy peers, young adults on RRT had lower quality of life, which was worse for patients on dialysis (seven studies: standardized mean difference, −1.01; 95% confidence interval [95% CI], −1.32 to −0.70) compared with patients with transplants (nine studies: standardized mean difference, −0.42; 95% CI, −0.64 to −0.20). They were more likely to be unemployed (seven studies: relative risk, 1.89; 95% CI, 1.47 to 2.44) and live in the family home (two studies: relative risk, 1.84; 95% CI, 1.40 to 2.43). They were less likely to be married or have a partner (four studies: relative risk, 0.71; 95% CI, 0.53 to 0.95). Higher education (three studies: relative risk, 1.05; 95% CI, 0.73 to 1.51), alcohol abstinence (three studies: relative risk, 1.96; 95% CI, 0.84 to 4.67), and smoking status (two studies: relative risk, 0.72; 95% CI, 0.36 to 1.44) did not differ. Results were limited by high heterogeneity and a small evidence base, biased toward surviving patients.
Conclusions
Established kidney failure is associated with lower quality of life in young people and limited employment, independence, and relationships compared with healthy peers.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2017_10_19_CJASNPodcast_17_12_.mp3
Keywords: systematic review, meta-analysis, young adult, renal replacement therapy, kidney transplantation, dialysis, education, employment, relationships, mental health, lifestyle, Cross-Sectional Studies, Risk, Confidence Intervals, Language, Alcohol Abstinence, quality of life, Smoking, RRT, Unemployment, Uncertainty, Demography, Renal Insufficiency, Life Style
Introduction
Young adulthood is a distinct developmental period where physical growth stops but high-level brain function continues to develop (1). Young adulthood features growing independence, decision making, self-exploration, and experimentation. Young adults receiving RRT in high-income countries are mostly transplanted (70% of United States 0–21-year olds [2] and 73% of United Kingdom 18–24-year olds [3]) and must learn to integrate responsibility for managing their condition into their changing lives.
There are little data regarding psychosocial outcomes for young adults, an area not captured by most disease registers. Although young adults are known to be high risk for graft failure (4,5), the extent to which established kidney failure has affected their social status, mental health, and lifestyle remains unclear. The literature to date focuses on single-center cohorts of transplanted children followed to young adulthood and the process of transition to adult services (6,7). Those receiving dialysis, those presenting directly to adult services, and nonsurviving patients are under-represented.
We aimed to review the literature systematically to establish the sociodemographic, psychologic health, and lifestyle status of young adults receiving RRT. This information is important to determine whether patients lead comparable lives to their healthy peers. We hypothesized that young adults receiving RRT would be psychosocially disadvantaged compared with healthy peers.
Materials and Methods
We conducted this systematic review in accordance with Meta-Analysis of Observational Studies in Epidemiology criteria (8) rather than Preferred Reporting Items for Systematic Reviews and Meta-Analyses, because it is more appropriate for observational studies. We did not use a review protocol.
Eligibility Criteria
We used the criteria defined in Table 1 to select studies from our systematic search. We included all studies reporting sociodemographic, psychologic health, and lifestyle outcomes for young adults (defined as ages between 16 and 30 years old at the time of study) receiving long-term RRT. There is no consensual definition for young adulthood, and we chose a wide age range to ensure that we did not miss any important publications. We included all language types.
Table 1.
Systematic search criteria
| Variable | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Young adults ages between 16 and 30 yr old at the time of study | Average age of study participants <16 or >30 yr old, incalculable, or not reported |
| Exposure | ESRD requiring transplantation, hemodialysis, or peritoneal dialysis | CKD not requiring RRT; AKI |
| Outcome | Sociodemographic (e.g., education, employment, income, living situation, relationships) | |
| Psychologic health (e.g., quality of life, wellbeing, depression, anxiety) | ||
| Lifestyle (e.g., alcohol, tobacco, illicit drug use, participation in sport, antisocial behavior) | ||
| Study types | Randomized, controlled trials; cohort studies; cross-sectional studies; patient-control studies; ecologic studies; qualitative studies | Outcomes not self-reported (e.g., study of caregiver or clinician) |
| Publication types | Abstract and full text articles, theses, book chapters | Editorials, reviews, patient reports |
| Any language | ||
| Any year |
Search Strategy
Together with an information specialist, we devised a sensitive search strategy (Supplemental Material), which we applied to nine databases (Medline, EMBASE, PsycINFO, Applied Social Sciences Index and Abstracts [ProQuest], CINAHL (EBSCO), Web of Science, Scopus, Open Grey, and Cochrane Library). We ran our first search in July of 2015 and repeated the search in August of 2016 (for the latter, limiting results to those from the last year only). We also screened references of key articles obtained in our search.
Where studies did not present appropriate data (e.g., subgroup data not presented or study published in abstract form), we asked authors by email for the data if the study was carried out in the last 5 years; two of five study authors responded to these requests.
Study Selection, Data Collection, and Risk of Bias Appraisal
We imported all references into Endnote and then used this software to remove duplicate publications. A.J.H. screened the titles and abstracts of all citations to identify studies fulfilling the inclusion criteria. R.L.C. screened a random sample of 1000 titles and abstracts to ensure consistency. Concordance was 99%, and any disagreements were resolved by discussion. We used Google Translate to screen non-English abstracts and arranged formal translation of included non-English articles.
Where there were multiple versions of the same study, we selected the more comprehensive/substantive version (e.g., journal article over conference abstract or journal articles arising from theses). We recorded study characteristics and used the Newcastle–Ottawa Scale (NOS) to assess the risk of bias for all studies (9). A high NOS score (range from zero to nine) indicates a lower risk of bias. A.J.H. assessed bias in all included studies. R.L.C. independently assessed bias in a random sample of six (10%) of the included studies to ensure a fair appraisal. Any disagreements were resolved by discussion.
Summary Measures and Synthesis of Results
We examined studies fulfilling the inclusion criteria and collated whether studies reported common outcome measures (regardless of scales used) before deciding on which outcome measures were amenable to meta-analysis. If a study presented multiple scales for a single outcome, we chose the most frequently used scale for analysis. We compared the data across studies and devised a data collection form to obtain consistently reported data. Study review and data extraction were performed by A.J.H. R.L.C. independently extracted data from a random sample of six (10%) of the included studies to ensure consistency and accuracy. There was 100% concordance for data extraction.
Statistical Methods and Subgroup Analyses
For outcomes reported as proportions, we extracted these and calculated their 95% confidence intervals (95% CIs) by assuming a binomial distribution using the Wilson method (10), because no study reported these. We then performed a meta-analysis of the outcome proportion using a random effects model (using the method by DerSimonian and Laird), because we presumed that there would be marked between-study heterogeneity given methodologic differences. We visually examined effect estimates using forest plots and calculated heterogeneity statistics (I2 and 95% CI using a noncentral chi squared–based approach [11] and τ2). We did not stratify by modality at enrolment, because most studies were long-term follow-ups of pediatric patients with kidney transplants for whom current modality was not reported or the outcome proportion by modality was not provided. However, most studies reporting quality of life scale scores did so by modality, and therefore, one could compare subgroups.
For studies with control data, papers reported a proportion/percentage outcome and sample size for the kidney sample and provided comparative control data mostly using census/routine survey data (no reported sample size in seven cases; the number with outcome/total in three cases and the number with outcome/no total in one case). We converted outcome rates (95% CI) for the kidney sample as above. The statistics comparing the kidney sample with controls were limited; there was no comparison in six studies, the chi-squared test was used in two studies, P value was available only in two studies, and a standardized incidence ratio and P value were given in one study. Therefore, where the reference sample size was unknown (n=8), we calculated the risk ratio (RR; 95% CI) by dividing study group risk by control group risk. This assumed no sampling variation for the larger reference population, because these are on the basis of much larger samples and assume that all of the variability in the RR is determined by the sampling variability in the smaller clinical group. Our calculated RR matched the standardized incidence ratio where provided. Where the sample size was known (n=3), we accounted for reference population variability. We then inspected forest plots (log RR) and chose to undertake a random effects meta-analysis (as above) to derive the pooled RR (95% CI, I2 statistic and 95% CI, τ2).
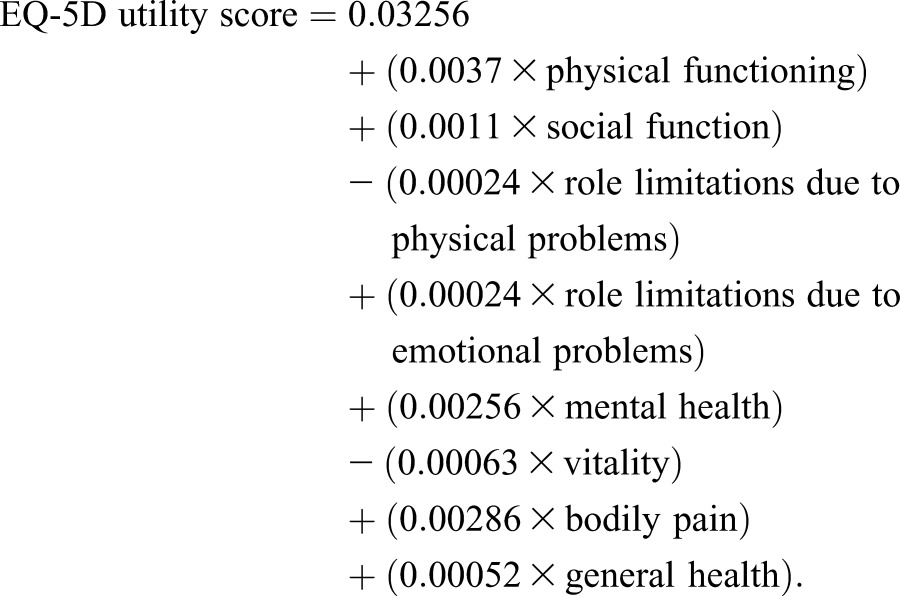
Quality of life is often assessed using the 36-Item Short Form Health Survey (SF-36) measure, which has four physical and four mental domains, or the EuroQoL-5 Dimensions (EQ-5D), which weights five domains (anxiety, pain, mobility, usual activities, and self-care) to produce a utility score with a maximum of 100. To summarize the quality of life data to a single domain or utility score, we converted mean SF-36 domains to an overall utility score using a model developed by Ara and Brazier (12), which is regarded as the best approach in the absence of individual participant–level data. This model was derived using ordinary least square regression models and weights each SF-36 domain to derive an EQ-5D utility score as follows:
 |
To derive the SD for the utility score, we used the model-based approach developed by Wyld et al. (13). This model derived a regression function using fractional polynomials of observed SDs against SDs from utility estimates in published studies as follows:
 |
We confirmed the validity of this model by showing similar model-derived SDs and reported SDs in our extracted data. Because the utility score is numerical, we performed a random effects meta-analysis using the Glass method (which standardizes using the reference group SD) to pool standardized mean differences (SMDs) using the mean scale score, SD, and sample size for patient and control groups stratified by modality. We also used this approach for the non–SF-36 quality of life scales. We derived both the overall heterogeneity statistics (I2 and 95% CI, τ2) as well as assessing for subgroup (transplant versus dialysis) heterogeneity to test for an interaction. If studies did not report a normative comparator, we still included them in the meta-analysis if we were able to find appropriate country- and age-specific control data (mean, SDs). We undertook several sensitivity analyses. We repeated the meta-analysis excluding the studies with derived control data to see if this altered our results qualitatively. We also performed separate meta-analyses by modality. Stratifying by modality in some cases meant including two subgroups from the same study. This does not affect the point estimate, because the data points were independent; however, because the two subgroups come from the same study, there is the potential for structural clustering, so that the SEMs for the pooled modality estimates may be underestimated. We investigated this by repeating the meta-analysis by modality but this time, meta-analyzing the treatment by modality interaction in studies that reported data for both patients with transplants and patients on dialysis. We assessed for small study effects using funnel plots, comparing by modality and excluding studies that did not report normative comparators (to avoid artificially inflating the effect estimate sample size). To help the reader contextualize the SMD results, we used normative data from the 2012 Health Survey for England (14) and backcalculated the differences in the SMD for the patients with kidney disease to an absolute EQ-5D utility using the reported SD.
For studies reporting other scale scores, we performed a random effects meta-analysis using the same methods as for the utility score analysis. We used Stata 14 for our analyses.
Results
Description of Included Studies
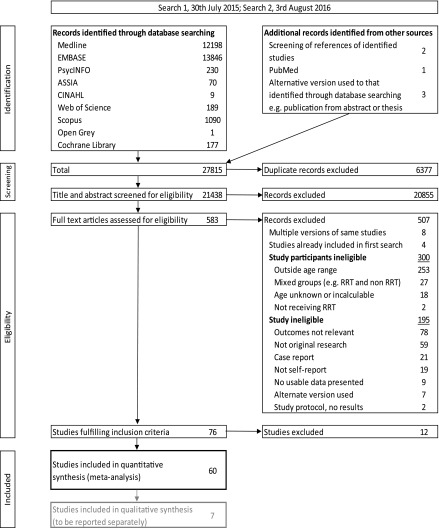
We included 60 studies in our quantitative review. We identified seven qualitative studies, which are not discussed further. Figure 1 shows the results of our systematic searches, and Supplemental Table 1 details the characteristics of the included studies. Table 2 summarizes the study attributes and highlights that most studies were small (median of 42 participants; interquartile range, 25–78), single center (75%), and cross-sectional (80%). One half of the studies examined young people transplanted in childhood. Studies were mainly from high-income countries (78%). Where reported, the recruitment, response rate, and key variable completeness of the various study types were reasonably high. However, the response rate was not reported in two thirds of surveys.
Figure 1.
Systematic search results for studies examining social, psychologic health, and lifestyle outcomes for young adults receiving RRT according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Table 2.
Summary features of studies included in quantitative synthesis
| Study Attributes | n | Percentage | Study Attributes (Continued) | Summary Statistic |
|---|---|---|---|---|
| Geography | Median (IQR) Newcastle–Ottawa Quality Assessment Scale scorea | |||
| Europe | 30 | 50 | Studies with control data (maximum score seven points, n=21) | 5 (5–5) |
| North America | 13 | 22 | Studies without control data (maximum score four points, n=39) | 2 (2–3) |
| Asia | 7 | 12 | All studies with score as percentage | 71% (50–75) |
| South America | 4 | 7 | Year | |
| Australasia | 2 | 3 | Range | 1978–2015 |
| International (United Kingdom and United States) | 2 | 3 | Mean±SD | 2004±10 |
| Africa | 2 | 3 | Median (IQR) | 2007 (1997–2012) |
| Publication type | No. of participants | |||
| Journal article | 51 | 85 | Range | 5–11,027 |
| Conference abstract | 5 | 8 | Mean±SD | 260±1418 |
| Thesis | 3 | 5 | Median (IQR) | 42 (25–78) |
| Book chapter | 1 | 2 | Recruitment/response rate or key variable completeness (%) | |
| Study design | Recruitment for long-term observation studies (n=19) | |||
| Cross-sectional | 48 | 80 | Not reported/unknown | 2 |
| Cohort study or clinical cohort | 12 | 20 | Median (IQR) | 100 (100–100) |
| Study sampling method | Recruitment for interview studies (n=14) | |||
| Convenience, single center | 45 | 75 | Not reported/unknown | 3 |
| Registry | 7 | 12 | Median (IQR) | 82 (70–90) |
| Multicenter | 6 | 10 | Response rate for survey studies (n=12) | |
| National recruitment via internet | 1 | 2 | Not reported/unknown | 8 |
| Not reported | 1 | 2 | Median (IQR) | 59 (44–83) |
| Study subject original modality | Key variable completeness for registry studies (n=2) | |||
| Transplant | 39 | 65 | Not reported/unknown | 0 |
| RRT | 18 | 30 | Median (IQR) | 99 (99–100) |
| HD | 1 | 2 | ||
| Dialysis | 1 | 2 | ||
| PD | 1 | 2 | ||
| Control data | 21 | 35 |
Not all percentages add up to 100 due to rounding. IQR, interquartile range; HD, hemodialysis; PD, peritoneal dialysis.
We used this tool to assess bias in all study types and modified it by omitting points where not applicable. For all studies, we omitted points for “demonstration that outcome of interest was not present at start of study” and “was follow-up long enough for outcomes to occur.” For uncontrolled studies, we also omitted points for “selection of the nonexposed cohort” and “comparability.” We compared total scores by study type and compared all studies using percentage scores.
Risk of Bias
The modified NOS scores are shown in Table 2. The risk of bias was higher in studies that did not report any normative comparator data. The overall scale median percentage score was 71% (interquartile range, 50–75). Because 80% of our identified publications were cross-sectional, they are subject to attrition bias; patients who may have died over follow-up are, by definition, not included, and hence, the observed results may be biased toward better outcomes. Two thirds of studies involved surveys and/or interviews, in which engaged patients are more likely to take part (selection bias). In addition, given the subjective nature of many of the outcomes, there may be recall and interviewer bias. Two thirds of studies involved patients with transplants; one half of the studies only recruited participants on the basis that they were transplanted as children, so that young adults presenting later may be under-represented. Most studies were European/North American, reflecting a geographic bias toward these health care systems. Three quarters of studies involved convenience sampling from single centers; here, center performance and clinician motivation may also bias results.
Observed Proportions
The pooled proportion estimates for sociodemographic, psychologic health, and lifestyle outcomes are shown in Table 3. Sociodemographic outcomes were commonly reported. There was considerable heterogeneity, which was unaffected by stratifying by modality at enrolment.
Table 3.
Pooled proportion estimates and 95% confidence intervals from weighted meta-analysis of observational studies by study numbers and size with heterogeneity statistics
| Outcome | n | No. of Studies | Proportion (95% CI) | I2, % (95% CI)a | τ2 | Refs. |
|---|---|---|---|---|---|---|
| Education and employment | ||||||
| Working | 2281 | 35 | 0.47 (0.40 to 0.53) | 90 (87 to 92) | 0.03 | 15–17,20,22–27,29,32,33,35–37,41,42,45,46,48–51,57,60,61,63,64,67,70,72,73,75,76 |
| Unemployed | 1705 | 29 | 0.19 (0.16 to 0.22) | 66 (46 to 76) | 0.005 | 15–17,22–26,29,30,32,33,35–37,41,42,44–49,51,61,67,70,73,75 |
| Studying | 1471 | 26 | 0.29 (0.23 to 0.36) | 91 (89 to 93) | 0.03 | 15,17,22–26,29,32,33,35–37,41,42,46,48,50,51,61,63,64,67,70,73,75 |
| Higher education | 1815 | 21 | 0.26 (0.18 to 0.34) | 95 (93 to 96) | 0.03 | 15–17,20,22,23,26,29,30,32,33,36,40,41,49,51,54,55,67,70,76 |
| Have a disability or registered disabled | 1252 | 13 | 0.15 (0.09 to 0.20) | 87 (79 to 91) | 0.008 | 23,24,26,29,33,35–37,44,49,69,70,72 |
| Basic/manual job | 366 | 5 | 0.44 (0.28 to 0.59) | 88 (72 to 93) | 0.03 | 23,24,33,36,37 |
| Relationships and living arrangements | ||||||
| Married/with partner | 1811 | 26 | 0.25 (0.19 to 0.31) | 90 (87 to 92) | 0.02 | 15–17,20,22,23,25,26,29,30,33,35,37,41,42,45,49–51,55,57,58,61,64,74,76 |
| Live in family home | 2357 | 24 | 0.56 (0.48 to 0.64) | 94 (93 to 95) | 0.04 | 15–17,20,22–27,29,33,35–37,47,49,51,59,64,67,69,73,74 |
| Lives in urban area | 11,724 | 4 | 0.67 (0.48 to 0.87) | 99 (99 to 99) | 0.04 | 34,52,68,71 |
| Psychologic health | ||||||
| Depression/anxiety | 449 | 13 | 0.30 (0.20 to 0.40) | 88 (82 to 92) | 0.03 | 15,17,29,32,38,42–45,48,54,56,67 |
| Self-rated health excellent or good | 243 | 6 | 0.85 (0.76 to 0.93) | 74 (16 to 87) | 0.008 | 15–17,29,45,50 |
| Dissatisfied with body image | 314 | 5 | 0.30 (0.16 to 0.44) | 88 (71 to 93) | 0.02 | 15,27,29,30,37 |
| Satisfied with life | 151 | 3 | 0.88 (0.83 to 0.93) | 0 (0 to 73) | <0.001 | 15–17 |
| Normal health, no complaints | 151 | 3 | 0.55 (0.42 to 0.68) | 59 (0 to 86) | 0.007 | 15–17 |
| Satisfied with personal relationships | 151 | 3 | 0.76 (0.56 to 0.96) | 89 (61 to 95) | 0.03 | 15–17 |
| Health never/seldom affects social life | 151 | 3 | 0.72 (0.53 to 0.92) | 86 (38 to 94) | 0.02 | 15–17 |
| Family support | ||||||
| Satisfied with family support | 300 | 6 | 0.79 (0.69 to 0.89) | 81 (53 to 90) | 0.01 | 15–17,52,56,67 |
| Disease never/seldom affects family life | 151 | 3 | 0.80 (0.51 to 1.08) | 96 (93 to 98) | 0.06 | 15–17 |
| Sexual function | ||||||
| Satisfied with sex life | 178 | 4 | 0.53 (0.30 to 0.77) | 92 (82 to 95) | 0.05 | 15–17,29 |
| Health no obstacle to sex life | 120 | 2 | 0.46 (0.37 to 0.54) | 0 | <0.001 | 15,17 |
| Lifestyle | ||||||
| Current smoker | 11,580 | 4 | 0.17 (0.07 to 0.28) | 96 (93 to 97) | 0.01 | 30,36,64,68 |
| Participate in sports | 274 | 4 | 0.46 (0.26 to 0.67) | 92 (82 to 95) | 0.04 | 17,30,35,76 |
| Sees friends or relatives at least weekly | 176 | 4 | 0.84 (0.78 to 0.91) | 27 (0 to 76) | 0.001 | 15–17,76 |
| Abstains from alcohol | 536 | 3 | 0.30 (0.03 to 0.57) | 98 (97 to 99) | 0.05 | 30,36,38 |
| Has a driving license | 501 | 3 | 0.65 (0.61 to 0.69) | 0 (0 to 73) | <0.001 | 25,36,64 |
| Attends social events at least weekly | 134 | 2 | 0.56 (0.43 to 0.70) | 64 | 0.006 | 15,17 |
95% CI, 95% confidence interval.
95% CI for I2 incalculable with one degree of freedom.
There were fewer studies examining psychologic health, with depression or anxiety being most frequently reported, although there was marked heterogeneity. Most of the data were pooled from three studies that studied patients with transplants using the same questionnaire (15–17), and apart from depression/anxiety and body image, all of the estimates were from studies examining patients with transplants. The apparent disparity between suboptimal mental health and good satisfaction in other areas of life may be explained by the small number of studies and patient modality.
In terms of lifestyle, there were again few studies and high heterogeneity for the proportions of those currently smoking, taking part in sports, and abstaining from alcohol. Young adults appeared to live healthy lifestyles.
Outcomes Relative to Healthy Controls
The pooled effect estimates of sociodemographic, psychologic health, and lifestyle outcomes compared with healthy controls are shown in Table 4. Few studies presented normative comparison data, and when pooled, they were very heterogeneous.
Table 4.
Pooled relative risk and standardized mean difference estimates and 95% confidence intervals from weighted meta-analysis relative to healthy controls by study numbers and size with heterogeneity statistics
| Outcome | n | No. of Studies | Effect Estimate (95% CI) | I2, % (95% CI)a | τ2 | Refs. |
|---|---|---|---|---|---|---|
| Sociodemographic | Risk Ratio | |||||
| Unemployed | 733 | 8 | 1.89 (1.47 to 2.44) | 49 (0 to 76) | 0.06 | 20,23,24,27,29,33,35–37 |
| Married/with partner | 594 | 4 | 0.71 (0.53 to 0.95) | 81 (21 to 91) | 0.06 | 20,22,30,36,37 |
| Higher education | 395 | 3 | 1.05 (0.73 to 1.51) | 81 (26 to 91) | 0.1 | 22,29,36 |
| Live in family home | 418 | 2 | 1.84 (1.40 to 2.43) | 40 | 0.02 | 36,37 |
| Lifestyle | ||||||
| Alcohol abstainer | 536 | 3 | 1.96 (0.84 to 4.67) | 90 (67 to 95) | 0.5 | 30,36,38 |
| Current smoker | 487 | 2 | 0.72 (0.36 to 1.44) | 94 | 0.2 | 30,36 |
| Psychologic health | SMD | |||||
| Quality of lifeb | 678 | 10 | −0.65 (−0.88 to −0.43) | 83 (74 to 88) | 0.2 | 30,31,35,39,40,53,62,65,66,72; control data from refs. 78–81 |
| Transplantc,d | 517 | 9 | −0.42 (−0.64 to −0.20) | 77 (54 to 86) | 0.09 | 30,31,35,39,40,53,62,65,72; control data from refs. 78,79,81 |
| Dialysisd | 161 | 7 | −1.01 (−1.32 to -0.70) | 62 (0 to 81) | 0.1 | 31,35,39,53,62,65,66; control data from refs. 79–81 |
| Positive affecte | 121 | 3 | 0.40 (−0.12 to 0.91) | 84 (15 to 93) | 0.2 | 28,32,74 |
| Negative affecte | 121 | 3 | 0.18 (−0.26 to 0.61) | 79 (12 to 90) | 0.2 | 28,32,74 |
| Self-perception/self-imagef | 86 | 2 | −0.31 (−1.08 to 0.47) | 84 | 0.3 | 21,38 |
Although clustering (potential correlation from including subgroups taken from the same study) may underestimate between-study variance, the overall quality of life result showed a compensatory larger variance in effect size (τ2 value). To overcome any effect of clustering, we also undertook a sensitivity analysis by meta-analyzing the treatment by modality interaction in studies that reported data for both patients with transplants and patients on dialysis (n=6). This showed a pooled difference in SMD between dialysis and transplant groups of −0.62 (95% CI, −0.88 to −0.37), I2=0% (95% CI, 0 to 61), and τ2<0.0001. This was equivalent to the difference in effect estimates between transplant and dialysis presented in the table. 95% CI, 95% confidence interval; SMD, standardized mean difference.
95% CI for I2 incalculable with one degree of freedom.
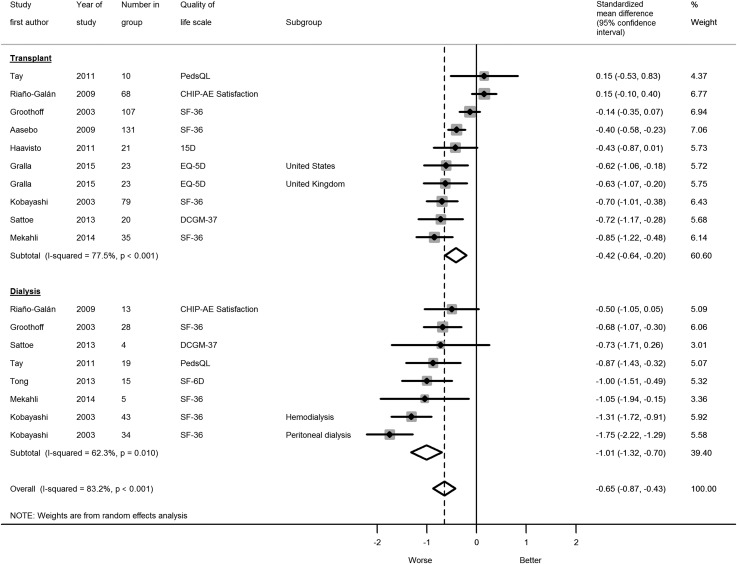
Quality of life scales and forest plot can be seen in Figure 2. 36-Item Short Form Health Survey scores were converted to utility scores using model EQ1 by Ara and Brazier (12), and SDs were derived using a model by Wyld et al. (13). If studies did not report a normative comparator and country- and age-specific control data were readily available, we included them in the meta-analysis. When performing the meta-analysis without control data sourced externally to the studies from the systematic search, the following results were obtained: five studies, overall SMD of −0.42 (95% CI, −0.66 to −0.17), I2=78% (95% CI, 51 to 88), and τ2=0.09; five studies, transplant SMD of −0.31 (95% CI, −0.60 to −0.02), I2=84% (95% CI, 56 to 91), and τ2=0.09; and three studies, dialysis SMD of −0.67 (95% CI, −0.97 to −0.38), I2=0% (95% CI, 0 to 73), and τ2<0.0001.
Young adults on RRT were more likely to be unemployed (eight studies: RR, 1.89; 95% CI, 1.47 to 2.44) and live in the family home (two studies: RR, 1.84; 95% CI, 1.40 to 2.43). They were less likely to be married/have a partner (four studies: RR, 0.71; 95% CI, 0.53 to 0.95). Higher education (three studies: RR, 1.05; 95% CI, 0.73 to 1.51), alcohol abstinence (three studies: RR, 1.96; 95% CI, 0.84 to 4.67), and smoking status (two studies: RR, 0.72; 95% CI, 0.36 to 1.44) did not differ.
Psychologic Health Scales
Only five of nine studies measuring quality of life in patients with transplants reported normative comparison data, and similarly, only three of seven studies in patients on dialysis reported these data (Table 4). The study sample sizes were small, particularly for patients on dialysis (median n=17). Heterogeneity was less when stratifying by modality but remained high. The SF-36 was the most frequently used scale (n=4). Seven studies reported relevant quality of life measures, which we could not include in the meta-analysis due to missing variability data (n=3) or control data (n=4).
Young adults on RRT had lower quality of life compared with their peers, which was worse for patients on dialysis (seven studies: SMD, −1.01; 95% CI, −1.32 to −0.70) compared with patients with transplants (nine studies: SMD, −0.42; 95% CI, −0.64 to −0.20) (Figure 2). This equates to the following absolute differences in EQ-5D utilities: mean utility for 16–30 year olds (n=1333) from the Health Survey for England 2012 was 93 of 100 (SD=14.7; 95% CI, 92.2 to 93.8) (14); the average adjusted score for patients with transplants is 6.2 points lower at 86.8 (95% CI, 83.6 to 90.1) and 14.8 points lower at 78.2 (95% CI, 73.6 to 82.7) for patients on dialysis. This modality effect was also seen when meta-analyzing the interaction terms (data are in Table 4). We found no evidence for small study effects (P=0.70), but analysis lacked power, because few studies were available for inclusion.
Figure 2.
Forest plot showing that compared with healthy peers, young adults on RRT had lower quality of life, which was worse for patients on dialysis. CHIP-AE Satisfaction, Child Health and Illness Profile–Adolescent Edition; 15D, 15-Dimension; DCGM-37, 37-item DISABKIDS Chronic Generic Module; EQ-5D, EuroQoL-5 Dimensions; PedsQL, Pediatric Quality of Life Inventory; SF-36, 36-Item Short Form Health Survey; SF-6D, 6-Dimension Short Form Health Survey.
We found no differences in the Positive and Negative Affect Schedule and the Rosenberg Self-Image Scale and Self-Perception Profile for Adolescents, although these were measured in few studies with small numbers of participants and subject to high heterogeneity. Five studies reported relevant self-concept, self-esteem, or self-image scale measures, which we could not include in the meta-analysis due to missing information (n=2) or a lack of control data (n=3). Three studies reported coping via the use of different multidomain scales and could not be combined in a meta-analysis.
Discussion
Key Findings
This systematic review establishes the negative social and psychologic effects (lower quality of life and limited employment, independence, and relationships) of ESRD on young adults compared with healthy controls and highlights the limitations of existing research. We have found a marked drop in quality of life, particularly for patients on dialysis compared with those who were transplanted. Young adults on RRT were also more likely to be unemployed, despite a similar proportion having higher education as in the general population (on the basis of only three studies). The effect estimates that we present are likely to be biased toward better outcomes, because many studies examined patients with transplants and may be subject to a healthy responder bias.
Comparison with Existing Studies
To our knowledge, this is the first quantitative systematic review of psychosocial and lifestyle outcomes for young adult patients on RRT. We found a similar drop in quality of life for dialysis compared with transplant as a previous systematic review in an older adult population (13); however, comparison is limited, because we calculated a Z score relative to the general population, whereas Wyld et al. (13) reported absolute utility scores.
Implications for Research and Clinical Care
This meta-analysis has identified some areas of concern regarding the long-term sociodemographic and psychologic outcomes for young adults with ESRD. However, one must be cautious in interpreting these results given the limitations highlighted below. These data, despite a small evidence base, suggest limited life chances and need further attention to establish how young adults receiving RRT function in society and potential barriers that they may face to establishing successful employment, independent living, and long-term relationships. It is possible that psychosocial outcomes may be more meaningful to patients than biochemical and intermediate outcomes often collected by registries. In the short term, we are undertaking a large-scale national multicenter study looking at a young adult population of all treatment modalities: The Surveying People Experiencing Young Adult Kidney Failure Study (18). The results may raise awareness in the clinical setting and shift the focus of outcomes onto those that matter most to patients, perhaps prompting disease registers to collect patient-centered outcomes. Ideally, researchers should undertake a large, prospective cohort study collecting baseline data in childhood, with repeat outcome measure data that span the transition into adulthood as well as later life. Comparative longitudinal population data, using the same methods, should also be available to facilitate interpretation. Such a study would be costly, be challenging, and take at least a decade to undertake, but it would provide a far more robust evidence base on the needs of this ESRD population.
Strengths and Limitations
The strengths of this review are the wide search criteria to ensure that all relevant studies were included, the inclusion of gray literature and non-English articles, and repeating the systematic search to capture new studies. Furthermore, we have gone to great lengths not to lose valuable data by contacting authors for other data, converting different outcomes to a common metric, and searching appropriate comparative data to provide additional measures of outcome differences.
There are also limitations to be considered. First, the evidence base was small. Second, in some cases, we had to assume no sampling variation in reference populations of unreported size. This results in artificially small 95% CIs around the difference. Nonetheless, in all cases where this assumption was made, the normative data were on the basis of census data or regional/national statistics, and therefore, this is a minor issue. Third, pooled proportions come from different studies and therefore, may not always be directly comparable due to reporting differences. Fourth, we combined mean domain scores into a single EQ-5D utility score for the SF-36 quality of life studies. Although we used established methods for this conversion and calculation of the score variability, these were validated in other datasets; however, where data were available, the predicted values seemed consistent with the observed. Fifth, many studies did not report subgroups data, such as sex and modality (except for quality of life), and therefore, we could not examine these variables for heterogeneity. Given the paucity of studies, we did not feel that exploration of between-study variability using metaregression would have been helpful; in addition, we were reassured that subgroups were statistically (but not structurally) independent when including subgroups as individual studies in meta-analysis. Sixth, because there were almost 20,000 abstracts to screen, we only checked a random sample of 1000 abstracts (5%) for inclusion and six studies (10%) for independent data extraction, and concordance rates were high in both cases.
Quantitative Assessment of Bias.
The nature of the studies in this area was such that we could only quantitate bias by modifying the NOS. For studies without comparator data, this lessened the scope of the bias assessment, and the risk of bias was higher. However, overall study quality seemed reasonable. In addition to the NOS, we summarized the study attributes in Table 2 and described the potential bias that these attributes suggest.
Justification of Exclusion.
We excluded studies where outcomes were reported by caregiver/family/health or educational professionals to focus on the patient experience, particularly from a psychologic perspective. Other reasons that studies were excluded are included in Figure 1—largely due to the reported average age of participants being outside the specified range.
Assessment of Quality of Included Studies.
The Oxford Centre for Evidence-Based Medicine (19) grades evidence from level 1a (e.g., homogenous systematic review) to level 5 (e.g., expert opinion without critical appraisal) and grades recommendations on the basis of such evidence from level A (consistent level 1 studies) to level D (inconsistent/inconclusive or level 5 evidence). According to this framework, overall, the studies in this review are from level of evidence 2b–4 and grade of recommendation B/C. In general, studies were descriptive, cross-sectional, and single center, with small numbers of participants. There were no prospective studies, although three studies were retrospective cohort studies. Although one third of studies presented normative data, these almost all used data collected for other purposes.
Generalization of the Conclusions.
Our review included young adults requiring RRT, and studies appropriately focused on patients with transplants, because they represent much of this age group in high-income countries, where the studies were mostly undertaken. Therefore, the effect estimates that we have presented are generalizable to patients with transplants in high-income countries, but apart from quality of life, they may not be generalizable to young adults receiving dialysis. The small number of studies and high heterogeneity of findings must also be stressed as well as the inability to explore demographic effects.
In summary, young adults receiving RRT have lower quality of life and limited employment, independence, and relationships compared with healthy controls. This review highlights the limitations of existing research and clarifies the key patient-reported outcomes to focus on in the future.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Penny Whiting for help in planning this review. We thank Dee Knipe, Mairead Murphy, and Penny Whiting for their help with creating and managing a database for references. We acknowledge Catherine Borwick, specialist librarian, for assistance with the search strategy. We thank the interlibrary loan service at the University of Bristol and study authors for providing full text articles. We are indebted to Heide Busse for German translation, Steve Clissold and Keisuke Suzuki for help with Japanese translation, José López-López for Spanish translation, and Marta Tazewell for Polish translation. We also thank Prof. Julian Higgins for advice on statistical aspects.
A.J.H. is funded on a Tony Wing Clinical Studentship from the British Kidney Patient Association and Kidney Research UK.
Funders had no role in study design, collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04760517/-/DCSupplemental.
References
- 1.Giedd JN: The teen brain: Insights from neuroimaging. J Adolesc Health 42: 335–343, 2008 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System : 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2016 [Google Scholar]
- 3.MacNeill SJ, Casula A, Shaw C, Castledine C: UK renal registry 18th annual report: Chapter 2 UK renal replacement therapy prevalence in 2014: National and centre-specific analyses. Nephron 132[Suppl 1]: 41–68, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Foster BJ: Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol 30: 567–576, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Van Arendonk KJ, James NT, Boyarsky BJ, Garonzik-Wang JM, Orandi BJ, Magee JC, Smith JM, Colombani PM, Segev DL: Age at graft loss after pediatric kidney transplantation: Exploring the high-risk age window. Clin J Am Soc Nephrol 8: 1019–1026, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson AR: Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol 14: 469–472, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Harden PN, Walsh G, Bandler N, Bradley S, Lonsdale D, Taylor J, Marks SD: Bridging the gap: An integrated paediatric to adult clinical service for young adults with kidney failure. BMJ 344: e3718, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses, Ottawa, ON, Canada, Ottawa Hospital Research Institute, 2014 [Google Scholar]
- 10.Newcombe RG: Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med 17: 857–872, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis JP, Patsopoulos NA, Evangelou E: Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335: 914–916, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ara R, Brazier J: Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 11: 1131–1143, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Wyld M, Morton RL, Hayen A, Howard K, Webster AC: A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 9: e1001307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UK Data Service: Health Survey for England 2012, London, United Kingdom, NatCen Social Research, Department of Epidemiology and Public Health, University College London, 2014 [Google Scholar]
- 15.Morel P, Almond PS, Matas AJ, Gillingham KJ, Chau C, Brown A, Kashtan CE, Mauer SM, Chavers B, Nevins TE, Dunn DL, Sutherland DER, Payne WD, Najarian JS: Long-term quality of life after kidney transplantation in childhood. Transplantation 52: 47–53, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Krmar RT, Eymann A, Ramirez JA, Ferraris JR: Quality of life after kidney transplantation in children. Transplantation 64: 540–541, 1997 [DOI] [PubMed] [Google Scholar]
- 17.El-Husseini A, Hassan R, Sobh M, Ghoneim M: The effects of gender on health-related quality of life in pediatric live-donor kidney transplantation: A single-center experience in a developing country. Pediatr Transplant 14: 188–195, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hamilton AJ: SPEAK Study Website—Information and Updates. UK Renal Registry, 2017. Available at: https://www.renalreg.org/projects/speak/. Accessed January 10, 2017
- 19.Center for Evidence-Based Medicine : Levels of Evidence (March 2009), Oxford, United Kingdom, University of Oxford, 2009 [Google Scholar]
- 20.Ehrich JH, Rizzoni G, Broyer M, Brunner FP, Brynger H, Fassbinder W, Geerlings W, Selwood NH, Tufveson G, Wing AJ: Rehabilitation of young adults during renal replacement therapy in Europe. 2. Schooling, employment, and social situation. Nephrol Dial Transplant 7: 579–586, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Prather SJ: The association between family and psychosocial variables and the treatment adherence in adolescent kidney transplant patients [dissertation]. San Diego: United States International University; 1992
- 22.Ayonayon HN: Pediatric renal transplant survivors: Adult transitions [dissertation]. Berkeley: California School of Professional Psychology at Alameda; 1997
- 23.Querfeld U, Korten B, Naumann G, Michalk DV: Medical and psychosocial rehabilitation of young adults receiving renal replacement therapy since childhood: A single-centre experience. Nephrol Dial Transplant 12: 33–37, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Offner G, Latta K, Hoyer PF, Baum HJ, Ehrich JH, Pichlmayr R, Brodehl J: Kidney transplanted children come of age. Kidney Int 55: 1509–1517, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Wingen AMF, Feldhoff C: [Quality of life following renal transplantation in childhood]. Zentralbl Chir 124: 74–78, 1999 [PubMed] [Google Scholar]
- 26.Olausson B, Hansson S, Wennerström M, Olausson M, Friman S: Quality of life after paediatric kidney transplantation: A single-centre experience. Transplant Proc 33: 2446–2448, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Rosenkranz J, Reichwald-Klugger E, Oh J, Turzer M, Mehls O, Schaefer F: Psychosocial rehabilitation and satisfaction with life in adults with childhood-onset of end-stage renal disease. Pediatr Nephrol 20: 1288–1294, 2005 [DOI] [PubMed] [Google Scholar]
- 28.de Castro EK, Moreno-Jiménez B, Rodríguez-Carvajal R: Psychological well-being in adults transplanted in childhood. Pediatr Transplant 11: 272–278, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kärrfelt HM, Berg UB: Long-term psychosocial outcome after renal transplantation during childhood. Pediatr Transplant 12: 557–562, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Aasebø W, Homb-Vesteraas NA, Hartmann A, Stavem K: Life situation and quality of life in young adult kidney transplant recipients. Nephrol Dial Transplant 24: 304–308, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Riaño-Galán I, Málaga S, Rajmil L, Ariceta G, Navarro M, Loris C, Vallo A: Quality of life of adolescents with end-stage renal disease and kidney transplant. Pediatr Nephrol 24: 1561–1568, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Rafie S: The psychosocial impact of renal transplantation on adolescent and young adult recipients [dissertation]. Palo Alto: Pacific Graduate School of Psychology; 2011
- 33.Rocha S, Fonseca I, Silva N, Martins LS, Dias L, Henriques AC, Faria S, Costa T, Rocha L, Cabrita A, Mota C: Impact of pediatric kidney transplantation on long-term professional and social outcomes. Transplant Proc 43: 120–124, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Ritchie AG, Clayton PA, Mackie FE, Kennedy SE: Nationwide survey of adolescents and young adults with end-stage kidney disease. Nephrology (Carlton) 17: 539–544, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Mekahli D, Ledermann S, Gullett A, Rees L: Evaluation of quality of life by young adult survivors of severe chronic kidney disease in infancy. Pediatr Nephrol 29: 1387–1393, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Mellerio H, Alberti C, Labèguerie M, Andriss B, Savoye E, Lassalle M, Jacquelinet C, Loirat C; French Working Group on the Long-Term Outcome of Transplanted Children : Adult social and professional outcomes of pediatric renal transplant recipients. Transplantation 97: 196–205, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Reynolds JM, Morton MJ, Garralda ME, Postlethwaite RJ, Goh D: Psychosocial adjustment of adult survivors of a paediatric dialysis and transplant programme. Arch Dis Child 68: 104–110, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morton MJ, Reynolds JM, Garralda ME, Postlethwaite RJ, Goh D: Psychiatric adjustment in end-stage renal disease: A follow up study of former paediatric patients. J Psychosom Res 38: 293–303, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Groothoff JW, Grootenhuis MA, Offringa M, Gruppen MP, Korevaar JC, Heymans HS: Quality of life in adults with end-stage renal disease since childhood is only partially impaired. Nephrol Dial Transplant 18: 310–317, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Haavisto A, Jalanko H, Sintonen H, Holmberg C, Qvist E: Quality of life in adult survivors of pediatric kidney transplantation. Transplantation 92: 1322–1326, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Fine RN, Malekzadeh MH, Pennisi AJ, Ettenger RB, Uittenbogaart CH, Negrete VF, Korsch BM: Long-term results of renal transplantation in children. Pediatrics 61: 641–650, 1978 [PubMed] [Google Scholar]
- 42.Poznanski EO, Miller E, Salguero C, Kelsh RC: Quality of life for long-term survivors of end-stage renal disease. JAMA 239: 2343–2347, 1978 [DOI] [PubMed] [Google Scholar]
- 43.Velasco de Parra ML, González L, Santoyo E: [Evaluation of psychological and social rehabilitation of the child with chronic renal failure]. Bol Med Hosp Infant Mex 37: 51–62, 1980 [PubMed] [Google Scholar]
- 44.Simmons RG, Marine SK, Simmons RL: Gift of Life: The Effect of Organ Transplantation on Individual, Family, and Societal Dynamics, Piscataway, NJ, Transaction Publishers, 1977 [Google Scholar]
- 45.Lee HM, Mendez-Picon G, Posner MP: The status of rehabilitation, morbidity, and mortality of long-term survivors of pediatric kidney transplants. Transplant Proc 21: 1989–1991, 1989 [PubMed] [Google Scholar]
- 46.Sato K, Akahoshi K, Suzuki Y, Fukuyama Y, Isomoto A: Psychiatric problems of children and adolescents who have had living kidney transplants [in Japanese]. Jpn J Child Adolesc Psychiatry 31: 327–350, 1990 [Google Scholar]
- 47.Roscoe JM, Smith LF, Williams EA, Stein M, Morton AR, Balfe JW, Arbus GS: Medical and social outcome in adolescents with end-stage renal failure. Kidney Int 40: 948–953, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Bocheńska M, Bednorz R, Niezbrzycka-Andrzejewska K, Wikiera I, Morawska Z: [Psychological aspects of the treatment of children with terminal renal failure by repeated hemodialysis]. Wiad Lek 45: 28–31, 1992 [PubMed] [Google Scholar]
- 49.Gämperli A, Leumann E, Neuhaus TJ, Schlumpf R, Largiadèr F: [25 years of dialysis and kidney transplantation in children and adolescents]. Schweiz Med Wochenschr 126: 77–85, 1996 [PubMed] [Google Scholar]
- 50.Park CH, Kim DY, Kim KS, Cho WH, Park SB, Kim HC: Clinical and psychosocial consequences of renal transplantation in children. Transplant Proc 28: 1604–1606, 1996 [PubMed] [Google Scholar]
- 51.Haberal M, Bereket G, Karakayali H, Arslan G, Moray G, Bilgin N: Pediatric renal transplantation in Turkey: A review of 56 cases from a single center. Pediatr Transplant 4: 293–299, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Fernández de Preliasco MV, Viera MJ, Sebelli P, Rodríguez Rilo L, Sánchez GA: Compliance with medical and dental treatments in children and adolescents renal transplant patients. Acta Odontol Latinoam 15: 21–27, 2002 [PubMed] [Google Scholar]
- 53.Kobayashi A, Itoh Y, Honda M, Yoshiiya K: Quality of life assessed with SF-36 health survey in pediatric ESRD patients [in Japanese]. Med J Kobe Univ 63: 39–45, 2003 [Google Scholar]
- 54.Penkower L, Dew MA, Ellis D, Sereika SM, Kitutu JM, Shapiro R: Psychological distress and adherence to the medical regimen among adolescent renal transplant recipients. Am J Transplant 3: 1418–1425, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Cetingok M, Winsett RP, Hathaway DK: A comparative study of quality of life among the age groups of kidney transplant recipients. Prog Transplant 14: 33–38, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Feinstein S, Keich R, Becker-Cohen R, Rinat C, Schwartz SB, Frishberg Y: Is noncompliance among adolescent renal transplant recipients inevitable? Pediatrics 115: 969–973, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Wu ZX, Yang SL, Wu WZ, Cai JQ, Wang QH, Wang D, Gao X, Liao LM, Tan JM: The long-term outcomes of pediatric kidney transplantation: A single-centre experience in China. Pediatr Transplant 12: 215–218, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Ferraresso M, Ghio L, Raiteri M, Belingheri M, Beretta C, Martina V, Edefonti A, Berardinelli L: Pediatric kidney transplantation: A snapshot 10 years later. Transplant Proc 40: 1852–1853, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Tielen M, van Staa AL, Jedeloo S, van Exel NJ, Weimar W: Q-methodology to identify young adult renal transplant recipients at risk for nonadherence. Transplantation 85: 700–706, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Schiavelli R, Merino D, Gambina V, Avella F, Gahan G, Khoury M, Alvarenga E, Cuevas C: Transición de la atención pediátrica a la del adulto en trasplante renal. Rev Nefrol Dial Transpl 30: 10–14, 2010 [Google Scholar]
- 61.Feinstein S, Ben-Shalom E, Becker-Cohen R, Rinat C, Meislish N, Frishberg Y: Not leaving home-continued care of adolescent and young adult kidney transplant recipients at the pediatric nephrology clinic. Pediatr Nephrol 26: 1591–1731, 2011 [Google Scholar]
- 62.Tay LS, Wan D, Aw M, Kim YH: Examining Health-Related Quality of Life (HRQoL), disease-specific quality of life, and coping behaviors in adolescents with renal disease. Asia Pac Psychiatry 3: 204–211, 2011 [Google Scholar]
- 63.Haddiya I, Rhou H, Ezaitouni F, Ouzeddoun N, Bayahia R, Benamar L: [Peritoneal dialysis in patients under twenty years: Experience in a Moroccan university hospital]. Pan Afr Med J 12: 45, 2012 [PMC free article] [PubMed] [Google Scholar]
- 64.Tozzi AE, Mazzotti E, Di Ciommo VM, Dello Strologo L, Cuttini M: Quality of life in a cohort of patients diagnosed with renal failure in childhood and who received renal transplant. Pediatr Transplant 16: 840–845, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Sattoe JN, Jedeloo S, van Staa A: Effective peer-to-peer support for young people with end-stage renal disease: A mixed methods evaluation of Camp COOL. BMC Nephrol 14: 279, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong A, Wong G, McTaggart S, Henning P, Mackie F, Carroll RP, Howard K, Craig JC: Quality of life of young adults and adolescents with chronic kidney disease. J Pediatr 163: 1179–1185.e5, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Gralla J, Murray P, Harden P, Wiseman A: Impact of renal transplantation on employment opportunities in young adults. Transplantation 14 [Suppl S3]: 808–849, 2014 [Google Scholar]
- 68.Johns TS, Estrella MM, Crews DC, Appel LJ, Anderson CA, Ephraim PL, Cook C, Boulware LE: Neighborhood socioeconomic status, race, and mortality in young adult dialysis patients. J Am Soc Nephrol 25: 2649–2657, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis H, Marks SD: Differences between paediatric and adult presentation of ESKD in attainment of adult social goals. Pediatr Nephrol 29: 2379–2385, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Murray PD, Dobbels F, Lonsdale DC, Harden PN: Impact of end-stage kidney disease on academic achievement and employment in young adults: A mixed methods study. J Adolesc Health 55: 505–512, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Connelly J, Pilch N, Oliver M, Jordan C, Fleming J, Meadows H, Baliga P, Nadig S, Twombley K, Shatat I, Taber D: Prediction of medication non-adherence and associated outcomes in pediatric kidney transplant recipients. Pediatr Transplant 19: 555–562, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Gralla J, Murray P, Wiseman A, Harden P: Education and employment after renal transplantation in young adults: An International Study. Am J Transplant 15 [Suppl 3]: 1, 2015 [Google Scholar]
- 73.Lewis H, Arber S: Impact of age at onset for children with renal failure on education and employment transitions. Health (London) 19: 67–85, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Massey EK, Meys K, Kerner R, Weimar W, Roodnat J, Cransberg K: Young adult kidney transplant recipients: Nonadherent and Happy. Transplantation 99: e89–e96, 2015 [DOI] [PubMed] [Google Scholar]
- 75.Patel R, Murray PD, Moiseev A, Kalachik A, Harden PN: Impact of end stage kidney disease on education and employment in young adults: A comparison between Belarus and the UK. Nephrol Dial Transplant 29 [Suppl 3]: iii539–iii566, 2014 [Google Scholar]
- 76.Rus RBPJ, Battelino N, Kersnik Levart T, Novljan G: Long-term outcome of pediatric renal transplantation in Slovenia. Presented at the 8th Congress on Pediatric Transplantation, IPTA, San Francisco, CA, March 28–31, 2015 [Google Scholar]
- 77.Center for Evidence-Based Medicine : Study Designs, Oxford, United Kingdom, University of Oxford, 2016 [Google Scholar]
- 78.Ware J, Snow K, Kosinski M, Gandek B: SF-36 Health Survey: Manual and Interpretive Guide, Boston, The Health Institute, New England Medical Center, 1993 [Google Scholar]
- 79.Shiroiwa T, Fukuda T, Ikeda S, Igarashi A, Noto S, Saito S, Shimozuma K: Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res 25: 707–719, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norman R, Church J, van den Berg B, Goodall S: Australian health-related quality of life population norms derived from the SF-6D. Aust N Z J Public Health 37: 17–23, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Heng Mei Ling M: Examining the Impact of Fatigue, Age, Coping & Social Support on the Health-Related Quality of Life of Pediatric Cancer Patients in Southeast Asia [dissertation]. Singapore: National University of Singapore; 2010
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.