Abstract
Background and objectives
Joubert syndrome is a genetically heterogeneous ciliopathy associated with >30 genes. The characteristics of kidney disease and genotype-phenotype correlations have not been evaluated in a large cohort at a single center.
Design, setting, participants, & measurements
We evaluated 97 individuals with Joubert syndrome at the National Institutes of Health Clinical Center using abdominal ultrasonography, blood and urine chemistries, and DNA sequencing.
Results
Patients were ages 0.6–36 years old (mean of 9.0±7.6 years old); 41 were female. Mutations were identified in 19 genes in 92 patients; two thirds of the mutations resided in six genes: TMEM67, C5orf42, CC2D2A, CEP290, AHI1, and KIAA0586. Kidney disease was detected in 30%, most commonly in association with the following genes: CEP290 (six of six), TMEM67 (11 of 22), and AHI1 (three of six). No kidney disease was identified in patients with mutations in C5orf42 (zero of 15) or KIAA0586 (zero of six). Prenatal ultrasonography of kidneys was normal in 72% of patients with kidney disease. Specific types of kidney disease included nephronophthisis (31%), an overlap phenotype of autosomal recessive polycystic kidney disease/nephronophthisis (35%), unilateral multicystic dysplastic kidney (10%), and indeterminate-type cystic kidney disease (24%). Early-onset hypertension occurred in 24% of patients with kidney disease. Age at ESRD (n=13) ranged from 6 to 24 years old (mean of 11.3±4.8 years old).
Conclusions
Kidney disease occurs in up to one third of patients with Joubert syndrome, most commonly in those with mutations in CEP290, TMEM67, and AHI1. Patients with mutations in C5orf42 or KIAA0586 are less likely to develop kidney disease. Prenatal ultrasonography is a poor predictor of kidney involvement in Joubert syndrome. Unilateral multicystic dysplastic kidney and autosomal recessive polycystic kidney disease–like enlarged kidneys with early-onset hypertension can be part of the Joubert syndrome kidney phenotype.
Keywords: genetic renal disease; cystic kidney; ciliopathy; nephronophthisis; polycystic kidney disease; Joubert syndrome 1; Polycystic Kidney, Autosomal Recessive; Multicystic Dysplastic Kidney; Prospective Studies; Kidney Diseases, Cystic; Eye Abnormalities; kidney; Retina; Kidney Failure, Chronic; Mutation; Phenotype; Sequence Analysis, DNA; hypertension; Genetic Association Studies; Ultrasonography, Prenatal; Ciliopathies; Pregnancy; Abnormalities, Multiple; Cerebellum
Introduction
Fibrocystic kidney disease is a common feature in many ciliopathies, including Meckel, Bardet–Biedl, and Joubert syndromes (1,2). First described in 1969 (3), Joubert syndrome (OMIM 213300) is defined on the basis of a characteristic set of cerebellum and midbrain abnormalities that collectively result in the diagnostic “molar tooth sign” on axial brain images (4) (Figure 1). The incidence of Joubert syndrome is estimated to range between 1:80,000 and 1:100,000 (5). Core clinical features of Joubert syndrome observed shortly after birth include hypotonia and episodic tachypnea and/or apnea followed later by developmental delay, ocular motor apraxia, truncal ataxia, and speech apraxia (5,6). Most patients have extraneurologic involvement that can include polydactyly, chorioretinal colobomas, retinal degeneration, congenital hepatic fibrosis, and/or fibrocystic kidney disease (5,7). This clinical heterogeneity led to the use of the term Joubert syndrome and related disorders, which include Senior–Løken (nephronophthisis and retinal degeneration) syndrome and COACH (colobomas, “oligophrenia” for cognitive impairment, ataxia, cerebellar vermis hypoplasia, and hepatic fibrosis) syndrome (8). For simplicity, the term Joubert syndrome refers to all patients with the “molar tooth sign,” including patients with or without non-neurologic disease (6). In this article, we use Joubert syndrome to include Senior–Løken and COACH syndromes.
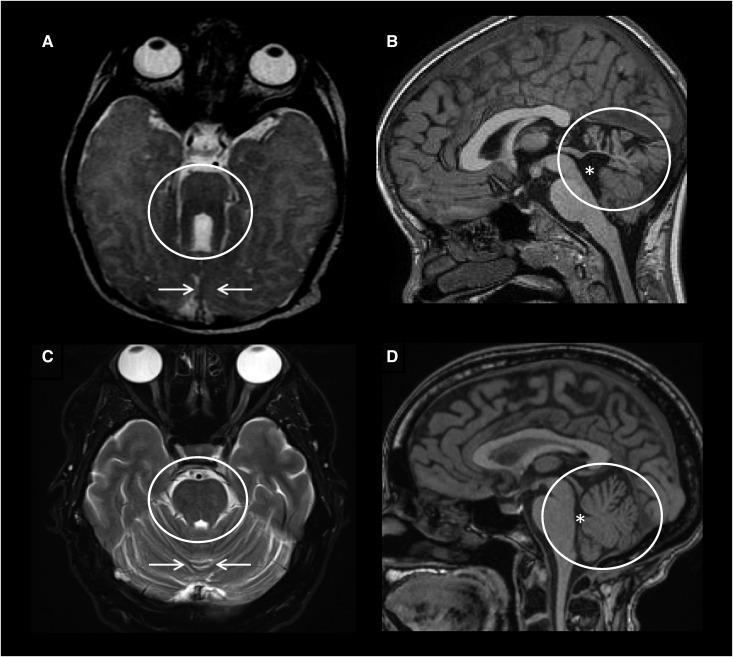
Figure 1.
Clinical diagnosis of Joubert syndrome is made based on the characteristic “molar tooth sign” on brain imaging. Axial brain magnetic resonance imaging (MRI) obtained at the pontomesencephalic junction showing (A) the molar tooth sign (circle) and hypoplastic cerebellar vermis (arrows) compared with (C) normal. Midsagittal brain MRI showing (B) hypoplasia and dysplasia of the cerebellar vermis (circle) and enlarged fourth ventricle (asterisk) with rostral displacement of the fastigium compared with (D) normal.
Like other syndromic ciliopathies, Joubert syndrome is characterized by extreme genetic heterogeneity, with >30 causative genes (5). The frequency of kidney disease in individual gene–specific subtypes of Joubert syndrome remains unknown; estimates range from 25% to 27% (9,10). Most publications on kidney involvement in the disease have been limited to small case series or retrospective multicenter reports on the basis of questionnaires and medical records review (7,9,11–13). In 1992, Saraiva and Baraitser (9) claimed that kidney disease and retinal disease in Joubert syndrome were closely linked and that only patients with retinal dystrophy were at risk for fibrocystic kidney disease. However, many individuals with Joubert syndrome and kidney disease but no retinal degeneration have since been reported (5,7). Although the nephronophthisis in Senior–Løken syndrome (14) and cystic dysplastic kidneys in Arima syndrome (7) are relatively well described, the kidney disease in other patients with Joubert syndrome is often reported in nonspecific terms, noting only absence or presence of “kidney cysts” and neglecting data on features, such as BP, kidney function, and delineation of the exact type of the fibrocytic kidney disease. Although many of the over 30 causative Joubert syndrome genes, including CEP290, NPHP1, AHI1, OFD1, RPGRIP1L, CC2D2A, TMEM67, TMEM216, TMEM138, and TMEM237, have been associated with kidney disease, correlations between genotype and kidney disease remain largely unknown (5,15,16).
Here, we describe the characteristics of kidney disease in 97 patients with Joubert syndrome prospectively evaluated at a single center. We detail kidney ultrasonography, magnetic resonance imaging (MRI) of the kidneys, and kidney function tests in the context of the molecular genetic diagnosis as well as other organ involvement, including liver disease and ophthalmologic features.
Materials and Methods
Patients
All patients were prospectively evaluated at the National Institutes of Health (NIH) Clinical Center between 2003 and 2014 under the intramural NIH research protocol “Clinical and Molecular Investigations Into Ciliopathies” (www.clinicaltrials.gov; trial NCT00068224) approved by the National Human Genome Research Institute Institutional Review Board. The study was advertised by the Joubert Syndrome & Related Disorders Foundation. Patients and/or their parents gave written, informed consent.
Enrollment required a clinical diagnosis of Joubert syndrome on the basis of the pathognomonic “molar tooth sign” on brain MRI. For recruitment, the study was advertised to individuals with Joubert syndrome and their families by the Joubert Syndrome and Related Disorders Foundation as a natural history study aiming to describe the individual organ system involvement in Joubert syndrome, including kidney disease. To minimize ascertainment bias, all travel, lodging, and other participation costs as well as clinical and laboratory evaluations were sponsored by the NIH. A total of 120 patients from 105 families who reported to be diagnosed with Joubert syndrome on the basis of brain MRI applied. Brief phone interviews answering parents’ questions on risks and benefits of the study evaluations were performed by the senior investigator (M.G.-A.) on all 120 individuals with Joubert syndrome from 105 families; no patients were excluded at this stage. Fifteen patients could not travel to the NIH Clinical Center in Bethesda, Maryland, in some patients because of the severity of neurologic symptoms. The remaining 105 patients with Joubert syndrome from 90 families underwent week-long clinical evaluations at the NIH Clinical Center. Eight patients from six families (including three patients with the classic kidney and retina findings of Senior–Løken syndrome) were excluded, because they did not have the “molar tooth sign” on our review of their brain MRI images (17). The remaining 97 patients were included in this study.
All patients were evaluated by the senior investigator (M.G.-A.); evaluations included past medical history, family history, physical examination, review of past medical records, review of brain magnetic resonance images, high-resolution complete abdominal ultrasonography (all patients), abdominal MRI (13 patients who were able to cooperate for the study without sedation), complete ophthalmologic examination, and comprehensive blood and urine chemistries. Because abdominal MRI was performed in only 13 patients, ultrasonography-based kidney length, which was available for all patients, was used to evaluate kidney size.
eGFR was calculated using pediatric [eGFR =39.8× (height (meters)/Scr)0.456(1.8/cystatin C)0.418(30/BUN)0.0791.076men(height (meters)/1.4)0.179] (18) and adult [eGFR =175× (Scr)−1.154× (age)−0.203×(0.742 if a woman) ×(1.212 if black)] (19) formulas. Blood samples for DNA were collected from all patients and parents when available.
Kidney disease was defined on the basis of eGFR and/or abnormal findings on kidney ultrasonography, including increased echogenicity, increased or decreased kidney size, and/or cystic changes. Liver disease was defined by elevated liver enzymes and/or increased echogenicity of the liver and/or splenomegaly on abdominal ultrasonography. Retinal degeneration was on the basis of typical findings on retinal examination after dilation of pupils. All available brain magnetic resonance images were qualitatively evaluated by a team of five physicians, including two pediatric neuroradiologists, one adult neuroradiologist, one pediatric neurologist, and one pediatric clinical geneticist (M.G.-A.), as a group to achieve consensus (17).
Molecular Inversion Probes, Whole Exome, and Sanger Sequencing
The coding exons of 27 genes associated with Joubert syndrome were sequenced by combining a molecular inversion probe capture method and next generation sequencing as previously described (20). In addition, exome sequencing was performed using the HiSeq2000 (Illumina, San Diego, CA) (21) and analyzed as previously described (20). Additional details are in Supplemental Material.
Statistical Methods
Ranges, averages, and SDs were calculated. Serum cystatin C was not available in four children (Supplemental Table 1, patients 26, 31, 82, and 83) due to problems in handling of blood specimens. For these patients, eGFR was calculated using an alternative formula on the basis of height and serum creatinine [eGFR =0.413× (height in centimeters/Scr)] (22).
Results
Patient Cohort
Supplemental Table 1 shows the molecular genetic findings in 97 patients as well as their major clinical features, including kidney disease, retinal degeneration, coloboma, liver disease, and polydactyly. There were 41 females and 56 males. Ages ranged from 0.6 to 36 years old (mean of 9.0±7.6 years old) (Supplemental Table 1). Nine families had two siblings (families 6, 7, 8, 18, 24, 29, 36, 65, and 78) and two families had three siblings with Joubert syndrome (families 16 and 66) evaluated at the NIH. In addition, four other families were represented in this study by a single patient (Table 1, families 5, 14, 34, and 44), and family 66 had another affected deceased child.
Table 1.
Comparison of patients with Joubert syndrome with and without kidney disease
| Kidney Disease | No Kidney Disease | |
|---|---|---|
| No. of patients | 29/97 (30%) | 68/97 (70%) |
| Age, yr, mean±SD | 9.6±6.5 | 8.8±8.0 |
| Females, males | 11, 18 | 30, 38 |
| Genea | ||
| TMEM67 | 11 | 11 |
| CEP290 | 6 | 1 |
| AHI1 | 3 | 3 |
| CC2D2A | 2 | 8 |
| INPP5E | 2 | 2 |
| NPHP1 | 1 | 0 |
| RPGRIP1L | 1 | 0 |
| TMEM216 | 1 | 1 |
| TMEM237 | 1 | 0 |
| C5orf42 | 0 | 15 |
| KIAA0586 | 0 | 6 |
| MKS1 | 0 | 5 |
| TMEM231 | 0 | 3 |
| CSPP1 | 0 | 2 |
| KIAA0753 | 0 | 2 |
| OFD1 | 0 | 2 |
| CELSR2 | 0 | 1 |
| KIF7 | 0 | 1 |
| B9D1 | 0 | 1 |
| Unknown | 1 | 4 |
| Polydactyly | 2/29 (7%) | 10/68 (15%) |
| Coloboma | 13/29 (45%) | 17/68 (25%) |
| Retinal degeneration | 11/29 (38%) | 16/68 (24%) |
| Liver involvement | 18/29 (62%) | 24/68 (35%) |
Number of patients with and without kidney disease.
The molecular genetic cause was identified in 19 different Joubert syndrome genes in all but five families; detailed molecular genetic findings of this cohort were reported separately (20) (Supplemental Table 1). Mutations for two thirds of the families resided in six genes: TMEM67 (20%), C5orf42 (14%), CC2D2A (11%), CEP290 (8%), AHI1 (7%), and KIAA0586 (7%). Each of the remaining 13 genes accounted for a small proportion of the families ranging from 1% to 6%: MKS1 (6%), INPP5E (4%), NPHP1 (1%), TMEM216 (2%), OFD1 (2%), CSPP1 (2%), TMEM231 (1%), KIF7 (1%), B9D1 (1%), RPGRIP1L (1%), TMEM237 (1%), KIAA0753 (1%), and CELSR2 (1%).
Retinal degeneration was detected in 20%, coloboma was detected in 30%, polydactyly was detected in 13%, and liver involvement was detected in 43% (Supplemental Table 1).
Kidney Disease
Kidney involvement was identified in 29 patients; in the remaining 68 patients, kidney ultrasonography and eGFR were normal, and there was no hypertension or chronic anemia. (Tables 1 and 2). Four patients with kidney disease (Table 2, patients 9, 16, 53, and 73) underwent kidney transplantation before their NIH evaluation. Of the remaining 25 patients with kidney involvement, 24 had abnormalities on kidney ultrasonography; 13 of these 24 had decreased eGFR (Table 2). One patient had decreased eGFR with normal kidney ultrasonography.
Table 2.
Characteristics of kidney disease in 29 patients with Joubert syndrome and kidney involvement
| Patient No./ Family No./ NIH Ciliopathy No. | Sex/Age at NIH Evaluation, yr | Gene | Prenatal Ultrasonography | NIH Kidney US | Laterality of Kidney Disease | Kidney Length at NIH evaluationa (Right, Left in SD) | GFR, ml/min per 1.73 m2 | Early-Onset Hypertensionb | Kidney Histopathology | Specific Type of Kidney Disease |
|---|---|---|---|---|---|---|---|---|---|---|
| 3/3/97 | m/3.9 | TMEM67 | Prenatal US normal. Oligohydramnios at delivery, enlarged kidneys palpable at birth | Diffuse moderate hyperechogenicity, loss of CMD, small cysts in cortex and medulla | Bilateral | −3.6, −4.3 | 28 (12)c | + | NP | ARPKD-NPHP |
| 4/4/548 | m/4.5 | TMEM67 | N | Loss of CMD | Bilateral | −0.1, +1.5 | 146 | − | NP | NPHP |
| 5/5/216 | f/4.9 | TMEM67 | 18 wk, oligohydramnios, enlarged diffusely hyperechoic cystic kidneys (affected sibling’s prenatal US showed large cystic kidneys, oligohydramnios) | Several cysts in left kidney, normal echogenicity and CMD | Bilateral | −0.2, at mean | 69 (12)c | + | NP | ARPKD-NPHP |
| 8/7/271 | f/6.7 | TMEM67 | 20 wk, enlarged diffusely hyperechoic kidneys with scattered cysts, no oligohydramnios | Diffuse moderate hyperechogenicity, loss of CMD, cysts in cortex and medulla | Bilateral | +0.6, −0.2 | 27 (8)c | + | Extracted right kidney: histopathologic findings consistent with “autosomal recessive polycystic kidney disease,” interstitial nephritis, and glomerulosclerosis | ARPKD-NPHP |
| 9/7/272 | m/9.3 | TMEM67 | N | Tx | Bilateral | Tx | Tx (7) | + | Extracted right kidney: moderate chronic interstitial nephritis, glomerulosclerosis, tubular atrophy, and nephrocalcinosis as well as cysts, mostly accumulated at the CM junction but also scattered throughout the cortex and medulla | ARPKD-NPHP |
| 10/8/557 | m/6.8 | TMEM67 | N | Diffuse mild hyperechogenicity, loss of CMD | Bilateral | −2.5, −1.9 | 129 | − | NP | NPHP |
| 11/8/559 | m/17 | TMEM67 | N | Diffuse moderate hyperechogenicity, loss of CMD, medullary cysts | Bilateral | −0.5, −1.3 | 59 | + | NP | ARPKD-NPHP |
| 13/10/238 | m/8.2 | TMEM67 | 19 wk, enlarged diffusely hyperechoic kidneys with loss of CMD, progressive oligohydramnios | Diffuse moderate hyperechogenicity, loss of CMD, cysts in cortex and medulla | Bilateral | +2.6, +2.3 | 38 (10)c | + | NA | ARPKD-NPHP |
| 16/13/303 | f/15 | TMEM67 | N | Tx | Bilateral | Tx | Tx (13) | + | Extracted right kidney (51 g, 8.5×4.4×2.8 cm); extensive glomerulosclerosis, tubular atrophy, and interstitial fibrosis with severe chronic inflammation without any evidence of dysplasia. A few thin-walled tubular cysts at CM junction | ARPKD-NPHP |
| 17/14/252 | f/15 | TMEM67 | N (affected sibling’s prenatal US showed polycystic kidneys) | Multiple cysts in cortex and medulla of right kidney, normal echogenicity and CMD | Bilateral | −1.8, −0.9 | 83 | − | NP | Cystic, unable to classify |
| 18/15/309 | m/17 | TMEM67 | N | Multiple cysts in cortex and medulla, normal echogenicity and CMD | Bilateral | −0.2, +1.3 | 106 | − | NP | Cystic, unable to classify |
| 40/32/575 | f/2.3 | CC2D2A | N | Medullary hyperecogenicity | Bilateral | −1.8, −0.5 | 119 | − | NP | NPHP |
| 43/35/185 | m/3.6 | CC2D2A | N | Loss of CMD | Bilateral | −3.4, −2.0 | 66 | − | NP | NPHP |
| 48/39/480 | m/0.9 | CEP290 | N | Multiple cysts in cortex and medulla of right kidney, normal echogenicity and CMD | Bilateral | −1.7, −2.8 | 90 | − | NP | Cystic, unable to classify |
| 49/40/552 | m/4.3 | CEP290 | N | Multiple cysts in cortex and medulla of both kidneys, normal echogenicity and CMD | Bilateral | −0.7, −1.4 | 115 | − | NP | Cystic, unable to classify |
| 50/41/373 | m/4.4 | CEP290 | 20 wk, left multicystic dysplastic kidney; right kidney normal | Left multicystic dysplastic kidney without any normal parenchyma, several cortical cysts in right kidney | Unilateral (left) | +1.9, atrophic MCDK | 55 (6)c | − | NP | Unilateral MCDK |
| 52/43/412 | m/10 | CEP290 | N | Multiple medullary cysts, normal echogen | Bilateral | −0.9, −0.9 | 35 | − | NP | NPHP |
| 53/44/213 | f/13 | CEP290 | 19 wk, hyperechogenic kidneys (resolved in third trimester) | Tx | Bilateral | Tx | Tx (9) | − | NA | NPHP |
| 54/45/441 | f/24 | CEP290 | N | Multiple medullary cysts, moderate hyperechogenicity | Bilateral | +0.3, −0.9 | 21 (24)c | − | NP | NPHP |
| 56/47/517 | m/3.3 | AHI1 | 19 wk, left multicystic dysplastic kidney; right kidney normal | Left kidney could not be visualized, right kidney normal | Unilateral (left) | +1.7, atrophic MCDK | 108 | − | NP | Unilateral MCDK |
| 59/50/540 | f/18 | AHI1 | N | Left kidney with several large cysts, right kidney normal size with multiple cysts and moderate hyperechogenicity | Asymmetrical (left earlier onset than right) | +1.9, −0.7 | 92 | − | NP | Cystic, unable to classify, (asymmetric) |
| 60/51/228 | f/21 | AHI1 | N | Left multicystic dysplastic kidney, right kidney normal (14.9 cm) | Unilateral (left) | +4.3, atrophic MCDK | 219 | − | NP | Unilateral MCDK |
| 72/63/372 | m/3.2 | INPP5E | 8 mo, oligohydramnios | Multiple medullary cysts, loss of CMD, moderate hyperechogenicity | Bilateral | +3.9, +3.7 | 137 | − | NP | ARPKD-NPHP |
| 73/64/352 | m/15 | INPP5E | N | Tx | Bilateral | Tx | Tx (6) | − | NP | NPHP |
| 79/67/438 | m/3.4 | NPHP1 | N | Medullary cysts and echogenic foci in medullary pyramids | Bilateral | −1.0, +1.3 | 117 | − | NP | NPHP |
| 80/68/396 | f/9 | TMEM216 | N | N | Bilateral | +0.5, −1.64 | 52 | − | NA | Unable to classify (associated with bladder dysfunction) |
| 88/76/360 | m/21 | RPGRIP1L | N | Diffuse moderate hyperechogenicity, loss of CMD, cysts in medulla and cortex | Bilateral | +2.0, +1.3 | 33 | − | NP | ARPKD-NPHP |
| 89/77/474 | f/4.5 | TMEM237 | 20 wk, enlarged (three times normal size) kidneys with markedly increased echogenicity | Diffuse moderate hyperechogenicity, loss of CMD, echogenic foci and cysts in medulla and cortex | Bilateral | +1.3, −1.8 | 52 | − | NP | ARPKD-NPHP |
| 97/84/358 | m/9.1 | Unknown | N | Moderate hyperechogenicity especially in the cortex, single cortical cyst in right kidney | Bilateral | −0.5. −0.5 | 34 (15)c | − | NP | Cystic, unable to classify |
NIH, National Institutes of Health; US, renal ultrasonography; m, male; CMD, corticomedullary differentiation; NP, not performed; ARPKD-NPHP, autosomal recessive polycystic kidney disease-nephronophthisis; N, normal; NPHP, nephronophthisis; f, female; Tx, transplanted (age at transplantation in years); CM, corticomedullary; NA, not available; MCDK, multicyctic dysplastic kidney.
Kidney length on the basis of US measurement.
Hypertension diagnosed before any decrease in GFR.
These patients received renal transplantation after the NIH visit; ages at transplantation in years are indicated in parentheses.
Kidney Transplantation
At the time that this paper was written, 13 of the 29 patients with Joubert syndrome and kidney involvement had reached ESRD at ages 6–24 years old (mean of 11.3±4.8 years old); seven patients have undergone transplantation after their NIH evaluation (Table 2, patients 3, 5, 8, 13, 50, 54, and 97), and two other patients (Table 2, patients 52 and 80) reached ESRD at age 12 years old but have not yet been transplanted.
Kidney-Related Findings on Prenatal Ultrasonography
Prenatal kidney ultrasonography was abnormal in only eight (28%) of the 29 patients with kidney disease (Table 2, patients 5, 8, 13, 50, 53, 56, 72, and 89). In two other affected individuals (Supplemental Table 1, patients 17 and 42), prenatal ultrasonography was normal, but their mothers had other affected pregnancies with enlarged diffusely cystic kidneys, resulting in fetal demise. In four patients (Table 2, patients 5, 8, 13, and 89), enlarged hyperechogenic kidneys with or without discrete cysts and/or oligohydramnios were seen at gestational age 19–20 weeks. The mother of patient 5 had another pregnancy with similar ultrasonography findings at 19 weeks. In patient 53, who required kidney transplantation at age 9 years old, prenatal ultrasonography at 19 weeks showed hyperechogenic kidneys that returned to normal on repeat ultrasonography in the third trimester. Patient 72 had polyhydramnios during the eighth month of pregnancy. In patient 3, prenatal ultrasonography was normal, but oligohydramnios was diagnosed at delivery; the infant’s kidneys were palpable at birth. In two patients (50 and 56), the kidney abnormality was unilateral; ultrasonography at the 20th week of gestation in each infant showed left multicystic dysplastic kidney, and the right kidney was normal.
Kidney Imaging Findings at the NIH Clinical Center
The most common abnormality on kidney ultrasonography was increased echogenicity involving both cortex and medulla, resulting in loss of corticomedullary differentiation with or without discrete cysts (11 of 24 patients with abnormal ultrasonography). In five of these 11 patients (Table 2, patients 3, 5, 8, 13, and 89), the kidneys were perinatally enlarged; one of these patients still had enlarged kidneys at the time of NIH evaluation at age 8 years old (Table 2, patient 13). In the remaining four, kidney size normalized or became smaller over time (Figure 2, A and B). Patient 60 with left multicystic dysplastic kidney had an enlarged right kidney likely due to compensatory hypertrophy; in the remaining 24 patients with kidney disease, kidney size was either normal or small (Table 2). In four patients (Table 2, patients 40, 52, 54, and 79), cysts and/or hyperechogenicity were confined to medulla (Figure 2D). Asymmetric kidney disease was identified in four patients (Table 2, patients 50, 56, 59, and 60), including three with left multicystic dysplastic kidney (Figure 2, E and F). Patient 56’s nuclear scan at age 12 months old failed to show any kidney function of the left kidney (Figure 2F), and ultrasonography at 19 months showed a normal right kidney and a small left-sided hypoechoic mass that may have represented an atrophic kidney; his kidney function and voiding cystourethrogram were normal. Patient 59’s yearly ultrasonography examinations between ages 1 and 7 years old were completely normal. At age 7 years old, the only abnormal finding was a small cortical cyst at the upper pole of the left kidney; annual ultrasonography imaging between ages 7 and 13 years old remained otherwise normal. At age 17 years old, both kidneys were hyperechogenic; the left kidney (length at −0.7 SD) had several large cysts in the upper pole, whereas the right kidney was relatively larger (length at +1.9 SD), with a few small cortical cysts.
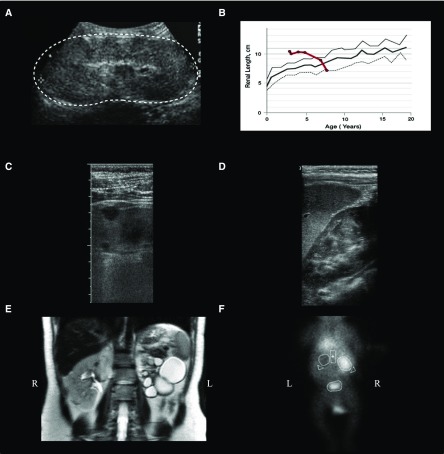
Figure 2.
Kidney disease in patients with Joubert syndrome include autosomal recessive PKD-like disease and unilateral multicytic dysplastic kidneys. (A) Ultrasonographic image of patient 9 at age 4 years old showing enlarged and hyperechogenic left kidney (outlined by the dots) with loss of corticomedullary differentiation. (B) Patient 9’s kidney length (bold red line; average of right and left) plotted against age in comparison with normal mean (bold gray line) and +2 SD (gray line). (C) High-resolution ultrasonographic image of patient 88’s left kidney displaying cysts in cortex. (D) Ultrasonography image of patient 40’s left kidney showing medullary hyperechogenicity. (E) T2-weighted coronal magnetic resonance image of patient 60 showing a left multicystic dysplastic kidney and normal right kidney. (F) 99mTc nuclear scan (coronal image) of patient 56 at age 12 months old showing no function of his left multicystic dysplastic kidney. This patient’s prenatal ultrasonography at 19 weeks of gestation showed a left multicystic dysplastic kidney and a normal right kidney. On ultrasonography at age 3 years old, his left kidney could not be visualized, but his right kidney was normal.
Kidney Histopathology Findings
Histopathology of three kidney explants (Table 2, patients 8, 9, and 16) showed severe tubular atrophy, interstitial fibrosis, chronic inflammation, variable degrees of glomerulosclerosis, and nephrocalcinosis as well as cysts, mostly accumulated at the corticomedullary junction but also scattered throughout the cortex and medulla.
Early-Onset Hypertension
Of the 29 patients with kidney disease, seven had early-onset hypertension diagnosed shortly after birth or within the first years of life before any measurable decrease in eGFR (Table 2). Patient 9 was screened at age 3 years old after his sister’s diagnosis of “polycystic kidneys”; he had cardiomyopathy secondary to undiagnosed early-onset severe hypertension, which resolved after treatment.
Discussion
The frequency of kidney disease in our Joubert syndrome cohort was 30%, slightly higher than published estimates of 25%–27% (9,10), perhaps a reflection of our comprehensive evaluations performed specifically to detect kidney disease. The percentage of individuals with kidney disease may also increase with age; our cohort’s mean age was 9 years old.
Nephronophthisis was present in 31% of our patients with Joubert syndrome and kidney disease, and it resembled the kidney disease of nonsyndromic nephronophthisis (23,24). Clinically, kidney size was normal or small, with increased echogenicity and a few cysts at the corticomedullary junction. Of the nine patients with Joubert syndrome and nephronophthisis, two had Senior–Løken syndrome with retinal degeneration due to mutations in CEP290 andINPP5E; the seven patients with nephronophthisis and no retinal disease had mutations in CC2D2A, TMEM67, and NPHP1.
A hybrid autosomal recessive polycystic kidney disease (PKD)-nephronophthisis pattern of kidney disease was present in 35% of patients with Joubert syndrome and kidney disease. One characteristic feature of autosomal recessive PKD (25) but not nephronophthisis (24), present in seven patients, was early-onset severe hypertension (before any decrease in eGFR). Another feature of autosomal recessive PKD, enlarged kidneys, was seen in five patients, making the early differentiation of Joubert syndrome and autosomal recessive PKD challenging. In fact, three patients (8,9,and 13) were originally diagnosed as having autosomal recessive PKD and carried this diagnosis until their NIH evaluation under our autosomal recessive PKD study at ages 6, 9, and 8 years old; at that time, their mild oculomotor apraxia and developmental delays made us suspect Joubert syndrome. An additional patient (patient 3) was misdiagnosed as having autosomal recessive PKD in addition to his known diagnosis of Joubert syndrome, which was the cause of his polycystic kidneys. Before making a prenatal diagnosis of autosomal recessive PKD, cerebellar abnormalities and polydactyly should be excluded, because renal disease in Joubert syndrome can be indistinguishable from autosomal recessive PKD. Only 13% of patients with Joubert syndrome have polydactyly (Table 1), and therefore, a careful evaluation for brain anomalies is essential for prenatal differentiation of Joubert syndrome from autosomal recessive PKD. In addition, if a child diagnosed as having autosomal recessive PKD manifests developmental delay and/or ocular motor apraxia, a brain MRI should be performed, because patients with Joubert syndrome with mild cerebellar involvement may be misdiagnosed as having autosomal recessive PKD. The combination of coloboma, kidney disease, and polydactyly strongly suggests a ciliopathy, specifically Joubert syndrome. Molecular studies can also be helpful, because seven of the ten patients in the autosomal recessive PKD-nephronophthisis group had mutations in TMEM67 and enlarged, autosomal recessive PKD–like kidneys; indeed, mutations in TMEM67 also cause Meckel syndrome, another ciliopathy with cystic kidney disease (1).
Our most surprising finding was unilateral multicystic dysplastic kidney in three patients with Joubert syndrome (patients 50, 56, and 60), two of whom had completely normal contralateral kidney and normal eGFR at ages 3 (patient 56) and 20 years old (patient 60) (Figure 2, E and F, Table 2). Both of these patients had mutations in AHI1. Although unilateral multicystic dysplastic kidney occurs as part of many syndromes, asymmetric kidney disease is unexpected in ciliopathies (1,23). We are aware of multicystic dysplastic kidney reported in Joubert syndrome in the medical literature: one Iranian patient with Joubert syndrome and right-sided multicystic dysplastic kidney (26) and one with either unilateral kidney agenesis (7) or unilateral multicystic dysplastic kidney misdiagnosed as kidney agenesis due to resolution of the atrophic kidney (27). Of note, patient 60 in our cohort was part of a previously reported series (family K8062) (28). Recently, mutations in HNF1β and TBX18 have been identified in rare families with multicystic dysplastic kidney (29–31). Our patients, however, had mutations in CEP290 and AHI1 as single-gene causes of syndromic multicystic dysplastic kidney associated with Joubert syndrome.
Modifier genes may influence the occurrence of kidney disease in Joubert syndrome (32). All 11 sibling sets evaluated at the NIH were concordant for kidney disease. However, in two families, there was history of a deceased sibling who was discordant for presence of kidney disease (Supplemental Table 1, Table 3, families 34 and 66). In family 34 (Supplemental Table 1), patient 42 had no significant kidney disease at age 3.2 years old, despite having an affected brother who passed away perinatally due to cystic kidneys. Similarly, in family 66, a fourth child passed away at birth with polycystic kidneys (33), whereas three other affected siblings (Supplemental Table 1, patients 76–78) had normal kidney imaging and function at ages 15 months old and 4 years and 9 months old (twins). It is possible that these patients may develop kidney disease as they get older.
Table 3.
Molecular genetic and clinical findings of the 11 sibling sets with Joubert syndrome
| Patient No. | Family No. | NIH Ciliopathy No. | Sex | Age, yr | Gene | Polydactyly | Coloboma | Retinal Dystrophy | Liver Disease | Kidney Disease |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 6 | 302 | m | 5.1 | TMEM67 | − | + | − | + | − |
| 7 | 301 | m | 9.5 | − | + | − | + | − | ||
| 8 | 7 | 271 | f | 6.7 | TMEM67 | − | − | − | + | + |
| 9 | 272 | m | 9.3 | − | − | − | + | + | ||
| 10 | 8 | 557 | m | 6.8 | TMEM67 | − | + | − | + | + |
| 11 | 559 | m | 16.7 | − | + | − | + | + | ||
| 19 | 16 | 562 | m | 22.9 | TMEM67 | − | + | − | + | − |
| 20 | 561 | m | 24.9 | − | + | − | + | − | ||
| 21 | 560 | m | 29.6 | − | + | − | + | − | ||
| 23 | 18 | 488 | m | 0.6 | C5orf42 | − | − | − | − | − |
| 24 | 487 | f | 5.3 | − | − | − | − | − | ||
| 30 | 24 | 501 | m | 10 | C5orf42 | + | − | − | + | − |
| 31 | 500 | f | 11.8 | + | − | − | + | − | ||
| 36 | 29 | 482 | f | 24.5 | C5orf42 | + | − | − | + | − |
| 37 | 481 | m | 27.6 | + | − | − | + | − | ||
| 44 | 36 | 577 | f | 13.1 | CC2D2A | − | − | − | − | − |
| 45 | 576 | f | 15.7 | − | − | − | − | − | ||
| 74 | 65 | 7504 | f | 19 | INPP5E | − | − | + | − | − |
| 75 | 7503 | f | 21 | − | − | + | − | − | ||
| 76a | 66 | 520 | f | 1.2 | TMEM231 | − | − | +/− | +/− | − |
| 77 | 518 | f | 4.8 | − | − | + | − | − | ||
| 78 | 519 | f | 4.8 | − | − | + | − | − | ||
| 90 | 78 | 390 | f | 1.9 | KIAA0753 | − | − | − | − | − |
| 91 | 389 | m | 7 | − | − | − | − | − |
NIH, National Institutes of Health; m, male; f, female.
Because of the young age of this patient, her retinal examination was not conclusive, and liver involvement classification, which was made on the basis of liver hyperechogenicity, was not definitive, because liver can be physiologically hyperechogenic during the first years of life.
Prenatal ultrasonography was abnormal in 28% of patients with Joubert syndrome and kidney disease, representing a surprisingly low prenatal detection rate of kidney disease in Joubert syndrome. In another multisystem ciliopathy, Bardet–Biedl syndrome (34), Forsythe et al. (34) reported a prenatal detection rate of 64% for kidney disease. This discrepancy in prenatal detection rates of kidney disease of patients with Joubert syndrome and Bardet–Biedl syndromes may be due to the variation in the expertise of the prenatal ultrasonography specialists as well as differences in the nature of kidney disease among different ciliopathies.
We can derive several important conclusions from this study. First, kidney disease affects up to one third of patients with Joubert syndrome and is more common in those with pathogenic variants in CEP290, TMEM67, and AHI1 genes; it was not observed in patients with mutations in C5orf42 or KIAA0586. Second, the severity and progression of kidney disease seemed comparable across Joubert syndrome genotypes, with most patients requiring kidney transplantation in childhood or young adulthood. Third, prenatal ultrasonography does not predict kidney disease well in Joubert syndrome; it was abnormal in only 28% of patients with Joubert syndrome and kidney disease. Moreover, the prenatal kidney ultrasonography findings in Joubert syndrome are indistinguishable from autosomal recessive PKD, and therefore, fetuses with hyperechogenic kidneys should have careful imaging for other abnormalities, especially brain anomalies; in addition, polydactyly, if present, can be a helpful sign of Joubert syndrome, although its absence does not exclude the condition. Fourth, early-onset hypertension, before kidney function is decreased, occurred in 24% of patients with Joubert syndrome and kidney disease, particularly those with enlarged, autosomal recessive PKD–like, diffusely hyperechogenic kidneys. Fifth, in families with multiple children with Joubert syndrome, siblings are largely concordant for kidney disease, although exceptions exist. On the basis of these conclusions, we make several recommendations for surveillance. All patients with Joubert syndrome should have BP measurement, complete abdominal ultrasonography, liver and kidney function tests, and complete blood counts at the time of diagnosis and annually thereafter. Patients with Joubert syndrome and unilateral multicystic dysplastic kidney should be radiologically assessed for other congenital anomalies of the kidney and urinary tract, such as vesicoureteral reflux or contralateral ureteropelvic junction obstruction (27,35,36).
Although this study represents the largest number of subjects with Joubert syndrome evaluated at a single center, the size of the cohort is still relatively small. Gene-phenotype correlations were not possible, except for the most common genetic causes. Our cohort is likely enriched for patients with Joubert syndrome and kidney and liver diseases, because the comprehensive evaluations performed at the NIH Clinical Center may have attracted patients with these manifestations. However, our study also offered other investigations, including neurocognitive testing, DNA sequencing, sleep studies, and echocardiograms, which may have minimized this bias. Patients with Joubert syndrome and severe disease, such as those who required mechanical ventilation, may be under-represented, because they could not travel to Bethesda, Maryland. In addition, the overall frequency of kidney disease as well as other extraneurologic manifestations may have been underestimated by this cross-sectional study, because these manifestations may develop as the patient gets older.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the Joubert Syndrome and Related Disorders Foundation for their extensive support and the individuals with Joubert syndrome and their families who generously participated in this investigation.
D.A.D. was supported by grant R01NS064077, the University of Washington Intellectual and Developmental Disabilities Research Center, grant U54HD083091 Genetics Core 6845 and subproject 6849, and private donations from families of children with Joubert syndrome. This research was supported by the Intramural Research Program of the National Human Genome Research Institute and the National Institutes of Health (NIH) Clinical Center, NIH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “A Perspective on Inherited Kidney Disease: Lessons for Practicing Nephrologists,” on pages 1914–1916.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05660517/-/DCSupplemental.
References
- 1.Gunay-Aygun M: Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet 151C: 296–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunay-Aygun M, Parisi MA, Doherty D, Tuchman M, Tsilou E, Kleiner DE, Huizing M, Turkbey B, Choyke P, Guay-Woodford L, Heller T, Szymanska K, Johnson CA, Glass I, Gahl WA: MKS3-related ciliopathy with features of autosomal recessive polycystic kidney disease, nephronophthisis, and Joubert Syndrome. J Pediatr 155: 386–392.e1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joubert M, Eisenring JJ, Robb JP, Andermann F: Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology 19: 813–825, 1969 [DOI] [PubMed] [Google Scholar]
- 4.Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D, Fennell E: Molar tooth sign in Joubert syndrome: Clinical, radiologic, and pathologic significance. J Child Neurol 14: 368–376, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Parisi M, Glass I: Joubert syndrome and related disorders. In: GeneReviews(R), edited by Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, Seattle, WA, University of Washington, 1993 [Google Scholar]
- 6.Romani M, Micalizzi A, Valente EM: Joubert syndrome: Congenital cerebellar ataxia with the molar tooth. Lancet Neurol 12: 894–905, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satran D, Pierpont ME, Dobyns WB: Cerebello-oculo-renal syndromes including Arima, Senior-Löken and COACH syndromes: More than just variants of Joubert syndrome. Am J Med Genet 86: 459–469, 1999 [PubMed] [Google Scholar]
- 8.Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, Graham JM Jr., Maria BL, Barkovich AJ, Dobyns WB: Molar tooth sign of the midbrain-hindbrain junction: Occurrence in multiple distinct syndromes. Am J Med Genet A 125A: 125–134, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Saraiva JM, Baraitser M: Joubert syndrome: A review. Am J Med Genet 43: 726–731, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Bachmann-Gagescu R, Dempsey JC, Phelps IG, O’Roak BJ, Knutzen DM, Rue TC, Ishak GE, Isabella CR, Gorden N, Adkins J, Boyle EA, de Lacy N, O’Day D, Alswaid A, Ramadevi A R, Lingappa L, Lourenço C, Martorell L, Garcia-Cazorla À, Ozyürek H, Haliloğlu G, Tuysuz B, Topçu M, Chance P, Parisi MA, Glass IA, Shendure J, Doherty D; University of Washington Center for Mendelian Genomics : Joubert syndrome: A model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet 52: 514–522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildebrandt F, Nothwang HG, Vossmerbäumer U, Springer C, Strahm B, Hoppe B, Keuth B, Fuchshuber A, Querfeld U, Neuhaus TJ, Brandis M: Lack of large, homozygous deletions of the nephronophthisis 1 region in Joubert syndrome type B. APN Study Group. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Pediatr Nephrol 12: 16–19, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Steinlin M, Schmid M, Landau K, Boltshauser E: Follow-up in children with Joubert syndrome. Neuropediatrics 28: 204–211, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Boycott KM, Parboosingh JS, Scott JN, McLeod DR, Greenberg CR, Fujiwara TM, Mah JK, Midgley J, Wade A, Bernier FP, Chodirker BN, Bunge M, Innes AM: Meckel syndrome in the Hutterite population is actually a Joubert-related cerebello-oculo-renal syndrome. Am J Med Genet A 143A: 1715–1725, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Warady BA, Cibis G, Alon U, Blowey D, Hellerstein S: Senior-Loken syndrome: Revisited. Pediatrics 94: 111–112, 1994 [PubMed] [Google Scholar]
- 15.Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F: The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38: 674–681, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Utsch B, Sayer JA, Attanasio M, Pereira RR, Eccles M, Hennies HC, Otto EA, Hildebrandt F: Identification of the first AHI1 gene mutations in nephronophthisis-associated Joubert syndrome. Pediatr Nephrol 21: 32–35, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Poretti A, Snow J, Summers AC, Tekes A, Huisman TAGM, Aygun N, Carson KA, Doherty D, Parisi MA, Toro C, Yildirimli D, Vemulapalli M, Mullikin JC, NISC Comparative Sequencing Program, Cullinane AR, Vilboux T, GahlWA, Gunay-Aygun M: Joubert syndrome: Neuroimaging findings in 110 patients in correlation with cognitive function and genetic cause. J Med Genet 54: 521–529, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilboux T, Doherty DA, Glass IA, Parisi MA, Phelps IG, Cullinane AR, Zein W, Brooks BP, Heller T, Soldatos A, Oden NL, Yildirimli D, Vemulapalli M, Mullikin JC, Nisc Comparative Sequencing P, Malicdan MC, Gahl WA, Gunay-Aygun M: Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med 19: 875–882, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL, Bignell HR, Boutell JM, Bryant J, Carter RJ, Keira Cheetham R, Cox AJ, Ellis DJ, Flatbush MR, Gormley NA, Humphray SJ, Irving LJ, Karbelashvili MS, Kirk SM, Li H, Liu X, Maisinger KS, Murray LJ, Obradovic B, Ost T, Parkinson ML, Pratt MR, Rasolonjatovo IM, Reed MT, Rigatti R, Rodighiero C, Ross MT, Sabot A, Sankar SV, Scally A, Schroth GP, Smith ME, Smith VP, Spiridou A, Torrance PE, Tzonev SS, Vermaas EH, Walter K, Wu X, Zhang L, Alam MD, Anastasi C, Aniebo IC, Bailey DM, Bancarz IR, Banerjee S, Barbour SG, Baybayan PA, Benoit VA, Benson KF, Bevis C, Black PJ, Boodhun A, Brennan JS, Bridgham JA, Brown RC, Brown AA, Buermann DH, Bundu AA, Burrows JC, Carter NP, Castillo N, Chiara E Catenazzi M, Chang S, Neil Cooley R, Crake NR, Dada OO, Diakoumakos KD, Dominguez-Fernandez B, Earnshaw DJ, Egbujor UC, Elmore DW, Etchin SS, Ewan MR, Fedurco M, Fraser LJ, Fuentes Fajardo KV, Scott Furey W, George D, Gietzen KJ, Goddard CP, Golda GS, Granieri PA, Green DE, Gustafson DL, Hansen NF, Harnish K, Haudenschild CD, Heyer NI, Hims MM, Ho JT, Horgan AM, Hoschler K, Hurwitz S, Ivanov DV, Johnson MQ, James T, Huw Jones TA, Kang GD, Kerelska TH, Kersey AD, Khrebtukova I, Kindwall AP, Kingsbury Z, Kokko-Gonzales PI, Kumar A, Laurent MA, Lawley CT, Lee SE, Lee X, Liao AK, Loch JA, Lok M, Luo S, Mammen RM, Martin JW, McCauley PG, McNitt P, Mehta P, Moon KW, Mullens JW, Newington T, Ning Z, Ling Ng B, Novo SM, O’Neill MJ, Osborne MA, Osnowski A, Ostadan O, Paraschos LL, Pickering L, Pike AC, Pike AC, Chris Pinkard D, Pliskin DP, Podhasky J, Quijano VJ, Raczy C, Rae VH, Rawlings SR, Chiva Rodriguez A, Roe PM, Rogers J, Rogert Bacigalupo MC, Romanov N, Romieu A, Roth RK, Rourke NJ, Ruediger ST, Rusman E, Sanches-Kuiper RM, Schenker MR, Seoane JM, Shaw RJ, Shiver MK, Short SW, Sizto NL, Sluis JP, Smith MA, Ernest Sohna Sohna J, Spence EJ, Stevens K, Sutton N, Szajkowski L, Tregidgo CL, Turcatti G, Vandevondele S, Verhovsky Y, Virk SM, Wakelin S, Walcott GC, Wang J, Worsley GJ, Yan J, Yau L, Zuerlein M, Rogers J, Mullikin JC, Hurles ME, McCooke NJ, West JS, Oaks FL, Lundberg PL, Klenerman D, Durbin R, Smith AJ: Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456: 53–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun DA, Hildebrandt F: Ciliopathies. Cold Spring Harb Perspect Biol 9: a028191, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokman M, Lilien M, Knoers N: Nephronophthisis. In: GeneReviews(R), edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, Seattle, WA, University of Washington, 1993 [PubMed] [Google Scholar]
- 25.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman M, Graf J, Bryant JC, Kleta R, Garcia A, Edwards H, Piwnica-Worms K, Adams D, Bernardini I, Fischer RE, Krasnewich D, Oden N, Ling A, Quezado Z, Zak C, Daryanani KT, Turkbey B, Choyke P, Guay-Woodford LM, Gahl WA: Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clin J Am Soc Nephrol 5: 972–984, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malaki M, Nemati M, Shoaran M: Joubert syndrome presenting as unilateral dysplastic kidney, hypotonia, and respiratory problem. Saudi J Kidney Dis Transpl 23: 325–329, 2012 [PubMed] [Google Scholar]
- 27.Schreuder MF, Westland R, van Wijk JA: Unilateral multicystic dysplastic kidney: A meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol Dial Transplant 24: 1810–1818, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Parisi MA, Doherty D, Eckert ML, Shaw DW, Ozyurek H, Aysun S, Giray O, Al Swaid A, Al Shahwan S, Dohayan N, Bakhsh E, Indridason OS, Dobyns WB, Bennett CL, Chance PF, Glass IA: AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet 43: 334–339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivante A, Kleppa MJ, Schulz J, Kohl S, Sharma A, Chen J, Shril S, Hwang DY, Weiss AC, Kaminski MM, Shukrun R, Kemper MJ, Lehnhardt A, Beetz R, Sanna-Cherchi S, Verbitsky M, Gharavi AG, Stuart HM, Feather SA, Goodship JA, Goodship TH, Woolf AS, Westra SJ, Doody DP, Bauer SB, Lee RS, Adam RM, Lu W, Reutter HM, Kehinde EO, Mancini EJ, Lifton RP, Tasic V, Lienkamp SS, Jüppner H, Kispert A, Hildebrandt F: Mutations in TBX18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J Hum Genet 97: 291–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama M, Nozu K, Goto Y, Kamei K, Ito S, Sato H, Emi M, Nakanishi K, Tsuchiya S, Iijima K: HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 25: 1073–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Hasui M, Kaneko K, Tsuji S, Isozaki Y, Kimata T, Nozu Y, Nozu K, Iijima K: Different phenotypes of HNF1ß deletion mutants in familial multicystic dysplastic kidneys. Clin Nephrol 79: 484–487, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Zaki MS, Sattar S, Massoudi RA, Gleeson JG: Co-occurrence of distinct ciliopathy diseases in single families suggests genetic modifiers. Am J Med Genet A 155A: 3042–3049, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maglic D, Stephen J, Malicdan MC, Guo J, Fischer R, Konzman D, Mullikin JC, Gahl WA, Vilboux T, Gunay-Aygun M; NISC Comparative Sequencing Program : TMEM231 Gene conversion associated with Joubert and Meckel-Gruber syndromes in the same family. Hum Mutat 37: 1144–1148, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Forsythe E, Sparks K, Best S, Borrows S, Hoskins B, Sabir A, Barrett T, Williams D, Mohammed S, Goldsmith D, Milford DV, Bockenhauer D, Foggensteiner L, Beales PL: Risk factors for severe renal disease in bardet-biedl syndrome. J Am Soc Nephrol 28: 963–970, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.al-Khaldi N, Watson AR, Zuccollo J, Twining P, Rose DH: Outcome of antenatally detected cystic dysplastic kidney disease. Arch Dis Child 70: 520–522, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eickmeyer AB, Casanova NF, He C, Smith EA, Wan J, Bloom DA, Dillman JR: The natural history of the multicystic dysplastic kidney--Is limited follow-up warranted? J Pediatr Urol 10: 655–661, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.