Introduction
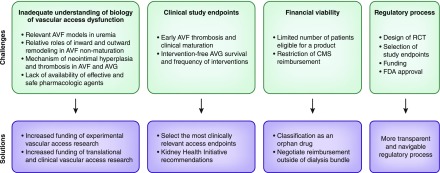
Vascular access dysfunction impedes the provision of effective hemodialysis. Dysfunction of arteriovenous fistulas (AVFs) largely reflects maturational failure, whereas dysfunction of arteriovenous grafts (AVGs) is mainly driven by recurrent stenosis and thrombosis at the venous anastomosis. There is a pressing need for new therapies for vascular access dysfunction, which contributes substantially to patient morbidity and mortality, requires corrective procedures, incurs catheter dependency, and imposes staggering health care costs. This commentary highlights salient challenges in meeting this need (Figure 1).
Figure 1.
There are several challenges to optimizing vascular access outcomes, and this figure offers potential solutions to overcome them. AVF, arteriovenous fistula; AVG, arteriovenous graft; CMS, Centers of Medicare and Medicaid Services; FDA, Food and Drug Administration; RCT, randomized clinical trial.
Inadequate Understanding of the Biology of Vascular Access Dysfunction
Models of human disease are indispensable in understanding relevant pathobiology and uncovering new therapeutic strategies. Preclinical studies of vascular access dysfunction on the basis of rodent AVF models have advanced our understanding by identifying vasculopathic and vasoprotective molecules and elucidating the basis for neointimal hyperplasia (1). Challenges in translating information from such models are several. First, as for any model, the species specificity of the findings so derived may limit their applicability to human vascular access dysfunction. In this regard, studies of vascular access dysfunction in larger animals (such as pigs) may be more relevant to human vascular access dysfunction, but their costs, needed technical expertise, and regulatory hurdles are such that smaller animal (rodents) are preferentially used. In addition to the issue of species specificity, it should be noted that, until recently, many experimental AVF studies were conducted in animals without uremia, and uremia is now recognized as a substantive contributor to vascular access dysfunction (2). Second, pharmacologic interventions, such as statins, which generally benefit human cardiovascular disease and may promote rodent AVF maturation, may not prove salutary in human AVFs. For example, although there are no randomized clinical trials (RCTs) examining the efficacy of statins in human AVFs, an observational study found no association between statin use and AVF maturation (3).
Third, AVF models have largely focused on neointimal hyperplasia (inward remodeling) in limiting maturation. Prior observations in human AVFs support neointimal hyperplasia in maturational failure. Indeed, an ongoing RCT is evaluating the efficacy of sirolimus-eluting implants in preventing AVF nonmaturation. This study posits that adventitial delivery of sirolimus (an antiproliferative agent) would interrupt processes that drive neointimal hyperplasia (NCT02513303). However, the indispensability of neointimal hyperplasia and inward remodeling in causing AVF nonmaturation has recently been questioned. For example, in patients undergoing a two-stage AVF, venous neointimal hyperplasia, despite a fourfold increase after AVF creation, does not correlate with AVF nonmaturation (4). Similarly, in a large observational study, although AVF nonmaturation associated with postoperative stenosis, the majority of AVFs with postoperative stenosis still matured (5). This inability of inward remodeling to entirely explain AVF nonmaturation has underscored the importance of outward remodeling in AVF maturation. Outward remodeling achieves a sustained increase in luminal diameter because of vasodilation and structural vascular enlargement and offsets the luminal encroachment caused by neointimal hyperplasia and inward remodeling; conversely, an AVF with inadequate outward remodeling may not mature even without neointimal hyperplasia (6). Promoting AVF maturation thus requires strategies that not only inhibit inward remodeling but more importantly, promote outward remodeling. The latter can be achieved, for example, by periadventitial application of elastase to disrupt the adventitial elastic layer, thereby promoting AVF dilation (NCT02414841). Investigation of AVF models should thus seek out therapeutic strategies that promote outward remodeling.
Fourth, challenges in translating preclinical studies may include the lack of clinical availability of pharmacologic agents targeting the pathway identified in AVF models. Fifth, unanticipated adverse effects of therapeutic strategies derived from experimental studies may limit their clinical use. For example, an RCT evaluating the efficacy of paclitaxel wraps in preventing AVG failure was terminated due to increased implant site infections (NCT01033357).
Failure to Select Appropriate Study End Points in Clinical Trials of Vascular Access Dysfunction
Inappropriate end points may belie either the true efficacy or inefficacy of a given therapy as illustrated by the following examples. First, the Dialysis Access Consortium Fistula Trial examined whether daily clopidogrel (versus placebo) prevented early AVF failure (7). Although clopidogrel significantly reduced AVF thrombosis within 6 weeks (the primary end point), it did not significantly increase AVF suitability for dialysis within 6 months (the secondary end point). Thus, the antithrombotic effect of clopidogrel did not culminate in improved AVF functionality, the more clinically relevant end point. Second, the Fish Oil Inhibition of Stenosis in Hemodialysis Grafts Study evaluated whether twice daily fish oil therapy prevented AVG failure (8). Fish oil administration did not significantly reduce the proportion of AVGs with thrombosis or an intervention within 1 year of follow-up (the primary end point), but it significantly lowered AVG thrombosis frequency (the secondary end point). Despite this beneficial effect on AVG thrombosis, a clinically relevant end point, the study would have been considered negative, because the primary end point was unaltered. Third, a recent trial compared deployment of graft stents with conventional balloon angioplasty for treatment of perianastomotic AVG stenosis (9). The primary end point, treatment area patency 6 months after intervention, was significantly higher for the stent graft group, thereby leading to Food and Drug Administration (FDA) approval of the use of graft stents for this clinical indication. However, the likelihood of AVG thrombosis tended to be higher in the stent graft arm, indicating that this intervention did not prevent the more clinically meaningful end point.
The choice of clinical end points also dictates how many patients are needed for RCT enrollment as well as the interpretation and applicability of the findings. For example, evaluating whether a novel drug can improve AVF maturation may be undertaken in patients who are pre-ESRD, patients who have already initiated hemodialysis, or both populations. The conventional definition of AVF maturation (reproducible AVF cannulation for 1 month of dialysis) necessitates dialysis initiation. However, only 60%–70% of patients with CKD who undergo predialysis AVF placement initiate dialysis within 1 year. If the study design for this drug is restricted to patients on dialysis, the findings may not apply to patients undergoing AVF creation predialysis. However, if patients who are pre-ESRD were included in the study and the time period selected to achieve AVF maturation was 1 year or less, the needed sample size (and cost) would substantially increase, because 30%–40% of patients who are pre-ESRD would not be initiated on hemodialysis 1 year after AVF creation.
Inadequate Reimbursement to Justify the Cost of Developing or Using New Products
A third barrier is the relatively small population of patients on hemodialysis. There are approximately 27 million patients with heart disease in the United States, making development of new therapies for heart disease financially attractive. In contrast, there are approximately 450,000 patients on hemodialysis, and therefore, the profit margin for new therapies is relatively limited; drugs for vascular access dysfunction may thus be considered “orphan drugs.” Approximately 1 million Americans receive a coronary stent annually, whereas only about 35,000 receive a dialysis access stent. In patients with coronary artery disease, drug-eluting stents are superior to bare metal stents, raising the possibility that drug-eluting stents may also be useful for treatment of vascular access stenosis. However, a medical product company may shy away from supporting the evaluation of a drug-eluting stent for vascular access dysfunction because of the extraordinary costs of such RCTs and the uncertain return on investment, because the target patient population is comparatively small.
Medical reimbursement in the United States for outpatient hemodialysis also affects the economic viability of novel therapies. For example, a Canadian RCT showed that tissue plasminogen activator (tPA) instilled weekly into the dialysis catheters and heparin twice weekly after dialysis were superior to conventional instillation of heparin locks thrice weekly in preventing catheter dysfunction (10). Weekly catheter instillation of tPA costs approximately $150 ($50 per hemodialysis session). However, the current Centers of Medicare and Medicaid Services (CMS) dialysis reimbursement bundle (approximately $232 per session) includes all drugs provided during dialysis (http://www.nephrologynews.com/cms-releases-final-rule-2017-esrd-payment-bundle/). Inadequate reimbursement for weekly prophylactic tPA locks likely explains why this proven therapy is not the standard of care in the United States.
Challenges in Patient Recruitment
Uremia adversely affects virtually all organs and tissues. Novel therapies uncovered in AVF models, and investigated in phase 1 and 2 clinical trials, still pose the risk of adverse effects in larger trials of uremic patients. Exclusion criteria in trials involving patients with CKD may be more stringent because of concerns of patient safety. This may limit patient recruitment, as would other features of the CKD population, such as increased disability and depression and decreased resilience and motivation.
Arduous Regulatory Process
Key regulatory steps include selection of appropriate end points, designing the RCT, negotiating the study design and end points with the FDA, raising money from venture capitalists for study support, subcontracting with a clinical research organization to conduct the RCT, identifying and recruiting the study centers, performing and analyzing the study, and presenting the results to the FDA. Failure to complete all of these steps successfully would preclude approval of a new drug to prevent vascular access dysfunction. The relative lack of development of effective therapies for vascular access dysfunction reflects the enormity of underlying challenges, the spectrum of which includes the pathobiology of the disease, translational uncertainty, trial design and recruitment, financial exigencies, and regulatory hurdles. Cognizance of this overarching perspective may aid in linking initiatives in specific aspects of this field in the hope that a more encompassing and integrated approach would lead to effective therapies for this intractable disease.
Disclosures
M.A. is a consultant for CorMedix.
Acknowledgments
K.A.N. is supported by grant R01 DK 70124 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). M.A. is supported by grant R01DK085027 from the NIDDK.
The content of this article does not reflect the views or opinions of The American Society of Nephrology (ASN) or the Clinical Journal of the American Society of Nephrology (CJASN). Responsibility for the information and views expressed therein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA: Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang L, Grande JP, Hillestad ML, Croatt AJ, Barry MA, Katusic ZS, Nath KA: A new model of an arteriovenous fistula in chronic kidney disease in the mouse: Beneficial effects of upregulated heme oxygenase-1. Am J Physiol Renal Physiol 310: F466–F476, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisoni R, Barker-Finkel J, Allon M: Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol 5: 1447–1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabbara M, Duque JC, Martinez L, Escobar LA, Wu W, Pan Y, Fernandez N, Velazquez OC, Jaimes EA, Salman LH, Vazquez-Padron RI: Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis 68: 455–464, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Litovsky S: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothuizen TC, Wong C, Quax PH, van Zonneveld AJ, Rabelink TJ, Rotmans JI: Arteriovenous access failure: More than just intimal hyperplasia? Nephrol Dial Transplant 28: 1085–1092, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI; Dialysis Access Consortium Study Group: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lok CE, Moist L, Hemmelgarn BR, Tonelli M, Vazquez MA, Dorval M, Oliver M, Donnelly S, Allon M, Stanley K; Fish Oil Inhibition of Stenosis in Hemodialysis Grafts (FISH) Study Group: Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: A randomized controlled trial. JAMA 307: 1809–1816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskal ZJ, Trerotola S, Dolmatch B, Schuman E, Altman S, Mietling S, Berman S, McLennan G, Trimmer C, Ross J, Vesely T: Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med 362: 494–503, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Hemmelgarn BR, Moist LM, Lok CE, Tonelli M, Manns BJ, Holden RM, LeBlanc M, Faris P, Barre P, Zhang J, Scott-Douglas N; Prevention of Dialysis Catheter Lumen Occlusion with rt-PA versus Heparin Study Group: Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med 364: 303–312, 2011 [DOI] [PubMed] [Google Scholar]