In December of 2014, the Organ Procurement and Transplant Network (OPTN) implemented the first major change to kidney allocation policy in several decades. Now, with 2 years of follow-up, we can reflect on the kidney allocation system (KAS) and determine both the magnitude and effect of the policy’s intended and unintended consequences. Although the changes to the KAS achieved many stated goals, some features of KAS were designed to be easily adjusted. The new KAS introduced a concept of disease severity and exposure to dialysis as well as longevity matching to increase utility as important allocation criteria. With an increasing emphasis on reducing kidney discards, innovative allocation pathways may be required to ensure that the right kidney gets to the right recipient in the right amount of time.
Progress
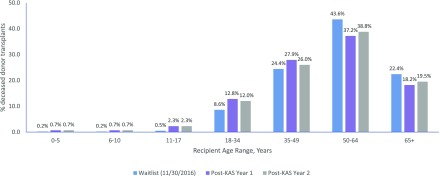
The new KAS ushered in some novel concepts in kidney allocation, going beyond just listing time as the primary driver of priority. Longevity matching (1) (prioritizing kidneys with kidney donor profile index [KDPI] <20 to candidates with the longest estimated post-transplant survival [<20]) has decreased large mismatches in longevity and promises to increase the utility of the system, while balancing equity in access by maintaining unrestricted access to the other 80% of donated kidneys. However, the introduction of longevity matching raised significant concern about the potential for decreased access to transplant among older candidates. Although there was an initial decrease in the rate of transplantation for patients 50–64 and over 65 years old during the first year of KAS, in the second year, both groups saw an increase in the rate of transplantation, although not back to the rate before 2014 (Figure 1) (2).
Figure 1.
Deceased donor transplants by recipient age for the time period 1 year post-KAS, 2 years post-KAS, and compared to their percentage makeup of the overall waitlist as of November 30, 2016 (reprinted from Ref. 6 on the basis of OPTN data as of July 16, 2017). KAS, kidney allocation system; OPTN, Organ Procurement and Transplant Network.
Introducing dialysis time in addition to listing time as the measure of waiting time was a large step forward for equity. Transplants in candidates with 5–10 years of dialysis time increased from 21% pre-KAS to 35% in the first year post-KAS and then decreased down to 34% in the second year as the bolus effect eased. The same was true for candidates with 10+ years on dialysis, with a large bolus effect increasing from 5% to 19% in the first 4 months of KAS and now tapering back down to 6% after transplanting the candidates with significant accumulated dialysis time. This change in waiting time calculation also improved equity with regards to ethnicity. Black candidates saw an increase in transplants, likely due to the change to dialysis waiting time (3).
Another major success of the new KAS was improved access for the most highly sensitized candidates. Before the KAS, there was significant disparity in waiting time and access to kidney transplants for highly sensitized patients, with calculated panel reactive antibody (CPRA) 98%–100% candidates receiving very few offers. The KAS currently gives national priority to candidates with CPRA scores of 100%, regional priority to candidates with CPRA scores of 99%, and local priority to candidates with CPRA scores of 98%.
In fact, offer rates increased sharply for CPRA 95%+ patients and decreased sharply for CPRA 80%–84% patients post-KAS. However, in post-KAS year 2 versus year 1, offer rates declined significantly for CPRA 100% patients and have increased for 80%–96% CPRA candidates (2).
Pre-KAS, 3% of transplants went to CPRA 99%–100% recipients; this rate is significantly lower than their representation on the waitlist (7%). However, this has increased greatly post-KAS: year 1 =13% and year 2 =10%. There was an initial bolus effect that is tapering over time and has yet to reach steady state. CPRA 99%–100% recipients are receiving disproportionately lower KDPI kidneys. Post-KAS (both years), >30% of CPRA 99%–100% recipients received a KDPI 0%–20% kidney, whereas only 21% of lower CPRA recipients received one of these highest longevity kidneys. However, this disproportionality was true even before KAS.
Pitfalls
Outcomes
The expected increase in overall utility in the system (more life-years realized with the same supply of kidneys through longevity matching) has been tempered in the short term by the decrease in 1-year graft survival thought to be due to allocation to highly sensitized candidates and patients with long dialysis exposure. This initial bolus effect is decreasing for both those groups (2). Also, likely contributing to the decrement in graft survival was an increase in delayed graft function, which increased from 24% of transplants pre-KAS to 29% in the first year post-KAS and decreased back down to 28% in year 2. The system will need to reach a steadier state before realizing the gains from longevity matching, which was estimated to add roughly 8000 additional life-years from each year of donated organs (1).
Highly Sensitized Candidates
There has been concern that CPRA 98%–100% candidates now receive too much priority and that they are receiving the best-quality kidneys, have shorter waiting times (although not supported by the OPTN data) and perhaps, should not be prioritized over first time transplant recipients. Ultimately, programs determine who is eligible for transplant. The ethics of who is more worthy of transplant is an ongoing debate and beyond the scope of the KAS. One could argue that a better-quality kidney with a better match may result in more longevity in this population. The United Network for Organ Sharing (UNOS) Kidney and HLA Committees are carefully monitoring the data and examining CPRA levels that are receiving very few to no offers to determine the true need of prioritization for regional or national allocation. After steady state is achieved, careful consideration of adjusting priority points may be warranted.
High KDPI Kidneys and Discards
Before the KAS, kidneys from KDPI>85% donors were offered locally first, and consenting candidates were rank ordered only according to waiting time. The goal of the KAS is to expedite placement of high-KDPI kidneys while decreasing discards by expanding offers first to a combined regional and local unit.
At 2 years, the number of transplants involving KDPI 86%–100% donor kidneys has decreased from 9% pre-KAS to 8% year 1 and 8% year 2 post-KAS. The overall kidney discard rate increased from 19% post-KAS year 1 to 20% post-KAS year 2. KDPI 21%−34% kidneys saw a decrease in discard rate in the most recent year, whereas KDPI 35%−85% kidneys discard rates increased again. Reasons for discard are mostly similar in post-KAS years 1 and 2. However, there was an obvious increase in list exhaustion and a subsequent decrease in biopsy findings and “other” (2).
The low utilization and high discard rate of KDPI>85% kidneys remain a challenge. Despite good intentions, the local and regional allocation scheme has not resulted in an increase in transplants or a decline in discard rates. The UNOS Kidney Committee is currently proposing a dual allocation project that crosses into the KAS by changing sequence D (KDPI>85%) to include dual allocation after single allocation at the local, regional, and national levels.
A Path Forward
Despite increased numbers of donors in recent years, the mismatch between supply and demand remains large. Thus, efforts have been focused on reducing the number of discarded organs. Rates of organ discard did not change significantly after implementation of the KAS. The discard rate approaches 65% for kidneys with a KDPI 85+ (2). Although the discard rate is lower for kidneys with lower KDPI, the actual number of those discarded kidneys is still quite large. There is agreement that not all discarded kidneys could or should be used. However, there is disagreement in where that line should be drawn and how to motivate candidates and transplant programs to reduce discards. There is also significant variability among center utilization of kidneys at risk for discard. Efforts, like the ongoing Health Resources & Services Administration–sponsored Collaborative Innovation and Improvement Network (COIIN) project at UNOS, are aimed at increasing utilization of higher-risk kidneys (in the example of COIIN, kidneys with KPDI>50%) (4).
Although the ultimate reason for organ discard is often hard to decipher with the current set of refusal codes, long cold ischemic time is a major contributing factor. Thus, efforts to increase the efficiency of allocation have been entertained. One such idea would involve preferential allocation to centers with high utilization of kidneys deemed at risk for discard. Once defined (5), these kidneys could be fast tracked to centers with experience and interest in transplanting these organs determined by prior acceptance rates of these types of kidneys over a 24-month period at that center. This, however, raises some challenging questions. By allocating to a center rather than a candidate at a center, the possibility arises that a candidate is unaware that their chosen center does not perform these higher-risk transplants. More frequently, the tradeoff of speed over quality must be presented to candidates, particularly those with poor survival on dialysis. Often, these are older candidates. Such a system would require accurate estimates of waiting list mortality, accurate estimates of waiting times to transplant not just in a region or donor service area but at an individual center, and accurate estimates of patient and graft survival after high-risk kidney transplantation. These tools are currently available but are not likely with the degree of granularity that most clinicians would like. One challenge is attempting to estimate graft and patient outcomes with kidneys that are currently being discarded. This limits our ability to generate an expected outcome, which is usually on the basis of similar transplants in similar patients in the recent past. We will need, as a transplant community, to accept some unknowns and loosen the regulations on transplant outcomes that are currently cited as a reason to avoid performing higher-risk transplants. As we define the true margin of kidney transplantation, we would like to provide some assurances for candidates willing to take that risk. However, many of these patients are marginal candidates for a first transplant, and therefore, guaranteeing some type of safety net for retransplantation in the event of primary graft nonfunction is likely not feasible. If these policy changes were to be adopted, the transplant and regulatory communities would have to agree to move beyond our current comfort zone in terms of acceptable outcomes. With focus on the high risk of mortality in patients never transplanted, we might be able to find our way.
Disclosures
None.
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
The content of this article does not reflect the views or opinions of The American Society of Nephrology or the Clinical Journal of the American Society of Nephrology. Responsibility for the information and views expressed therein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Friedewald JJ, Samana CJ, Kasiske BL, Israni AK, Stewart D, Cherikh W, Formica RN: The kidney allocation system. Surg Clin North Am 93: 1395–1406, 2013 [DOI] [PubMed] [Google Scholar]
- 2.UNOS: Two-Year Analysis Shows Effects of Kidney Allocation System, 2017. Available at: https://www.transplantpro.org/news/two-year-analysis-shows-effects-of-kidney-allocation-system/. Accessed June 14, 2017
- 3.UNOS: Odds for Receiving a Kidney Transplant Now Equal for Black, White and Hispanic Candidates, 2017. Available at: https://www.unos.org/odds-equal-of-kidney-transplant-for-minorities/. Accessed July 13, 2017
- 4.UNOS: UNOS COIIN PROJECT, 2017. Available at: https://optn.transplant.hrsa.gov/resources/coiin/. Accessed June 14, 2017
- 5.Massie AB, Desai NM, Montgomery RA, Singer AL, Segev DL: Improving distribution efficiency of hard-to-place deceased donor kidneys: Predicting probability of discard or delay. Am J Transplant 10: 1613–1620, 2010 [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]