Abstract
Diabetic kidney disease develops in approximately 40% of patients who are diabetic and is the leading cause of CKD worldwide. Although ESRD may be the most recognizable consequence of diabetic kidney disease, the majority of patients actually die from cardiovascular diseases and infections before needing kidney replacement therapy. The natural history of diabetic kidney disease includes glomerular hyperfiltration, progressive albuminuria, declining GFR, and ultimately, ESRD. Metabolic changes associated with diabetes lead to glomerular hypertrophy, glomerulosclerosis, and tubulointerstitial inflammation and fibrosis. Despite current therapies, there is large residual risk of diabetic kidney disease onset and progression. Therefore, widespread innovation is urgently needed to improve health outcomes for patients with diabetic kidney disease. Achieving this goal will require characterization of new biomarkers, designing clinical trials that evaluate clinically pertinent end points, and development of therapeutic agents targeting kidney-specific disease mechanisms (e.g., glomerular hyperfiltration, inflammation, and fibrosis). Additionally, greater attention to dissemination and implementation of best practices is needed in both clinical and community settings.Introduction
Keywords: diabetic nephropathy, structural changes, natural history, pathogenesis, altered renal hemodynamics, novel therapies, diagnosis
It took more than three millennia from the first description of diabetes in 1552 BC to the recognition of an association between diabetes and kidney disease, but it took only several decades for diabetic kidney disease (DKD) to become the leading cause of ESRD in the United States (1,2). This microvascular complication develops in approximately 30% of patients with type 1 diabetes mellitus (DM1) and approximately 40% of patients with type 2 diabetes mellitus (DM2) (2,3).
The increasing prevalence of DKD parallels the dramatic worldwide rise in prevalence of diabetes (4,5). In the United States, the prevalence of diabetes among adults increased from 9.8% in the 1988–1994 time period to 12.3% in the 2011–2012 time period (6). Worldwide, in the year 2015, 415 million people were estimated to have diabetes; by 2040, prevalence is projected to increase to 642 million, with disproportionate growth in low- to middle-income countries (7). The driving force behind the escalating prevalence of diabetes is the global pandemic of obesity (4). Between the years 1980 and 2000, the overall prevalence of obesity in adults snowballed from 15% to 31% in the United States (8). By 2013–2014, the adjusted prevalence of obesity was up to 35% among men and 40% among women (9).
Kidney disease attributed to diabetes is a major but under-recognized contributor to the global burden of disease. Between 1990 and 2012, the number of deaths attributed to DKD rose by 94% (10). This dramatic rise is one of the highest observed for all reported chronic diseases (11). Notably, most of the excess risk of all-cause and cardiovascular disease (CVD) mortality for patients with diabetes is related to the presence of DKD (12).
Risk Factors
DKD risk factors can conceptually be classified as susceptibility factors (e.g., age, sex, race/ethnicity, and family history), initiation factors (e.g., hyperglycemia and AKI), and progression factors (e.g., hypertension, dietary factors, and obesity) (Table 1) (13). Two of the most prominent established risk factors are hyperglycemia and hypertension.
Table 1.
Risk factors for diabetic kidney disease
| Risk Factor | Susceptibility | Initiation | Progression |
| Demographic | |||
| Older age | + | ||
| Sex (men) | + | ||
| Race/ethnicity (black, American Indian, Hispanic, Asian/Pacific Islanders) | + | + | |
| Hereditary | |||
| Family history of DKD | + | ||
| Genetic kidney disease | + | ||
| Systemic conditions | |||
| Hyperglycemia | + | + | + |
| Obesity | + | + | + |
| Hypertension | + | + | |
| Kidney injuries | |||
| AKI | + | + | |
| Toxins | + | + | |
| Smoking | + | + | |
| Dietary factors | + | + | |
| High protein intake | + | + |
DKD, diabetic kidney disease.
Hyperglycemia
In normoalbuminuric patients with DM1, poor glycemic control is an independent predictor of progression to development of proteinuria (albuminuria) and/or ESRD (14). Two landmark trials conducted with patients with early-stage DM1 or DM2 showed that intensive blood glucose control early in the course of disease exhibits a long-lasting favorable effect on the risk of DKD development (15,16). This “legacy effect,” also named “metabolic memory,” suggests that early intensive glycemic control can prevent irreversible damage, such as epigenetic alterations, associated with hyperglycemia (17). In patients with DM1, an intensive glucose control intervention targeting a hemoglobin A1C (HbA1C) level ≤7% reduced the 9-year risks of developing microalbuminuria and macroalbuminuria by 34% and 56%, respectively, compared with standard care (18). After a median follow-up of 22 years, the intensive therapy group had approximately 50% lower risk of a low eGFR (<60 ml/min per 1.73 m2), and the average rate of eGFR loss was significantly reduced from 1.56 ml/min per 1.73 m2 per year with standard therapy to 1.27 ml/min per 1.73 m2 per year with intensive therapy (19). Similarly, in patients with newly diagnosed DM2, 10 years of an intensive glycemic control intervention targeting an HbA1C of 7% produced a 24% reduction in development of microvascular complications, including DKD, compared with conventional therapy (20,21). After 12 years, intensive glycemic control resulted in a 33% reduction in the risk of development of microproteinuria or “clinical grade” proteinuria and a significant reduction in the proportion of patients with a doubling of the blood creatinine level (0.9% versus 3.5%) relative to the conventional therapy group (20,21).
Hypertension
In patients with newly diagnosed DM2, treating to a target BP of <150/85 mmHg over a median of 15 years resulted in a significant 37% risk reduction of microvascular complications compared with that in patients treated to a target of <180/105 mmHg. Each 10-mmHg increase in mean systolic BP was associated with a 15% increase in the hazard ratio for development of both micro- and macroalbuminuria and impaired kidney function defined as eGFR<60 ml/min per 1.73 m2 or doubling of the blood creatinine level (22). Broadly, a baseline systolic BP >140 mmHg in patients with DM2 has been associated with higher risk of ESRD and death (23,24).
Structural Changes
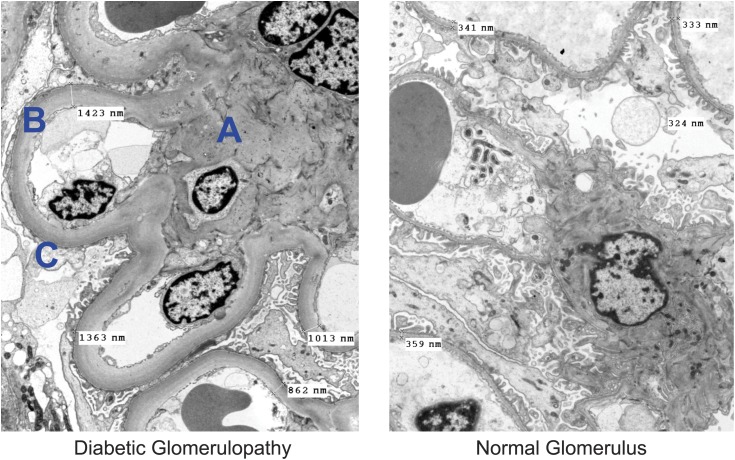
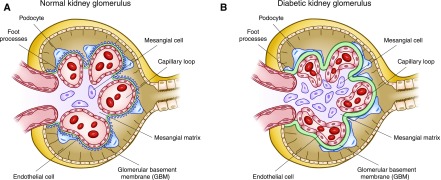
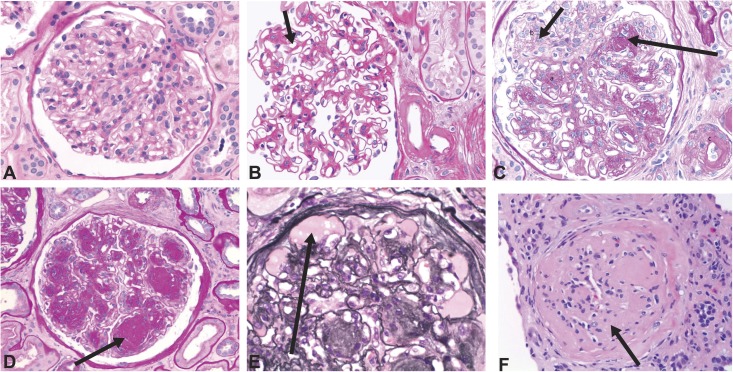
Development of DKD is associated with many alterations in the structure of multiple kidney compartments. The earliest consistent change is thickening of glomerular basement membrane, which is apparent within 1.5–2 years of DM1 diagnosis. It is paralleled by capillary and tubular basement membrane thickening (14,25,26) (Figure 1). Other glomerular changes include loss of endothelial fenestrations, mesangial matrix expansion, and loss of podocytes with effacement of foot processes (Figure 2). Mesangial volume expansion is detectable within 5–7 years after DM1 diagnosis (14,25,27,28). Segmental mesangiolysis is observed with progression of diabetes and thought to be associated with development of Kimmelstiel–Wilson nodules and microaneurysms, which often present together (29,30) (Figure 3). The exudative lesions result from subendothelial deposits of plasma proteins, which form periodic acid–Schiff-positive and electron-dense deposits and accumulate in small arterial branches, arterioles, and glomerular capillaries as well as microaneurysms. These deposits can result in luminal compromise (e.g., hyaline arteriosclerosis). Similar subepithelial deposits are seen in Bowman’s capsule (capsular drop lesion) and proximal renal tubules. In later stages of diabetes, interstitial changes and glomerulopathy coalesce into segmental and global sclerosis (31). In patients with DM1, GFR, albuminuria, and hypertension are strongly correlated with mesangial expansion and somewhat less strongly associated with glomerular basement membrane width (31) (Figure 4).
Figure 1.
Electron microscope images of structural changes in diabetic kidney disease. Structural changes in diabetic glomerulopathy found with electron microscopy. A indicates marked expansion of the mesangium. B indicates marked diffuse thickening of capillary basement membranes (to three times the normal thickness in this case). C indicates segmental effacement of the visceral epithelial foot processes. Original magnification, ×3500.
Figure 2.
Normal kidney morphology and structural changes in diabetes mellitus. Diabetic kidney disease induces structural changes, including thickening of the glomerular basement membrane, fusion of foot processes, loss of podocytes with denuding of the glomerular basement membrane, and mesangial matrix expansion.
Figure 3.
Diabetic glomerulopathy. Changes in glomerular histology in diabetic glomerulopathy (A) Normal glomerulus. (B) Diffuse mesangial expansion with mesangial cell proliferation. (C) Prominent mesangial expansion with early nodularity and mesangiolysis. (D) Accumulation of mesangial matrix forming Kimmelstiel–Wilson nodules. (E) Dilation of capillaries forming microaneurysms, with subintimal hyaline (plasmatic insudation). (F) Obsolescent glomerulus. A–D and F were stained with period acid–Schiff stain, and E was stained with Jones stain. Original magnification, ×400.
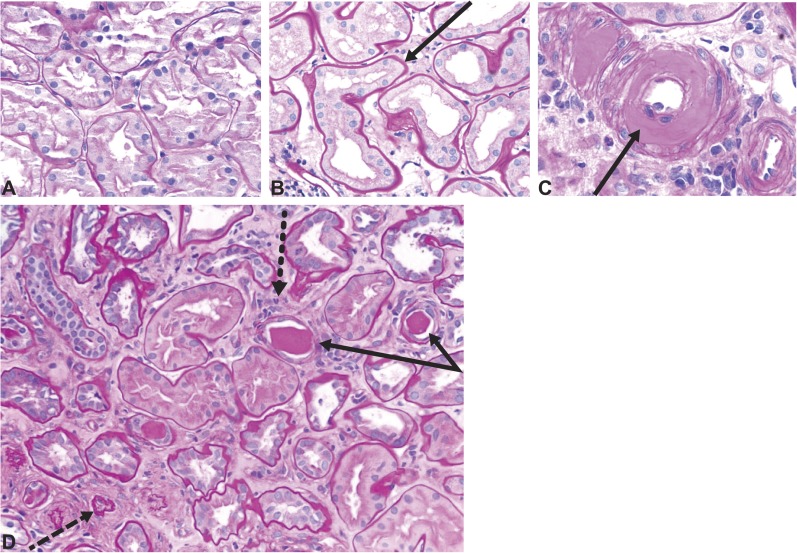
Figure 4.
Tubulointerstitial changes and arteriolar hyalinosis in diabetic kidney disease. Tubulointerstitial changes in diabetic kidney disease. (A) Normal renal cortex. (B) Thickened tubular basement membranes and interstitial widening. (C) Arteriole with an intimal accumulation of hyaline material with significant luminal compromise. (D) Renal tubules and interstitium in advancing diabetic kidney disease, with thickening and wrinkled tubular basement membranes (solid arrows), atrophic tubules (dashed arrow), some containing casts, and interstitial widening with fibrosis and inflammatory cells (dotted arrow). All sections were stained with period acid–Schiff stain. Original magnification, ×200.
Renal structure changes in patients with DM2 are similar to those seen in DM1, but they are more heterogeneous and less predictably associated with clinical presentations (32). Early renal pathology studies described a high prevalence of nondiabetic glomerular disease in the patients with DM2 population, probably because of selection bias: patients who were diabetic and underwent biopsies tended to have atypical presentations of DKD. Conclusions from more recent biopsy studies are more conservative, estimating <10% prevalence of non-DKD in patients with diabetes and albuminuria (24).
Factors underlying the different presentation of DKD in DM2 may include the unreliable timing of DM2 onset compared with DM1, with potentially longer exposure to hyperglycemia before diagnosis; an older patient population; and a higher burden of atherosclerosis. Additionally, many patients with DM2 are treated with renin-angiotensin system inhibitors before diagnosis of diabetes. An international consensus working group has provided a pathologic classification system to address the heterogeneity of DKD presentation, which includes scoring of glomerular, interstitial, and vascular lesions (Tables 2 and 3) (33).
Table 2.
International pathologic classification of glomerular changes in diabetic kidney disease
| Class | Description | Inclusion Criteria |
| 1 | Mild or nonspecific light microscopy changes and electron microscopy–proven GBM thickening | GBM>395 nm in women and >430 nm in men 9 yr of age and older; biopsy does not meet any of the criteria mentioned below for classes 2–4 |
| 2a | Mesangial expansion, mild | Mild mesangial expansion in >25% of the observed mesangium; biopsy does not meet criteria for class 3 or 4 |
| 2b | Mesangial expansion, severe | Severe mesangial expansion in >25% of the observed mesangium; biopsy does not meet criteria for class 3 or 4 |
| 3 | Nodular sclerosis (Kimmelstiel–Wilson lesion) | At least one convincing Kimmelstiel–Wilson lesion; biopsy does not meet criteria for class 4 |
| 4 | Advanced diabetic glomerulosclerosis | Global glomerular sclerosis in >50% of glomeruli; lesions from classes 1–3 |
Degree of mesangial expansion: mild mesangial expansion occupies an area smaller than the area of the capillary lumen. Severe mesangial expansion occupies an area greater than the area of the capillary lumen (33). GBM, glomerular basement membrane.
Table 3.
International classification of interstitial and vascular lesions in diabetic kidney disease
| Type of Lesion and Criteria | Score |
| IFTA, % | |
| Absent | 0 |
| <25 | 1 |
| 25–50 | 2 |
| >50 | 3 |
| Interstitial inflammation | |
| Absent | 0 |
| Infiltration only in relation to IFTA | 1 |
| Infiltration in areas without IFTA | 2 |
| Vascular lesions arteriolar hyalinosis | |
| Absent | 0 |
| At least one area of arteriolar hyalinosis | 1 |
| More than one area of arteriolar hyalinosis | 2 |
| Presence of large vessels arteriosclerosis | |
| No intimal thickening | 0 |
| Intimal thickening less than thickness of media | 1 |
| Intimal thickening greater that thickness of media | 2 |
IFTA, interstitial fibrosis and tubular atrophy.
Natural History
The paradigm of the natural history of DKD continues to evolve. In many patients, DKD clearly does not follow the classic pattern of glomerular hyperfiltration progressing to persistent albuminuria associated with hypertension and declining GFR (34) (Figure 5). The United Kingdom Prospective Diabetes Study (UKPDS) offered a unique opportunity to observe the natural history of DKD in patients with DM2 from early in the course of diabetes. Of enrolled patients, approximately 2% per year progressed from normo- to microalbuminuria and from micro- to macroalbuminuria. At a median of 15 years after diagnosis, 40% of participants developed albuminuria, and 30% developed eGFR<60 ml/min per 1.73 m2 or doubling of the blood creatinine level (22,35). It is noteworthy that 60% of those developing kidney functional impairment did not have preceding albuminuria, and 40% never developed albuminuria during the study (22). This finding underscores the fact that albuminuria is a dynamic, fluctuating condition rather than a linearly progressive process. For example, in the Multifactorial Intervention for Patients with Type 2 Diabetes Study, 31% of participants with microalbuminuria progressed to macroalbuminuria, whereas 31% regressed to normoalbuminuria during 7.8 years of follow-up. Another 38% remained microalbuminuric during this time period (36). Recent clinical data from over 20,000 patients with DM1 show lower frequencies of low eGFR (<60 ml/min per 1.73 m2) and albuminuria in this population; 19±13 years after diagnosis, frequencies of low eGFR and albuminuria were 8% and 19%, respectively (37).
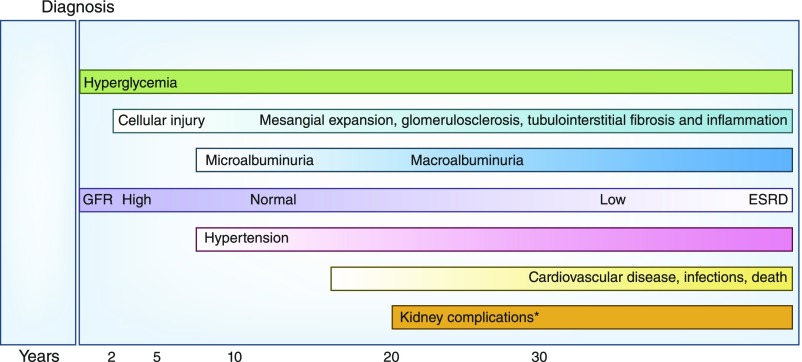
Figure 5.
Conceptual model of the natural history of diabetic kidney disease. Duration of diabetes, in years, is presented on the horizontal axis. Timeline is well characterized for type 1 diabetes mellitus; for type 2 diabetes mellitus, timeline may depart from the illustration due to the variable timing of the onset of hyperglycemia. *Kidney complications: anemia, bone and mineral metabolism, retinopathy, and neuropathy.
In step with the changing paradigm of the natural history of DKD, emerging evidence suggests that the clinical presentation of DKD is altering. A comparison of DKD presentation in adults with diabetes during the time periods between 1988 and 1994 and between 2009 and 2014 shows that the prevalence of albuminuria as a manifestation of DKD decreased from 21% to 16%, that low eGFR (<60 ml/min per 1.73 m2) increased from 9% to 14%, and that severely reduced eGFR (<30 ml/min per 1.73 m2) increased from 1% to 3% (38). Furthermore, lack of albuminuria or low eGFR may not necessarily preclude structural DKD. A recent autopsy study found a considerably higher prevalence of DKD diagnosed histologically compared with that indicated by clinical laboratory testing. Of 168 patients with DM1 or DM2, 106 exhibited histopathologic changes characteristic of DKD. Albuminuria or low eGFR was absent in 20% (20 of 106) of patients throughout life. Moreover, structural changes were highly variable and encompassed almost all histopathologic classes of DKD (39).
In later stages of DKD, as GFR declines, both kidney- and nonkidney-related DKD complications develop. Anemia and bone and mineral metabolism disorders often develop earlier in DKD than in other types of CKD. Predominant tubulointerstitial disease is associated with damage to the peritubular interstitial cells that produce erythropoietin. As a result, patients with diabetes may be prone to erythropoietin deficiency and are nearly twice as likely to have anemia compared with patients with nondiabetic CKD and comparable eGFR (40). Insulin is a cofactor for parathyroid hormone release; therefore, insulin deficiency and/or resistance may be associated with lower parathyroid hormone levels than in other types of CKD (41), which may predispose patients with DKD to adynamic bone disease.
Deaths due to CVDs and infections are highly prevalent and compete with progression to ESRD. In the UKPDS, the overall death rate after onset of DKD in those with blood creatinine levels >2 mg/dl or those receiving kidney replacement therapy was nearly 20% per year (35). Follow-up data from 2003 showed crude 1-year mortality of patients on dialysis ranging from 6.6% in Japan to 21.5% in the United States (42). Patients on dialysis over age 75 years old are 3.9 times more likely to die than their counterparts in the general population (43).
Pathophysiology of DKD
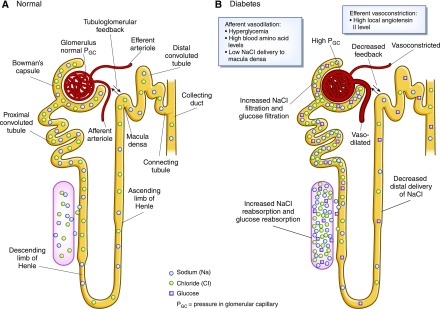
Critical metabolic changes that alter kidney hemodynamics and promote inflammation and fibrosis in early diabetes include hyperaminoacidemia, a promoter of glomerular hyperfiltration and hyperperfusion, and hyperglycemia (44–47) (Figure 6). In DM2, systemic hypertension and obesity also contribute to glomerular hyperfiltration via mechanisms, such as high transmitted systemic BP and glomerular enlargement (47). Glomerular hyperfiltration is a well characterized consequence of early diabetes. Overall, it is observed in 10%–40% or up to 75% of patients with DM1 and up to 40% of patients with DM2 (48). Mechanisms underlying glomerular hyperfiltration in diabetes are incompletely understood (48); however, one plausible mechanism is increased proximal tubular reabsorption of glucose via sodium–glucose cotransporter 2, which decreases distal delivery of solutes, particularly sodium chloride, to the macula densa (49,50). The resulting decrease in tubuloglomerular feedback may dilate the afferent arteriole to increase glomerular perfusion, while concurrently, high local production of angiotensin II at the efferent arteriole produces vasoconstriction. The overall effect is high intraglomerular pressure and glomerular hyperfiltration (47,49) (Figure 7).
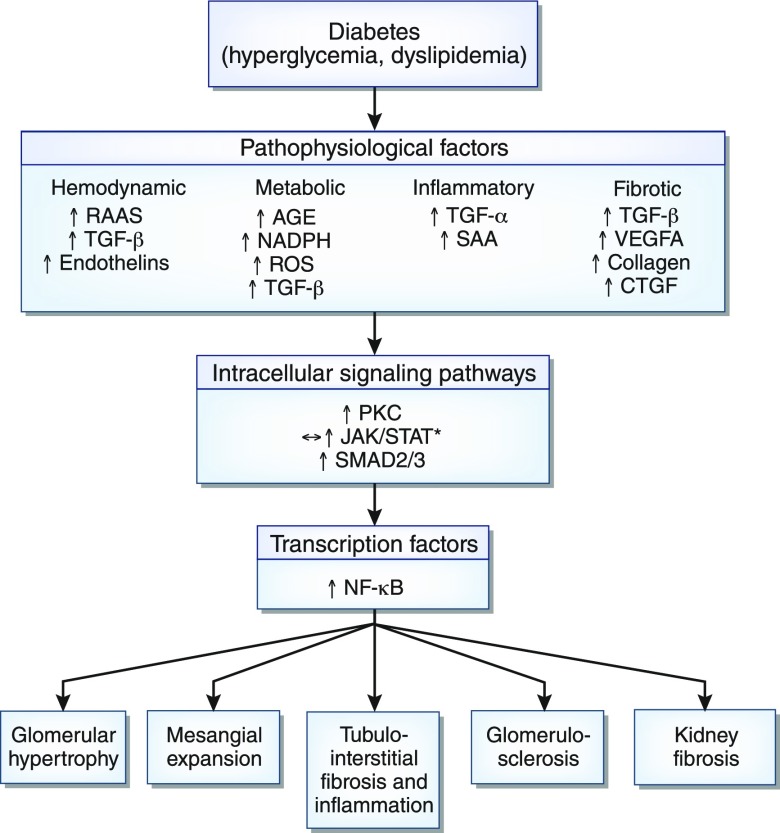
Figure 6.
Different pathways and networks involved in the initiation and progression of diabetic kidney disease. AGE, advanced glycation end product; CTGF, connective tissue growth factor; JAK-STAT, Janus kinase/signal transducer and activator of transcription; PKC, protein kinase C; RAAS, renin-angiotensin-aldosterone system; ROS, reactive oxygen species; SAA, serum amyloid A; VEGF-A, vascular endothelial growth factor A. *JAK/STAT signaling can be unchanged (↔) or upregulated (↑) in early and later stages of diabetes, respectively.
Figure 7.
Normal and diabetic nephron with altered renal hemodynamics.
Diagnosis of DKD
The clinical diagnosis of DKD is on the basis of measurement of eGFR and albuminuria along with clinical features, such as diabetes duration and presence of diabetic retinopathy (51,52). DKD is identified clinically by persistently high urinary albumin-to-creatinine ratio ≥30 mg/g and/or sustained reduction in eGFR below 60 ml/min per 1.73 m2 (53). Screening for DKD should be performed annually for patients with DM1 beginning 5 years after diagnosis and annually for all patients with DM2 beginning at the time of diagnosis. In patients with albuminuria, the presence of diabetic retinopathy is strongly suggestive of DKD. The preferred test for albuminuria is a urinary albumin-to-creatinine ratio performed on a spot sample, preferably in the morning (51,52). The eGFR is calculated from the serum creatinine concentration. Although the Chronic Kidney Disease-Epidemiologic Prognosis Initiative equation is more accurate, particularly at eGFR levels in the normal or near-normal range, the Modification of Diet in Renal Disease equation is typically reported by clinical laboratories (52). Confirmation of albuminuria or low eGFR requires two abnormal measurements at least 3 months apart. If features atypical of DKD are present, then other causes of kidney disease should be considered. Atypical features include sudden onset of low eGFR or rapidly decreasing eGFR, abrupt increase in albuminuria or development of nephrotic or nephritic syndrome, refractory hypertension, signs or symptoms of another systemic disease, and >30% eGFR decline within 2–3 months of initiation of a renin-angiotensin system inhibitor (53).
Treatment of DKD
Prevention of diabetic complications, particularly DKD, by long-term intensive glycemic control from early in the course of diabetes is well established for DM1 and DM2 (19,22). However, intensive glucose control after onset of complications or in longstanding diabetes has not been shown to reduce risk of DKD progression or improve overall clinical outcomes. Targeting low HbA1C (6%–6.9%) compared with standard therapy in this population did not reduce risk of cardiovascular (CV) or microvascular complications but increased the risk of severe hypoglycemia (54–56). Furthermore, an analysis of patients with DM2 and early-stage CKD showed 30% and 40% higher risks for all-cause mortality and CV mortality, respectively, with intensive glycemic control compared with standard therapy (57). The finding that intensive glycemic control incurs great risk of hypoglycemia and does not benefit the risk of CVD or all-cause mortality has been sustained over the long term (8–10 years). A small benefit of intensive glycemic control on the risk of ESRD was observed, but the absolute number of patients was minute (58). A stratified analysis showed that the greatest benefit of intensive glycemic control for preventing ESRD was seen in participants without kidney disease at study entry, further supporting the concept that intensive glycemic control initiated during early diabetes can prevent DKD (59).
The American Diabetes Association recommends that targets for glycemia should be tailored to age, comorbidities, and life expectancy of individual patients. More stringent goals, such as HbA1C<6.5%, may be reasonable for patients with shorter duration of diabetes, younger age, absence of complications, and a longer life expectancy. To the contrary, less stringent goals of HbA1C<8% are recommended for patients with longstanding diabetes, older age, micro- and macrovascular complications, and limited life expectancy (51). Similarly, the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative and the Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend a target HbA1c of about 7.0% to prevent or delay progression of the microvascular complications of diabetes. However, patients at risk for hypoglycemia, such as those with diabetes and CKD, should not be treated to an HbA1c target of <7.0% (53).
For management of hypertension, the Eighth Joint National Committee (JNC-8) recommended initiation of pharmacologic treatment at a systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg, with treatment goals less than these levels. In the general hypertensive population, including those with diabetes, initial antihypertensive treatment may include a thiazide-type diuretic, a calcium channel blocker, an angiotensin-converting enzyme (ACE) inhibitor, or an angiotensin receptor blocker (ARB). In black patients with diabetes, the JNC-8 recommends initial treatment with a thiazide diuretic or calcium channel blocker. The same BP targets are recommended for those with CKD irrespective of diabetes status. In patients who are diabetic with high levels of albuminuria, the medication regimen should include an ACE inhibitor or an ARB alone or in combination with medication from another drug class (60). The KDIGO guidelines recommend use of an ACE or an ARB and a BP goal <130/80 mmHg in all patients with CKD and albuminuria irrespective of diabetes status (52). There is unambiguous evidence that renin-angiotensin system blockade with either an ACE inhibitor or an ARB reduces the progression of DKD in patients with macroalbuminuria (61). However, combination therapy (an ACE inhibitor and an ARB administered together) increases the risk of serious side effects, primarily hyperkalemia and AKI, and offers no clinical benefits (62,63).
Following the liberalized JNC-8 recommendations, target BP goals have been challenged by results of the Systolic BP Intervention Trial (SPRINT). The SPRINT included 9361 nondiabetic participants with hypertension and high CV risk. Participants were randomized to either an intensive (<120 mmHg) or standard (<140 mmHg) systolic BP goal. The trial was terminated early after a median of 3.26 years, because rates of the primary outcome (myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from CV causes) and all-cause mortality were reduced by 25% and 27%, respectively, in the intensively treated group compared with the standard regimen group. These results held across prespecified subgroups defined according to CKD stage, age >75 years old, sex, race, previous CVD, and baseline levels of systolic BP (64,65).
In contrast to the SPRINT, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial, which included 4733 patients with diabetes at high risk for CV events, showed that achieving the same systolic BP targets (<120 versus <140 mmHg) did not have a statistically significant effect on the risk of nonfatal myocardial infarction, nonfatal stroke, death from CV cause, or death from any causes (66). One of the possible explanations for this incongruent finding is that the ACCORD Trial was underpowered to show between-group differences, because CV morbidity and mortality occurred at substantially lower rates than predicted. However, in the SPRINT participants who had CKD at study entry, intensive BP treatment did not reduce incidence of ESRD, cause a 50% decline in eGFR, or cause ≥30% decline in eGFR to a value of <60 ml/min per 1.73 m2. Furthermore, hospitalizations or emergency room visits for AKI occurred more frequently in the intensive treatment group than the standard regimen group (4.4% versus 2.6%; hazard ratio, 1.71) (64,67). Similarly, the ACCORD Trial detected a signal suggestive of a possible negative effect of intensive BP control on kidney function. Even among participants who had normal kidney function at baseline, instances of eGFR≤30 ml/min per 1.73 m2 were almost doubled in the intensive treatment group (99 in the intensive treatment group versus 52 in the standard treatment group; P<0.001) (66).
Novel Therapies and Approaches
Despite current approaches to management of diabetes and hypertension and use of ACE inhibitors and ARB, there is still large residual risk in DKD. Novel agents targeting mechanisms, such as glomerular hyperfiltration, inflammation, and fibrosis, have been a major focus for development of new treatments. Agents that have shown promise include ruboxistaurin, a protein kinase C-β inhibitor (68); baricitinib, a selective Janus kinase 1 and Janus kinase 2 inhibitor (69); pentoxifylline, an anti-inflammatory and antifibrotic agent (70); atrasentan, a selective endothelin A receptor antagonist (71,72); and finerenone, a highly selective nonsteroidal mineralocorticoid receptor antagonist (Table 4) (73). However, thus far, there are no available phase 3 clinical trial data for these agents, and none are approved for use in DKD.
Table 4.
Studies of novel treatments for diabetic kidney disease
| Name of the Study | Tested Intervention/Drugs | Study Population | Outcomes |
| Tuttle et al., 2005 (68) | Ruboxistaurin (PKC inhibitor) | DM2, macroalbuminuria | Decreased albuminuria, stabilized kidney function |
| PIONEER (81) | PYR-311 (anti-AGE treatment) | DM2, HTN, 1.3≤SCr≤3.0 mg/dl, protein-to-creatinine ratio ≥1200 mg/g | Halted |
| PREDIAN (70) | Pentoxifylline (anti-inflammatory, antifibrotic action) | DM2, eGFR=15–60 ml/min per 1.73, UAE>300 mg/24 h | Pentoxifylline group: eGFR decline 4.3 ml/min per 1.73 m2 less than control group; mean difference in albuminuria of 21% |
| Study to Test Safety and Efficacy of Baricitinib in Participants with Diabetic Kidney Disease (69) | Baricitinib, JAK1/2 inhibitor | DM2, eGFR=20–75 ml/min per 1.73 m2, macroalbuminuria | Albuminuria reduction by 40% in the highest treatment group; no effect on eGFR |
| RADAR and RADAR/JAPAN (71) | Atrasentan (ETA) | DM2, eGFR=30–75 ml/min per 1.73 m2, UACR=300–3500 mg/g | 35% Reduction of albuminuria |
| SONAR, ongoing (72) | Atrasentan (ETA) | HTN, eGFR=15–90 ml/min per 1.73 m2, UACR>30<5000 mg/g | Ongoing |
| PERL, ongoing (82) | Allopurinol (xanthine oxidase) | DM1, eGFR=40–99 ml/min per 1.73 m2, UAE=18–5000 mg/d | Ongoing |
| ARTS-DN, 2015 (83) | Finerenone (steroid mineralocorticoid receptor antagonist) | DM2, UACR≥30 mg/g, eGFR>30 ml/min per 1.73 m2 | No difference in eGFR, 17%–40% albuminuria reduction dose dependent |
SCr is in milligrams per deciliter. Protein to creatinine ratio is in milligrams per gram. eGFR is in milliliters per minute per 1.73 m2. UAE is in milligrams per day. UACR is in milligrams per gram. PKC, protein kinase C; DM2, diabetes mellitus type 2; PIONEER, A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Evaluate the Safety and Efficacy of Pyridorin (Pyridoxamine Dihydrochloride) in Subjects With Nephropathy Due to Type 2 Diabetes; PYR-311, pyridoxamine-311; AGE, advance glycation end product; HTN, hypertension; SCr, serum creatinine; PREDIAN, Effect of Pentoxifylline on Renal Function and Urinary Albumin Excretion in Patients with Diabetic Kidney Disease; UAE, urine albumin excretion; JAK1/2, Janus kinases 1/2; RADAR, Reducing Residual Albuminuria in Subjects With Diabetes and Nephropathy With AtRasentan—A Phase 2b, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate Safety and Efficacy; RADAR/JAPAN, RADAR in Japan; ETA, endothelin A; UACR, urinary albumin-to-creatinine ratio; SONAR, A Randomized, Multicountry, Multicenter, Double-Blind, Parallel, Placebo-Controlled Study of the Effects of Atrasentan on Renal Outcomes in Subjects With Type 2 Diabetes and Nephropathy; PERL, A Pilot Study of Allopurinol to Prevent GFR Loss in Type 1 Diabetes; DM1, diabetes mellitus type 1; ARTS-DN, A Randomized, Double-blind, Placebo-controlled, Multi-Center Study to Assess the Safety and Efficacy of Different Oral Doses of BAY94-8862 in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Nephropathy.
Since the year 2008, the US Food and Drug Administration has mandated that new antihyperglycemic therapies seeking approval for the treatment of DM2 must show CV safety. Three agents within the glucagon-like peptide-1 receptor agonist class of medications, lixisenatide, liraglutide, and semaglutide, currently have CV outcome trial data available. The Evaluation of Lixisenatide in Acute Coronary Syndrome Trial showed that the addition of lixisenatide to standard care did not significantly alter the rate of major CV events (74). In contrast, in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) Study and the Trial to Evaluate Cardiovascular and Other Long-Term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6), fewer participants reached the primary composite CV end point in the liraglutide and semaglutide groups compared with those receiving placebo (hazard ratio, 0.87; P=0.01 for superiority and hazard ratio, 0.74; P<0.001 for noninferiority, respectively) (75,76). Notably, similar benefits on CV outcomes were observed in the LEADER Study and the SUSTAIN-6 subsets with moderate to severe CKD. Studies in patients with DKD have additionally shown that liraglutide lowered albuminuria levels in patients with normal kidney function or early-stage CKD and showed improved glycemic control in CKD stage 3 (75). Recently released data from clinical trials of semaglutide and dulaglutide consistently show reduced risk of albuminuria onset and progression (75,76). The consistency of these data across glucagon-like peptide-1 receptor agonists persuasively suggests a class effect of protection from DKD. The mechanisms of action may be multifactorial and include glycemic control, weight control, and direct effects on the kidney.
In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients, an sodium-glucose cotransporter 2 inhibitor, empaglifozin, also showed significantly lowered rates of death from CVD causes (38% relative risk reduction), hospitalization for heart failure (35% relative risk reduction), and death from any cause (32% relative risk reduction) compared with placebo (77,78). Analysis of prespecified secondary outcomes showed that empaglifozin also slowed progression of DKD and lowered rates of clinically relevant kidney outcomes among patients with CKD stages 2–4 (78) (Table 5).
Table 5.
Kidney outcomes in clinical trials of newer antihyperglycemic therapies
| Name of the Study | Tested Intervention/Drugs | Study Population | Outcomes |
| SAVOR-TIMI (84) | Saxagliptin (DPP-4 inhibitor) | DM2, HbA1c≥6.5%, high risk for CV events | Improvement in and/or less deterioration in ACR categories from baseline to end of trial (P=0.02, P<0.001, and P=0.05 for normoalbuminuria, microalbuminuria, and macroalbuminuria, respectively); no changes in eGFR |
| CARMELINA (85) | Linagliptin (DPP-4 inhibitor) | DM2, 6.5%≥HbA1c≤10%, albuminuria, macrovascualar complications, eGFR>15 ml/min per 1.73 m2 | In progress, estimated completion in January of 2018 |
| LEADER (75) | Liraglutide (GLP-1 receptor agonist) | DM2, HbA1c>7%, eGFR<60 ml/min per 1.73 m2, CV coexisting disease | Lower incidence of nephropathy (new-onset albuminuria, doubling of SCr and CrCl<45 ml/min per 1.73 m2; need for RRT, death to renal causes [1.5 number of events per 100 patients per year versus 1.9 number of events per 100 patients per year; P=0.003]) |
| AWARD-7, (86) | Dulaglutide (GLP-1 receptor agonist) | DM2, 7.5%≥HbA1c≤10.5%, 15≥eGFR≤60 ml/min per 1.73 m2 | In progress, estimated completion in July of 2018 |
| EMPA-REG OUTCOME (78) | Empaglifozin (SGLT-2 inhibitor) | DM2, eGFR≥30 ml/min per 1.73 m2, high CV risk | 44% Relative risk reduction of doubling of SCr (1.5% versus 2.6%); 38% relative risk reduction of progression to macroalbuminuria (11.2% versus 16.2%); 55% relative risk reduction of initiation of RRT (0.3% versus 0.6%); slowing GFR decline (annual decrease 0.19±0.11 versus 1.67±0.13 ml/min per 1.73 m2; P<0.001) |
| CREDENCE (87) | Canaglifozin (SGLT-2 inhibitor) | DM2, 6.5%≥HbA1c≤12%, high CV risk, 300 mg/g≥UACR≤5000 mg/g, 30≥eGFR≤90 ml/min per 1.73 m2 | In progress, estimated completion in June of 2019 |
eGFR is in milliliters per minute per 1.73 m2. UACR is in milligrams per gram. SAVOR-TIMI, Does Saxagliptin Reduce the Risk of Cardiovascular Events When Used Alone or Added to Other Diabetes Medications; DPP-4, dipeptidyl peptidase-4 inhibitor; DM2, diabetes mellitus type 2; HbA1c, hemoglobin A1c; CV, cardiovascular; ACR, albumin-to-creatinine ratio; CARMELINA, Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; GLP-1, glucagon-like peptide-1; SCr, serum creatinine; CrCl, creatinine clearance; AWARD-7, A Study Comparing Dulaglutide With Insulin Glargine on Glycemic Control in Participants With Type 2 Diabetes (T2D) and Moderate or Severe Chronic Kidney Disease (CKD); EMPA-REG OUTCOME, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; SGLT-2, sodium-glucose cotransporter 2; CREDENCE, Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy; UACR, urine albumin-to-creatinine ratio.
Population-Based Approaches
Success of this strategy has been shown by recently available data from the Centers for Disease Control. A 54% decrease in diabetes-related kidney failure occurred between the years 1996 and 2013 among American Indians, a group with a historically high prevalence of diabetes and DKD. Interventions leading to this change included systematic implementation of guidelines for treatment of hypertension and diabetes, regular albuminuria testing, use of ACE inhibitors and ARBs, services to support nutrition, physical activity, and diabetes education (79).
Conclusion
Since the discovery of insulin in the 1920s, research has made significant strides toward understanding and improving the clinical management of diabetes. Although these advances have meaningfully improved outcomes for diabetes complications, such as CVD, these improvements have not translated nearly as well to DKD or ESRD (80). In response, the International Society of Nephrology has convened a Global Kidney Health Initiative to call attention to kidney diseases overall. Key collaborative stakeholders in the quest to fight DKD should include patients, health care providers and payers, advocacy groups, scientists, and governmental agencies. Advocacy and a call to action are essential to effective dissemination and implementation of current best practices. Using public health and population approaches in clinical practice and promoting meaningful and strategic research will be key to improving health outcomes for people with diabetes and DKD.
Disclosures
K.R.T. has received consulting fees from Eli Lilly and Company, Amgen, Noxxon Pharma, and Boehringer Ingelheim. The other authors have no disclosures.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Cameron JS: The discovery of diabetic nephropathy: From small print to centre stage. J Nephrol 19[Suppl 10]: S75–S87, 2006 [PubMed] [Google Scholar]
- 2.USRDS: United States Renal Data System Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 3.Reutens AT: Epidemiology of diabetic kidney disease. Med Clin North Am 97: 1–18, 2013 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization: Global Status Report on Noncommunicable Diseases, Geneva, Switzerland, World Health Organization, 2014 [Google Scholar]
- 5.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menke A, Casagrande S, Geiss L, Cowie CC: Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 314: 1021–1029, 2015 [DOI] [PubMed] [Google Scholar]
- 7. International Diabetes Federation: Diabetes Atlas, 7th Ed., Brussels, Belgium, IDF Executive Office, 2015.
- 8.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL: Overweight and obesity in the United States: Prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord 22: 39–47, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL: Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284–2291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY-M, Yang C-W: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH: Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24: 302–308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taal MW: Risk factors and chronic kidney disease. In: Brenner and Rector's The Kidney, 10th Ed., edited by Skorecki K, Amsterdam, Elsevier, 2015, pp 669–692.e7 [Google Scholar]
- 14.Caramori ML, Parks A, Mauer M: Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 24: 1175–1181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications (DCCT) Research Group: Effect of intensive therapy on the development and progression of diabetic nephropathy in the diabetes control and complications trial. Kidney Int 47: 1703–1720, 1995 [DOI] [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 17.Tonna S, El-Osta A, Cooper ME, Tikellis C: Metabolic memory and diabetic nephropathy: Potential role for epigenetic mechanisms. Nat Rev Nephrol 6: 332–341, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Nathan DM; DCCT/EDIC Research Group: The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 37: 9–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DCCT/EDIC Research Group; de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B: Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Bilous R: Microvascular disease: What does the UKPDS tell us about diabetic nephropathy? Diabet Med 25[Suppl 2]: 25–29, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group: Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM; RENAAL Study Group: Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Arch Intern Med 163: 1555–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JB, Mangili R, Moore J Jr., Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ: Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J Am Soc Nephrol 16: 3027–3037, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Fioretto P, Mauer M: Histopathology of diabetic nephropathy. Semin Nephrol 27: 195–207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi I, Agrawal U, Amitabh V, Jain AK, Saxena S: Thickness of glomerular and tubular basement membranes in preclinical and clinical stages of diabetic nephropathy. Indian J Nephrol 18: 64–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drummond K, Mauer M; International Diabetic Nephropathy Study Group: The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Osterby R, Tapia J, Nyberg G, Tencer J, Willner J, Rippe B, Torffvit O: Renal structures in type 2 diabetic patients with elevated albumin excretion rate. APMIS 109: 751–761, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Kida H, Takeda S, Yoshimura M, Yokoyama H, Koshino Y, Hattori N: Mesangiolysis in diabetic glomeruli: Its role in the formation of nodular lesions. Kidney Int 34: 389–396, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Stout LC, Kumar S, Whorton EB: Focal mesangiolysis and the pathogenesis of the Kimmelstiel-Wilson nodule. Hum Pathol 24: 77–89, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Rossing PFP, Feldt-Rasmussen B, Parving HH: Diabetic nephropathy. In: Brenner and Rector's The Kidney, 10th Ed., edited by Skorecki K, Chertow GM, Marsden PA, Yu ASL, Taal MW, Philadelphia, Elsevier, pp 1283–1381 [Google Scholar]
- 32.Fioretto P, Caramori ML, Mauer M: The kidney in diabetes: Dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia 51: 1347–1355, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Tervaert TWC, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA; Renal Pathology Society: Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Mogensen CE, Christensen CK, Vittinghus E: The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32[Suppl 2]: 64–78, 1983 [DOI] [PubMed] [Google Scholar]
- 35.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Gaede P, Tarnow L, Vedel P, Parving H-H, Pedersen O: Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 19: 2784–2788, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Pacilli A, Viazzi F, Fioretto P, Giorda C, Ceriello A, Genovese S, Russo G, Guida P, Pontremoli R, De Cosmo S; AMD-Annals Study Group: Epidemiology of diabetic kidney disease in adult patients with type 1 diabetes in Italy: The AMD-Annals initiative [published online ahead of print December 9, 2016]. Diabetes Metab Res Rev doi:10.1002/dmrr.2873 [DOI] [PubMed] [Google Scholar]
- 38.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316: 602–610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klessens CQF, Woutman TD, Veraar KAM, Zandbergen M, Valk EJJ, Rotmans JI, Wolterbeek R, Bruijn JA, Bajema IM: An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int 90: 149–156, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Thomas MC, Cooper ME, Rossing K, Parving H-H: Anaemia in diabetes: Is there a rationale to TREAT? Diabetologia 49: 1151–1157, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Moseley KF: Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes 19: 128–135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foley RN, Hakim RM: Why is the mortality of dialysis patients in the United States much higher than the rest of the world? J Am Soc Nephrol 20: 1432–1435, 2009 [DOI] [PubMed] [Google Scholar]
- 43.USRDS: United States Renal Data System Annual Data Report: Mortality, Ann Arbor, MI, USRDS, 2015 [Google Scholar]
- 44.Tuttle KR, Bruton JL: Effect of insulin therapy on renal hemodynamic response to amino acids and renal hypertrophy in non-insulin-dependent diabetes. Kidney Int 42: 167–173, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Tuttle KR, Bruton JL, Perusek MC, Lancaster JL, Kopp DT, DeFronzo RA: Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Engl J Med 324: 1626–1632, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Tuttle KR, Puhlman ME, Cooney SK, Short RA: Effects of amino acids and glucagon on renal hemodynamics in type 1 diabetes. Am J Physiol Renal Physiol 282: F103–F112, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Grabias BM, Konstantopoulos K: The physical basis of renal fibrosis: Effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol 306: F473–F485, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ: The impact of hyperfiltration on the diabetic kidney. Diabetes Metab 41: 5–17, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Tuttle KR: Back to the future: Glomerular hyperfiltration and the diabetic kidney. Diabetes 66: 14–16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Anonymous: Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care 39[Suppl 1]: S4–S5, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME: Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care 37: 2864–2883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Kidney Foundation: KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60: 850–886, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F; ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr., Probstfield JL, Simons-Morton DG, Friedewald WT; Action to Control Cardiovascular Risk in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators: Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Papademetriou V, Lovato L, Doumas M, Nylen E, Mottl A, Cohen RM, Applegate WB, Puntakee Z, Yale JF, Cushman WC; ACCORD Study Group: Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int 87: 649–659, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Wong MG, Perkovic V, Chalmers J, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, MacMahon S, Mancia G, Marre M, Matthews D, Neal B, Poulter N, Rodgers A, Williams B, Zoungas S; ADVANCE-ON Collaborative Group: Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care 39: 694–700, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr., Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I; ACCORD Trial Group: Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 376: 419–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS, Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving H-H, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Mann JFE, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET Investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators: Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 64.SPRINT Research Group, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT: A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perkovic V, Rodgers A: Redefining blood-pressure targets--SPRINT starts the marathon. N Engl J Med 373: 2175–2178, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group: Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rocco MV, Cheung AK: A SPRINT to the finish, or just the beginning? Implications of the SPRINT results for nephrologists. Kidney Int 89: 261–263, 2016 [DOI] [PubMed] [Google Scholar]
- 68.Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW: The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 28: 2686–2690, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Brosius FC, Tuttle KR, Kretzler M: JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59: 1624–1627, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del Castillo N, Rivero A, Getino MA, García P, Jarque A, García J: Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J Am Soc Nephrol 26: 220–229, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danielle P: A Randomized, Multicountry, Multicenter, Double-Blind, Parallel, Placebo-Controlled Study of the Effects of Altrasentan on Renal Outcomes in Subjects with Type 2 Diabetes and Nephropathy SONAR: Study of Diabetic Nephropathy with Atrasentan NCTO1858532. Available at: ClinicalTrials.gov. Accessed April 28, 2017 [Google Scholar]
- 73.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group: Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA 314: 884–894, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators: Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 373: 2247–2257, 2015 [DOI] [PubMed] [Google Scholar]
- 75.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee, LEADER Trial Investigators: Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834–1844, 2016 [DOI] [PubMed] [Google Scholar]
- 77.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 78.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators: Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 79. Bullock A, Burrows NR, Narva AS, Sheff K, Hora L, Lekiachvili A, Cain H, Espey D: Vital signs: Decrease in incidence of diabetes-related end-stage renal disease among American Indians/Alaska natives — United States, 1996–2013. MMWR Morb Mortal Wkly Rep 66: 26–32, 2017. [DOI] [PMC free article] [PubMed]
- 80.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L: Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370: 1514–1523, 2014 [DOI] [PubMed] [Google Scholar]
- 81.A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Evaluate the Safety and Efficacy of Pyridorin (Pyridoxamine Dihydrochloride) in Subjects with Nephropathy Due to Type 2 Diabetes (PIONEER) NCTO2156843. Available at: ClinicalTrials.gov. Accessed April 27, 2017 [Google Scholar]
- 82.Maahs DM, Caramori L, Cherney DZI, Galecki AT, Gao C, Jalal D, Perkins BA, Pop-Busui R, Rossing P, Mauer M, Doria A; PERL Consortium: Uric acid lowering to prevent kidney function loss in diabetes: The preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep 13: 550–559, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM: Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 314: 884–894, 2015 [DOI] [PubMed] [Google Scholar]
- 84.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, Rozenberg A, Yanuv I, Stahre C, Ray KK, Iqbal N, Braunwald E, Scirica BM, Raz I: Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care 40: 69–76, 2017 [DOI] [PubMed] [Google Scholar]
- 85. Cardiovascular and Renal Microvascular Outcome Study with Linagliptin in Patients with Type 2 Diabetes Mellitus (CARMELINA) NCT01897532, 2016. Available at: ClinicalTrials.gov. Accessed April 27, 2017.
- 86. A Study Comparing Dulaglutide with Insulin Glargine on Glycemic Control in Participants with Type 2 Diabetes (T2D) and Moderate or Severe Chronic Kidney Disease (CKD) (AWARD-7) NCT01621178, 2017. Available at: ClinicalTrials.gov, 2017. Accessed April 27, 2017.
- 87. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) NCT02065791, 2017. Available at: ClinicalTrials.gov. Accessed April 27, 2017.