Abstract
Polymer mimics of antifreeze proteins are emerging as an exciting class of macromolecular cryoprotectants for the storage of donor cells and tissue. Poly(vinyl alcohol), PVA, is the most potent polymeric ice growth inhibitor known, but its mode of action and the impact of valency (DP) are not fully understood. Herein, tandem RAFT polymerization and column chromatography are used to isolate oligomers with dispersities <1.01 to enable the effect of molecular weight distribution, as well as length, to be probed. It is found that polymers with equal number average molecular weight, but lower dispersity, have significantly less activity, which can lead to false positives when identifying structure-property relationships. The minimum chain length for PVA’s unique activity, compared to other non-active poly-ols was identified. These results will guide the design of more active inhibitors, better cryopreservatives and a deeper understanding of synthetic and biological antifreeze macromolecules.
Antifreeze proteins and antifreeze glyco proteins (AF(G)Ps) have evolved the unique function of binding to and inhibiting the growth of ice crystals.1–3 The ability to slow ice growth has major technological applications in aerospace, wind farms4 and in the cryopreservation of donor cells and tissue, which are traditionally stored using toxic organic solvents.5 Synthetic mimics of AF(G)Ps have recently emerged, which have few structural similarities but can reproduce AF(G)P properties.6,7 The most desirable property for cryopreservation is ice recrystallization inhibition (IRI) which prevents ice-growth during thawing.8 Various IRI active materials and their use in cryopreservation have been reported including poly(vinyl alcohol)9–11 poly(ampholytes)12,13, amphipathic metallo-helices14 and graphene oxide derivatives.15 Whilst the interactions between antifreeze proteins and ice has led to identification of ice-binding domains3,16 how the most active synthetic polymer mimic, PVA, functions remains unclear, but amphipathicity appears to play a role.14,15 Inada et al.17 and Gibson and coworkers18 have demonstrated that PVA’s activity increases with increasing molecular weight. Most other poly-ols (and most polymers) have essentially zero IRI activity highlighting that the presence of hydroxyls is not the key motif for activity.19,20 Budke and Koop have postulated that a minimum of 10 repeat units are essential for PVA to function, based on lattice matching with ice crystal surfaces.21 This is supported by statistical co-polymerizations of PVA (spacing out the hydroxyls) which dramatically reduces IRI activity,18 but block copolymerization is a tolerated modification.22,23 Externally triggerable IRI’s have been obtained which use supramolecular self-assembly to increase the DP of PVA from 10 to 30, and hence the activity of PVA ‘turns on’.24 However, identification of the minimum chain length for activity is non-trivial due to the inherent dispersity of polymers meaning even those derived from controlled radical polymerization are complex mixtures.
Recent advances in single-monomer insertion,25 and attempts to obtain sequence-regulated synthetic polymers26–28 have highlighted the importance of minor sequence modifications on emergent macromolecular properties such as self-assembly29 single chain folding30 and lectin binding.31 Stubbs et al. have demonstrated that alternating poly(ampholyte) co-polymers have superior ice recrystallization inhibition activity relative to random copolymers.32 Hawker and coworkers have recently introduced the use of silica gel column chromatography to purify low-molecular weight (DP<20) polymers derived from radical polymerization such that near-monodisperse fractions can be easily isolated, dramatically reducing dispersity.33 Using this method, the self-organization of oligo(methyl methacrylate)-block-oligo(dimethylsiloxane) was found to be markedly different from those with increased dispersity. Considering the above, here we report the synthesis and purification of ultra-low dispersity oligo(vinyl alcohol) and quantitative evaluation of the impact of each fraction on its IRI activity. We show a profound effect of the chain length distribution, with tiny amounts of high molecular weight material, even in ‘narrow’ polymers derived from RAFT, having dramatic effects on ice interaction.
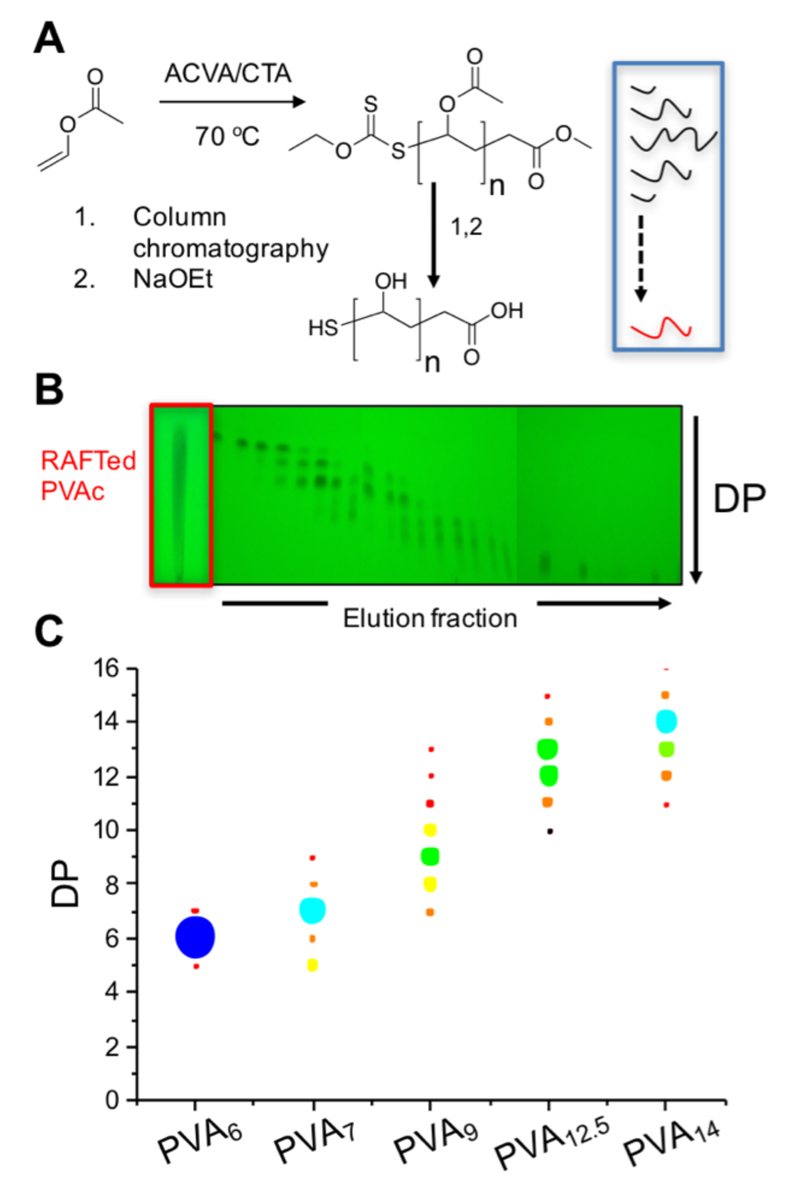
To obtain well-defined PVA, RAFT/MADIX polymerization34 of vinyl acetate was conducted using methyl (ethoxy carbonthioyl) sulfanyl acetate as the chain transfer agent (CTA) and azobis(4-cyanovaleric acid) (ACVA) as the radical source, Figure 1A.18 RAFTed polymers with average DPs from 6 – 13 were subjected to column chromatography using a procedure from Hawker et al., with monitoring by thin layer chromatography (Figure 2B).33 Fractions enriched in single oligomers were obtained, resulting in significant narrowing of their dispersity in size exclusion chromatography (ESI). The PVAcs were analysed by electrospray ionisation mass spectrometry, enabling molecular weight and distribution to be calculated. From these, a range were selected with PVAc dispersities < 1.01, covering a range DPs; in terms of the purity of the major peak this is an increase from <10% to >60%. PVA was generated by deacetylation with sodium ethoxide followed by purification with ion-exchange resins. 1H NMR and infrared spectroscopy (Supp. Info.) confirmed quantitative removal of the acetate groups. Fractionated chain lengths > 15 could not be isolated as the method is only suitable for oligomers.33
Figure 1.
A) Synthesis of PVA by RAFT/MADIX polymerization; B) Thin layer chromatography of columned polymers; C) Chain length distribution frequency of polymers used here.
Figure 2.
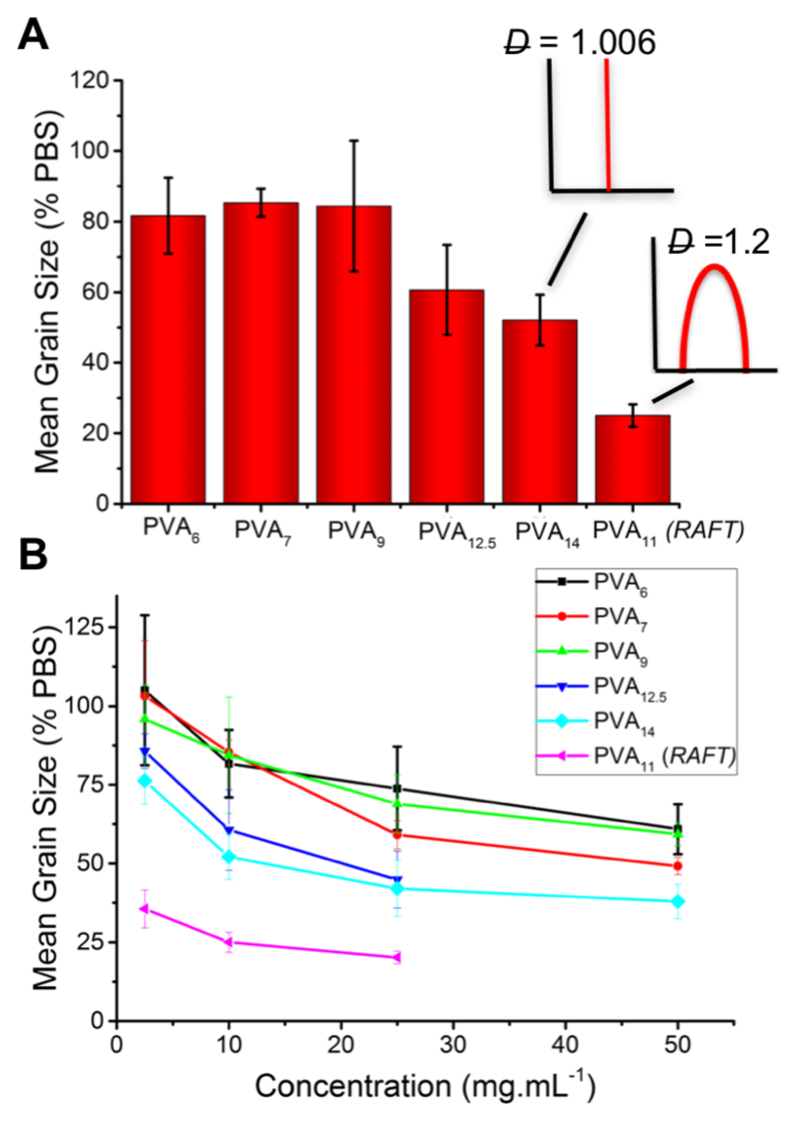
IRI activity of polymers. A) Comparison of IRI activity of purified versus RAFTed PVA at 10 mg.mL-1; B) Variable concentration IRI activity. Error bars represents ± S.D from minimum of 3 repeats.
A ‘splat’ assay was used to evaluate the ice recrystallization inhibition (IRI) activity of the oligomers.35 A 10 μL droplet at each concentration in PBS was dropped onto a chilled (-80 °C) glass slide, before being transferred to a cold stage set at -8 °C, under N2. After 30 minutes the wafer was imaged and the mean grain size (MGS) was calculated relative to a PBS control, with smaller numbers indicating more activity. Figure 2A compares the IRI activity of RAFTed PVA11 compared to the low-dispersity polymers at 10 mg.mL-1. As expected the shortest polymers (PVAs 6-9) had low activity with MGS ~ 80 %, identical to a negative control (poly(ethyleneglycol)). However, PVA14 which had a higher number average molecular weight than RAFTed PVA11, had lower activity (50 % MGS versus 20 %). To a first approximation, this is contrary to previous results which show that increased Mn increases activity.18,24 However, it provides the first evidence that high molecular weight fractions, even within a RAFTed polymer distribution, dominates the IRI activity. To probe this in more detail, the entire library was evaluated across a full range of concentrations, Figure 2B. At all concentrations, PVA11 with higher dispersity was significantly more active than all other fractions.
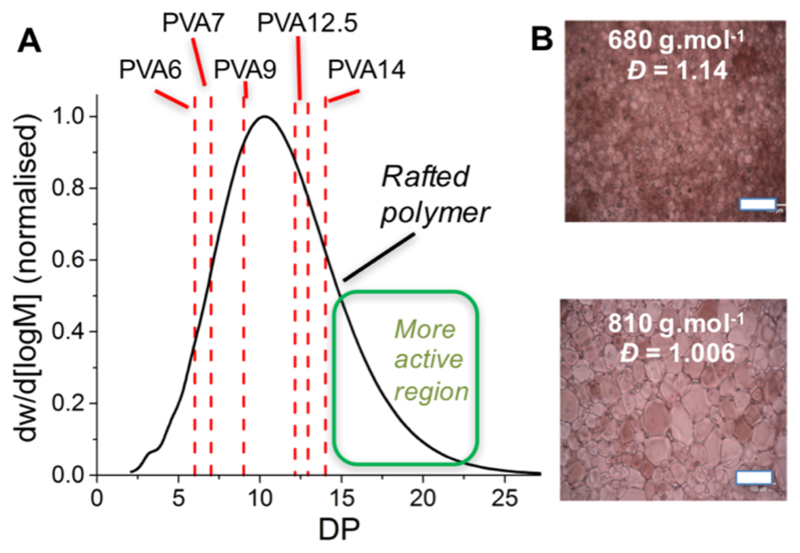
The above data shows that small fractions of high molecular weight impurities dominate the observed IRI. For translation to biomedical applications, understanding the role of the entire distribution is essential, particularly to reduce dosage through removal of inactive components. Figure 3 depicts the molecular weight distribution of the polymers purified here, compared to the starting distribution from a RAFTed polymer. Example ice wafers of two polymers with similar Mn but different dispersity are shown in Figure 3B
Figure 3.
Distribution of PVA11 by RAFT compared to the narrow fractions isolated here. Scale bar is 100 μm.
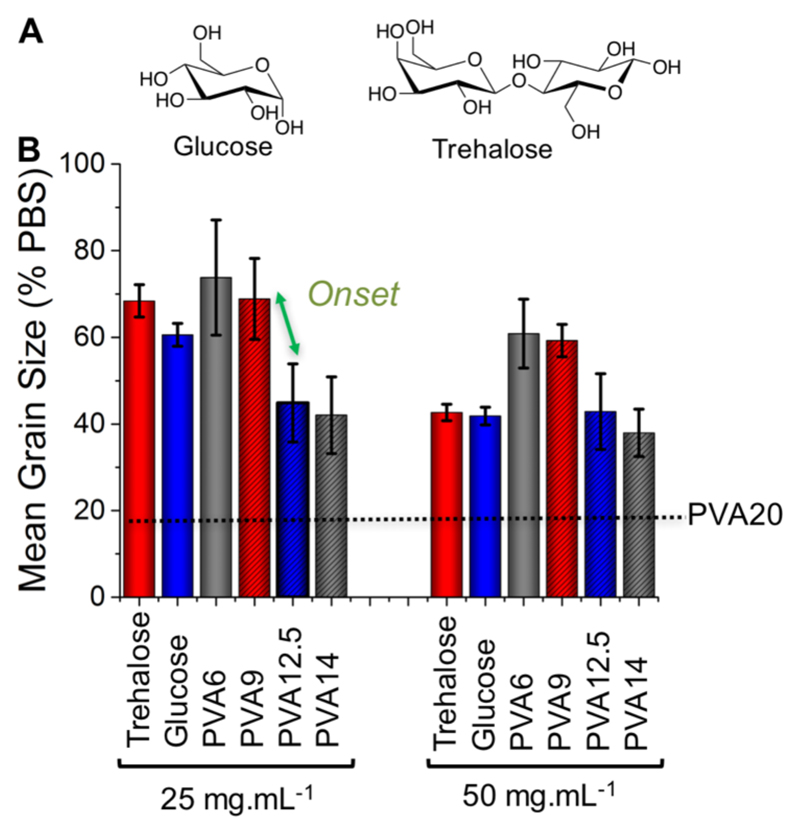
The above data raises the question of when does PVA become IRI active compared to other poly-ols? PVA is unique in its IRI activity compared to other poly-ols.19,36 Koop has hypothesized that the hydroxyl spacing in PVA presents a match to the primary and secondary prism faces of ice, and that a minimum of approximately 10 units is required for activity.21 To determine the critical IRI chain length, a mono and di-saccharide were used as controls. Saccharides have regularly spaced hydroxyls and hence can be considered as oligomers, to compare against PVA.37 At 50 mg.mL-1 glucose (OH = 4), and trehalose (OH = 8) had essentially identical activity to PVA6, PVA7 and PVA9, indicating that at this length there was no specific IRI activity. (It should be noted that these high concentrations any additive will have some weak, non-specific growth inhibition.) However, PVA12.5 and PVA14 were significantly more active and maintained activity to lower concentrations (25 mg.mL-1), indicating this is the threshold for activity. It is important to note that these are still vastly less active than e.g. PVA20 which functions at 20 X lower concentrations. The use of ultra-low dispersity polymers to identify the minimum chain length will help to understand the unique activity of PVA and to develop new IRI active macromolecules for translational biomedical applications, as well as enabling a link to modelling/simulation of this complex interface.
In conclusion, we have demonstrated that column chromatography can be used to access ultra-low dispersity PVA’s, with dispersity values as low as 1.005. Using these oligomers, the critical chain length for biomimetic ice recrystallization inhibition activity was identified, with PVA showing onset (‘switch on’) of activity at 12 units. Such insight will help improve our theoretical understanding of this extremely complex interface. It also provides insight into the role of dispersity in emergent applications of polymers; in this case, the high molecular weight tail dominates all observed ice interacting properties and will guide the use of substantially lower therapeutic doses of polymers by removing inactive (or too active) fractions.
Supplementary Material
Associated Content
Experimental procedures and characterization data, including mass spectrometry and size exclusions chromatography analysis is included in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 4.
IRI activity of sugars verses PVA. A) sugars used; B) Comparison of IRI activity of saccharides verses PVAs. Error bars represents ± S.D from minimum of 3 repeats.
Table 1.
RAFTed and column-purified polymers
| Entry | Mn(d) (g.mol-1) |
DP (-) |
Đ (-) |
|---|---|---|---|
| RAFT-PVA6 | 540 | 6 | 1.03(a) |
| RAFT-PVA11 | 680 | 11 | 1.14(a) |
| RAFT-PVA13 | 820 | 13 | 1.10(a) |
| PVA6 | 460 | 6 | 1.005(b) |
| PVA7 | 500 | 7 | 1.009(b) |
| PVA9 | 590 | 9 | 1.01(b) |
| PVA12.5 | 745 | 12.5(c) | 1.007(b) |
| PVA14 | 810 | 14 | 1.006(b) |
Data from SEC of PVAc precursors, expressed as PVA equivalent for comparison;
Calculated from ESI-MS;
DP 12 and 13 were equal as the major peaks;
Mn is PVA equivalent not PVAc
Acknowledgment
M.I.G. holds an ERC starting grant (CRYOMAT 638661). The Royal Society are also thanked for funding the cryo-microscopes used in this study.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- (1).Capicciotti CJ, Malay D, Ben RN. Ice Recrystallization Inhibitors: From Biological Antifreezes to Small Molecules. In: Wilson P, editor. Recent Developments in the Study of Recrystallization. InTech; 2013. p. 232. [Google Scholar]
- (2).Harding MM, Anderberg PI, Haymet ADJ. “Antifreeze” Glycoproteins from Polar Fish. Eur J Biochem. 2003;270(7):1381–1392. doi: 10.1046/j.1432-1033.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- (3).Davies PL. Trends in Biochemical Sciences. Elsevier; Nov, 2014. Ice-Binding Proteins: A Remarkable Diversity of Structures for Stopping and Starting Ice Growth; pp. 548–555. [DOI] [PubMed] [Google Scholar]
- (4).Parent O, Ilinca A. Anti-Icing and de-Icing Techniques for Wind Turbines: Critical Review. Cold Reg Sci Technol. 2011;65(1):88–96. [Google Scholar]
- (5).Fowler A, Toner M. Cryo-Injury and Biopreservation. Ann N Y Acad Sci. 2005;1066:119–135. doi: 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- (6).Gibson MI. Slowing the Growth of Ice with Synthetic Macromolecules: Beyond Antifreeze(glyco) Proteins. Polym Chem. 2010;1(8):1141–1152. [Google Scholar]
- (7).Leclère M, Kwok BK, Wu LK, Allan DS, Ben RN. C-Linked Antifreeze Glycoprotein (C-AFGP) Analogues as Novel Cryoprotectants. Bioconjug Chem. 2011;22(9):1804–1810. doi: 10.1021/bc2001837. [DOI] [PubMed] [Google Scholar]
- (8).Capicciotti CJ, Kurach JDR, Turner TR, Mancini RS, Acker JP, Ben RN. Small Molecule Ice Recrystallization Inhibitors Enable Freezing of Human Red Blood Cells with Reduced Glycerol Concentrations. Sci Rep. 2015;5:9692. doi: 10.1038/srep09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Deller RC, Pessin JE, Vatish M, Mitchell DA, Gibson MI. Enhanced Non-Vitreous Cryopreservation of Immortalized and Primary Cells by Ice-Growth Inhibiting Polymers. Biomater Sci. 2016;47:935–945. doi: 10.1039/c6bm00129g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mitchell DE, Lovett JR, Armes SP, Gibson MI. Combining Biomimetic Block Copolymer Worms with an Ice-Inhibiting Polymer for the Solvent-Free Cryopreservation of Red Blood Cells. Angew Chem Int Ed. 2016;55(8):2801–2804. doi: 10.1002/anie.201511454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Deller RC, Vatish M, Mitchell DA, Gibson MI. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat Commun. 2014;5:3244. doi: 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- (12).Matsumura K, Hyon SH. Polyampholytes as Low Toxic Efficient Cryoprotective Agents with Antifreeze Protein Properties. Biomaterials. 2009;30(27):4842–4849. doi: 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- (13).Mitchell DE, Cameron NR, Gibson MI. Rational, yet Simple, Design and Synthesis of an Antifreeze-Protein Inspired Polymer for Cellular Cryopreservation. Chem Commun. 2015;51(65):12977–12980. doi: 10.1039/c5cc04647e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Mitchell DE, Clarkson G, Fox DJ, Vipond RA, Scott P, Gibson MI. Antifreeze Protein Mimetic Metallohelices with Potent Ice Recrystallization Inhibition Activity. J Am Chem Soc. 2017;139(29):9835–9838. doi: 10.1021/jacs.7b05822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Geng H, Liu X, Shi G, Bai G, Ma J, Chen J, Wu Z, Song Y, Fang H, Wang J. Graphene Oxide Restricts Growth and Recrystallization of Ice Crystals Communications Angewandte. Angew Chem Int Ed. 2017;56(4):997–1001. doi: 10.1002/anie.201609230. [DOI] [PubMed] [Google Scholar]
- (16).Scotter AJ, Marshall CB, Graham LA, Gilbert JA, Garnham CP, Davies PL. The Basis for Hyperactivity of Antifreeze Proteins. Cryobiology. 2006;53(2):229–239. doi: 10.1016/j.cryobiol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- (17).Inada T, Lu SS. Inhibition of Recrystallization of Ice Grains by Adsorption of Poly(vinyl Alcohol) onto Ice Surfaces. Cryst Growth Des. 2003;3(5):747–752. [Google Scholar]
- (18).Congdon T, Notman R, Gibson MI. Antifreeze (Glyco)protein Mimetic Behavior of Poly(vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules. 2013;14(5):1578–1586. doi: 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- (19).Gibson MI, Barker CA, Spain SG, Albertin L, Cameron NR. Inhibition of Ice Crystal Growth by Synthetic Glycopolymers: Implications for the Rational Design of Antifreeze Glycoprotein Mimics. Biomacromolecules. 2009;10(2):328–333. doi: 10.1021/bm801069x. [DOI] [PubMed] [Google Scholar]
- (20).MacDonald MJ, Cornejo NR, Gellman SH. Inhibition of Ice Recrystallization by Nylon-3 Polymers. ACS Macro Lett. 2017;6(7):695–699. doi: 10.1021/acsmacrolett.7b00396. [DOI] [PubMed] [Google Scholar]
- (21).Budke C, Koop T. Ice Recrystallization Inhibition and Molecular Recognition of Ice Faces by Poly(vinyl Alcohol) ChemPhysChem. 2006;7(12):2601–2606. doi: 10.1002/cphc.200600533. [DOI] [PubMed] [Google Scholar]
- (22).Congdon TR, Notman R, Gibson MI. Influence of Block Copolymerization on the Antifreeze Protein Mimetic Ice Recrystallization Inhibition Activity of Poly(vinyl Alcohol) Biomacromolecules. 2016;17(9):3033–3039. doi: 10.1021/acs.biomac.6b00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).DeLuca CI, Comley R, Davies PL. Antifreeze Proteins Bind Independently to Ice. Biophys J. 1998;74(3):1502–1508. doi: 10.1016/S0006-3495(98)77862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Phillips DJ, Congdon TR, Gibson MI. Activation of Ice Recrystallization Inhibition Activity of Poly(vinyl Alcohol) Using a Supramolecular Trigger. Polym Chem. 2016;7(9):1–13. doi: 10.1039/C5PY01948F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Haven JJ, Vandenbergh J, Kurita R, Gruber J, Junkers T. Efficiency Assessment of Single Unit Monomer Insertion Reactions for Monomer Sequence Control: Kinetic Simulations and Experimental Observations. Polym Chem. 2015;6(31):5752–5765. [Google Scholar]
- (26).Lutz J-F. An Introduction to Sequence-Controlled Polymers. Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties. 2014;1170:1. [Google Scholar]
- (27).Engelis NG, Anastasaki A, Nurumbetov G, Truong NP, Nikolaou V, Shegiwal A, Whittaker MR, Davis TP, Haddleton DM. Sequence-Controlled Methacrylic Multiblock Copolymers via Sulfur-Free RAFT Emulsion Polymerization. Nat Chem. 2017;9(2):171–178. doi: 10.1038/nchem.2634. [DOI] [PubMed] [Google Scholar]
- (28).Hibi Y, Ouchi M, Sawamoto M. A Strategy for Sequence Control in Vinyl Polymers via Iterative Controlled Radical Cyclization. Nat Commun. 2016;7:11064. doi: 10.1038/ncomms11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Van Genabeek B, De Waal BFM, Gosens MMJ, Pitet LM, Palmans ARA, Meijer EW. Synthesis and Self-Assembly of Discrete Dimethylsiloxane-Lactic Acid Diblock Co-Oligomers: The Dononacontamer and Its Shorter Homologues. J Am Chem Soc. 2016;138(12):4210–4218. doi: 10.1021/jacs.6b00629. [DOI] [PubMed] [Google Scholar]
- (30).Schmidt BVKJ, Fechler N, Falkenhagen J, Lutz J-F. Controlled Folding of Synthetic Polymer Chains through the Formation of Positionable Covalent Bridges. Nat Chem. 2011;3(3):234–238. doi: 10.1038/nchem.964. [DOI] [PubMed] [Google Scholar]
- (31).Ponader D, Maffre P, Aretz J, Pussak D, Ninnemann NM, Schmidt S, Seeberger PH, Rademacher C, Nienhaus GU, Hartmann L. Carbohydrate-Lectin Recognition of Sequence-Defined Heteromultivalent Glycooligomers. J Am Chem Soc. 2014;136(5):2008–2016. doi: 10.1021/ja411582t. [DOI] [PubMed] [Google Scholar]
- (32).Stubbs C, Lipecki J, Gibson MI. Regio-Regular Alternating Polyampholytes Have Enhanced Biomimetic Ice Recrystallization Activity Compared to Random Copolymers and the Role of Side Chain Verses Main Chain Hydrophobicity. Biomacromolecules. 2017;18(1):295–302. doi: 10.1021/acs.biomac.6b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lawrence J, Lee SH, Abdilla A, Nothling MD, Ren JM, Knight AS, Fleischmann C, Li Y, Abrams AS, Schmidt BVKJ, Hawker MC, et al. A Versatile and Scalable Strategy to Discrete Oligomers. J Am Chem Soc. 2016;138(19):6306–6310. doi: 10.1021/jacs.6b03127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Moad G, Rizzardo E, Thang SH. Radical Addition-Fragmentation Chemistry in Polymer Synthesis. Polymer. 2008:1079–1131. [Google Scholar]
- (35).Congdon T, Notman R, Gibson MI. Antifreeze (Glyco)protein Mimetic Behavior of Poly(vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules. 2013;14(5):1578–1586. doi: 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- (36).Casillo A, Parrilli E, Sannino F, Mitchell DE, Gibson MI, Marino G, Lanzetta R, Parrilli M, Cosconati S, Novellino E, Randazzo A, et al. Structure-Activity Relationship of the Exopolysaccharide from a Psychrophilic Bacterium: A Strategy for Cryoprotection. Carbohydr Polym. 2017;156:364–371. doi: 10.1016/j.carbpol.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Deller RC, Congdon T, Sahid MA, Morgan M, Vatish M, Mitchell DA, Notman R, Gibson MI. Ice Recrystallisation Inhibition by Polyols: Comparison of Molecular and Macromolecular Inhibitors and Role of Hydrophobic Units. Biomater Sci. 2013;1(5):478–485. doi: 10.1039/c3bm00194f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.