Abstract
The 2016 update of the WHO Classification of Tumours of the Central Nervous System has redefined a number of tumors. Embryonal tumor with multilayered rosettes, C19MC-altered is one such tumor entity which has been newly defined on the basis of a characteristic molecular alteration. We report, to our knowledge, the first case of this rare pediatric brain neoplasm in the Pakistani population. An 8-month-old girl was presented with vomiting and left-sided ptosis, and magnetic resonance imaging scan showed a cerebellar tumor. Histologically, a highly cellular population of primitive cells was seen alternating with hypocellular neuropil-rich regions containing multilayered true rosettes and cells with glial and neuronal differentiation. Amplification of 19q13. 42 chromosome region on fluorescence in situ hybridization analysis confirmed the diagnosis. Post-operative radiological examination revealed widespread central nervous system involvement. Adjuvant treatment was not offered due to complications. Patient expired a week after diagnosis.
Keywords: Central nervous system–primitive neuroectodermal tumor, embryonal tumor with multilayered rosettes, multilayered rosettes, C19MC, LIN28A
Introduction
Embryonal tumor with multilayered rosettes (ETMR), C19MC-altered is a recently described tumor entity included in the latest update (revised fourth edition) of WHO Classification of Tumours of the Central Nervous System.1 It encompasses a group of three morphologically distinct embryonal tumors which were described as separate entities in the 2007 fourth edition of the World Health Organization (WHO) blue book.2 These include embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma (EBL) and medulloepithelioma (MEPL). The basis for merging these hitherto separate tumor entities is a unique molecular signature, that is, C19MC locus amplification which is common to these entities. Morphologically, the majority of these tumors share the presence of multilayered rosettes and correspond histologically to WHO grade IV.1 They usually affect children before the age of 4 years and can occur throughout the brain. The behavior is extremely aggressive and the prognosis is dismal.3
We herein report the first case of ETMR from Pakistan with histologic features of ETANTR, characteristic amplification of the C19MC locus and positive LIN28A immunohistochemical (IHC) expression.
Case presentation
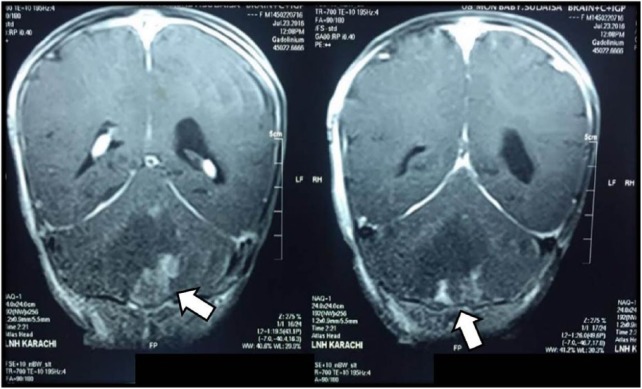
An 8-month-old girl presented in the pediatric clinic with complaints of vomiting and drooping of left eyelid for 3 weeks. On general physical examination, she was found to have bradycardia and hypotension. On neurological examination, ptosis of left eyelid and papilledema were noted. Examination of other systems was normal. Magnetic resonance imaging (MRI) of brain showed an ill-defined hypodense lesion with mild, patchy peripheral enhancement involving the left cerebellar hemisphere. The lesion measured 4.5 × 4 cm2 and was causing near complete obliteration of the fourth ventricle with moderate to severe dilatation of lateral and third ventricles (Figure 1). A ventriculoperitoneal shunt was placed to relieve the raised intracranial pressure.
Figure 1.
An ill-defined hypodense lesion (arrows) with mild peripheral patchy enhancement is involving the cerebellar hemisphere. The lesion is reaching up to the roof of fourth ventricle resulting in its near complete obliteration with moderate to severe dilatation of third and fourth ventricles.
The patient underwent a posterior fossa craniotomy. Intra-operatively, a tan white, soft, moderately vascular, solid tumor was seen at the roof of the fourth ventricle. Gross total resection of the tumor was attempted but the postoperative MRI showed evidence of residual tumor.
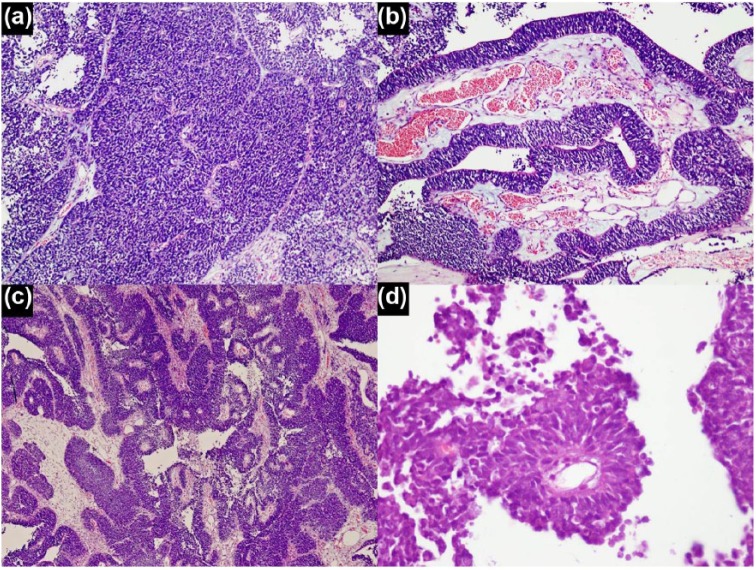
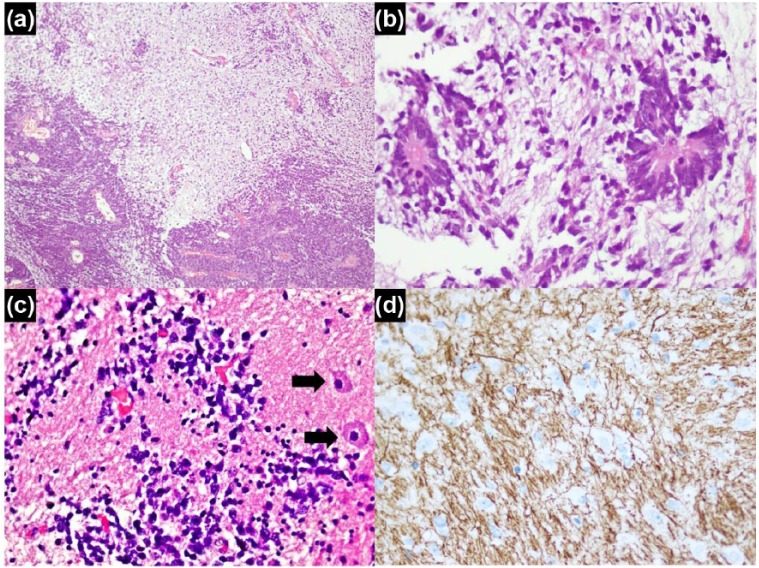
Histologically, the tumor predominantly showed highly cellular areas composed of primitive cells arranged in diffuse sheets, pseudopapillae and broad trabeculae (ribbon-like arrangement). Hypocellular areas showing abundant neuropil containing clusters of primitive cells as well as differentiated cells with glial and neuronal differentiation was also seen. The primitive cells showed increased nuclear to cytoplasmic ratio, scant cytoplasm, and round to oval nuclei with frequent mitotic figures and apoptosis. Necrosis was also seen in the cellular areas. In hypocellular areas with abundant neuropil, true rosettes lined by multiple layers of primitive cells were seen. Some of these rosettes had empty central lumina, while others demonstrated a central core of fibrillary material (Figures 2 and 3). In cellular areas, primitive cells were arranged around vessels to form pseudorosettes. True rosettes were not seen in the cellular areas. The primitive cell population showed diffuse expression for IHC stains vimentin, CD99, INI-1 and p53. Patchy expression was also observed for IHC stains synaptophysin and chromogranin A, while patchy dot-like expression was seen for epithelial membrane antigen (EMA) and neurofilament. Ki-67 (Mib-1) proliferative index was markedly raised (70%–80%) in the cellular areas. Cytokeratins, glial fibrillary acidic protein (GFAP), neuron-specific enolase (NSE) and desmin were negative in the primitive cells. In areas with trabecular arrangement, special stain periodic acid–Schiff (PAS) did not highlight any outer membrane. Neuropil-rich areas showed positive expression for IHC stains GFAP, synaptophysin, NSE and neurofilament. Ki-67 (Mib-1) proliferative index was low (<1%) in these areas. The cells forming true rosettes showed positive expression for Vimentin, CD99 and INI-1 IHC stains and a high Ki-67 (Mib-1) proliferative index (70%–80%). Histological features were characteristic for the entity previously defined as ETANTR. The slides and blocks were sent to The Hospital for Sick Children, Toronto, Ontario, Canada for expert consult and molecular confirmation. Fluorescence in situ hybridization (FISH) analysis demonstrated amplification of 19q13.41 chromosome region. Tumor cells also exhibited diffuse positive expression for IHC stain LIN28A. Hence, the diagnosis of ETMR, C19MC-altered was confirmed.
Figure 2.
Histological patterns of primitive cell population: (a) hypercellualr areas showing primitive cells arranged in sheets, (b) trabecular arrangement, (c) trabeculae, pseudopapillae and neural tube–like structures and (d) perivascular pseudorosettes.
Figure 3.
Neuropil-rich areas: (a) abrupt transition of hypercellualr areas with neuropil-rich areas, (b) multilayered rosettes lined by primitive cells, central area contains neurofibrillary material, (c) few primitive cells and few gangliocytic cells (arrows) against fibrillary background and (d) neurofilament IHC stain in neuropil-rich background.
On the sixth postoperative day, the patient developed paraplegia. MRI scan showed widespread central nervous system (CNS) involvement and drop metastases involving cerebellopontine angle, prepontine region, as well as cervical, thoracic, lumbar and sacral spine. High-dose chemotherapy was suggested initially but due to intermittent respiratory distress, family opted for comfort care only. Patient expired a week after diagnosis.
Discussion
In the 2016 update of the WHO Classification of Tumors of the Central Nervous System, the term “CNS embryonal neoplasm” encompasses a heterogeneous group of highly aggressive, malignant tumors which are composed of primitive cells with distinct morphological and molecular features. ETMR is one of the rarest members of this group. Before the pioneering studies by Korshunov et al.4 were published, the tumors now included in ETMR group were distributed into three entities on the basis of histological features. Clinical features and prognosis of these three tumor entities were very similar. The tumors termed as ETANTR were composed of a primitive cell component arranged in sheets and mature glial and/or neuronal component with easily appreciable background neuropil. Scattered multilayered rosettes were also an integral component of this tumor type.5 Tumors termed EBL were composed of sheets of primitive cells and frequent multilayered (ependymoblastic) rosettes. MEPLs were composed of primitive cells arranged in papillae, tubules and trabeculae with deposition of PAS positive outer membrane at one of the surfaces, resembling primitive neural tube. Multilayered rosettes were also seen in these tumors.6,7 Thus, the presence of primitive cell population and multilayered rosettes were shared by these tumors.8 Li et al. discovered the unique C19MC amplification in a subset of CNS primitive neuroectodermal tumors (PNETs) with aggressive behavior.9 Korshunov et al. performed genetic analysis on 97 such tumors and identified a common genetic alteration, that is, amplification of C19MC locus on chromosome 19 in 93% cases. This alteration which was common and specific to these three tumor entities became the unifying molecular signature for these tumors.3 Based on these findings, EBL and ETANTR are now considered two ends of a histologic spectrum in which EBL represents the undifferentiated and ETANTR the differentiated end.10 The 2016 WHO CNS update has redefined and regrouped a significant number of CNS neoplasms by incorporating their molecular characteristics. For ETMR, presence of C19MC amplification is mandatory for diagnosis, and diagnosis can be made in the presence of this amplification even if multilayered rosettes are not seen. In those cases in which molecular testing cannot be performed, the presence of multilayered rosettes is mandatory and such cases should be diagnosed as embryonal tumors with multilayered rosettes, not otherwise specified (NOS).1
Apart from a few large studies, most of the published literature about this entity comprise small case series and case reports.3,8,11–13 The majority of these tumors occur in the first 2 years of life. They can involve any part of the CNS but over two-thirds occur in the supratentorial region, that is, in the cerebral hemispheres. The presenting symptoms depend on the areas of the brain affected by the tumor. However, hydrocephalus is the most common symptom.11
Other embryonal neoplasms of CNS are included in the differential diagnosis of these tumors as sheets of primitive neoplastic cells and rosettes (unilayered) are also seen in other embryonal tumors. Presence of multilayered rosettes is a histologic feature of utmost diagnostic utility as it is specific to tumors belonging to the ETMR group. Presence of a differentiated neuronal/glial component with abundant neuropil background is an additional helpful feature in cases with ETANTR morphology. However, on limited biopsy material, the sampled tissue might comprise primitive neoplastic population only, and the distinction then relies solely on IHC stains or molecular testing. LIN28A is a sensitive and relatively specific IHC marker for this entity.12,14 These tumors behave very aggressively, and the prognosis is dismal with average survival of 12 months. Local recurrence is frequent and widespread dissemination has also been observed. Treatment modalities include surgery, radiation and high-dose chemotherapy. Gross total resection and radiation are factors associated with a better outcome.1,3,14
Conclusion
ETMR is a highly aggressive CNS embryonal neoplasm with extremely poor prognosis. It should be considered in the differential diagnosis of pediatric brain tumors. On histological examination, multilayered rosettes should be carefully searched for.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Korshunov A, McLendon RE, Judkins AR, et al. Embryonal tumour with multilayered rosettes, C19MC-altered. In: Louis DN, Ohgaki H, Wiestler OD, et al. (eds) WHO classification of tumours of the central nervous system. 4th ed Lyon: IARC Press, 2016, pp. 201–205. [Google Scholar]
- 2. McLendon RE, Judkins AR, Eberhart CG, et al. CNS primitive neuroectodermal tumours (PNETs). In: Louis DN, Ohgaki H, Wiestler OD, et al. (eds) WHO classification of tumours of the central nervous system. 4th ed Lyon: IARC Press, 2007, pp. 141–146. [Google Scholar]
- 3. Korshunov A, Sturm D, Ryzhova M, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol 2014; 128(2): 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korshunov A, Remke M, Gessi M, et al. Focal genomic amplification at 19q13.42 comprises a powerful diagnostic marker for embryonal tumors with ependymoblastic rosettes. Acta Neuropathol 2010; 120(2): 253–260. [DOI] [PubMed] [Google Scholar]
- 5. Eberhart CG, Brat DJ, Cohen KJ, et al. Pediatric neuroblastic brain tumors containing abundant neuropil and true rosettes. Pediatr Dev Pathol 2000; 3(4): 346–352. [DOI] [PubMed] [Google Scholar]
- 6. Judkins AR, Ellison DW. Ependymoblastoma: dear, damned, distracting diagnosis, farewell!*. Brain Pathol 2010; 20(1): 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buccoliero AM, Castiglione F, Innocenti DR, et al. Embryonal tumor with abundant neuropil and true rosettes: morphological, immunohistochemical, ultrastructural and molecular study of a case showing features of medulloepithelioma and areas of mesenchymal and epithelial differentiation. Neuropathology 2010; 30(1): 84–91. [DOI] [PubMed] [Google Scholar]
- 8. Nobusawa S, Orimo K, Horiguchi K, et al. Embryonal tumor with abundant neuropil and true rosettes with only one structure suggestive of an ependymoblastic rosette. Pathol Int 2014; 64(9): 472–477. [DOI] [PubMed] [Google Scholar]
- 9. Li M, Lee KF, Lu Y, et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitiveneuroectodermal brain tumors. Cancer Cell 2009; 16(6): 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wesseling P. Embryonal tumor with multilayered rosettes (ETMR): signed, sealed, delivered …. Acta Neuropathol 2014; 128(2): 305–308. [DOI] [PubMed] [Google Scholar]
- 11. Horwitz M, Dufour C, Leblond P, et al. Embryonal tumors with multilayered rosettes in children: the SFCE experience. Childs Nerv Syst 2016; 32(2): 299–305. [DOI] [PubMed] [Google Scholar]
- 12. Edmonson CA, Weaver KJ, Kresak J, et al. Embryonal tumor with multilayered rosettes of the fourth ventricle: case report. J Neurosurg Pediatr 2015; 7: 1–5. [DOI] [PubMed] [Google Scholar]
- 13. Ceccom J, Bourdeaut F, Loukh N, et al. Embryonal tumor with multilayered rosettes: diagnostic tools update and review of the literature. Clin Neuropathol 2014; 33(1): 15–22. [DOI] [PubMed] [Google Scholar]
- 14. Korshunov A, Ryzhova M, Jones DT, et al. LIN28A immunoreactivity is a potent diagnostic marker of embryonal tumor with multilayered rosettes (ETMR). Acta Neuropathol 2012; 124(6): 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]