Abstract
The blood–brain barrier (BBB) regulates differing needs of the various brain regions by controlling transport of blood-borne components from the neurovascular circulation into the brain parenchyma. The mechanisms underlying region-specific transport across the BBB are not completely understood. Previous work showed that pericytes are key regulators of BBB function. Here we investigated whether pericytes influence BBB permeability in a region-specific manner by analysing the regional permeability of the BBB in the pdgf-bret/ret mouse model of pericyte depletion. We show that BBB permeability is heterogeneous in pdgf-bret/ret mice, being significantly higher in the cortex, striatum and hippocampus compared to the interbrain and midbrain. However, we show that this regional heterogeneity in BBB permeability is not explained by local differences in pericyte coverage. Region-specific differences in permeability were not associated with disruption of tight junctions but may result from changes in transcytosis across brain endothelial cells. Our data show that certain brain regions are able to maintain low BBB permeability despite substantial pericyte loss and suggest that additional, locally-acting mechanisms may contribute to control of transport.
Keywords: Blood–brain barrier, neurovascular unit, pericytes, extravasation, permeability
Introduction
The vascular network of the central nervous system (CNS) is responsible for providing an optimal environment within the brain parenchymal space. In addition to its role in nutrient supply, the CNS vasculature is a key component of the blood–brain barrier (BBB). The BBB is a multi-cellular structure formed by astrocytes, pericytes, neurons and endothelial cells and is characterized by a low vascular permeability which prevents most blood-borne molecules from entering the brain parenchyma.1,2 The low permeability of the BBB is achieved by the presence of endothelial tight intercellular junctions and a low transcellular diffusion and transcytosis rate.3,4 Interestingly, multiple studies have shown that BBB permeability for specific molecules is heterogeneous throughout the brain.5–8 For example, in rats, brain uptake of insulin is higher in the hippocampus than in the cortex.7 These changes in insulin uptake could not be attributed to differences in insulin receptor expression levels. Additionally, specialized brain regions such as the circumventricular organs9 or the sub-ependymal zone10 also have higher permeability than the rest of the brain, likely due to changes in vesicular transport or tight junction architecture. The signals that regulate such heterogeneous permeability of the BBB during physiological conditions are currently unknown.
The permeability of the BBB can be compromised in a brain region-dependent manner during aging or in pathophysiological conditions. In aging, magnetic resonance imaging (MRI) analysis in humans revealed that the BBB is compromised initially in the hippocampus.11 In Alzheimer's disease, BBB permeability is increased in both the hippocampus and the frontal cortex,12 whereas in Fahr's disease, brain calcification in the basal ganglia is linked to BBB disruption.13 In all these conditions, changes in permeability have been associated with pericyte malfunction and reduced pericyte coverage. These results, together with extensive work with mouse models of pericyte depletion,14–16 suggested that pericytes play an important role in controlling BBB permeability. Therefore, we addressed the question whether pericytes are the underlying cause for brain region-specific differences in BBB permeability by using homozygous mice with a mutation in the retention motif of pdgf-β (pdgf-bret/ret). These mice are a well characterized model of pericyte depletion, with at least an 80% reduction in pericyte coverage of the brain vasculature network.15 By using three independent and complementary techniques, including ex vivo Evans Blue brain distribution and in vivo magnetic resonance imaging (MRI) as well as immunohistochemistry to study endogenous components, we demonstrated that the permeability of the BBB is significantly increased in the cortex, striatum and hippocampus compared to the interbrain and midbrain of pdgf-bret/ret mice. In this mouse model, increased BBB permeability did not correlate with the extent of pericyte loss in the different brain regions. Our finding that the regional heterogeneity of BBB permeability persists despite the substantial reduction in pericyte coverage suggests that additional, locally-acting mechanisms regulate transport across the BBB.
Materials and methods
Mice
pdgf-bret/ret mice used for characterizing BBB permeability of different brain regions were previously described.15,17 We used mice between 11 and 19 months old to measure brain-region specific changes in BBB permeability. Importantly, the pattern of extravasation and/or accumulation was similar between 12 and 19 months old mice (Supplementary Figure 1); 6 and 22 months old mice were only used for biochemical experiments. TauPS2APP (3Tg) × pdgf-bret/ret mice used for confirming the localization of endogenous immunoglobulins in the brain parenchyma were previously described.18 Ethical approval for this study was provided by the Federal Food Safety and Veterinary Office of Switzerland. All animal experiments were conducted in strict adherence to the Swiss federal ordinance on animal protection and welfare as well as according to the rules of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), and with the explicit approval of the local veterinary authorities (License BS2763 and BS1902). All experiments are reported in compliance with the ARRIVE guidelines.
Brain preparation and immunofluorescence
Brain processing was performed as previously described.18 Briefly, 12–19 months-old pdgf-bret/wt or pdgf-bret/ret mice were euthanized with CO2 and transcardially perfused with PBS at 37℃. Brain protein extracts were prepared by homogenizing the tissue in Krebs-bicarbonate buffer. Homogenates were then incubated overnight at 4℃ with RIPA lysis buffer; 5 µg of protein were loaded in a 10% Bis-Tris gel to perform standard SDS-PAGE. mIgG was detected using a goat polyclonal anti-mouse IgG coupled to horse-radish peroxidase (Perkin Elmer). Actin was detected using a mouse monoclonal anti β-actin antibody (clone AC-15, Abcam) and was used as a loading control. To prepare brain sections for immunofluorescence, PBS perfusion was followed by perfusion with 2% paraformaldehyde (PFA). The brain was then removed and incubated overnight in 2% PFA at 4℃ before sectioning. Brains were included in 2% agarose and 100-µm sagittal sections were cut using a Leica VT1000M vibratome. Sections were stored at −20℃ in 1:1 PBS/Glycerol. For quantification of endogenous mIgG in whole brain sections, the brain was fresh-frozen and 10-µm sagittal sections were prepared using a Leica CM3050S cryostat and mounted on HistoBond glass slides. Floating sections were processed for immunofluorescence by washing with PBS and permeabilization with PBS 0.3% Triton X-100 and 10% donkey serum for blocking. Primary antibodies were diluted in 5% donkey serum in PBS and incubated with sections for 72 h at 4℃, followed by washing with PBS and 1 h incubation at room temperature with appropriate fluorescently-labelled secondary antibodies (Donkey anti-goat, donkey anti-rabbit, or donkey anti-rat IgG coupled to AlexaFluor488, 555, or 647, from LifeTechnologies) in 5% donkey serum in PBS. Frozen sections were rehydrated in PBS, fixed with 100% acetone, blocked with 1% Bovine Serum Albumin/Ovalbumine/Normal Goat Serum in PBS and incubated with an appropriate fluorescently-labelled antibody. In both cases, sections were washed with PBS, stained with 1 µg/ml DAPI and mounted using DAKO Fluorescent Mounting medium on glass slides with a 0.17 mm coverslip. Table 1 shows the list of antibodies used for the study.
Table 1.
List of validated antibodies for immunofluorescence staining of brain sections.
| Cell type or antigen | Antibody manufacturer |
|---|---|
| Pericytes (CD13) | Goat polyclonal, R&D Systems |
| Tight junctions (ZO-1) | Rabbit polyclonal, Life Technologies |
| Basal lamina (CollagenIV) | Rabbit polyclonal, serotec Goat polyclonal, BioRad |
| mIgG | Donkey anti-mouse IgG (H+L) AlexaFluor594, Life Technologies |
| Pan-Neuronal marker | Rabbit Neuro-Chrom Pan Neuronal Marker AlexaFluor488, Millipore |
| Microglia (Iba-1) | Rabbit polyclonal, Wako |
| Astrocytes (GFAP) | Rabbit polyclonal, DAKO |
| Tbr1 | Rabbit polyclonal, Abcam |
| Ctip2 | Rat monoclonal 25B6, Abcam |
Evans blue injection
A solution of 2% Evans Blue in 0.9% saline solution was injected intravenously at a dose of 6 ml/kg into the tail vein of pdgf-bret/wt or pdgf-bret/ret mice. Three hours after injection, mice were euthanized with pentobarbital and perfused with saline solution followed by 4% PFA. The brain was then removed and incubated overnight in 4% PFA at 4℃ before sectioning as described above.
Microscopy
Imaging of whole-brain frozen sections was performed using a Metafer 4 slide scanning system (MetaSystems) with a 10X/0.45 NA objective and sequential exposure using appropriate filters for red and blue emission. Individual frames were stitched together using the default parameters of the Metafer software to generate mosaic images. High-resolution confocal imaging was performed using a Leica TCS SP8 microscope with a HC PL APO 40×/1.3 oil objective. Images were 12-bit with 1024 × 1024 pixels. Imaging of Evans Blue in brain sections was performed using a Leica TCS SP8 microscope with a HC PL Fluotar 10X/0.3 NA objective. Images were 12-bit with 512 × 512 pixels. Evans Blue was excited at 555 nm and detected at 670–795 nm. In all cases, illumination intensity and detector gain settings were optimized to minimize pixel saturation and maximize dynamic range.
Image quantification
To estimate the distribution of Evans Blue and mIgG in different brain regions, brain sections were manually segmented using the DAPI-stained nuclei signal as a reference. The mean fluorescence intensity of Evans Blue and mIgG was calculated using FIJI. Brain slices from four to five different mice per genotype were analysed.
Pericyte coverage was estimated as previously described.12 Briefly, an absolute intensity threshold mask was first applied to the CollagenIV signal to estimate the area (Col) of the image covered by capillaries. Next a conditional CollagenIV AND CD13 intensity threshold mask was applied to estimate the area (CD) of the image covered by pericytes on capillaries. Pericyte coverage was estimated from the ratio CD/Col. Values were calculated from five randomly selected images from individual brain sections each covering a volume of 280 × 280 × 18 µm3 for three mice per genotype. For visual inspection, 3D reconstruction and background subtraction were performed using Imaris. The quantification of mIgG-positive intracellular vesicles, accumulation at the basement membrane and brain parenchyma was performed as previously described.18
The fraction of cells accumulating mIgG corresponding to neurons, microglia and astrocytes was estimated by immunolabeling these cell types using a pan-neuronal marker (Neuro-Chrom, Millipore), Iba1 and GFAP, respectively, and dividing them by the total number of mIgG-positive cells. Cells were counted manually by using mosaic images of individual sections from three different pdgf-bret/ret mice.
In vivo magnetic resonance imaging
In vivo magnetic resonance imaging of the brain was performed under shallow isoflurane (Abbott, Baar, Switzerland) anaesthesia, which was delivered to the animals via a face mask in a carrier gas composed of oxygen and air (1:5 v/v). Anaesthetised animals were placed in the MRI scanner and secured in a stereotaxic head frame. Respiratory rate, body temperature, and inspired/exhaled oxygen and CO2 levels were continuously monitored using a PowerLab data acquisition system (ADInstruments, Spechbach, Germany), as previously described.19 Body temperature was maintained at 37℃ by a feedback-regulated electric heating blanket. For permeability measurements of the BBB, 0.5 mmol/kg of the MRI contrast agent Gd-DTPA (Bayer, Leverkusen, Germany) was administered 150 min prior to the MRI investigation into a lateral tail vein of the awake animal.
Magnetic resonance imaging was carried out on a Bruker Biospec 94/20 MR system (Bruker Biospin, Ettlingen, Germany) equipped with a volume resonator for signal excitation and a head coil for signal reception. Images were acquired from 16 contiguous coronal planes with a field of view of 20 × 20 mm2, and a slice thickness of 0.6 mm. Quantitative T1-maps were obtained using an inversion-recovery snapshot FLASH sequence with eight inversion times (TR/TE=4000/1.6 ms, matrix 128 × 64, 12 averages).20 Cerebral perfusion was assessed by continuous arterial spin-labelling (CASL)21 with single-slice centred-RARE readout (TR/TE = 3,750 ms/5.4 ms, RARE factor = 32, matrix 128 × 64, labelling pulse 3 s, post labelling delay 0.4 s, 2 averages).
Images of individual animals were co-registered with SPM5 (Welcome trust Centre for Neuroimaging, London, UK) to an in-house mouse brain template and overlaid with a digital atlas delineating regions of interest. Tissue relaxivity and cerebral perfusion were calculated voxel-wise based on the series of T1-weighted images and CASL images, respectively.19 A relaxivity of 4.1 L×mmol−1s−1 was assumed for Gd-DTPA to allow conversion of the change in tissue relaxivity into local Gd-DTPA concentration.
Statistics
For mIgG, Evans Blue, capillary diameter and pericyte coverage measurements, the data were analyzed using a repeated measures two-way ANOVA (genotype × brain region) followed by pair-wise comparisons between brain regions of pdgf-bret/ret using Fisher's LSD test with n = 5 different mice per genotype for IgG, n = 4 mice per genotype for Evans Blue and n = 3 for pericyte coverage. For MRI experiments of Gd-DTPA accumulation, two separate cohorts of n = 4 + 10 pdgf-bret/ret and 7 + 10 pdgf-bret/wt mice were analyzed brain region-wise using two-way ANOVAs (genotype x study) followed by Welch's t-test. Blood perfusion measurements in n = 8 pdgf-bret/ret mice and 8 pdgf-bret/wt mice were analyzed with repeated measures two-way ANOVAs followed by pair-wise comparisons with Welch's t-test corrected for a false discovery rate of 0.15. IgG vesicle number and intensity in the brain parenchyma and basal lamina were analysed using repeated measures two-way ANOVAs (genotype × brain region) followed by Fisher's LSD test with n = 30 capillary sections from three different mice. The specific values from the multiple statistical comparisons are included in Appendix in Supplementary Material.
Results
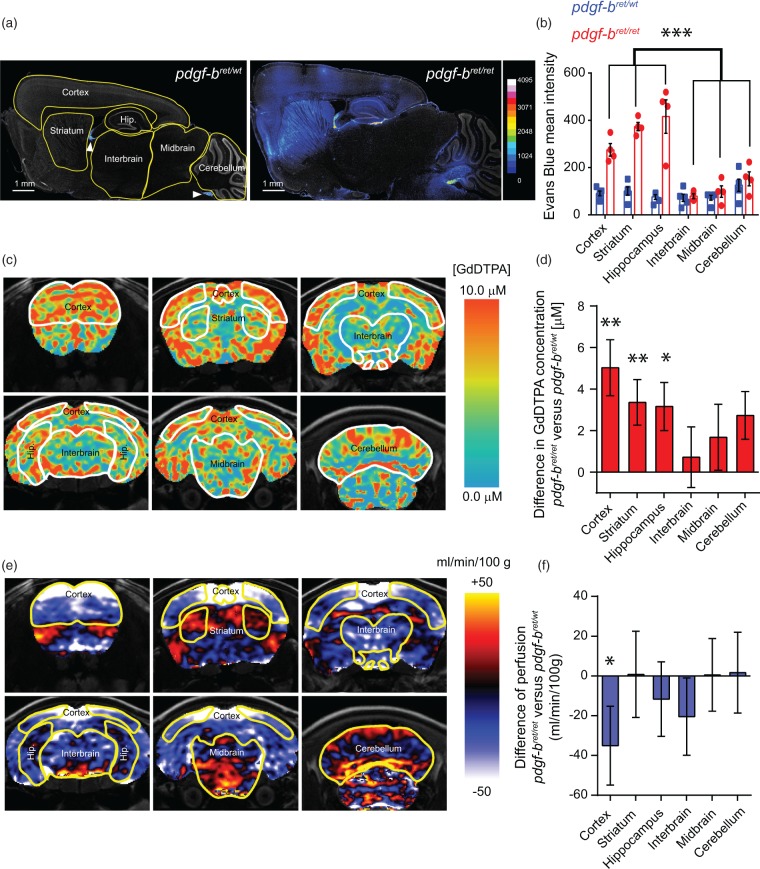
To evaluate the permeability of the BBB in different brain regions upon pericyte loss, we injected Evans Blue to pdgf-bret/wt and pdgf-bret/ret mice and analysed its distribution 3 hours post-injection using a recently described fluorescence microscopy-based protocol.22 With this method, we quantified the mean fluorescence intensity of Evans Blue in different brain regions (cortex, striatum, hippocampus, interbrain, midbrain and cerebellum) (Figure 1(a)). In control mice, the fluorescence intensity of Evans Blue was generally below the detection limit in the brain parenchyma except for small regions including the meninges, ventricles and the choroid plexus (Figure 1(a)). On the other hand, in pdgf-bret/ret mice, there was a significant extravasation of Evans Blue in the cortex, striatum and hippocampus but not in the interbrain, midbrain or cerebellum, which exhibited intensity values similar to control mice (Figure 1(a) and (b)). These results suggest that the permeability of the BBB is heterogenous throughout the brain of pdgf-bret/ret mice.
Figure 1.
Regional heterogeneity of blood–brain barrier permeability upon pericyte depletion. (a) Representative mosaic images of increased Evans Blue accumulation in pdgf-bret/wt and pdgf-bret/ret mice. The segmentation of brain regions used for subsequent quantification is shown in yellow. Evans Blue fluorescent signal is displayed with a 16-color scheme using the full dynamic range of the 12-bit images. DAPI-stained nuclei are shown in grey. Arrowheads point to the choroid plexus and meninges. (b) Quantification of Evans Blue mean intensity from whole-brain sections of pdgf-bret/wt (blue) or pdgf-bret/ret (red) mice as shown in (a). Points show individual sections from 5 pdgf-bret/wt and 4 pdgf-bret/ret mice, bars show the mean and error bars show S.E.M. ***, p < 0.0001 by repeated measures two-way ANOVA and pair-wise comparisons between different brain regions of pdgf-bret/ret mice with Fisher's LSD test. (c) MRI group images of selected coronal sections displaying the mean difference of Gd-DTPA accumulation in the brains of pdgf-bret/ret mice versus pdgf-bret/wt mice as a proxy for BBB extravasation. Gd-DTPA accumulation was evaluated in vivo based on changes in tissue relaxivity as measured by non-invasive MRI and is color-coded according to the scale shown to the right. (d) Quantification of the mean difference of Gd-DTPA accumulation in selected brain regions of pdgf-bret/ret mice versus pdgf-bret/wt mice. Bars show the mean value estimated from 14 pdgf-bret/ret mice and 17 pdgf-bret/wt mice. Error bars show SEM. **p < 0.009, *p < 0.05 from ANOVA and Welch's t-test. (e) Selected coronal sections displaying the mean difference in cerebral blood perfusion between 12 month old pdgf-bret/ret mice and pdgf-bret/wt mice. The values were estimated from perfusion MRI and are displayed using the color scheme shown to the right. (f) Quantification of the mean difference in cerebral blood perfusion in pdgf-bret/ret versus pdgf-bret/wt in the same brain regions measured in (c). Bars show the mean value estimated from 8 pdgf-bret/ret mice and 8 pdgf-bret/wt mice. Error bars show SEM. *p = 0.05 from ANOVA (corrected for a FDR = 0.15) and Welch's t-test.
Next, we sought to confirm the region-specific differences in BBB permeability in living pdgf-bret/ret mice using magnetic resonance imaging (MRI), a technique with demonstrated translational potential.11 MRI was performed upon intravascular administration of the contrast agent Gd-DTPA. Extravasation of Gd-DTPA into the parenchyma leads to an increase in tissue relaxivity which is proportional to the accumulation of the contrast agent and can be quantified by T1 MRI. Figure 1(c) shows grouped images that represent the mean difference of Gd-DTPA concentration throughout the brain. In agreement with the Evans Blue measurements, we found accumulation of Gd-DTPA preferentially in the cortex, striatum and hippocampus, whereas lower levels were detected in the interbrain and midbrain (Figure 1(c) and (d)). In the cerebellum, we detected an increase of Gd-DTPA due to a region-dependent signal with higher levels in the dorsal region and lower levels in the ventral region (Figure 1(c)).
The extent of accumulation and/or extravasation of Gd-DTPA in the brain parenchyma reflect the intrinsic permeability of the BBB but may also depend on regional blood flow. To exclude that the differences of Gd-DTPA accumulation arise from changes in blood flow, we measured the regional vascular irrigation of the brain parenchyma by quantitative perfusion MRI. We observed a significantly lower blood perfusion in the cortex of pdgf-bret/ret mice, in agreement with the proposed role of pericytes in the regulation of blood flow in this brain region.23 In contrast, we observed no major changes in the striatum, hippocampus, interbrain, midbrain or cerebellum (Figure 1(e) and (f)). This result suggests that the differences in Gd-DTPA distribution in the brain parenchyma of pdgf-bret/ret mice are not directly caused by changes in blood flow but instead reflect the altered intrinsic permeability of the BBB of different brain regions. The measurements on the accumulation of Gd-DTPA in the brain parenchyma confirm the differential distribution of Evans Blue in the brains of pdgf-bret/ret mice and provide further support to the use of MRI for the assessment of local changes in BBB permeability.11 Together, these results show that BBB permeability is not homogeneous upon pericyte loss but exhibits differences in specific brain regions.
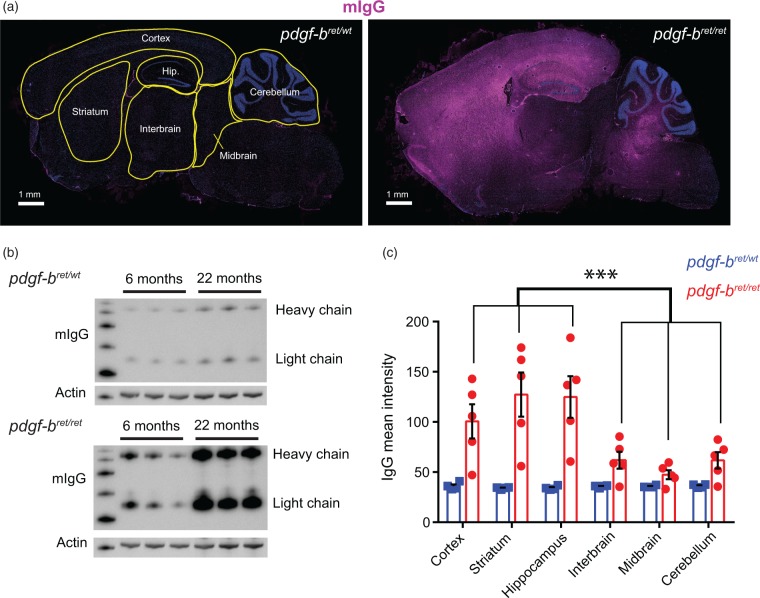
To further confirm the heterogeneous permeability of the BBB in pdgf-bret/ret mice, we analysed the accumulation of endogenous mouse immunoglobulin G (mIgG) throughout the brain. In agreement with previous results,16 mIgG accumulated in the brain of pdgf-bret/ret mice in an age-dependent manner but not in control mice (Figure 2(b)). To investigate the spatial distribution of mIgG accumulation, we performed immunohistochemistry using a secondary antibody specific against mIgG and imaged whole-brain sections of pdgf-bret/wt and pdgf-bret/ret mice. We quantified the mean fluorescence intensity corresponding to mIgG in the cortex, striatum, hippocampus, interbrain, midbrain and cerebellum (Figure 2(a) and (c)). In pdgf-bret/ret mice, we detected a significant accumulation of endogenous mIgG specifically in the cortex, striatum and hippocampus. In agreement with our measurements of Evans Blue extravasation, the fluorescence intensity of mIgG in the interbrain, midbrain, and cerebellum was significantly lower compared to the cortex, striatum and hippocampus (Figure 2(a) and (c)). Altogether, these data show that the permeability of the BBB to an endogenous large molecule is increased in the cortex, hippocampus and striatum upon pericyte loss.
Figure 2.
Regional differences in the accumulation of endogenous mouse immunoglobulins upon pericyte depletion. (a) Representative mosaic images of whole-brain sections of 12 month old pdgf-bret/wt or pdgf-bret/ret mice. The segmentation of brain regions used for subsequent quantification is shown in yellow. Endogenous mIgG is shown in magenta, DAPI-stained nuclei are shown in blue. Scale bar, 1 mm. (b) Western Blot against mouse IgG of whole-brain lysates from pdgf-bret/wt (upper panel) or pdgf-bret/ret (lower panel) mice at 6 or 22 months of age. Actin is shown as a loading control. (c) Quantification of IgG mean intensity from whole-brain sections of 12 month old pdgf-bret/wt (blue) or pdgf-bret/ret (red) mice as shown in (a). Points show individual sections from 5 mice, bars show the mean and error bars show S.E.M. ***, p < 0.0001 by repeated measures two-way ANOVA and pairwise comparisons between different brain regions of pdgf-bret/ret mice using Fisher's LSD test.
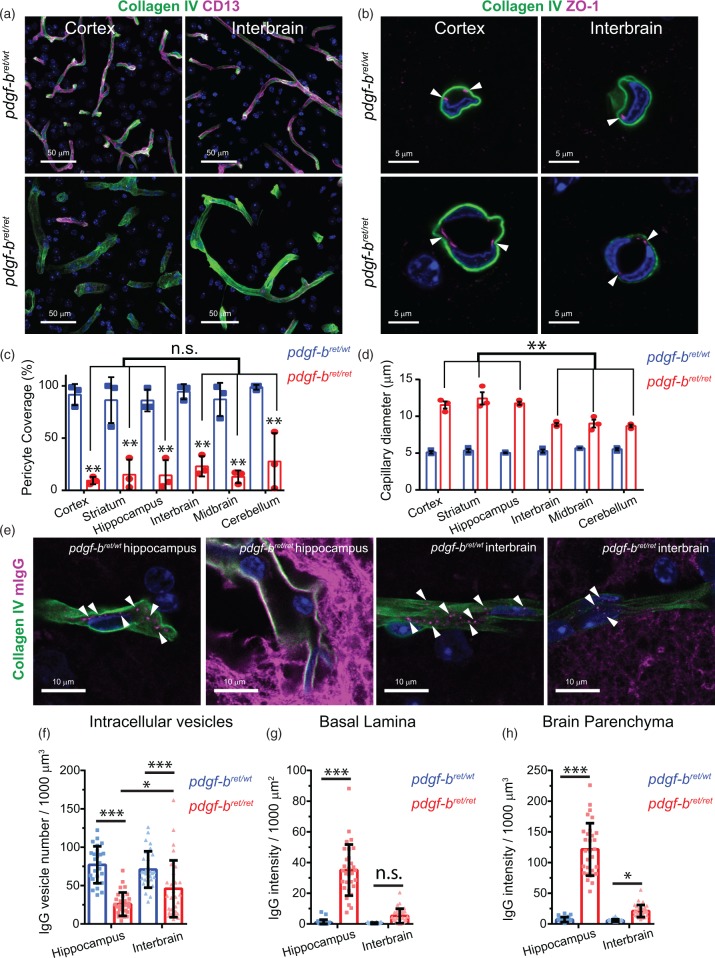
Previous studies found differences in residual pericyte coverage of capillaries between cortex, striatum and cerebellum in pdgf-bret/ret mice.15,24 Therefore, the brain-region specific differences in BBB permeability of pdgf-bret/ret mice may only reflect local differences in pericyte coverage. To address this possibility, we used high resolution microscopy to quantify the pericyte coverage of capillaries by measuring the area of CollagenIV colocalizing with the pericyte marker CD13 (Figure 3(a) and (c)). This method was previously used to investigate differences in pericyte coverage between brain regions in mice25 or in Alzheimer's disease brain samples.12 For each mouse, we measured a total volume of 7 × 106 µm3 of brain tissue. We found that pericyte coverage in control pdgf-bret/wt mice was homogenous throughout the brain with an average value of 90%. In pdgf-bret/ret mice, pericyte coverage was reduced to 20% throughout the brain (Figure 3(c)). These values are in agreement with previous results,15,25 thus validating our quantifications. The small differences between brain regions (∼10% for the cortex, ∼15% for striatum, hippocampus and midbrain, and ∼25% for the interbrain and cerebellum), were not significant (p > 0.19) (Figure 3(c)). Pericyte coverage in the cerebellum was highly variable, with one mouse showing coverage over 50%. Previous work showed that BBB permeability was not affected by a 50% reduction in pericyte coverage.15 This is in agreement with the lack of mIgG accumulation in the cerebellum as the pericyte coverage may still be above a critical threshold.26 On the other hand, extravasation and/or accumulation of Evans Blue, Gd-DTPA and mIgG in pdgf-bret/ret mice were significantly lower in the interbrain and midbrain compared to the cortex, striatum and hippocampus even though all areas had similar reduction in pericyte coverage (Figure 3(c)). Therefore, these data suggest that local differences in pericyte coverage alone cannot explain the heterogeneity of the BBB permeability in pdgf-bret/ret mice.
Figure 3.
Changes to the neurovascular unit upon pericyte loss. (a) Representative images of capillaries (marked by CollagenIV, green) covered by pericytes (marked by CD13, magenta) in the cortex and interbrain of 19 months old pdgf-bret/wt (upper panels) or pdgf-bret/ret (lower panels) mice. The images show a 3D reconstruction of the imaged tissue volume after background subtraction using a Collagen IV intensity mask. Scale bar, 50 µm. (b) Representative cross-sections of capillaries marked by Collagen IV (green) and the tight junctional marker ZO-1 (magenta) in the cortex and interbrain of 19 months old pdgf-bret/wt (upper panels) or pdgf-bret/ret (lower panels) mice. Arrowheads point to the localization of tight junctions in these capillaries. Scale bar = 5 µm. (c) Estimation of pericyte coverage in different brain regions quantified as the percentage of the total CollagenIV area covered by CD13. Points show mean coverage values for individual mice, bars show the mean and error bars show S.E.M. for three mice. **p < 0.0001 by repeated measures two-way ANOVA and pairwise comparison between the same brain region in pdgf-bret/wt or pdgf-bret/ret mice using Fisher's LSD test. (d) Measurement of capillary cross-section diameter in different brain regions. Points show mean capillary diameter values for individual mice, bars show the mean and error bars show S.E.M. for three mice. **p < 0.0001 by repeated measures two-way ANOVA and pairwise comparison between different brain regions of pdgf-bret/ret mice using Fisher's LSD test. (e) Representative high-resolution single optical sections of the hippocampus and interbrain of pdgf-bret/wt and pdgf-bret/ret mice showing a single capillary marked with collagenIV (green) and the distribution of mIgG (magenta) within intracellular vesicles, the basal lamina or the brain parenchyma. Arrowheads point to individual intracellular vesicles within capillaries. Scale bar = 10 µm. In all images, nuclei are labelled with DAPI (blue). (f–h) Quantification of the number of mIgG-positive intracellular vesicles (f) and the total mIgG intensity in the basal lamina (g) and the brain parenchyma (h). Points represent individual capillaries. Bars show the mean and error bars the S.D. of 30 individual capillaries from three different mice. *p < 0.05; **p < 0.001; ***p < 0.0001 by Fisher's LSD test.
Next, we investigated the mechanisms that could contribute to the brain region-specific extravasation of blood-borne components in the brain parenchyma. We and others previously showed by electron microscopy that tight junctions of cortical capillaries are still present in pdgf-bret/ret mice.15,18 We confirmed these previous observations by examining the junctional marker ZO-1 using confocal microscopy. We found that the localization of ZO-1 was similar in capillaries of the cortex and of the interbrain of both pdgf-bret/wt and pdgf-bret/ret mice (Figure 3(b)). These data suggest that the heterogenous BBB permeability in pdgf-bret/ret mice is not due to defects in tight junction formation.15,18 We also found that the diameter of the capillaries was significantly increased in all brain regions of pdgf-bret/ret mice, but more pronounced in the cortex, striatum and hippocampus (Figure 3(d)).
Next we assessed whether pericyte loss differently impacts mIgG trancytosis across the BBB in a region-specific manner. We previously showed that pericyte loss increases the transcytosis of mIgG in brain endothelial cells resulting in (i) a decrease in the number of mIgG-positive intracellular vesicles within brain endothelial cells (BECs), (ii) an accumulation of mIgG within the basal lamina of capillaries and, (iii) an accumulation of mIgG into the brain parenchymal space of the cortex.18 Therefore, we measured these parameters in the hippocampus and the interbrain of pdgf-bret/wt and pdgf-bret/ret mice to investigate whether changes in mIgG transcytosis exist in different brain regions. We found that the number of mIgG-positive intracellular vesicular structures was significantly reduced in the hippocampus and in the interbrain of pdgf-bret/ret mice compared to pdgf-bret/wt (Figure 3(e) and (f)). However, the reduction in the total number of vesicles was more pronounced in the hippocampus (66% reduction) compared to the interbrain (33% reduction). In addition, the total number of intracellular vesicular structures within BECs in pdgf-bret/ret mice was significantly lower in the hippocampus compared to the interbrain (*, p = 0.003). In the basal lamina and the brain parenchyma of pdgf-bret/ret mice, the mIgG intensity was increased in both the hippocampus and the interbrain compared to pdgf-bret/wt mice but showed region-specific differences. In the basal lamina, we found a significant increase in mIgG intensity only in the hippocampus. In the brain parenchyma, we found a 17-fold increase in the hippocampus, whereas we found a 3-fold increase in the interbain. Altogether, these data suggest that the transcytosis of mIgG in the hippocampus is significantly enhanced compared to the interbrain in pdgf-bret/ret mice. These data suggest that the heterogeneous permeability of the BBB upon pericyte loss may arise from local differences in the regulation of intracellular transport.
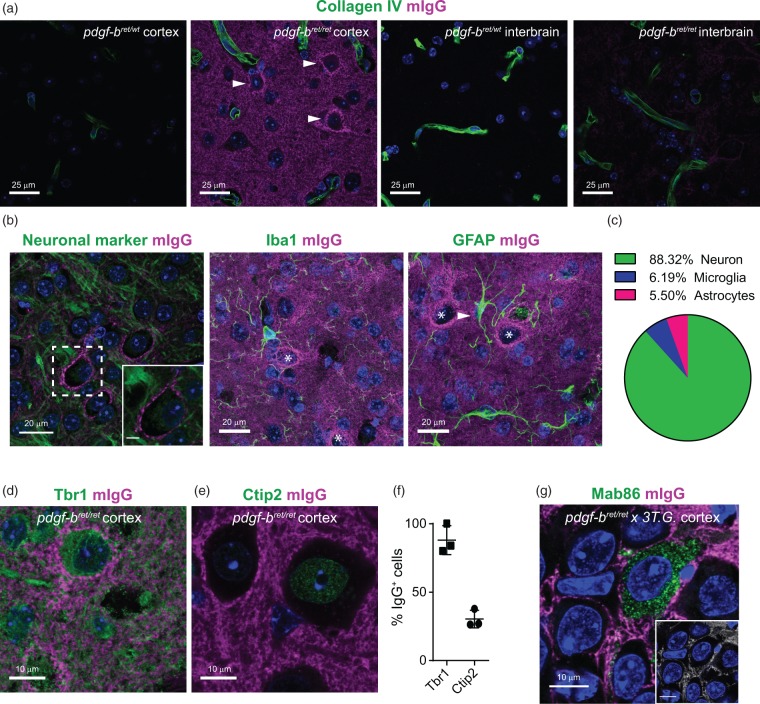
In the brain regions with permeable BBB, endogenous mIgG were distributed in the extracellular space and did not accumulate within cells. However, we found that some mIgG specifically distributed around the soma of cells in the cortex (Figure 4(a)). The majority of these mIgG-positive cells were neurons (88.32%), whereas only a small percentage correspond to microglia (6.19%) and astrocytes (5.5%) (Figure 4(b) and (c)). The accumulation of mIgG around neurons was widespread, since we estimated that ∼16% of cortical neurons were mIgG-positive. No neurons in the interbrain region of pdgf-bret/ret mice showed such mIgG distribution (Figure 4(a)). Interestingly, the distribution of mIgG around cortical neurons was selective, as nearly 100% of mIgG-positive cells expressed the cortical marker Tbr1, whereas only 30% expressed the deep-layer cortical marker Ctip2.27 (Figure 4(d) to (f)). Such selective accumulation at the plasma membrane of specific cortical neurons suggests that a fraction of mIgG present in the brain parenchyma of pdgf-bret/ret mice could be auto-antibodies against neuronal proteins. To confirm that mIgG were restricted to the extracellular space, we injected the 3Tg × pdgf-bret/ret mice17 with a monoclonal IgG, Mab86, known to be internalized specifically by neurons with tau pathology in the hippocampus.28 In these mice, we found that mIgG did not colocalize intracellularly with Mab86 in neurons (Figure 4(g)). This observation suggests that mIgG are not internalized by neurons but instead distribute in the parenchymal space and accumulate at the plasma membrane of some cortical neurons.
Figure 4.
Accumulation of endogenous mouse immunoglobulins in a specific subpopulation of cortical neurons. (a) Representative images of the cortex or interbrain of 19 months old pdgf-bret/wt or pdgf-bret/ret mice. Capillaries are marked by CollagenIV in green and mIgG in magenta. Arrowheads point to the distinct accumulation of mIgG signal in the periphery of a subpopulation of cells in the cortex of pdgf-bret/ret mice. The signal in the interbrain of pdgf-bret/ret mice was below our detection limit with these imaging settings. Scale bar = 25 µm. (b) Representative images of mIgG distribution in the brain parenchyma and in the periphery of a cell positive for a general pan-neuronal marker (Neuro-chrom, green) but not in the periphery of microglia (Iba1, green) or astrocytes (GFAP, green). Scale bar = 20 µm. The inset is a zoom-in to the cell body to highlight the distribution of mIgG at the membrane. Stars indicate cells accumulating mIgG, arrowhead indicates accumulation of mIgG on an astrocytic process. Scale bar = 5 µm. (c) Pie-chart displaying the fractions of cell-types accumulating mIgG on their membranes. (d–e) Representative images of mIgG accumulation in the periphery of Tbr1+ (d) but not in Ctip2+ (e) neurons in the cortex of pdgf-bret/ret mice. In both panels, neuronal markers are shown in green. (f) Quantification of the fraction of cells accumulating mIgG in the periphery which are also positive for Tbr1 or Ctip2. Individual points show the average fraction of 10 areas in individual slices from 3 mice. Error bars show the S.E.M. In all images, DAPI-stained nuclei are shown in blue. (g) Representative image of parenchymal localization of mIgG (magenta) and intracellular localization of the monoclonal antibody Mab86 (green) in the hippocampus of pdgf-bret/ret crossed with the 3TG mouse model of Alzheimer's disease. The inset shows the colocalized pixels between the Mab86 and mIgG channels and highlights the lack of mIgG intracellular localization. Scale bar = 10 µm.
Discussion
In this study we used various independent and complementary techniques to investigate the heterogeneity of BBB permeability throughout the whole mouse brain and in specific brain regions. It is well established that loss of PDGF-BB/PDGFRB signalling due to pdgfb and/or pdgfrB gene mutation or deletion results in loss of pericytes and vascular defects in the central nervous system.14–16 A detectable increase in BBB permeability was observed only when pericyte coverage of capillaries was below 50%.15 We found, on average, an 80% reduction of pericyte coverage of the vasculature network in the pdgf-bret/ret mice. With the exception of the cerebellum, pericyte loss was relatively uniform across brain regions. We therefore anticipated that BBB permeability in all brain regions analysed would also increase in a uniform manner. Measurements of Evans Blue extravasation, Gd-DTPA extravasation by MRI and mIgG accumulation showed collectively that BBB permeability is heterogeneous throughout the brain despite relatively homogeneous pericyte loss. Specifically, the cortex, the striatum and the hippocampus showed increased permeability to blood-borne components, whereas it remained significantly lower in the interbrain, the midbrain and to some extent in the cerebellum. Altogether, these data suggest that, in specific brain regions of pdgf-bret/ret mice, additional factors besides the extent of pericyte loss may influence the permeability of the BBB. Different mouse models of pericyte loss have been used to show the key role of these cells for the regulation of BBB permeability. These models exibit phenotypic differences including, for example, brain calcifications in specific brain regions.24 It remains to be shown that heterogeneity of BBB permeability is conserved in other mouse models of pericyte loss.24
One possible explanation for the heterogeneity of the BBB could come from the fact that pericytes exhibit phenotypic variability29,30 and specialized sub-populations of pericytes may be differentially distributed throughout the brain.31 Indeed, pericytes in the forebrain have a neuroectoderm origin, whereas pericytes within the midbrain have a mesodermal origin.32 Therefore, even though the total number of pericytes and their coverage of the neurovasculature in pdgf-bret/ret mice are similar in different brain regions, their phenotype and function may be different. This phenotypic diversity could lead to local differences in the regulation of BBB transport and extravasation of blood-borne molecules.
Alternatively, it is possible that pericytes are not the sole regulators of BBB permeability throughout the brain. We found that the diameter of capillaries and transcytosis were significantly increased in a region-specific manner. On the other hand, pericyte coverage of pdgf-bret/ret capillaries was similar in all brain regions we examined. Together, these results suggest that the induction and maintenance of a tight BBB phenotype could involve other factors in addition to pericytes, which may vary between different brain regions. Differences in the cellular and/or molecular composition of the neurovascular unit may also contribute to the tightness of the BBB. For example, different molecular pathways may regulate the transcytosis process in BECs in a brain region-specific manner. Detailed molecular profiling of the neurovascular unit33 combined with systematic high resolution imaging of intracellular transport across the BBB18 will be required to characterize the mechanisms regulating BBB heterogeneity under physiological and pathophysiological conditions.
As a consequence of increased BBB permeability, mIgG accumulated over time in specific brain regions including cortex, hippocampus and striatum. In the cortex, one specific sub-population of neurons accumulated mIgG on their plasma membrane. Such pattern of mIgG accumulation is indicative of auto-antibodies against neuronal proteins as recently described in S100B knock-out mice.34 These animals had an age-dependent increase in BBB permeability and also presented brain-reactive auto-antibodies accumulating around cortical neurons.34 One mechanism for auto-antibody generation is the release of cellular antigens by dying cells.35 For instance, influx of neurotoxic blood-borne components such as fibrin into the brain parenchyma triggers neuronal death that may increase neuronal antigen release.16,36 The presence of auto-antibodies specifically around Tbr1-positive neurons suggests that these neurons may be more susceptible to cellular damage and antigen release following increased BBB permeability. Reactive antibodies can further increase neuronal damage in multiple neurological diseases.37 Therefore, a consequence of increased BBB permeability for auto-antibodies may be the exacerbation of neurotoxicity in specific brain regions. Identifying the cellular and/or molecular mechanisms that maintain a low BBB permeability to blood-borne components despite severe pericyte loss (e.g., at the interbrain) could provide valuable insights on how to reduce neurotoxicity.
Therapeutic antibodies targeting key molecules and/or pathways involved in the progression of neurodegenerative diseases are currently in development.28,38 Our observations on the differential permeability of the BBB raise the possibility that not all brain regions may be equally accessible to therapeutic antibodies. Therefore, successful development of therapeutic strategies for the CNS will require further investigation into the mechanisms regulating the heterogeneity of BBB permeability in different brain regions.
Supplementary Material
Acknowledgments
We thank Christer Betsholz for providing the pdgf-bret/ret mice and a critical reading of the manuscript. We thank Julie Lambotte, Stephanie Schöppenthau and Sébastien Debilly for excellent technical assistance. We thank Thomas Bielser, Francoise Gerber and Juerg Messer for their technical support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RV is supported by the Roche Postdoctoral Fellowship (RPF) program (2014–2016).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
The project was designed by LC. The manuscript was prepared by RV, BK, LO, FG, HL, POF and LC. RV performed all microscopy experiments and subsequent image analysis and quantification. BK performed MRI experiments and analysis. MA and CK performed initial immunohistochemistry and biochemistry experiments, respectively. LO carried out the in vivo work. FG provided the Mab86 antibody. RV and LC analysed the experimental data. Correspondence to be addressed to LC.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Chow BW, Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci 2015; 38: 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z, Nelson AR, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell 2015; 163: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bock M, Van Haver V, Vandenbroucke RE, et al. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 2016; 64: 1097–1123. [DOI] [PubMed] [Google Scholar]
- 4.Preston JE, Joan Abbott N, Begley DJ. Transcytosis of macromolecules at the blood-brain barrier. Adv Pharmacol [Review] 2014; 71: 147–163. [DOI] [PubMed] [Google Scholar]
- 5.Banks W, Kastin AJ, Jaspan JB. Regional variation in transport of pancreatic polypeptide across the blood-brain barrier of mice. Pharmacol BiochemBehav 1995; 51: 139–147. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab 2000; 278: E1158–E1165. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides 1998; 19: 883–889. [DOI] [PubMed] [Google Scholar]
- 8.Moinuddin A, Morley JE, Banks WA. Regional variations in the transport of interleukin-1α across the blood-brain barrier in ICR and aging SAMP8 mice. Neuroimmunomodulation 2000; 8: 165–170. [DOI] [PubMed] [Google Scholar]
- 9.Coomber BL, Stewart PA. Morphometric analysis of CNS microvascular endothelium. Microvasc Res 1985; 30: 99–115. [DOI] [PubMed] [Google Scholar]
- 10.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008; 3: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengillo JD, Winkler EA, Walker CT, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol 2013; 23: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller A, Westenberger A, Sobrido MJ, et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet 2013; 45: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 14.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 16.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grueninger F, Bohrmann B, Czech C, et al. Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol Dis 2010; 37: 294–306. [DOI] [PubMed] [Google Scholar]
- 18.Villasenor R, Ozmen L, Messaddeq N, et al. Trafficking of endogenous immunoglobulins by endothelial cells at the blood-brain barrier. Sci Rep 2016; 6: 256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruns A, Kunnecke B, Risterucci C, et al. Validation of cerebral blood perfusion imaging as a modality for quantitative pharmacological MRI in rats. Magn Reson Med 2009; 61: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 20.Haase A, Frahm J, Matthaei D, et al. FLASH imaging. Rapid NMR imaging using low flip-angle pulses. J Magn Reson 1986; 67: 258–266. [DOI] [PubMed] [Google Scholar]
- 21.Williams DS, Grandis DJ, Zhang WG, et al. Magnetic-resonance-imaging of perfusion in the isolated rat-heart using spin inversion of arterial water. Magn Reson Med 1993; 30: 361–365. [DOI] [PubMed] [Google Scholar]
- 22.Jaffer H, Adjei IM, Labhasetwar V. Optical imaging to map blood-brain barrier leakage. Sci Rep 2013; 3: 3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanlandewijck M, Lebouvier T, Andaloussi Mae M, et al. Functional characterization of germline mutations in PDGFB and PDGFRB in primary familial brain calcification. PLoS One 2015; 10: e0143407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkler EA, Sengillo JD, Bell RD, et al. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 2012; 32: 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enge M. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 2002; 21: 4307–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molyneaux BJ, Arlotta P, Menezes JR, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 2007; 8: 427–437. [DOI] [PubMed] [Google Scholar]
- 28.Collin L, Bohrmann B, Gopfert U, et al. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain 2014; 137(Pt 10): 2834–2846. [DOI] [PubMed] [Google Scholar]
- 29.Attwell D, Mishra A, Hall CN, et al. What is a pericyte? J Cereb Blood Flow Metab 2016; 36: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 1991; 113: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartmann DA, Underly RG, Grant RI, et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015; 2: 041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crouch EE, Liu C, Silva-Vargas V, et al. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J Neurosci 2015; 35: 4528–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Brown EV, Acharya NK, et al. Age-dependent increase of blood-brain barrier permeability and neuron-binding autoantibodies in S100B knockout mice. Brain Res 2016; 1637: 154–167. [DOI] [PubMed] [Google Scholar]
- 35.Zaenker P, Gray ES, Ziman MR. Autoantibody production in cancer – The humoral immune response toward autologous antigens in cancer patients. Autoimmun Rev 2016; 15: 477–483. [DOI] [PubMed] [Google Scholar]
- 36.Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 2012; 3: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diamond B, Honig G, Mader S, et al. Brain-reactive antibodies and disease. Annu Rev Immunol 2013; 31: 345–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niewoehner J, Bohrmann B, Collin L, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014; 81: 49–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.