Abstract
Recirculation, from arterial inflow routes through venous outflow pathways, was conceptualized in stroke research 50 years ago. As new technologies were developed, blocked arteries could be reopened, capillaries could be reperfused, and the use of recanalization and reperfusion grew to dominate therapeutic strategies. These approaches overwhelmingly focused on restoration of arterial and capillary inflow, but not on veins even though venous disorders may initiate or exacerbate brain injury. In this commentary, we advance the term “recirculation” after “recanalization” and “reperfusion” as a primary concept of stroke pathophysiology that targets the restoration of both the arterial and venous cerebral circulations.
Keywords: Recanalization, recirculation, reperfusion, stroke pathophysiology, stroke treatment
Re-introducing recirculation
Many stroke investigators have presumed that because venules and veins have extremely thin walls, increased arterial blood flow will force venules and veins to passively dilate and therefore venous hemodynamics are not generally considered during treatment of acute arterial stroke. Many textbooks and schematics of stroke pathophysiology focus on small arteries, arterioles, capillaries, neurons, astrocytes, and pericytes,1 but offer little or no mention of venules and veins.2 The function of venules and veins, however, is especially critical during severe stroke, and their dysfunction may initiate and contribute significantly to brain injuries. In ischemic stroke patients, an absence of venous filling is associated with poor outcomes and brain edema formation, even though reperfusion can reduce brain injury.3 Therefore, we propose to add “recirculation” after the terms “recanalization” and “reperfusion,” both of which are focused primarily on arterial and capillary blood flow. The use of “recirculation” as a term that includes both the arterial and venous aspects of a stroke event appeared as early as 1975, when Zimmermann and Hossmann4 published their study of a monkey cerebral ischemia model. Thus, we propose that “recirculation” again should define involvement of both the arterial and venous components in stroke, and emphasize that clinical assessment of stroke patients should include the “entire” circulation in designing strategies for improving outcomes.
Historical overview: Monro-Kellie doctrine and starling resistors
In the early 1900s, pioneering work by the English physiologist Ernest Starling introduced multiple concepts fundamental to modern understanding of cardiac physiology, including the Frank–Starling Law of the heart.5 A key concept essential to these early studies of cardiac function was the “Starling resistor,” an idea that attributed control of vascular resistance to vascular compression by extravascular forces, such as those encountered by coronary vessels during systole.6 These perspectives helped emphasize that any limit on tissue volume, such as those imposed by the cranium, could amplify flow-induced changes in tissue pressure and venous compression. In contrast to the coronary circulation, however, where flow is greatest during diastole, flow in the cerebral circulation is near-continuous throughout all of both systole and diastole, which has unique implications for interactions between arterial inflow and venous outflow.
Prior to the work of Starling, understanding of the regulation of intracranial pressure (ICP) was dominated mainly by the Monro-Kellie Doctrine, established in the early 1800s,7 which dictated that a change in the volume of any component within the fixed volume of the cranium must be accompanied by an opposite change in another intracranial constituent. Because the cerebral ventricles were large and fluid-filled, a common early view was that these elements could change volume to compensate for changes in cerebral blood flow and volume.8 However, integration of the tissue pressure ideas introduced by Starling implicated cerebral venous compression as a major, but graded, response to increased ICP.
Starling resistors in stroke pathophysiology
Given that severe stroke results from multiple different arterial etiologies, it is not certain how venous pressure, flow, and injury contribute to brain pathology. To explore this question, Hallenbeck hypothesized that cerebral veins function as Starling resistors following cerebral ischemia and reported that venous compression was a common element of responses to even mild cerebral ischemia.9 Hallenbeck’s work motivated other studies that expanded the view of cerebral veins as Starling resistors, and demonstrated that this mechanism was functionally important even under physiological (non-ischemic) conditions.10,11 Additional work further identified veins at the external margin of the cranium that contained multiple layers of smooth muscle, were adrenergically innervated, and could enhance ICP through venoconstriction.12 Together, these results argued strongly against the view that cerebral veins were merely passive structures, and for the view that veins participated actively in regulation of both cerebral perfusion and ICP.13
Venous pathophysiology after acute arterial stroke
Relevant to patients with elevated ICP, the Starling resistor theory predicts that increased ICP may not compress small arteries or arterioles due to their greater pressure and thick layers of smooth muscle and adventitia, but may compress capillaries, venules, and even veins,2,9,11 including cerebral bridging veins prior to their entry into the superior sagittal sinus.14 In this case, even though venous lumen diameters may change little on average, total venous blood outflow can actually decrease.14 Correspondingly, Uhl et al.15 reported compressed venules and low capillary densities in subarachnoid hemorrhage (SAH) patients, and that subsequent craniotomy to clip aneurysms ultimately decreased ICP and increased both venous flow and capillary filling.15 Mursch et al.16 reported that the overall outcome of SAH was more closely related to cerebral venous outflow than to arterial vasospasm.
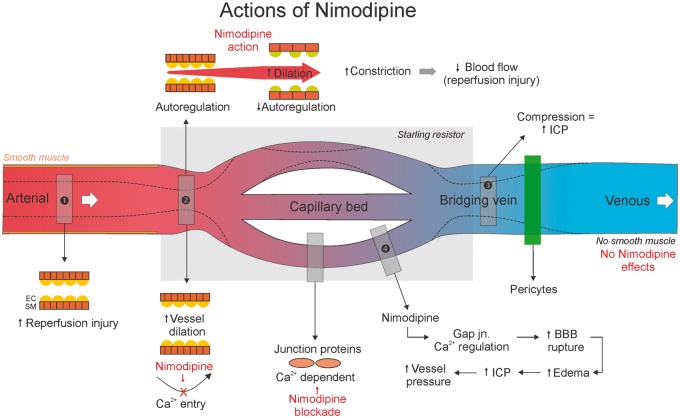
Clinical use of nimodipine and other dihydropyridines to improve cerebral perfusion after SAH and TBI17 may aggravate injury if venous pressure is elevated18 (Figure 1). Dihydropyridines dilate cerebral arteries and arterioles by blocking voltage-dependent calcium channels in arterial smooth muscle.19 However, there are few smooth muscle cells in venules, small cerebral veins, or bridging veins.20 Thus, nimodipine increases arterial inflow but does not dilate veins, which augments extravascular pressure on the venous system. For patients with developmental abnormalities of the sinuses, venous thromboses, or elevated ICP (e.g. after SAH), Starling resistor theory predicts that anatomical limits on venous outflow will lead to brain swelling when arterial flow is significantly increased. Interestingly, “vasogenic brain edema” has been reported in SAH patients after administering nimodipine.21
Figure 1.
In patients with brain swelling and venous sinus narrowing, administration of Nimodipine may aggravate brain swelling. Nimodipine dilates artery but does not increase venous blood flow directly, because there are few smooth muscle cells in veins. Pericytes are also involved in venous flow. EC: endothelial cell; SM: smooth muscle.
In addition, several studies related to stroke clearly suggest that multiple factors influence, and are prognostic for, cerebral venous thrombosis including edema, migraine, and hormonal etiologies.22,23 Endovascular treatment in a subset of patients also can provide improved outcomes.22 Furthermore, quantitative neuromimaging is now possible using susceptibility-weighted imaging (SWI); Dempfle et al.24 were able to quantitate venous volume and demonstrated that improved microcirculation following cerebral venous thrombosis may be prognostic.
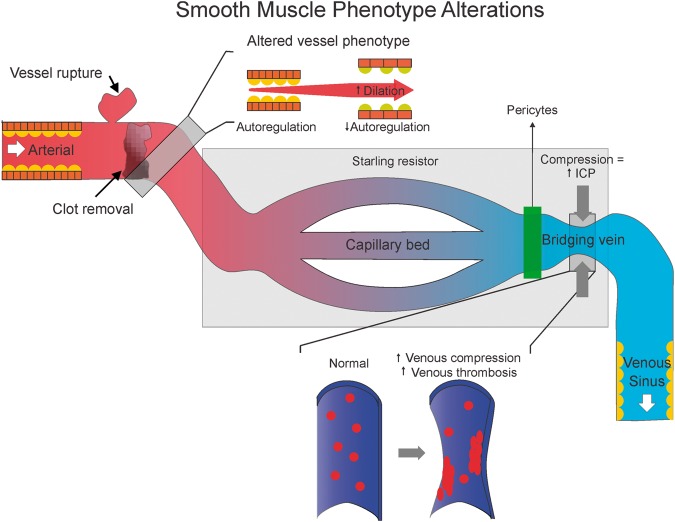
Vasotrophic factors released in response to arterial occlusion, hemorrhage, or vasoconstriction could modulate the phenotype of cells in both the arterial and venous vascular wall.25 Damage of the arterial wall, rupture of blood cells during hemorrhagic stroke, and/or diminished arterial pressure during ischemic stroke can trigger these changes.26–28 Similarly, ischemic injury can also alter the function of intramural and pericapillary pericytes, which in turn can also modulate smooth muscle and endothelial phenotype, structure, and reactivity.29–32 In turn, this phenotypic transformation could alter venous structure, inflammatory state, and the probability for thrombus formation, which could further increase outflow resistance and precipitate BBB damage, brain edema, raised ICP, and further venous compression27,33,34 (Figure 2). Venous endothelial injury and phenotypic transformation also could further aggravate local inflammation, increase probability for thrombus formation, and worsen stroke-related injury.
Figure 2.
Smooth muscle phenotype may change after a stroke and leads to loss of autoregulation. Venous endothelial cells can undergo phenotypic changes that promote venous thrombosis formation. In addition, pericytes are also involved in venous flow.
In parallel, vascular smooth muscle can transform from a contractile to a secretary/proliferative phenotype, yielding cells that are primarily non-contractile. Phenotypic changes may induce loss of cerebral autoregulation, due to arteries that no longer contract to restrict the flow of blood into the brain’s circulation when arterial pressure increases (Figure 2). Consistent with this idea, the myogenic response in the cerebral circulation typically is impaired following ischemic and hemorrhagic stroke, and impaired autoregulation is associated with a weak myogenic response.35 This point is particularly important for ischemic stroke patients who have recently undergone thrombectomy; over-perfusion can occur when occluded arteries are recanalized.36
Venous sinus hypoplasia (asymmetrical development) may be another precipitating factor that amplifies the increases in ICP and venous compression associated with stroke. Up to 50% of humans have a developmental abnormality of a venous sinus (e.g. transverse sinus) rendering it atretic or hypoplastic. Some patients exhibit asymmetrical abnormalities of a bilateral sinus, and others present multiple sinuses that are also only partially developed.37 The poorly developed venous sinuses or internal jugular veins of such individuals may not be able to accommodate the drastically enhanced blood flow typical of reperfusion after stroke, especially when other pathological conditions such as ICP elevation, brain edema, or use of dihydropyridines are involved in the same patient. Increased arterial inflow without an accompanying increase in venous outflow will increase venous pressure and the risk for brain swelling. Because arterial inflow must always equal the sum of venous outflow and fluid extravasation, atretic venous sinuses have the potential to reduce total cerebral perfusion, reduce the arterial-venous pressure gradient, and promote formation of vasogenic edema. Correspondingly, Yu et al.38 reported that after massive cerebral infarct, immediate malignant brain edema and death occurred in patients with abnormalities of the venous sinuses such as hypoplasia or occlusions that compromised cranial venous drainage.
Recirculation
Together, elevation of ICP, brain edema, massive brain infarction, smooth muscle and endothelium phenotype changes, use of dihydropyridines (e.g. nimodipine), and venous sinus hypoplasia, especially when several of these factors are combined, may increase venous pressure, enhance BBB disruption, and further elevate ICP, forming a vicious cycle that ultimately affects patients’ outcomes (Figures 1 and 2). Moreover, multiple studies have suggested that collateral flow, when present and independent of canalization, is sufficient to improve clinical and imaging outcomes, such as smaller lesion volumes, decreased penumbra, etc.39,40 In addition, timing of collateralization (or as we suggest, “recirculation”) appears to be crucial, as Yeo et al (2016) reported that delayed “recirculation” was not beneficial and perhaps even detrimental. Therefore, in patients with severe stroke, venous pressure and blood flow should be evaluated carefully before deciding on a therapeutic strategy. All of these points support the re-introduction of the concept of “recirculation,” after recanalization and reperfusion, to emphasize arterial and venous blood flow harmony in stroke pathophysiology. Therefore, stroke investigators need to remain mindful of cerebral venules and veins when considering patients’ treatments and experimental studies.
Future research directions
Neuroimaging of arterial involvement in stroke-related brain injury has become commonplace, but few options are available to image cerebral veins. The size of each voxel in either CT or MR imaging is adequate for large veins, but precludes accurate sampling of small veins. SWI has been used with some success to image the venous vasculature.41 Haacke and colleagues42 reported that SWI imaging of venous oxygenation in stroke patients was predictive of patient outcomes, suggesting that monitoring venous oxygenation could be beneficial. Similarly, T2*-weighted fluid-attenuated inversion recovery (FLAIR*) has been reported to provide high contrast for identifying parenchymal veins.43 In translational studies, two-photon microscopy has been used to differentiate arterial from venous effects.44 Hopefully these and other approaches in future studies will help address how oxygenation changes in cerebral venous blood in the context of recirculation/reperfusion.
Unfortunately, the standard external compression treatment regimen for insufficient venous flow in the periphery is not tenable for the brain because it is inside the rigid skull and cerebral veins do not have valves to prevent backflow. Pharmacological approaches to increase cerebral venous flow include the use of anticoagulants (heparin), thinning agents to reduce blood viscosity, and osmotherapy (e.g. mannitol, hypertonic saline, loop diuretics) to both decrease blood viscosity and draw water from the brain down osmotic gradients.45,46 Future goals include endovascular treatments for sinus thrombosis47 and sinus stenosis48 that target smaller veins or even venules to improve venous drainage via either chemical or mechanical approaches.
Although most experimental studies target arterial aspects of recirculation in stroke, a few have focused on the venous side.49 In contrast to studies of cerebral artery occlusion, which are methodologically more consistent among different laboratories, studies of venous thrombosis models are at an early stage of development and lack standardization, which greatly complicates comparison of results from different laboratories. Furthermore, most published studies have focused either on arterial or venous components, and very few have examined both arterial and venous components in studies of recirculation. Therefore, a goal of the present commentary is to emphasize that both arterial and venous components should be considered in studies of acute ischemic and hemorrhagic stroke.
Overall, the “recirculation” concept strongly suggests that stroke treatment paradigms need to address venous outflow from the brain in relation to arterial inflow. Therefore, to minimize potential brain swelling and reperfusion injury for severe stroke patients, we need to consider carefully venous pressure and outflow, potential arterial smooth muscle and venous endothelial phenotype changes, possible pre-existing venous sinus hypoplasia, and in particular, if nimodipine will be used.
Funding
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author(s) acknowledge financial support for the research, authorship, and/or publication of this article by NIH P01 NS082184-01.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke 2015; 10: 143–152. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Khatibi N, Zhang JH. Vascular neural network: the importance of vein drainage in stroke. Transl Stroke Res 2014; 5: 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Lai Y, Ding X, et al. Absent filling of ipsilateral superficial middle cerebral vein is associated with poor outcome after reperfusion therapy. Stroke 2017; 48: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann V, Hossmann KA. Resuscitation of the monkey brain after one hour's complete ischemia. II. Brain water and electrolytes. Brain Res 1975; 85: 1–11. [DOI] [PubMed] [Google Scholar]
- 5.Glower DD, Spratt JA, Snow ND, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 1985; 71: 994–1009. [DOI] [PubMed] [Google Scholar]
- 6.Ursino M, Lodi CA. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J Appl Physiol 1997; 82: 1256–1269. [DOI] [PubMed] [Google Scholar]
- 7.Macintyre I. A hotbed of medical innovation: George Kellie (1770–1829), his colleagues at Leith and the Monro-Kellie doctrine. J Med Biogr 2013; 22: 93–100. [DOI] [PubMed] [Google Scholar]
- 8.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 2001; 56: 1746–1748. [DOI] [PubMed] [Google Scholar]
- 9.Hallenbeck JM, Bradley ME. Experimental model for systematic study of impaired microvascular reperfusion. Stroke 1977; 8: 238–243. [DOI] [PubMed] [Google Scholar]
- 10.Luce JM, Huseby JS, Kirk W, et al. A Starling resistor regulates cerebral venous outflow in dogs. J Appl Physiol Respir Environ Exerc Physiol 1982; 53: 1496–1503. [DOI] [PubMed] [Google Scholar]
- 11.Chopp M, Portnoy HD, Branch C. Hydraulic model of the cerebrovascular bed: an aid to understanding the volume-pressure test. Neurosurgery 1983; 13: 5–11. [DOI] [PubMed] [Google Scholar]
- 12.Pearce WJ, Bevan JA. Retroglenoid venoconstriction and its influence on canine intracranial venous pressures. J Cereb Blood Flow Metab 1984; 4: 373–380. [DOI] [PubMed] [Google Scholar]
- 13.Stolz E, Rusges DA, Hoffmann O, et al. Active regulation of cerebral venous tone: simultaneous arterial and venous transcranial Doppler sonography during a Valsalva manoeuvre. Eur J Appl Physiol 2010; 109: 691–697. [DOI] [PubMed] [Google Scholar]
- 14.Nemoto EM. Dynamics of cerebral venous and intracranial pressures. Acta NeurochirSuppl 2006; 96: 435–437. [DOI] [PubMed] [Google Scholar]
- 15.Uhl E, Lehmberg J, Steiger HJ, et al. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery 2003; 52: 1307–1315. discussion: 1315–1357. [DOI] [PubMed] [Google Scholar]
- 16.Mursch K, Wachter A, Radke K, et al. Blood flow velocities in the basal vein after subarachnoid haemorrhage. A prospective study using transcranial duplex sonography. Acta Neurochir 2001; 143: 793–799. discussion: 799–800. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JH. Vascular neural network in subarachnoid hemorrhage. Transl Stroke Res 2014; 5: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pluta RM, Hansen-Schwartz J, Dreier J, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 2009; 31: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Famaey N, Ying Cui Z, Umuhire Musigazi G, et al. Structural and mechanical characterisation of bridging veins: a review. J Mech Behav Biomed Mater 2015; 41: 222–240. [DOI] [PubMed] [Google Scholar]
- 21.Ryu CW, Koh JS, Yu SY, et al. Vasogenic edema of the Basal Ganglia after intra-arterial administration of nimodipine for treatment of vasospasm. J Korean Neurosurg Soc 2011; 49: 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salottolo K, Wagner J, Frei DF, et al. Epidemiology, endovascular treatment, and prognosis of cerebral venous thrombosis: US center study of 152 patients. J Am Heart Assoc 2017; 6: pii: e005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duman T, Uluduz D, Midi I, et al. A Multicenter study of 1144 patients with cerebral venous thrombosis: the VENOST study. J Stroke Cerebrovasc Dis 2017; 26: 1848–1857. [DOI] [PubMed] [Google Scholar]
- 24.Dempfle AK, Harloff A, Schuchardt F, et al. Longitudinal volume quantification of deep medullary veins in patients with cerebral venous sinus thrombosis: venous volume assessment in cerebral venous sinus thrombosis using SWI. Clin Neuroradiol Epub ahead of print 6 June 2017. DOI: 10.1007/s00062-017-0602-z. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Wang HM. Effects of cobalt chloride on phenotypes of normal human saphenous vein smooth muscle cells. Int J Clin Exp Med 2014; 7: 4933–4941. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JH, Badaut J, Tang J, et al. The vascular neural network – a new paradigm in stroke pathophysiology. Nat Rev Neurol 2012; 8: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poittevin M, Lozeron P, Hilal R, et al. Smooth muscle cell phenotypic switching in stroke. Transl Stroke Res 2014; 5: 377–384. [DOI] [PubMed] [Google Scholar]
- 28.Shimamura N, Ohkuma H. Phenotypic transformation of smooth muscle in vasospasm after aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2014; 5: 357–364. [DOI] [PubMed] [Google Scholar]
- 29.Cai W, Liu H, Zhao J, et al. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res 2017; 8: 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhaus AA, Couch Y, Sutherland BA, et al. Novel method to study pericyte contractility and responses to ischaemia in vitro using electrical impedance. J Cereb Blood Flow Metab 2017; 37: 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Underly RG, Levy M, Hartmann DA, et al. Pericytes as inducers of rapid, matrix metalloproteinase-9-dependent capillary damage during ischemia. The J Neurosci 2017; 37: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massberg S, Enders G, Matos FC, et al. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood 1999; 94: 3829–3838. [PubMed] [Google Scholar]
- 34.Kharbanda RK, Peters M, Walton B, et al. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 2001; 103: 1624–1630. [DOI] [PubMed] [Google Scholar]
- 35.Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. J Am Soc Nephrol 1990; 1: 53–57. [DOI] [PubMed] [Google Scholar]
- 36.Telischak NA, Wintermark M. Imaging predictors of procedural and clinical outcome in endovascular acute stroke therapy. Neurovasc Imag 2015; 1: 4–12. [Google Scholar]
- 37.Zivadinov R, Chung CP. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 2013; 11: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu W, Rives J, Welch B, et al. Hypoplasia or occlusion of the ipsilateral cranial venous drainage is associated with early fatal edema of middle cerebral artery infarction. Stroke 2009; 40: 3736–3739. [DOI] [PubMed] [Google Scholar]
- 39.Cho TH, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015; 46: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 40.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsui YK, Tsai FY, Hasso AN, et al. Susceptibility-weighted imaging for differential diagnosis of cerebral vascular pathology: a pictorial review. J Neurol Sci 2009; 287: 7–16. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Hu J, Miao Y, et al. In vivo measurement of oxygenation changes after stroke using susceptibility weighted imaging filtered phase data. PLoS One 2013; 8: e63013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sati P, George IC, Shea CD, et al. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012; 265: 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih AY, Driscoll JD, Drew PJ, et al. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab 2012; 32: 1277–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabinstein AA. Treatment of cerebral edema. Neurologist 2006; 12: 59–73. [DOI] [PubMed] [Google Scholar]
- 46.Raslan A, Bhardwaj A. Medical management of cerebral edema. Neurosurg Focus 2007; 22: E12. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui FM, Dandapat S, Banerjee C, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke 2015; 46: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 48.Starke RM, Wang T, Ding D, et al. Endovascular treatment of venous sinus stenosis in idiopathic intracranial hypertension: complications, neurological outcomes, and radiographic results. Sci World J 2015; 2015: 140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yenigun M, Junemann M, Gerriets T, et al. Sinus thrombosis-do animal models really cover the clinical syndrome? Ann Transl Med 2015; 3: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]