Abstract
Introduction
Major depressive disorder (MDD) is thought to impact negatively on cognitive function; however, the relationship has not been well explored.
Objective
This study examined the association between depression severity and global cognitive function and memory in subjects with severe, treatment-resistant MDD.
Methods
We enrolled 66 subjects with SCID-I diagnosed unipolar MDD in a multi-center trial to assess the efficacy and neurocognitive effects of electroconvulsive therapy (ECT). We measured depression severity with the 24-item Hamilton Rating Scale for Depression (HRSD24). Neuropsychologic measures included the Mini Mental State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT), and the Complex Figure Test (CFT). Correlational and regression analyses were conducted to explore associations between depression severity and cognitive function.
Results
The mean age of the subjects was 53.6 years (SD=15.8), 65% were female, and mean HRSD24 was 33.9 (SD=6.7). Mean demographic-corrected T-scores for each neurocognitive measure were in the average to borderline range, and HRSD24 values were unrelated to performance on the MMSE, RAVLT immediate and delayed recall, and CFT immediate and delayed recall.
Conclusion
In this sample of severely depressed subjects referred for ECT, depression severity was unrelated to global cognitive function or memory. Future research should examine the interactions between other depressive characteristics and neurocognitive function.
Keywords: Major depressive disorder, neuropsychology, auditory verbal learning test, cognitive, Mini Mental State Examination, Error Analysis
INTRODUCTION
Major depressive disorder (MDD) is a serious, chronic, and debilitating disease [1–3] that may negatively impact neurocognitive function [4, 5]. However, there is limited information regarding what aspects of depression affect cognitive function. Thus, it is important to understand what specifically about MDD is affecting cognitive abilities [6]. One such depressive factor that has received attention is the severity of the major depressive episode.
Severity of the depressive episode has been inconsistently associated with neurocognitive functioning, particularly global cognitive function and memory [7]. Examining differences among 38 patients with MDD and 69 control patients, Gualtieri et al. [7] noted that patients with depression performed worse in the domains of global function, cognitive flexibility, complex attention, and vigilance. Some patients who required hospitalization for their depression (i.e., an indication of more severe depression) have been reported to demonstrate higher rates of neurocognitive difficulties [8]. On the contrary, in a study of 23 patients with severe depression [9], no association was found between depression severity and neuropsychologic performance. Similarly, depression severity, as measured by patient self-report with the Beck Depression Inventory [10] in 115 depressed outpatients, was unrelated to objective or subjective neuropsychologic measures [8].
Conflicting reports in the literature may be a result of a variety of factors. For example, severity of depressive episode might impact only particular aspects of memory or cognition. A study that compared patients with MDD to those with minor depression found that the former group was significantly more impaired on a test of working memory [11]. A meta analysis by Burt et al. [12] found depression was associated with declarative memory impairment, particularly verbal immediate recall. In addition, they found that age moderated the level of memory impairment in patients with depression. On the other hand, an investigation by Austin and colleagues found no association between depression severity and immediate verbal recall [13].
The differences between the above investigations draw attention to the incongruity of the relationship between severity of depressive episodes and neurocognitive function. Thus, we sought to test the assumption that the severity of the depressive episode would negatively affect neurocognitive function in patients with severe MDD. To this end, we studied severely depressed patients referred for electroconvulsive therapy (ECT) in the Consortium for Research in ECT (CORE) “Three Electrode Placement to Optimize ECT” study [14] to determine whether severity of the depressive episode was associated with neuropsychologic performance. We hypothesized that the severity of the depressive episode would be negatively associated with performance on measures of global cognitive function, as well as verbal and visual learning and memory.
METHODS
Study Overview
The population and methods of the CORE Comparing Three Electrode Placements to Optimize ECT trial are described in detail elsewhere [14]. In brief, this study was a multicenter, NIMH funded, randomized, double-masked, controlled trial carried out from 2001–2006. Outpatients and inpatients with either severe unipolar or bipolar depression, with or without psychosis, referred for ECT, were included in the CORE trial. Subjects were randomly assigned to one of three electrode placements (bifrontal, bitemporal, or right unilateral) during an acute course of ECT, and were treated until they achieved pre-specified remission criteria. This protocol was reviewed and approved by the Institutional Review Boards of all five participating academic clinical centers (The University of Texas Southwestern Medical Center (UTSW), Medical University of South Carolina (MUSC), Mayo Foundation, Hillside Hospital/Northshore Long Island Jewish Health System, and the University of Medicine and Dentistry of New Jersey (UMDNJ)).
Study Population
All study related risks, benefits, and adverse events were explained to study participants, who provided written consent before participation in the study. Male and female patients were included who received a Structured Clinical Interview for the DSM-IV (SCID-I) [15] diagnosis of unipolar MDD, had a pretreatment 24-item Hamilton Rating Scale for Depression (HRSD24) [16, 17] score of 21 or greater, had a clinical indication for ECT, were able to provide informed consent, and were able to cooperate with neuropsychologic testing.
Exclusion criteria included a lifetime history of bipolar disorder, depression with psychotic features, schizophrenia, schizoaffective disorder, mental retardation, and current primary diagnosis of anxiety, obsessive-compulsive disorder, or eating disorder. Participants were also excluded if they had a current diagnosis of delirium, dementia, amnestic disorder or any other major medical condition (i.e., heart disease) or central nervous system disease as well as active substance abuse or dependence. The above exclusion criteria were determined by a review of available medical records, physician assessment, and structured clinical interview. Patients with a baseline MMSE [18] score less than 23 (to decrease the possibility of including patients with dementia [19, 20]), or who received ECT within the past six months, were excluded.
Clinical Assessment Measures
Trained and certified clinical raters collected sociodemographic and clinical data at the screening/baseline visit. This information included prior and current course of psychiatric illness, current and past substance abuse/dependence, prior suicide attempts, general medical illnesses, and prior history of psychiatric treatment. The clinical rater and study psychiatrist completed the HRSD24, and their combined mean score was used to quantify severity of the depressive episode. As a secondary measure of depression severity, the patient completed the 30-item Inventory of Depressive Symptomatology-Self Report (IDS-SR30) [21, 22].
Neuropsychological Assessment Measures
Trained neuropsychometrists administered the neuropsychologic tests at baseline, per manualized procedures, before the patients received their first ECT treatment. Most neuropsychologic assessments took place in the morning in a private, distraction-free room. The MMSE was used to assess global cognitive function. The Rey Auditory Verbal Learning Test (RAVLT) [23, 24] and the Complex Figure Test (CFT; Rey-Osterrieth or Taylor) [25, 26] were used to measure verbal and visual learning and memory, respectively. To provide an estimate of premorbid intelligence, the Reading subtest of the Wide Range Achievement Test-3 (WRAT-3) [27, 28] was used. Raw scores were converted into demographic adjusted T-scores.
Statistical Analyses
Summary statistics were used to describe the sociodemographic and clinical characteristics of the population. Means and standard deviations are presented for continuous variables; percentages are presented for discrete variables. Pearson product-moment correlation coefficients were computed to examine associations between the magnitude of depression severity, as rated on the HRSD24 and IDS-SR30 and based on clinical characteristics (number of depressive episodes, length of current depressive episode), and neuropsychologic performance on the MMSE, RAVLT, and CFT. Correlations were interpreted based on standard guidelines [29]. Also, linear regression models were used to evaluate the prediction of neuropsychologic test performance from the HRSD24 total score (depression severity). Data were analyzed using SPSS version 16 for Windows (SPSS, Inc., Chicago, IL). The length of current depressive episode variable was log transformed to achieve normal distribution. Subjects with missing values were excluded from each analysis; no data were imputed. Statistical significance was defined as a two-sided p-value of less than 0.01.
RESULTS
Sociodemographic and Clinical Characteristics
Table 1 summarizes the sociodemographic and clinical characteristics of the sample (N=66). Most subjects were female, Caucasian, and married. The average years of education was 14.3 (SD=2.6), and most subjects were right handed. Of clinical relevance, most of the study subjects had several major depressive episodes and psychiatric hospitalizations consistent with recurrent MDD. The severity of the current depressive episode on average was in the high severe range as rated on both clinician rated (HRSD24; range of scores: 21–53) and patient rated (IDS-SR30; range of scores: 18–69) depression severity measures.
Table 1.
Sociodemographic and Clinical Characteristics of the Study Sample.
| Measure | Total Sample (N=66) |
|---|---|
| Age [mean (SD)] | 53.6 (15.8) |
| Female gender [N (%)] | 43 (65%) |
| Race [N (%)] | |
| White | 62 (94%) |
| Black | 4 (6%) |
| Employment status [N (%)] | |
| Employed | 15 (23.8%) |
| Unemployed | 8 (12.1%) |
| Retired | 17 (25.8%) |
| Other | 26 (38.3%) |
| Marital Status [N (%)] | |
| Married | 36 (54.5%) |
| Never Married | 10 (15.2%) |
| Divorced | 11 (16.7%) |
| Other | 9 (13.6) |
| Education, years [mean (SD)] | 14.3 (2.6) |
| Handedness [N (%)] | |
| Right | 59 (89.4%) |
| Left | 5 (7.6%) |
| Ambidextrous | 2 (3.0%) |
| Length of current MDE [weeks, mean (SD)] | 80.8 (155.9) |
| Number of MDEs [mean (SD)] | 4.2 (4.6) |
| Age of current MDE onset [mean (SD)] | 52.8 (16.3) |
| Total Psychiatric Hospitalizations | 3.1 (2.8) |
| Depression Severity [mean (SD)] | |
| HRSD24 | 33.9 (6.7) |
| IDS-SR30 | 45.9 (12.4) |
MDE=major depressive episode, HRSD24=24-item Hamilton Rating Scale for Depression, IDS-SR30=30-item Inventory of Depressive Symptomatology—self report
Neuropsychologic Performance of the Sample
The group had an estimated full scale IQ in the average range as evidenced on the WRAT-3 Reading subtest (Standard Score=100.5, SD=11.0). Overall, mean T-scores (Table 2) for each test were in the borderline to average range. Specifically, the mean T-score for the MMSE was 44.0 (SD=12.6), RAVLT immediate recall was 45.4 (SD=11.9), delayed recall was 41.3 (SD=12.8), and CFT immediate and delayed recall was 42.4 (SD=14.2) and 40.0 (SD=13.3), respectively.
Table 2.
Demographic Adjusted Neuropsychological Scores of the Study Sample.
| Measure | Standard Score1 |
|---|---|
| MMSE | 44.0 (12.6) |
| RAVLT | |
| Learning | 45.6 (10.3) |
| List B | 47.1 (8.4) |
| Immediate Recall | 45.4 (11.9) |
| Delayed Recall | 41.3 (12.8) |
| Perseverations | 3.6 (4.5) 2 |
| Intrusions | 2.8 (3.7) 2 |
| CFT | |
| Immediate Recall | 42.4 (14.2) |
| Delayed Recall | 40.0 (13.3) |
| WRAT-3 Reading Subtest | 100.5 (11.0) |
T-scores and Standard scores are demographic adjusted and are presented as mean (SD).
Raw score, worse performance is indicated by higher number
MMSE=Mini Mental State Examination, RAVLT=Rey Auditory Verbal learning Test, CFT=Complex Figure Test (Rey-Osterrieth or Taylor version), WRAT-3=Wide Range Achievement Test, 3rd Edition
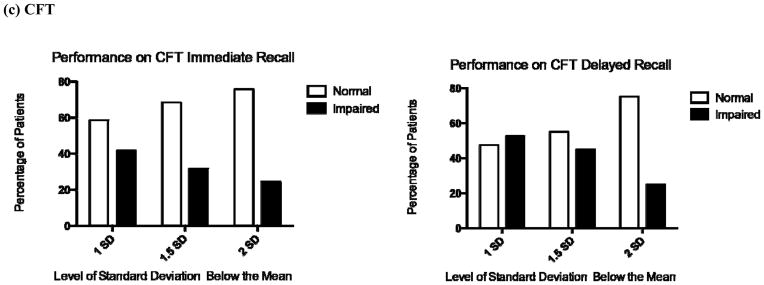
The percentage of the sample that showed impaired performance on the MMSE, RAVLT, or CFT varied based on the classification method used to determine impairment. Following standard guidelines [30], we classified impaired performance as a T-score either 1, 1.5, or 2 standard deviations below the mean (Figure 1). Overall, 58.7% of the sample showed no impairment on the MMSE, 70.5% showed no impairment on the RAVLT Delayed Recall, and 47.5% showed no impairment on the CFT Delayed Recall.
Figure 1. Percentage of Sample showing Impaired Performance on the MMSE, RAVLT, or CFT, based on Standard Guidelines.
Figure 1 shows the percentage of patients with normal or impaired performance on the respective measure. Impairment was defined by traditional standards as a demographic-adjusted T-score either 1 standard deviation (SD), 1.5 SD, or 2 SD below the mean. For example, on the MMSE, when impairment was defined as 1 SD below the mean, then 59% showed normal performance and 41% showed impaired performance. When impairment on the MMSE was defined as 1.5 SD below the mean, then 75% showed normal performance and 25% showed impaired performance. This shift in impairment from a higher percentage to a lower percentage reflects the use of a more conservative estimate of impairment.
MMSE=Mini Mental State Examination, RAVLT=Rey Auditory Verbal learning Test, CFT=Complex Figure Test (Rey-Osterrieth or Taylor version), SD=Standard Deviation
Association between Depression Severity and Neurocognitive Performance
No significant associations were found between depression severity, as objectively rated on the HRSD24, and performance on the MMSE, RAVLT, or CFT (Table 3 and Figure 2). When depression severity was examined based on a patient-rated instrument, however, some trends towards significant associations were found with neurocognitive performance. Specifically, depression severity as rated on the IDS-SR30 was significantly associated with lower performance on the interference list (list B) of the RAVLT (r=−.34, p=.01), and a trend was found for lower performance on the MMSE (r=−.29, p=.04). Regarding clinical characteristics, no significant associations were found between the number of depressive episodes or the length of the current depressive episode and performance on neurocognitive measures.
Table 3.
Pearson Product-Moment Correlations for Depression Severity and Global Cognitive and Memory Function in Depressed Patients.
| Number of MDE | Length of MDE | HRSD24 | IDS-SR30 | RAVLT List A | RAVLT List B | RAVLT IR | RAVLT DR | CFT IR | CFT DR | MMSE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of MDE | -- | ||||||||||
| Length of MDE | −.15 | -- | |||||||||
| HRSD24 | .12 | .03 | -- | ||||||||
| IDS-SR30 | .14 | .38 | .61 | -- | |||||||
| RAVLT List A | .24 | −.31 | −.03 | .16 | -- | ||||||
| RAVLT List B | −.09 | .29 | −.14 | −.34* | .08 | -- | |||||
| RAVLT IR | .15 | −.54 | −.11 | .03 | .69 | −.06 | -- | ||||
| RAVLT DR | .17 | −.33 | −.14 | .10 | .70 | .05 | .88 | -- | |||
| CFT IR | .03 | −.36 | −.22 | −.20 | .09 | .06 | .15 | .04 | -- | ||
| CFT DR | −.01 | −.09 | .01 | −.10 | −.04 | .14 | .02 | −.09 | .88 | -- | |
| MMSE | −.08 | −.35 | −.23 | −.29 | .18 | −.05 | .31 | .19 | .17 | .16 | -- |
MDE=Major Depressive Episode, HRSD24=24-item Hamilton Rating Scale for Depression, IDS-SR30=30-item Inventory of Depressive Symptomatology—self report, MMSE=Mini Mental State Examination, RAVLT=Rey Auditory Verbal learning Test, CFT=Complex Figure Test (Rey-Osterrieth or Taylor version), IR=Immediate Recall, DR=Delayed Recall
p=0.01
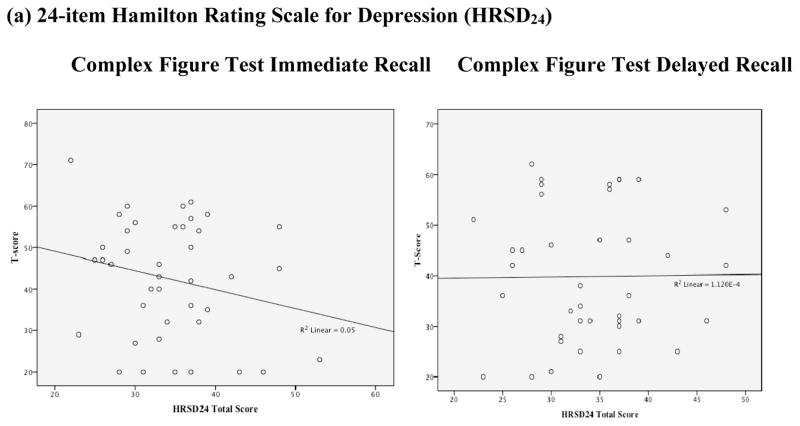
Figure 2. Scatterplots between Neurocognitive Performance and Depression Severity.

MMSE=Mini Mental State Examination, RAVLT=Rey Auditory Verbal learning Test, CFT=Complex Figure Test (Rey-Osterrieth or Taylor version)
Prediction of Neurocognitive Performance based on Depression Severity
In regression analyses, HRSD24 values did not significantly predict performance on the MMSE (R2=.05, p=.07), RAVLT immediate (R2=.01, p=.41) or delayed recall (R2=.02, p=.30), or CFT immediate (R2=.05, p=.16) or delayed recall (R2=.00, p=.95).
Neurocognitive Error Analysis
Examination of perseveration and intrusion errors from the RAVLT revealed the average number of perseverations and intrusions were 3.6 (SD=4.5) and 2.8 (SD=3.7), respectively. Depression severity as measured on the IDS-SR30 showed a trend to positively correlate with total RAVLT perseverations (r=.33, p=.02), and no significant association was found with intrusions (r=.04, p=.74), even when age and education were controlled. The association between depression severity as measured on the HRSD24 and RAVLT perseverations was also statistically nonsignificant.
DISCUSSION
Overall, global cognitive functioning and learning and memory scores were not related to depression severity. The HRSD24, a popular clinician-rating scale for depression, was not significantly correlated with the MMSE, Rey Auditory Verbal Learning Test, or the Complex Figure Test in this severely depressed sample referred for ECT. The IDS-SR30, a self-report measure of depression severity was negatively correlated with List B of the RAVLT, and showed a trend to negatively correlate with the MMSE. Based on standard guidelines [29], the correlation between the IDS-SR30 and List B of the RAVLT was considered to be low, and between the IDS-SR30 and the MMSE very low.
The MMSE is a widely accepted screening measure of global cognitive function that has been used in many studies. In the original article on the MMSE by Folstein et al. [31], the total score on the MMSE separated three diagnostic groups (dementia, depression, depression with cognitive impairment) and a control group. That study found that the MMSE was lower in patients with depression than in controls, although a later study suggested that the MMSE was not sensitive to the cognitive effects of depression [32], a finding similar to this current investigation. Alpert et al. [33] examined the performance of 148 depressed patients (age range = 18–65 years) on the MMSE and found no significant correlation between the MMSE and the HRSD17 (r = −0.11, p = .20). However, those patients differed from our sample as the subjects were non-hospitalized and had lower levels of depression severity. Based on these collective findings, it may be possible that the MMSE is insensitive to clinician- rated or patient- rated depression severity in severely depressed individual.
The RAVLT is a commonly used measure of verbal learning and memory in neuropsychologic research. Similarly, the CFT is a widely used measure of visuospatial memory. Contrary to common clinical belief, we found no relationship between the severity of the depressive episode and performance on the RAVLT or the CFT. A meta analysis of 122 studies of severely depressed patients with an age range of 19 to 84 years found that depression was related to memory impairment [34]. However, Rohling et al. [8] compared 115 patients with low depression (defined as a Beck Depression Inventory (BDI) [10] score of 10 or less) to a group of 112 with high depression (BDI score ≥ 25) and found no difference on the California Verbal Learning Test (CVLT) [35]. The difference between these studies may be due to methodologic factors. In their meta analysis, Burt et al. (1995) included diverse groups of patients with affective disorders, verbal memory was assessed with various measures, and they did not define or quantify severity of the depressive episode. Rohling et al. (2002) conducted a controlled study that directly compared performance on the CVLT in two defined groups, and with results similar to ours. Austin et al. [13] also found no association between depression and immediate recall on the RAVLT. Combined with our results, depression severity does not appear to be systematically related to verbal learning and memory.
To our knowledge, this is one of the first examinations of error on the RAVLT in a controlled, severely depressed patient cohort referred for ECT. Depression severity as rated by self-report, but not by clinician report, showed a trend to positively correlate with the number of perseverations. Based on the taxonomy of Sandson and Albert [36], these were recurrent preservations, which is repetition of a previously mentioned exemplar that is contained within the appropriate task. This type of perseveration has been associated with neurocircuitry in the posterior left hemisphere (Sandson & Albert [36]. We are unsure at this time of the implications of this finding as there is limited research regarding perseverative errors [37], but suggest that error be examined in future studies. It may be possible that speed of response, accuracy, and error, are three separate performance variables with differential implications [38]. For example, error rate relative to processing speed on the Trail Making Test has been found to be less affected by age, and may be a more sensitive indicator of impaired cognitive function [39, 40]. Further, Lezak et al. [41] reported that perseverations on a verbal list learning task may indicate either a problem in attention or mental flexibility. Thus, the examination of perseveration errors, in addition to other important variables, on the RAVLT in severely depressed patients may provide incremental information regarding which domain(s) of cognitive function are affected by depression.
The findings with the IDS-SR30 are somewhat discrepant from those with the HRSD24 including a negative association between the former, but not the latter with lower recall of words on the RAVLT interference list, and a trend to associate with lower performance on the MMSE and a greater number of perseverative errors on the RAVLT. These two depression severity measures are different in three ways that could explain the somewhat discrepant findings in this study. First, a clinical rater completed the HRSD24 and the subject completed the IDS-SR30. Second, the HRSD24 and the IDS-SR30 are comprised of different items with respect to quantity (24 vs. 30, respectively) and depressive symptom content. For example, the IDS-SR30, but not the HRSD24 assesses cognitive complaints. Item 15 (concentration/decision making) of the IDS-SR30 assesses concentration, attention, and decision making [21]. The IDS-SR30 also measures other depressive symptoms not assessed by the HRSD24 (i.e., atypical depressive symptoms, pain complaints). Third, the HRSD24 and the IDS-SR30 have different rating metrics. While the HRSD24 rates some items on a scale of 0 – 4 or 0 –2, the IDS-SR30 applies a rating of 0 –3 on all items. Thus, it would be useful in future investigations to use a depression severity measure that has parallel clinician-rated and patient-rated versions to control for confounds related to item content or rating.
Regarding clinical characteristics, this study revealed no significant relationships between the number of depressive episodes or the length of the current depressive episode and neurocognitive performance. This is consistent with other studies that also found no relationship between the number of depressive episodes, depressive episode length and neurocognitive function in patients with mild to moderate MDD [42–45]. For example, Reisheies & Neu [45] found no association between depressive episode length and performance on neurocognitive measures of attention, memory, or executive function. However, findings by Burt et al. [12] suggested that the number of depressive episodes can negatively impact neurocognitive function. Thus, clinicians and researchers should document depressive clinical characteristics to determine their impact, if any, on neurocognitive function.
The focus of this study was on the relationship between depression severity in a severely depressed cohort and neurocognitive function. As such, a limitation of the present study was the restricted range in depression severity and global cognitive function as measured on the HRSD24 and MMSE, respectively. Entry criteria into the study required a score of 21 or greater on the HRSD24 and a score of 23 or greater on the MMSE to rule out possible dementia, which may have limited the size of the correlations. However, we compared the lower (HRSD24 mean=26.7(2.3)) and upper (HRSD24 mean=40.6(4.5)) quartiles of the cohort to assess for neurocognitive effects of depression severity. Comparisons between the lower and upper quartiles showed no differences on the MMSE, RAVLT, or the CFT. This provides further support that severity of the depressive episode was unrelated to neurocognitive performance. Also, based on the data provided in the Alpert et al. [33] study, the inclusion of participants with lower HRSD24 scores would not necessarily have led to an association between depression severity and neurocognitive performance. A further limitation of this study was the relatively small sample size and inclusion of patients with severe MDD without psychotic features. Thus, these results may not generalize to other depressive patient cohorts. The strength of this analysis included a well-characterized cohort using standardized research diagnostic criteria for psychiatric diagnosis, quantification of depression severity on clinician rated and self-report symptom-severity instruments, and use of normative adjusted data for neuropsychologic scores. The sample in this study consisted of depressed patients referred for ECT, which underscores that this sample was severely depressed. We believe that this study provides important information regarding the effects of the most severe depression on neurocognitive function.
Although depression has been reported to negatively affect neurocognitive function in some cases [46], the severity of the depressive episode, based on clinical characteristics or depression severity measures, might not be related to the impaired cognitive function. Porter et al. [47] reported that patients with depression often demonstrate intact neurocognitive performance, which may be dependent on factors such as age and motivation. Variables such as psychomotor retardation and education have been found to moderate neuropsychologic test performance in some depressed individuals [48]. Thus, various factors (i.e., age, education, undiagnosed dementia) may affect the neurocognitive performance of patients with depression. To enhance the value of neurocognitive performance interpretation in research studies, it is critical to use normative adjusted data for interpretation of results. For instance, a raw score of 24 on the MMSE is thought to reflect normal performance [49], but in this sample of severely depressed patients, 17.5% showed impaired performance (conservatively defined as a total score 2 standard deviations below the mean) when the raw scores were adjusted based on normative data. This finding also demonstrates that while some of the patients in this study had impaired neurocognitive performance, it was unrelated to the severity of the depressive episode.
CONCLUSION
In conclusion, this is one of the first studies to show that the severity of the depressive episode is unrelated to the degree of global cognitive or memory difficulties in patients with severe depression. Severity of the depressive episode was not associated with and did not predict global cognitive or memory function. Future research is needed to understand the complex interaction between depression and neurocognitive function and we will follow up these findings with further analyses.
Focus Points.
The relationship between major depressive disorder and neurocognitive function is complex.
Studies of this complex relationship should include controlled methods with psychometrically sound clinical and neurocognitive instruments.
Depression severity may not affect global cognitive function, verbal learning and memory, or visual learning and memory, in patients with severe major depressive disorder.
Depression severity may be related to increased error on select neurocognitive measures.
Research focused on the interactions between other depressive characteristics and neurocognitive function is needed.
Acknowledgments
Funding
This work was supported by funding from the National Institutes of Health (NIH) grants MH 62355 (PI: Mustafa M. Husain), 62357 (PI: Keith Rasmussen), 62353 (PI: Georgios Petrides), and 67201 (PI: Charles H. Kellner). Also, this publication was supported by Grant Number KL2RR024983 (PI: Milton Packer) from the National Center for Research Resources (NCRR), a component of the NIH.
Portions of the information and analyses in this manuscript were presented at the 37th Annual Meeting of the International Neuropsychological Society. The authors thank the clinical raters and neuropsychometrists who collected the data, particularly Ms. Judy Shaw who was the Chief Neuropsychometrist for the CORE Lead Placement Trial. The authors also thank Dr. Charles Quinn and Dr. John Nelson for their helpful review of this manuscript.
References
- 1.Minor KL, Champion JE, Gotlib IH. Stability of DSM-IV criterion symptoms for major depressive disorder. Journal of Psychiatric Research. 2005;39:415–420. doi: 10.1016/j.jpsychires.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Moussavi S, et al. Depression, chronic diseases, and decriments in health: Results from the World Health Survey. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 3.Rush A. STAR*D: What have we learned? American Journal of Psychiatry. 2007;164(2):201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- 4.Zakzanis KK, Leach L, Kaplan E. Neuropsychological Differential Diagnosis. USA: Swets & Zeitlinger; 1999. [Google Scholar]
- 5.Shenal BV, Harrison DW, Demaree HA. The neuropsychology of depression: A literature review and preliminary model. Neuropsychology Review. 2003;13:33–42. doi: 10.1023/a:1022300622902. [DOI] [PubMed] [Google Scholar]
- 6.McClintock SM, et al. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. doi: 10.1037/a0017336. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: Patients on and off medication versus healthy comparison subjects. Journal of Neuropsychiatry Clinical Neuroscience. 2006;18:217–225. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Rohling ML, et al. Depressive symptoms and neurocognitive test scores in patients passing symptom validity tests. Archives of Clinical Neuropsychology. 2002;17:205–222. [PubMed] [Google Scholar]
- 9.Trichard C, et al. Time course of prefrontal lobe dysfunction in severely depressed in-patients: A longitudinal neuropsychological study. Psychological Medicine. 1995;25:79–85. doi: 10.1017/s0033291700028105. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Steer RA. Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 11.Elderkin-Thompson V, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Archives of Clinical Neuropsychology. 2003;18:529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 12.Burt T, et al. Learning and memory in bipolar and unipolar major depression: Effects of aging. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2000;13:246–253. [PubMed] [Google Scholar]
- 13.Austin MP, et al. Cognitive function in depression: A distinct pattern of frontal impairment in melancholia? Psychological Medicine. 1999;29:73–85. doi: 10.1017/s0033291798007788. [DOI] [PubMed] [Google Scholar]
- 14.Kellner CH, et al. Comparing bifrontal, bitemporal, and right unilateral electrode placement in ECT: A multisite study from CORE. British Journal of Psychiatry. doi: 10.1192/bjp.bp.109.066183. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, et al. User’s Guide for the Structured Clinical Interview for the DSM-IV Axis I Disorders-Research Version (SCID-I, Version 2.0) 1996. [Google Scholar]
- 16.Hamilton M. A rating scale for depression. Journal of Neruology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, Fanjiang G. MMSE: Mini-Mental State Examination Clinical Guide. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 19.Brown RG, et al. Cognitive function in depression: Its relationship to the presence and severity of intellectual decline. Psychological Medicine. 1994;24:829–847. doi: 10.1017/s0033291700028932. [DOI] [PubMed] [Google Scholar]
- 20.Lamberty GJ, Bieliauskas LA. Distinguishing between depression and dementia in the elderly: A review of neuropsychological findings. Archives of Clinical Neuropsychology. 1993;8:149–170. [PubMed] [Google Scholar]
- 21.Rush A, et al. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi MH, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychological Medicine. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 24.Rey A. L’Examen Clinique en psychologie. Paris: Press Universitaire de France; 1964. [Google Scholar]
- 25.Rey A, Osterrieth PA. Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy” and P.A. Osterrieth’s “The complex figure test”. In: Corwin J, Bylsma FW, translators. The Clinical Neuropsychologist. Vol. 7. 1993. pp. 3–21. [Google Scholar]
- 26.Taylor LB. Localisation of cerebral lesions by psychological testing. Clinical Neurosurgery. 1969;16:269–287. doi: 10.1093/neurosurgery/16.cn_suppl_1.269. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson GS. Wide Range Achievement Test (WRAT-3)Administration Manual. Wilmington: Wide Range, Inc; 1993. [Google Scholar]
- 28.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 29.Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill; 1994. [Google Scholar]
- 30.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for clinicians. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Forsell Y, Jorm AF, Winblad B. Assocation of age, sex, cognitive dysfunction, and disability with major depressive symptoms in an elderly sample. American Journal of Psychiatry. 1994;151:1600–1604. doi: 10.1176/ajp.151.11.1600. [DOI] [PubMed] [Google Scholar]
- 33.Alpert JE, et al. The Mini-Mental State Examination among adult outpatients with major depressive disorder. Psychotherapy and Psychosomatics. 1995;63:207–211. doi: 10.1159/000288961. [DOI] [PubMed] [Google Scholar]
- 34.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychological Bulletin. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 35.Delis DC, et al. The California Verbal Learning Test: Research Edition. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 36.Sandson J, Albert ML. Varieties of perseveration. Neuropsychologia. 1984;22:715–732. doi: 10.1016/0028-3932(84)90098-8. [DOI] [PubMed] [Google Scholar]
- 37.Hotz G, Helm-Estabrooks N. Perseveration Part I: A review. Brain Injury. 1995;9(2):151–159. doi: 10.3109/02699059509008188. [DOI] [PubMed] [Google Scholar]
- 38.Possin KL, et al. Is a perseveration a perseveration? An evaluation of cognitive error types in patients with subcortical pathology. Journal of Clinical and Experimental Neuropsychology. 2005;27:953–966. doi: 10.1080/13803390490919092. [DOI] [PubMed] [Google Scholar]
- 39.Wahlin RTB, et al. Trail Making Test performance in a community-based sample of healthy very old adults: effects of age on completion time, but not on accuracy. Archives of Gerontology and Geriatrics. 1996;22(1):87–102. doi: 10.1016/0167-4943(95)00681-8. [DOI] [PubMed] [Google Scholar]
- 40.Ashendorf L, et al. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Archives of Clinical Neuropsychology. 2008;23(2):129–137. doi: 10.1016/j.acn.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lezak MD, et al. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 42.Fossati P, Ergis AM, Allilaire JF. Problem-solving abilities in unipolar depressed patients: Comparison of performance on the modified version of the Wisconsin and the California sorting tests. Psychiatry Research. 2001;104:145–156. doi: 10.1016/s0165-1781(01)00307-9. [DOI] [PubMed] [Google Scholar]
- 43.Markela-Lerenc J, et al. Stroop performance in depressive patients: A preliminary report. Journal of Affective Disorders. 2006;94:261–267. doi: 10.1016/j.jad.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Delis DC, et al. Componential analysis of problem-solving ability: Performance of patients with frontal lobe damage and amnesic patients on a new sorting test. Neuropsychologia. 1992;30(8):683–697. doi: 10.1016/0028-3932(92)90039-o. [DOI] [PubMed] [Google Scholar]
- 45.Reischies FM, Neu P. Comorbidity of mild cognitive disorder and depression: A neuropsychological analysis. European Archives of Psychiatry and Clinical Neuroscience. 2000;250:186–193. doi: 10.1007/s004060070023. [DOI] [PubMed] [Google Scholar]
- 46.Airaksinen E, et al. Cognitive functions in depressive disorders: Evidence from a population-based study. Psychological Medicine. 2004;34:83–91. doi: 10.1017/s0033291703008559. [DOI] [PubMed] [Google Scholar]
- 47.Porter RJ, et al. Neurocognitive impairment in drug-free patients with major depressive disorder. British Journal of Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- 48.Den-Hartog HM, et al. Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: Testing the effort and cognitive speed hypotheses. Psychological Medicine. 2003;33:1443–1451. doi: 10.1017/s003329170300833x. [DOI] [PubMed] [Google Scholar]
- 49.Crum RM, et al. Population-based norms for the Mini-Mental State Examination byh age and education level. Journal of the American Medical Association. 1993;269:2386–2391. [PubMed] [Google Scholar]