Abstract
The present study examined the moderating role of sleep in the association between family demands and conflict and hypothalamic-pituitary-adrenal (HPA) axis functioning in a sample of ethnically diverse adolescents (n = 316). Adolescents completed daily diary reports of family demands and conflict for 15 days, and wore actigraph watches during the first 8 nights to assess sleep. Participants also provided five saliva samples for 3 consecutive days to assess diurnal cortisol rhythms. Regression analyses indicated that sleep latency and efficiency moderated the link between family demands and the cortisol awakening response. Specifically, family demands were related to a smaller cortisol awakening response only among adolescents with longer sleep latency and lower sleep efficiency. These results suggest that certain aspects of HPA axis functioning may be sensitive to family demands primarily in the context of longer sleep latency and lower sleep efficiency.
Keywords: Demands, Conflict, Cortisol, Sleep latency, Sleep efficiency, Adolescents
1. Introduction
The family environment during childhood and adolescence is important for the development of health. Youth from a family environment marked by socioeconomic disadvantage, a lack of support and structure, hostility, and conflict are at higher risk for developing poor mental and physical health outcomes such as depression, substance use, cardiovascular disease, hypertension, and obesity (Miller et al., 2011; Repetti et al., 2002). Family stress may contribute to physical health through its influence on the hypothalamic-pituitary-adrenal (HPA) axis. Stress modulates the production and release of cortisol, which follows a diurnal rhythm, peaking in the morning approximately 30–45 min after waking, and declining across the day until its nadir around midnight (Pruessner et al., 1997). A large body of work has linked family stress during childhood and adolescence to deviations from this pattern (Repetti et al., 2002). For instance, maternal separation has been linked to a larger cortisol awakening response (CAR) and a flatter diurnal cortisol slope (Kumari et al., 2012). Lower maternal involvement and warmth and poorer marital relationships have also been associated with a flatter diurnal slope and greater average cortisol output among youth (Pendry and Adam, 2007). However, not all individuals react similarly to their environment, and variation in biological stress responses partially depend on individual difference factors (Ellis and Boyce, 2011). Given its links to the HPA axis and regulation of cognitive and emotional functioning relevant to stress processes, sleep may be one such factor contributing to individual differences in sensitivity to family stress. The present study examined the role of sleep in the association between everyday family stress and adolescent HPA axis functioning.
The sleep-wake cycle and HPA secretion of cortisol largely depend on a circadian rhythm centrally regulated by the suprachiasmatic nuclei in the hypothalamus (Buckley and Schatzberg, 2005). Thus, HPA secretion of cortisol is intimately linked with sleep. During the first few hours of sleep, levels of cortisol remain low and subsequently rise throughout the sleep period until morning awakening (Balbo et al., 2010). Given these links, sleep difficulties have been associated with altered HPA functioning in both adults and youth. Among adults, experimentally-induced partial and total sleep loss increased evening levels of cortisol (Leproult et al., 1997; Spiegel et al., 1999) and total cortisol output (Wright et al., 2015). Additionally, circadian misalignment decreased total cortisol output and CAR (Griefahn and Robens, 2008; Wright et al., 2015), and trouble falling and staying asleep were related to a flatter diurnal slope (Kumari et al., 2009). Among youth, experimentally-induced acute sleep restriction resulted in a diminished CAR (Gribbin et al., 2012), and shorter sleep duration was related to a flatter diurnal slope (Zeiders et al., 2011), lower waking levels of cortisol (Vargas and Lopez-Duran, 2014), and a greater CAR (Lemola et al., 2015; Raikkonen et al., 2010; Vargas and Lopez-Duran, 2014). Lower sleep efficiency has also been linked to higher afternoon levels of cortisol (El-Sheikh et al., 2008) and total cortisol throughout the day (Raikkonen et al., 2010).
Importantly, sleep may moderate the effects of family stress on the HPA axis. The transactional theory of stress (Lazarus, 1966; Lazarus and Folkman, 1984) posits that the effects of stress depend on individuals’ cognitive appraisals of threat and resources to cope. Similarly and more specifically, the cognitive-contextual framework (Grych and Fincham, 1990) suggests that the effects of family stress on youths’ adjustment is mediated by youths’ appraisals. Notably, sleep is critical to cognitive appraisal and coping processes. In one study, poor and good sleepers reported equal number of stressful events, but poor sleepers perceived stressors to be more intense (Morin et al., 2003). In past experimental studies, sleep-deprived individuals rated mild stressors as more stressful (Minkel et al., 2012) and exhibited greater amygdala responses to negative emotional stimuli (Motomura et al., 2013). Inadequate sleep has also been linked to poorer executive function (Anderson et al., 2009) and emotion regulation (Baum et al., 2014; Mauss et al., 2013), both of which contribute to coping processes (Compton et al., 2011; Garnefski et al., 2001; Villegas and Cruz, 2015). Appraisals of stressors and coping strategies, in turn, contribute to HPA reactivity to and recovery from stress (Bohnen et al., 1991; Gaab et al., 2005; Olff et al., 2005). As a regulator of these processes, sleep may contribute to variability in the stress-HPA association.
The moderating role of sleep on the effects of stress during youth development has been previously proposed (El-Sheikh et al., 2010), and a growing body of literature supports this notion. For instance, peer victimization was more strongly related to internalizing symptoms among adolescents with more perceived sleep problems compared to those with fewer perceived sleep problems (Tu et al., 2015). Similarly, perceived discrimination was related to more depressive symptoms and externalizing behaviors among adolescents with shorter sleep duration and poorer perceived sleep quality (El-Sheikh et al., 2016; Yip, 2015). In regards to family functioning, adolescents exhibited more aggressive behaviors in the context of greater marital conflict and shorter subjective sleep duration and inconsistent sleep schedules (Lemola et al., 2012). In the context of family economic adversity and poor sleep (i.e., objective shorter sleep duration and low sleep efficiency), maternal psychological control was related to higher levels of depressive and anxiety symptoms among youth (El-Sheikh et al., 2010). By contrast, maternal sensitivity was related to fewer internalizing and externalizing symptoms for children who slept longer during infancy (Bordeleau et al., 2012) and to better executive functioning for children who had more consolidated sleep during infancy (Bernier et al., 2014). Although growing evidence supports sleep as a moderator of the effects of psychosocial stress, this body of work has focused primarily on socio-emotional outcomes. The present study builds on this work by examining sleep’s moderating role in the relation between family stressors and biological functioning, namely the HPA axis.
Prior work on the role of sleep in the effects of experimentally induced stress on HPA responses points to the possibility that sleep may also moderate the link between family stress and HPA axis functioning. Low sleep efficiency was associated with greater levels of cortisol after a laboratory social stressor among 8- and 9-year old children compared to their counterparts who had average or higher sleep efficiency (Raikkonen et al., 2010). Likewise, more awakenings after sleep onset was related to greater total cortisol output in response to a psychosocial stressor among kindergarten children (Hatzinger et al., 2008). Poorer perceived sleep quality in adults has also been associated with greater cortisol responses to the cold-pressor task (Goodin et al., 2012), a stress induction task that involves submerging a hand in ice-cold water. It is unknown whether these associations translate to adolescents and everyday experiences of family-related stress. The current study expands this work by shifting the focus to everyday experiences of family stressors.
We focused particularly on family demands and conflict given that experience of these aspects of family functioning may change during adolescence. Additionally, prior work on adults suggests that family demands and conflict are impactful daily stressors (Bolger et al., 1989). Family demands refer to activities, responsibilities, and expectations placed upon adolescents by their families (e.g., household chores) (Fuligni et al., 2009). Family conflict refers to behaviors or threats of verbal, psychological, or physical aggression among family members (Straus, 1979).
Family demands and conflict tend to increase during adolescence and become prevalent in adolescents’ everyday lives (Keith et al., 1990; Telzer and Fuligni, 2009a). As adolescents develop an increased sense of autonomy, parents may increasingly entrust adolescents with and expect them to assist in various household and family tasks. Although contributing to the family unit can serve as a means of maintaining family connectedness (Telzer and Fuligni, 2009a), it can also become burdensome, especially in the face of simultaneously increasing demands in the social and academic domains. Indeed, helping care for family has been associated with negative outcomes, including elevated inflammation, poorer academic achievement, and substance use (Fuligni et al., 2009; Telzer and Fuligni, 2009b; Telzer et al., 2014). The development of autonomy may also render adolescents more willing to overtly disagree with their parents (Smollar and Youniss, 1989). Consequently, frequency and intensity of conflict with parents increases over the course of adolescence (Smetana et al., 2006). Although this may be part of normative adolescent development, high levels of conflict with parents during adolescence can have negative ramifications, such as increased risk for depression, substance use, and risky sexual behavior (Herrenkohl et al., 2012; Lyerly and Huber, 2013).
The goal of the present study was to evaluate sleep as a moderator of the relation between family demands and conflict and HPA axis functioning during adolescence. We examined family demands and conflict separately given that they reflect different aspects of family functioning and may therefore have different implications for HPA functioning. In support of this notion, previous research has demonstrated that various family stressors are differentially associated with different aspects of HPA functioning (Kuhlman et al., 2015; Laurent et al., 2014; Lovallo et al., 2012). Examining family demands and conflict separately can thus provide a more nuanced understanding of specific facets of the family environment that are relevant for HPA activity.
We assessed sleep behaviorally using actigraphy and focused specifically on sleep duration, sleep efficiency, and sleep latency because these sleep parameters have been associated with health-relevant outcomes (e.g., prehypertension, parasympathetic functioning, pain, inflammation, insulin resistance) among youth (Hall et al., 2015; Javaheri et al., 2008; Matthews et al., 2012; Michels et al., 2013; Palermo et al., 2007; Rodríguez-Colón et al., 2015). We conceptualize sleep on a continuum, and use the term “poor sleep” to refer to shorter sleep duration, lower sleep efficiency, and longer sleep latency, as has been done in previous research (Doane and Thurston, 2014; Kahlhöfer et al., 2016).
Based on the prior theoretical and empirical work reviewed above, we hypothesized that sleep would moderate the relation between family demands and family conflict and HPA axis functioning. More specifically, adolescents who have poorer sleep would exhibit a relation between family demands and conflict and altered HPA axis functioning, reflected as greater or decreased total cortisol output and/or CAR, flatter diurnal slopes, and/or higher bedtime levels of cortisol. In contrast, better sleep was hypothesized to attenuate the effects of family stress on HPA axis functioning.
2. Methods
2.1. Participants
Participants were 316 adolescents (Mage = 16.40 years, SD = 0.74; 136 males and 180 females) from European (29.1%), Latino (41.8%), Asian (23.1%), and other (6.0%) ethnic backgrounds. Most of the adolescents from Latino and Asian backgrounds were from immigrant families, with 5.3% of Latino and 37.0% of Asian adolescents being first-generation (i.e., foreign-born), and with 54.5% of Latinos and 61.6% of Asian adolescents being second generation (i.e., US-born with at least one foreign-born parent). The majority (90.2%) of adolescents from European backgrounds were third generation or greater (i.e., adolescent and both parents US-born).
Participants were mostly from middle-class backgrounds: median household income reported by primary caregivers was $50,000 (range = $0–$825,000). Primary caregivers also indicated their own and their spouse’s highest level of education completed, using an 11-point scale (1 = some elementary school, 11 = graduated from medical, law, or graduate school). Averaging education across parents revealed that on average, adolescents’ parents completed some vocational or trade school (M = 7.21, SD = 1.80, range = 1.5–11). Approximately 14% of participants had parents with less than a high school diploma, 14.9% had parents with a high school diploma, 42.4% had parents who completed vocational trade school or some college, 16.8% had parents with a college degree, and 11.1% had parents who completed at least some medical, law, or graduate school.
2.2. Procedures
Adolescents and their primary caregivers were recruited from four high schools in the Los Angeles area via in-class presentations and study fliers and recruitment forms distributed in class and mailed to students’ homes. Families indicating interest on the recruitment forms were contacted and given more details about the study. Those who provided verbal parental consent were scheduled for an initial visit in participants’ homes or a local field research center. In the initial visit, study staff first obtained written consent, after which adolescents and their primary caregivers, usually biological mothers (89.5%), completed a set of questionnaires. Upon completion of the questionnaires, study staff provided participants with instructions for the daily diary portion of the study.
During the diary portion of the study, adolescents reported on their family demands, wore an actigraph watch, and provided saliva samples for cortisol. Each night for 15 consecutive days, adolescents completed four-page diary checklists of their social and emotional experiences. They were to complete the diary checklists before going to bed each night. During the first eight consecutive days, adolescents wore a wrist actigraph watch at night and completed morning diaries of their sleep from the previous night. During the first three days, participants provided saliva samples at 5 time points throughout the day: at waking, 15 min post-wake, 30 min post-wake, before dinner, and before bed. Although variables of interest were each assessed for a different number of days, it is important to note that we were interested in characterizing participants’ typical experiences in their daily lives. We used daily assessments to enhance measurement of their experiences, as conventional self-report measures are subject to cognitive and recall biases. Assessment of all variables was based on both weekdays and weekends. Each construct was assessed for a different number of days in order to minimize participant burden while following standard practice for assessing each construct.
To help ensure compliance, text messages were sent to adolescents throughout the day, reminding them when to complete the checklist, collect a saliva sample, and wear the actigraph watch. Adolescents were also provided with time stampers (Dymo Corporation, Stamford, CT) and stamping booklets that were used to indicate when nightly diary checklists and morning sleep reports were completed and when saliva samples were collected. Each page in the stamping booklet corresponded to a particular day and listed in temporal order the checklists and saliva samples that were to be completed. The electronic time stampers imprint the current date and time and were pre-programmed with a security code to prevent participants from altering the pre-set date and time. Adolescents were instructed to stamp the booklet beside the appropriate heading (e.g., morning sleep report, saliva at wake up, etc.) each time they provided data.
Study staff returned to participants’ homes at the end of the daily diary period to collect completed materials. Adolescents were compensated $50 and received two movie passes if their daily checklists were completed correctly and on time. Bilingual study staff were available to conduct study procedures in English, Spanish, or Chinese, and all study materials were available in these languages. Seven participants (2.2%) completed the study in Chinese. The UCLA Institutional Review Board approved all study procedures.
2.3. Measures
2.3.1. Family stress
Each night for 15 consecutive nights, adolescents completed diary reports of their family demands and family conflict. Assessment of family demands consisted of two items. Participants indicated whether they had a lot of work at home or had a lot of demands made by their families. This measure was adapted from prior adult work focusing on daily stress and well-being (Bolger et al., 1989), and has previously been associated with negative outcomes among adolescents, including poorer academic performance and school attendance (Flook and Fuligni, 2008) and shorter sleep duration (Fuligni and Hardway, 2006). A summary variable was computed to represent the proportion of days out of the 15 days that adolescents endorsed at least one of the two items. First, the two items were summed for each given day (range: 0–2). This variable was then recoded to indicate whether family demands occurred that day (0 or 1). Lastly, the average was taken across days, which resulted in a summary score that represented the proportion of days that included family demands.
Family conflict was assessed with three items. Adolescents indicated whether their parents argued or whether they argued with one of their parents or other family member. This measure has been used with adolescents, showing significant associations with psychological distress (Chung et al., 2009). The same process for creating the family demands summary variable was used to create a family conflict summary variable to index the proportion of days adolescents experienced any one of the three items.
The majority of adolescents (approximately 94.5%) completed daily checklists for at least 14 days. On average, adolescents completed 14.62 (SD = 1.45) of the 15 daily checklists. Of the completed diaries for any given day, the vast majority (97.1–99.3%) was judged to be compliant (i.e., completed before noon the following day). For 84.3% of adolescents, family stress summary variables were based on 15 days, and 13.4% and 2.2% of adolescents had diary variables computed based on 10–14 days and fewer than 10 days, respectively.
2.3.2. Sleep
Sleep was assessed using actigraphy (Micro Motionlogger Sleep Watch, Ambulatory Monitoring, Inc.), which measures movement to make inferences about sleep. Adolescents were instructed to wear the actigraph watch on their non-dominant wrists for eight consecutive nights. In addition, they were instructed to push a button on the actigraph watch to mark the following events: turning off the lights to sleep, getting out of bed in the middle of the night, and getting out of bed in the morning. Approximately 93% of adolescents (n = 294) wore the actigraph watches, and on average, these adolescents wore the actigraph watches for 6.58 out of 8 nights (SD = 1.45, Mdn = 7 nights). The majority wore the watches for at least 5 days: 28.9% wore them for 8 days, 33.3% wore them for 7 days, 23.1% wore them for 6 days, 4.4% wore them for 5 days, and 10.3% wore them for 4 or fewer days.
The software package Action4 (Ambulatory Monitoring, Inc.) was used to code and score actigraphy data. The first event marker indicating when lights were turned off to sleep and the last event marker indicating when participants got out of bed in the morning were used to determine the in-bed period. If event markers were absent on any given night, daily sleep reports were used.
Indices of sleep included sleep duration, efficiency, and latency. These indices were calculated by first scoring one-minute epochs using the Sadeh actigraph scoring algorithm (El-Sheikh et al., 2006; Sadeh et al., 1994; Wolfson et al., 2003). The first of at least three consecutive minutes of sleep were used to determine sleep onset time, and the last five or more consecutive minutes of sleep were used to determine sleep offset time (Acebo et al., 2005). Sleep periods were checked against the morning self-reports of the previous night’s sleep. Total sleep duration for each night was the total number of minutes scored as sleep during the in-bed period. Sleep efficiency was calculated as percentage of actual sleep during total time in bed, and sleep latency was the number of minutes taken to fall asleep. Sleep indices were averaged across the eight nights to compute adolescents’ mean sleep duration, sleep efficiency, and latency.
2.3.3. HPA-axis
Diurnal HPA-axis functioning was assessed by collecting five saliva samples using Salivettes (Sarstedt, Inc.) each day for three consecutive days. Participants were instructed to collect saliva before eating, drinking or brushing their teeth and to refrain from using tobacco products or consuming caffeinated products 30 min before saliva collection. Participants stored samples in their household refrigerators until research staff picked them up (typically 1–3 weeks). Samples were then stored in −80 °C until shipped to the Laboratory of Biological Psychology at the Technical University of Dresden, Germany where they were assayed for cortisol using high-sensitivity chemiluminescence-immunoassays (IBL International, Hamburg, Germany). The inter- and intra-assay coefficients of variation were below 8%.
Approximately 97.2% of participants (n = 307) provided at least one saliva sample across the three-day collection period, 96.2% (n = 304) provided all five samples for at least one day, and 86.1% (n = 272) provided all five samples on all three days. The majority of participants provided saliva samples on both weekdays and weekend days: 30.1% of participants provided samples on weekdays only.
Cortisol values greater than 60 nmol/L (n = 2) were set to missing. Cortisol values from two adolescents were also set to missing because these adolescents provided the same sampling time for all samples within a given day. Morning saliva samples that were considered non-compliant according to actigraphy-based estimations of wake time were also assigned as missing given that the estimation of CAR is sensitive to timing of samples relative to actual wake time (Dockray et al., 2008; Stalder et al., 2016). Samples were deemed non-compliant if they were provided past a 15-min window around the actigraph wake time, and around the 15- and 30-min mark after actigraph wake time. On any given day, 43–84 adolescents provided at least one non-compliant morning sample.
Cortisol values were log-transformed to correct for non-normality and used to compute total cortisol output (AUC), cortisol awakening response (CAR), cortisol decline over the day, and bedtime cortisol levels. All five samples were used to compute AUC with respect to ground using an established trapezoid formula (Pruessner et al., 2003). CAR was computed using two approaches found in previous HPA research (Rotenberg et al., 2012; Stalder et al., 2016). First, we subtracted wake sample from 30-min post-waking sample and divided by the fraction of hour between the samples. This reflects the rate of increase per hour (CAR). Second, we computed AUC with respect to increase using the three morning samples (CARi). Diurnal slope was computed by subtracting the 30-min post-waking sample from bedtime sample and dividing by the fraction of hour between the samples. We anchored the diurnal slope at the 30-min post-waking sample rather than the waking sample because anchoring the diurnal slope at the waking sample may yield less reliable estimates (Rotenberg et al., 2012). All indices were averaged across days.
Individual average HPA indices were missing for a particular day if any one of the relevant cortisol samples or sampling times was also missing. Thus, 70.8% of adolescents had values for AUC, 83.9% for CAR, 83.9% for diurnal slope, and 97.1% for bedtime cortisol. Of the participants who had values for HPA indices, 41.8–96.4% of adolescents’ HPA parameters were based on three sampling days. Approximately 2.61–35.74% had HPA parameters based on two sampling days, and 0.98–22.49% had HPA parameters based on one sampling day.
2.4. Statistical analysis
In order to reduce their influence on results, values beyond three SD’s on measures were set to be missing. Outlier screening identified four individuals below three SD’s on sleep efficiency, seven individuals above three SD’s on sleep latency, and three individuals below three SD’s on sleep duration. After excluding outliers and cortisol values from noncompliant saliva samples, 217 out of the 316 participants had complete data on all computed variables of interest and covariates. Less than 1% of adolescents had missing data for family stress variables, 7.6–9.2% for sleep indices, 2.9–21.2% for HPA indices, and up to 1.9% for covariates. Multiple imputation was conducted in order to minimize potential bias stemming from missing data. All study variables, potential confounds, and auxiliary variables were included in imputation models, and twenty datasets were generated.
A series of multiple linear regressions were conducted to examine whether sleep (i.e., duration, latency, and efficiency) moderated the association between family stress and the diurnal rhythm of the HPA axis. Potential confounding variables were first entered, followed by family stress, sleep, and then the family stress by sleep interaction term. Separate models were examined for each family stress, sleep, and HPA axis parameter. Observed significant interactions were followed up with tests of simple slopes using mean splits given that there were no individuals either above or below one standard deviation for the sleep indices.
Sociodemographic characteristics (i.e., age, gender, ethnicity, and parental education) and depressive symptoms were included as covariates in all models given that they have been associated with HPA axis functioning (DeSantis et al., 2007; Dowd et al., 2009; Stetler and Miller, 2011; Stroud et al., 2009; Uhart et al., 2006). Models also controlled for mean wake time across the three days of saliva sampling given that wake time has been related to diurnal functioning of the HPA axis (Zeiders et al., 2011).
Other potential covariates of cortisol include body mass index (BMI) (Champaneri et al., 2013), caffeine consumption (Lovallo et al., 2005), physical activity (Jacks et al., 2002), negative affect (Adam et al., 2006), and medication use. We examined whether these variables should be included as covariates. For medication use, 64 adolescents reported using some type of medication on at least one of the days on which saliva samples were collected. Medications included over the counter drugs such as aspirin, birth control, and prescription drugs for acne. Including these variables as covariates in the models did not alter results. Given that prior work has demonstrated differences in cortisol patterns on weekdays versus weekends (e.g., Karlamangla et al., 2013), we also examined whether weekday versus weekend should be included as a covariate. We tested mean differences in HPA indices between adolescents who provided saliva samples on weekend days and those who provided samples on weekdays only. We observed no mean differences, and controlling for weekday versus weekend did not alter results. Because controlling for these potential confounds did not change results, we report results from the more parsimonious models.
We also reran models using raw cortisol values and found that results remained unchanged. Although results from models using log-transformed HPA indices are reported, figures are based on models using raw cortisol values for interpretative and comparative purposes. All regression models were tested in the sample with complete cases (i.e., listwise deletion) and overall findings were the same. Given that missing data can bias estimates, the pooled estimates from regression analyses of the imputed datasets are reported. All analyses were conducted using Stata 12.1.
3. Results
Participant characteristics are presented in Table 1. On average, adolescents reported having family demands on 12% of days (i.e., 1.8 days) and family conflict on 20% of days (i.e., 3 days) during the 15-day daily diary period. Approximately 4.5% of adolescents reported having demands on at least 50% of the 15 days, 12.1% had demands 26–50% of days, and 38.6.% had demands on 1–25% of the days. Nearly 44.7% of adolescents reported having no family demands during the 15-day period. Family conflict occurred more frequently, with 9.6% of adolescents having family conflict on at least 50% of the 15 days, 22.4% had conflict 26–50% of the days, 44.7% had conflict on 1–25% of the days, and 23.6% reported having no family conflict during the 15-day period. In regards to sleep, adolescents obtained 7.46 h of sleep, took 10.73 min to fall asleep, and achieved over 90% sleep efficiency, on average. Approximately 32.7% of adolescents had sleep latency that was above the mean, 37.3% had below average levels of sleep efficiency, and 45.1% had below average levels of sleep duration.
Table 1.
Descriptive Statistics and Bivariate Correlations.
| Variables | Mean (SD) | Range | 1 Age | 2 Gender | 3 Parent education |
4 BMI | 5 Depressive Symptoms |
6 Family Demands |
7 Family Conflict |
8 Sleep Latency |
9 Sleep Duration |
10 Sleep Efficiency |
11 AUC | 12 CAR | 13 DCS | 14 Bed- time cortisol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 16.39 (0.74) | 14.50–20.50 | 0.03 | −0.13* | 0.07 | −0.03 | −0.10 | −0.01 | −0.08 | −0.15* | −0.10 | −0.05 | 0.07 | 0.08 | 0.01 | |
| 2. Gender | −0.11* | −0.03 | 0.17** | −0.05 | 0.14* | 0.07 | 0.17** | 0.26** | 0.20** | 0.10 | −0.04 | 0.16** | ||||
| 3. Parent education | 7.21 (1.80) | 1.50–11.00 | −0.11 | −0.03 | −0.01 | 0.10 | −0.08 | 0.10 | 0.06 | 0.02 | 0.04 | −0.06 | −0.06 | |||
| 4. BMI | 23.16 (5.01) | 14.68–47.58 | 0.02 | 0.05 | 0.03 | 0.12* | −0.21** | 0.19** | 0.04 | −0.04 | 0.00 | −0.01 | ||||
| 5. Depressive symptoms | 1.79 (0.53) | 1.00–3.40 | 0.23** | 0.15** | −0.03 | 0.01 | 0.01 | 0.18** | 0.14* | −0.02 | 0.06 | |||||
| 6. Family demands | 0.12 (0.17) | 0.00–1.00 | 0.19** | −0.04 | −0.11 | 0.05 | 0.00 | −0.06 | 0.06 | −0.03 | ||||||
| 7. Family conflict | 0.20 (0.21) | 0.00–1.00 | −0.05 | 0.07 | 0.05 | −0.03 | −0.05 | 0.08 | 0.11 | |||||||
| 8. Sleep latency | 10.73 (11.19) | 0.00–76.83 | −0.09 | −0.59** | −0.01 | −0.14* | 0.07 | −0.02 | ||||||||
| 9. Sleep duration | 447.60 (59.33) | 131.71–603 | 0.40** | −0.18** | 0.02 | −0.19** | −0.07 | |||||||||
| 10. Sleep efficiency | 93.33 (6.34) | 24.50–99.58 | 0.11 | 0.15* | −0.04 | 0.05 | ||||||||||
| 11. AUC | 25.88 (7.55) | 2.91–57.62 | 0.40** | 0.16** | 0.47** | |||||||||||
| 12. CAR | 0.46 (1.10) | −3.13–7.33 | −0.25** | −0.05 | ||||||||||||
| 13. DCS | −0.17 (0.06) | −0.32 −0.07 | 0.75** | |||||||||||||
| 14. Bedtime cortisol | 0.52 (0.76) | −1.55–3.38 |
Note.
p < 0.05,
p < 0.01.
All HPA indices are log-transformed. BMI = body mass index. CESD = Center for Epidemiologic Studies Depression Scale AUC = cortisol area under the curve. CAR = cortisol awakening response. DCS = diurnal slope.
Bivariate correlations among study variables are also presented in Table 1. Family conflict and demands were unrelated to indices of HPA axis functioning and sleep. By contrast, sleep was related to indices of the HPA axis. Specifically, longer sleep duration was related to lower AUC and a steeper diurnal slope. Poorer sleep efficiency and longer sleep latency were related to a shallower CAR.
3.1. Family demands
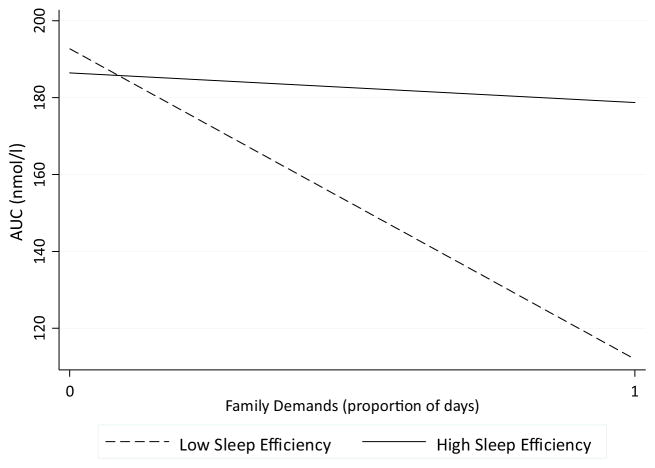
We first focused regression analyses on family demands and total cortisol output (i.e., AUC). As shown in Table 2 (column 3), the interaction between family demands and sleep efficiency approached statistical significance (p = 0.06, 95% CI [−0.07, 3.07]). As depicted in Fig. 1, greater family demands were related to lower AUC only among adolescents with lower sleep efficiency (b (SE) = −14.73 (6.93), β = −0.23, p = 0.04, 95% CI [−28.52, −0.93]). This association was non-significant among adolescents who had higher sleep efficiency (b (SE) = 0.29 (3.64), β = 0.01, p = 0.94 95% CI [−6.90, 7.48]). Adolescents with more family demands (i.e., above average) and lower sleep efficiency (i.e., below average) had a raw average AUC of 149.60 nmol/l whereas those with more family demands and higher sleep efficiency had a raw average AUC of 199.65 nmol/l.
Table 2.
Regression analyses predicting AUC as a function of daily family demands, sleep, and the interaction between daily demands and sleep.
| AUC
|
||||||
|---|---|---|---|---|---|---|
| Latency
|
Duration
|
Efficiency
|
||||
| b (SE) | β | b (SE) | β | b (SE) | β | |
| Intercept | 51.99 (14.68)*** | 54.07 (14.41)*** | 49.32 (14.63)*** | |||
| Age | −0.40 (0.72) | −0.03 | −0.81 (0.71) | −0.07 | −0.32 (0.72) | −0.03 |
| Gender | 2.01 (1.09)a | 0.11 | 2.56 (1.07)* | 0.14 | 1.74 (1.13) | 0.10 |
| Latino | −1.16 (1.32) | −0.07 | −1.83 (1.30) | −0.10 | −1.10 (1.31) | −0.06 |
| Asian | 0.18 (1.51) | 0.01 | 0.61 (1.51) | −0.03 | 0.11 (1.49) | 0.01 |
| Other | −1.18 (2.39) | −0.03 | −1.58 (2.36) | −0.04 | −1.29 (2.38) | −0.03 |
| Parent education | 0.11 (0.32) | 0.02 | 0.03 (0.31) | 0.01 | 0.13 (0.31) | 0.03 |
| Depressive symptoms | 2.95 (1.14)** | 0.18 | 2.77 (1.11)** | 0.17 | 3.09 (1.14)** | 0.18 |
| Wake time | −1.29 (0.40)*** | −0.20 | −1.00 (0.40)** | −0.16 | −1.23 (0.39)** | −0.19 |
| Family demands | −4.79 (2.24) | −0.09 | −4.75 (3.28) | −0.09 | −4.87 (3.21) | −0.09 |
| Sleep | −0.04 (0.07) | −0.04 | −0.04 (0.01)** | −0.22 | 0.10 (0.14) | 0.05 |
| Daily demands x Sleep | −0.57 (0.40) | −0.09 | 0.01 (0.07) | 0.01 | 1.50 (0.79)a | 0.12 |
Note.
p = 0.06,
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001.
Gender was coded such that male = 0 and female = 1. European Americans were coded as the reference group for ethnicity. The specific sleep parameter to which sleep refers is indicated in the top subheading.
Fig. 1.
The interaction between family demands and sleep efficiency on AUC. Family demands were related to decreased AUC only among adolescents with lower sleep efficiency (i.e., below the mean).
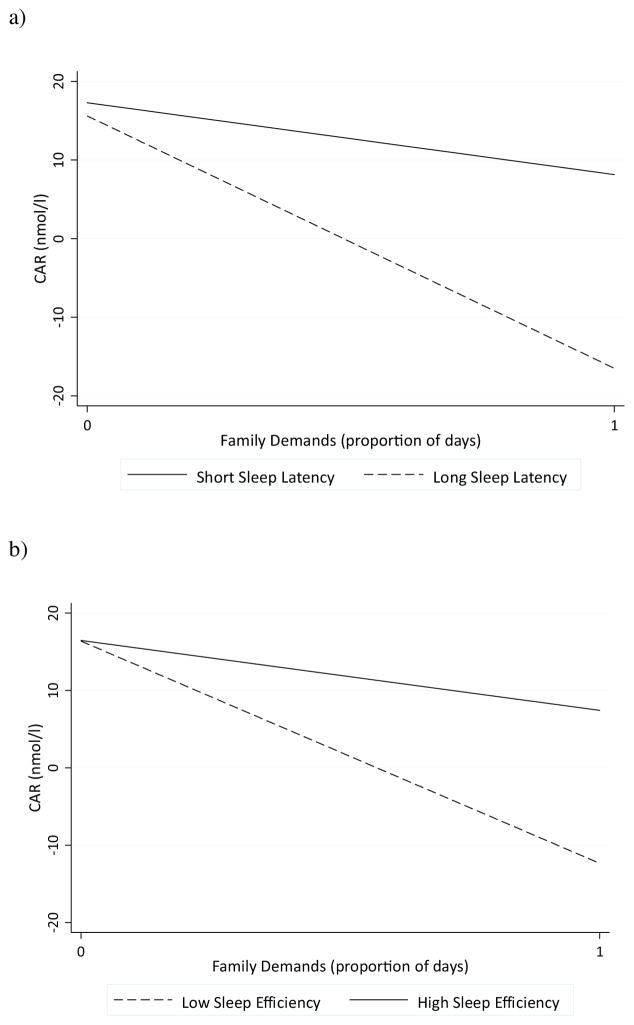
Regression analyses next focused on specific parameters of the diurnal cortisol profile (i.e., CAR, CARi, diurnal slope, bedtime levels). For CAR, there was a significant interaction between family demands and sleep latency (p = 0.01, 95% CI [−0.22, −0.03]) and sleep efficiency (p = 0.01, 95% CI [0.08, 0.48]), as presented in Table 3. As shown in Fig. 2a, family demands were related to a smaller CAR only among adolescents with longer sleep latency (b (SE) = −2.45 (0.93), β = −0.30, p = 0.01, 95% CI [−4.32, −0.59]). Family demands were unrelated to CAR among those with shorter sleep latency (b (SE) = −0.17 (.47), β = −0.03, p = 0.72, 95% CI [−1.10, 0.76]). Adolescents with more family demands and longer sleep latency had a raw average CAR of 7.89 nmol/l whereas those with more demands and shorter sleep latency had a raw average CAR of 12.68 nmol/l.
Table 3.
Regression analyses predicting CAR as a function of daily family demands, sleep, and the interaction between daily demands and sleep.
| CAR
|
||||||
|---|---|---|---|---|---|---|
| Latency
|
Duration
|
Efficiency
|
||||
| b (SE) | β | b (SE) | β | b (SE) | β | |
| Intercept | 3.15 (1.86) | 3.25 (1.91) | 2.77 (1.89) | |||
| Age | −0.11 (0.09) | −0.07 | −0.09 (0.09) | −0.06 | −0.08 (0.09) | −0.05 |
| Gender | 0.17 (0.13) | 0.07 | 0.17 (0.14) | 0.07 | 0.09 (0.14) | 0.04 |
| Latino | −0.10 (0.17) | 0.04 | −0.15 (0.17) | −0.07 | −0.10 (0.17) | 0.04 |
| Asian | 0.21 (0.19) | 0.08 | 0.21 (0.20) | 0.07 | 0.19 (0.19) | 0.07 |
| Other | −0.01 (0.29) | 0.001 | 0.07 (0.30) | −0.01 | −0.06 (0.31) | −0.01 |
| Parent education | 0.04 (0.04) | 0.06 | 0.04 (0.04) | 0.07 | 0.05 (0.04) | 0.07 |
| Depressive symptoms | 0.29 (0.13)* | 0.13 | 0.27 (0.14)* | 0.12 | 0.32 (0.13)* | 0.15 |
| Wake time | −0.09 (0.05) | −0.11 | −0.11 (0.05)* | −0.13 | −0.10 (0.05)* | −0.16 |
| Family demands | −1.04 (0.41)** | −0.15 | −0.90 (0.42)* | −0.13 | −1.03 (0.41)* | −0.15 |
| Sleep | −0.02 (0.01)** | −0.17 | 0.00 (0.00) | −0.01 | 0.03 (0.02) | 0.11 |
| Daily demands x Sleep | −0.12 (0.05)** | −0.15 | 0.00 (0.01) | 0.01 | 0.24 (0.10)** | 0.15 |
Note.
p < 0.05,
p ≤ 0.01.
Gender was coded such that male = 0 and female = 1. European Americans were coded as the reference group for ethnicity. The specific sleep parameter to which sleep refers is indicated in the top subheading.
Fig. 2.
The interaction between family demands and (a) sleep latency and (b) sleep efficiency on CAR. Family demands were related to a blunted CAR among adolescents who had high sleep latency (i.e., above the mean) and low sleep efficiency (i.e., below the mean).
Similarly, among adolescents who had lower sleep efficiency, greater family demands were associated with a blunted CAR (b (SE) = −3.00 (.89), β = −0.34, p = 0.001, 95% CI [−4.76, −1.24]; Fig. 2b). There was no significant association between family demands and CAR among adolescents with greater sleep efficiency (b (SE) = −0.27 (.46), β = −0.05, p = 0.57, 95% CI [−1.18, 0.64]). Among adolescents with more family demands, those who had lower sleep efficiency had a raw average CAR of 2.50 nmol/l whereas those with higher sleep efficiency had a raw average CAR of 15.07 nmol/l. None of the sleep indices moderated the association between family demands and CARi (p’s > 0.41–0.69), diurnal slope (p’s > 0.27–0.39), and bedtime cortisol levels (p’s = 0.25–0.89).
The interaction between family demands and sleep efficiency approached significance for AUC and was significant for CAR. Given that CAR was included in the computation of AUC, we examined whether differences in CAR drove the interaction effect between family demands and sleep efficiency on AUC by adding CAR to the model predicting AUC. When controlling for CAR, the interactions between family demands and sleep efficiency was no longer significant in predicting AUC (b (SE) = 0.02 (.13), β = 0.01, p = 0.87, 95% CI[−0.24, 0.29]).
3.2. Family conflict
We conducted the same set of analyses with family conflict as the key family stress variable. None of the sleep indices moderated the link between family conflict and HPA indices (p’s = 0.11–0.99).
3.3. Sensitivity analyses
Given that good estimation of typical sleep may require at least five nights of actigraphy data (Meltzer et al., 2012), we also tested the models while excluding adolescents with fewer than five nights of actigraphy data. Overall, results were not altered. The interaction between sleep efficiency and family demands on AUC became significant (b (SE) = 1.75 (.77), β = 0.14, p = 0.02, 95% CI [0.23, 3.28]). Both sleep latency and efficiency continued to interact with family demands to influence CAR.
We also tested models using temporally concurrent diary, actigraphy, and cortisol data given potential temporal issues that may influence results. There were no significant interactions between family stress variables and indices of sleep on HPA parameters.
4. Discussion
Research has established a link between family stress and alterations in the functioning of the HPA axis. However, the stress-HPA axis link has not always been observed and may depend on contextual and individual difference factors (e.g.,Hanson and Chen, 2010). In the present study, we found that sleep moderated the relation between family stress and HPA axis functioning. Specifically, family demands were related to a smaller CAR among adolescents who took longer to fall asleep and slept less efficiently. In light of previous work reporting CAR mean increases of 4.35–8.73 nmol/l (Bäumler et al., 2013; Bouma et al., 2009; O’Donnell et al., 2013; Wust et al., 2000), the current findings suggest that more family demands in the context of poor sleep may lead to a blunted CAR.
Although the primary function of the CAR is not entirely clear, it is believed that the CAR may be adaptive in that it prepares one to effectively cope with the anticipated stressors of the day (Adam et al., 2006; Fries et al., 2009). Indeed, prior work has shown that a greater CAR is associated with attenuated distress responses to stress (Powell and Schlotz, 2012). Under burnout conditions, however, the HPA axis exhibits hypocortisolism, characterized by a blunted CAR (Chida and Steptoe, 2009; Oosterholt et al., 2015). Given that poorer sleep can be a stressor itself and lead to greater feelings of fatigue, family demands in the context of poorer sleep may become excessively burdensome, thereby leading to a state of hypocortisolism similar to that observed in burnt out individuals. To the extent that a robust CAR is adaptive (i.e., facilitates coping) in the context of stress, adolescents with poorer sleep (i.e., longer latency or lower efficiency) and greater family demands may be at greater risk for the diseases to which stress contributes. Future research will need to determine whether adolescents with poor sleep and family demands continue to exhibit a blunted CAR over time.
The present findings highlight the role of sleep latency and efficiency as important modulators of the family demands-HPA axis link, raising the question of why sleep latency and efficiency may increase vulnerability to family demands. One possibility is that taking longer to fall asleep and sleeping less efficiently disrupts restorative processes of sleep. More specifically, sleep may be a coping mechanism that facilitates emotional and biological recovery from the challenges of the day and recalibrates systems to face challenges of the following day (Goldstein and Walker, 2014; Suchecki et al., 2012). As such, family demands in the context of taking longer to fall asleep and achieving lower sleep efficiency may lead to changes in HPA function.
Taking longer to fall asleep and sleeping less efficiently may also reflect cognitive and affective factors that render adolescents more susceptible to the effects of family demands. Cognitive and affective factors, including intrusive thoughts, negative affect, worry, and perception of threat, lead to a state of prolonged arousal or vigilance, delaying sleep onset (Dahl and Lewin, 2002; Hall et al., 1998; Kalmbach et al., 2014; Tang and Harvey, 2004; Wicklow and Espie, 2000; Wuyts et al., 2012; Zoccola et al., 2009) and decreasing sleep efficiency (Åkerstedt et al., 2007; Soderstrom et al., 2004). Taking longer to fall asleep and sleeping less efficiency, then, may indicate a decreased ability to regulate physiological, cognitive, and/or affective arousal (Dahl, 1996; Silk et al., 2007), which in turn, can disrupt physiological systems, including the HPA axis (Buchanan et al., 1999; Juster et al., 2012; Zoccola and Dickerson, 2012).
Sleep duration did not interact with family demands to influence HPA axis functioning in the present study. This is in line with prior work showing that lower sleep efficiency, but not sleep duration, among children was associated with altered cortisol levels in the context of a laboratory stressor (Raikkonen et al., 2010). This and the present study assessed naturalistic sleep and severely short sleep duration or extreme sleep loss may be necessary to observe the effects on the stress-HPA link. Indeed, total sleep deprivation has been shown to disrupt HPA axis responses to stress (Minkel et al., 2014). Perhaps, then, in the context of more severely short sleep duration, family demands might be related to altered HPA axis functioning.
Sleep latency and efficiency interacted only with family demands, but not family conflict, to influence CAR. This differential finding is not surprising given that family demands and family conflict were only modestly correlated, which suggests that these different family stressors do not co-occur within families. A prior study also found that sleep quality interacted only with harsh parenting, but not with marital conflict, to influence cognitive functioning (El-Sheikh et al., 2014). Together, this and the current study underscore the importance of examining multiple dimensions of family stress to better understand their implications for development.
Caution in interpreting results is warranted in light of the limitations of the present study. First, variables of interest were assessed at different times for varying duration. Although there may be concern over the temporal dynamics among these variables (e.g., Ross et al., 2014), daily assessment techniques are based on sampling experience and reflect one’s typical experience in his/her everyday life. Second, the correlational, cross-sectional nature of the study precludes conclusions regarding causality. However, past studies employing experimental designs have demonstrated that poor sleep increases biological sensitivity to stress (Franzen et al., 2011; Heffner et al., 2012; Minkel et al., 2014). Nevertheless, future studies should employ prospective designs to help clarify causal relations among naturally occurring stressors, mood, sleep and biological functioning.
Third, there is evidence suggesting that diurnal cortisol parameters based on three sampling days may not reflect individual differences in HPA axis functioning. In particular, studies have found low short-term stability in CAR and the diurnal slope and these HPA parameters are sensitive to situational factors that can result in their day-to-day fluctuations (Ross et al., 2014; Stalder et al., 2016; Wang et al., 2014). Evidence suggests better short-term stability for total daily cortisol output (Rotenberg et al., 2012; Wang et al., 2014). Suggestions on the number of sampling days have varied and depend on the specific HPA parameter. For CAR and AUC, researchers have recommended using at least three sampling days (Rotenberg et al., 2012; Stalder et al., 2016), which we followed in the present study. Although more sampling days may be optimal for other indices of the HPA axis, especially for diurnal slope (Rotenberg et al., 2012; Segerstrom et al., 2014), in large studies such as the current study, the number of sampling days is constrained by participant burden, concern over compliance, and financial resources.
Fourth, menstrual phase, which can affect HPA axis functioning (e.g., Kirschbaum et al., 1999), was not assessed in the present study. However, previous studies have also found that menstrual phase was unrelated to CAR among adolescents and adults (Bouma et al., 2009; Kudielka and Kirschbaum, 2003). Lastly, the generalizability of the findings are limited given that participants were sampled from the 10th and 11th grade students of four Los Angeles high schools. Given these limitations, future studies should examine the replicability of the present findings in other samples.
Despite these limitations, the present study extends previous research. The majority of past studies on the role of sleep on stress sensitivity among youth have focused on psychosocial outcomes (El-Sheikh et al., 2014; El-Sheikh et al., 2016; Lemola et al., 2012; Tu et al., 2015). Studies focusing on the HPA-axis have primarily relied on experimental inducement of acute stress and sleep loss (Goodin et al., 2012; Raikkonen et al., 2010), which may not reflect naturalistic experiences. Our study suggests that poor sleep during the adolescent years may potentiate the effects of naturally-occurring family-related stress on biological functioning. Of note is that even relatively low exposure to family demands in the context of relatively poor sleep may lead to HPA alterations during adolescence, thereby conferring early risk for poor health. This finding is similar to research demonstrating that discrimination during adolescence and young adulthood, although experienced infrequently, can leave a biological residue manifested by altered HPA functioning (Zeiders et al., 2012; Zeiders et al., 2014).
The present study suggests that family demands may not always result in compromised biological health among adolescents. Rather, only in the context of longer sleep latency and lower sleep efficiency are family demands related to changes in HPA axis function. To the extent that adolescents continue to take longer to fall asleep or sleep less efficiently and experience family demands, they may be at greater risk for developing poor mental and physical health outcomes related to altered HPA axis functioning. Conversely, taking less time to fall asleep and sleeping more efficiently in the face of family demands may render adolescents less susceptible to HPA alterations and related poor health outcomes.
Acknowledgments
Role of the funding sources
The sponsors of this research was not involved in the study design, collection and analysis of data, interpretation of findings, manuscript preparation, and decision to submit the manuscript for publication.
This research was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD062547) to AJF, National Science Foundation Graduate Research Fellowship Program (DGE-1144087) to JJC, UCLA California Center for Population Research, which is supported by the National Institute of Child Health and Human Development (R24-HD041022), and the UCLA Older Americans Independence Center, which is supported by the National Institute of Aging (P30-AG028748). The content does not necessarily represent the official views of the National Institute of Child Health and Human Development, National Science Foundation, National Institute of Aging, or the National Institutes of Health. This research was also supported by R01-AG034588; R01-AG026364; R01-CA160245-01; R01-CA119159; R01-HL095799; R01-DA032922-01; P30-AG028748 to MRI; and by the UCLA CTSI UL1TR000124, and the Cousins Center for Psychoneuroimmunology.
Footnotes
Conflicts of interest
None
Author contributions
JJC and AJF conceptualized the study and AJF oversaw data collection. JJC conducted data analyses, interpreted results, and drafted the manuscript with substantial contributions from AJF. All authors reviewed the manuscript, provided critical revisions, and approved the final version of the manuscript.
References
- Åkerstedt T, Kecklund G, Axelsson J. Impaired sleep after bedtime stress and worries. Biol Psychol. 2007;76:170–173. doi: 10.1016/j.biopsycho.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1-to 5-year-old children. Sleep. 2005;28:1568–1577. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. PNAS. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009;123:e701–e707. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler D, Kirschbaum C, Kliegel M, Alexander N, Stalder T. The cortisol awakening response in toddlers and young children. Psychoneuroendocrinology. 2013;38:2485–2492. doi: 10.1016/j.psyneuen.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:1–16. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55:180–190. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Bélanger MÈ, Tarabulsy GM, Simard V, Carrier J. My mother is sensitive, but I am too tired to know: infant sleep as a moderator of prospective relations between maternal sensitivity and infant outcomes. Infant Behav Dev. 2014;37:682–694. doi: 10.1016/j.infbeh.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Nicolson N, Sulon J, Jolles J. Coping style, trait anxiety and cortisol reactivity during mental stress. J Psychosom Res. 1991;35:141–147. doi: 10.1016/0022-3999(91)90068-y. [DOI] [PubMed] [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of daily stress on negative mood. J Pers Soc Psychol. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Bordeleau S, Bernier A, Carrier J. Maternal sensitivity and children’s behavior problems: examining the moderating role of infant sleep duration. J Clin Child Adolesc Psychol. 2012;41:471–481. doi: 10.1080/15374416.2012.686101. [DOI] [PubMed] [Google Scholar]
- Bouma EMC, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The trails study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, al’Absi M, Lovallo WR. Cortisol fluctuates with increases and decreases in negative affect. Psychoneuroendocrinology. 1999;24:227–241. doi: 10.1016/s0306-4530(98)00078-x. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Roux AD, Shrager S, Golden SH. Dirunal salivary cortisol is associated with body mass index and waist circumference: the multiethnic study of atherosclerosis. Obesity. 2013;21:E56–E63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chung GH, Flook L, Fuligni AJ. Daily family conflict and emotional distress among adolescents from Latin American, Asian, and European backgrounds. Dev Psychol. 2009;45:1406–1415. doi: 10.1037/a0014163. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A, Robinson MD. Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion. 2011;11:379–390. doi: 10.1037/a0021776. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol. 1996;8:3–27. [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Doane LD, Thurston EC. Associations among sleep, daily experiences, and loneliness in adolescence: evidence of moderating and bidirectional pathways. J Adolesc. 2014;37:145–154. doi: 10.1016/j.adolescence.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Mize J, Acebo B. Marital conflict and disruption of children’s sleep. Child Dev. 2006;77:31–43. doi: 10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Pyschol. 2008;27:26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Kelly RJ, Erath S. Maternal psychological control and child internalizing symptoms: vulnerability and protective factors across bioregulatory and ecological domains. J Child Psychol Psychiatry. 2010;51:188–198. doi: 10.1111/j.1469-7610.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Tu KM, Erath SA, Buckhalt JA. Family stress and adolescents’ cognitive functioning: sleep as a protective factor. J Fam Psychol. 2014;28:887–896. doi: 10.1037/fam0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Tu KM, Saini EK, Fuller-Rowell TE, Buckhalt JA. Perceived discrimination and youths’ adjustment: sleep as a moderator. J Sleep Res. 2016;25:70–77. doi: 10.1111/jsr.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: toward an understanding of sensitivity to developmental experiences and context. Dev Psychopathol. 2011;23:1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Flook L, Fuligni AJ. Family and school spillover in adolescents’ daily lives. Child Dev. 2008;79:776–787. doi: 10.1111/j.1467-8624.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, Buysse DJ. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Hardway C. Daily variation in adolescents’ sleep, activities, and psychological well-Being. J Res Adolesc. 2006;16:353–378. [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Irwin MR, Kiang L, Cole SW. Daily family assistance and inflammation among adolescents from Latin American and European backgrounds. Brain Behav Immun. 2009;23:803–809. doi: 10.1016/j.bbi.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V, Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Pers Individual Differences. 2001;30:1311–1327. [Google Scholar]
- Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91:36–41. doi: 10.1016/j.biopsycho.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribbin CE, Watamura SE, Cairns A, Harsh JR, LeBourgeois MK. The cortisol awakening response (CAR) in 2-to 4-year-old children: effects of acute nighttime sleep restriction, wake time, and daytime napping. Dev Psychobiol. 2012;54:412–422. doi: 10.1002/dev.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griefahn B, Robens S. The cortisol awakening response: a pilot study on the effects of shift work, morningness and sleep duration. Psychoneuroendocrinology. 2008;33:981–988. doi: 10.1016/j.psyneuen.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Grych JH, Fincham FD. Marital conflict and children’s adjustment: a cognitive-contextual framework. Psychol Bull. 1990;108:267–290. doi: 10.1037/0033-2909.108.2.267. [DOI] [PubMed] [Google Scholar]
- Hall M, Baum A, Buysse DJ, Prigerson HG, Kupfer DJ, Reynolds CF. Sleep as a mediator of the stress-immune relationship. Psychosom Med. 1998;60:48–51. doi: 10.1097/00006842-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Hall MH, Lee L, Matthews KA. Sleep duration during the school week is associated with C-reactive protein risk groups in healthy adolescents. Sleep Med. 2015;16:73–78. doi: 10.1016/j.sleep.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MD, Chen E. Daily stress, cortisol, and sleep: the moderating role of childhood psychosocial environments. Health Psychol. 2010;29:394–402. doi: 10.1037/a0019879. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, Stadelmann S, von Wyl A, von Klitzing K, Holsboer-Trachsler E. Electroencephalographic sleep profiles and hypothalamic–pituitary–adrenocortical (HPA)-activity in kindergarten children: early indication of poor sleep quality associated with increased cortisol secretion. J Psychiatric Res. 2008;42:532–543. doi: 10.1016/j.jpsychires.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Ng HM, Suhr JA, France CR, Marshall GD, Pigeon WR, Moynihan JA. Sleep disturbance and older adults’ inflammatory responses to acute stress. Am J Geriatr Psychiatry. 2012;20:744–752. doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrenkohl TI, Lee JO, Kosterman R, Hawkins JD. Family influences related to adult substance use and mental health problems: a developmental analysis of child and adolescent predictors. J Adolesc Health. 2012;51:129–135. doi: 10.1016/j.jadohealth.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks DE, Sowash J, Anning J, McGloughlin T, Andres F. Effect of exercise at three exercise intensities on salivary cortisol. J Strength Cond Res. 2002;16:286–289. [PubMed] [Google Scholar]
- Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Perna A, Marin MF, Sindi S, Lupien SJ. Timing is everything: anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adults. Stress. 2012;15:569–577. doi: 10.3109/10253890.2012.661494. [DOI] [PubMed] [Google Scholar]
- Kahlhöfer J, Karschin J, Breusing N, Bosy-Westphal A. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity. 2016;24:335–341. doi: 10.1002/oby.21326. [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Pillai V, Roth TL, Drake CL. The interplay between daily affect and sleep: a 2-week study of young women. J Sleep Res. 2014;23:636–645. doi: 10.1111/jsr.12190. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences—findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith JG, Nelson CS, Schlabach JH, Thompson CJ. The relationship between parental employment and three measures of early adolescent responsibility: family-related, personal, and social. J Early Adolesc. 1990;10:399–415. [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, Lopez-Duran NL. Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology. 2015;54:103–114. doi: 10.1016/j.psyneuen.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Head J, Bartley M, Stansfeld S, Kivimaki M. Maternal separation in childhood and diurnal cortisol patterns in mid-life: findings from the Whitehall II study. Psychol Med. 2012;1:1–11. doi: 10.1017/S0033291712001353. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, Leve LD. Stress system development from age 4.5 to 6: family environment predictors and adjustment implications of HPA activity stability versus change. Dev Psychobiol. 2014;56:340–354. doi: 10.1002/dev.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS. Psychological Stress and The Coping Process. McGraw-Hill; New York: 1966. [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; New York: 1984. [Google Scholar]
- Lemola S, Schwarz B, Siffert A. Interparental conflict and early adolescents’ aggression: is irregular sleep a vulnerability factor. J Adolesc. 2012;35:97–105. doi: 10.1016/j.adolescence.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Lemola S, Perkinson-Gloor N, Hagmann-von Arx P, Brand S, Holsboer-Trachsler E, Grob A, Weber P. Morning cortisol secretion in school-age children is related to the sleep pattern of the preceding night. Psychoneuroendocrinology. 2015;52:297–301. doi: 10.1016/j.psyneuen.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- Lovallo WR, Whitsett TL, al’Absi M, Sung BH, Vincent AS, Wilson MF. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosom Med. 2005;67:734. doi: 10.1097/01.psy.0000181270.20036.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012;71:344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly JE, Huber LRB. The role of family conflict on risky sexual behavior in adolescents aged 15–21. Ann Epidemiol. 2013;23:233–235. doi: 10.1016/j.annepidem.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35:1353–1358. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognit Emotion. 2013;27:567–576. doi: 10.1080/02699931.2012.727783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels N, Clays E, De Buyzere M, Vanaelst B, De Henauw S, Sioen I. Children’s sleep and autonomic function: low sleep quality has an impact on heart rate variability. Sleep. 2013;36:1939–1946. doi: 10.5665/sleep.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Simpson NS, Dinges DF. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J, Moreta MC, Muto J, Htaik O, Jones C, Basner M, Dinges D. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Pyschol. 2014;33:1430–1434. doi: 10.1037/a0034219. [DOI] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- Motomura Y, Kitamura S, Oba K, Terasawa Y, Enomoto M, Katayose Y, Hida A, Moriguchi Y, Higuchi S, Mishima K. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One. 2013;8:e56578. doi: 10.1371/journal.pone.0056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, O’Connor TG. Prenatal maternal mood is associated with altered diurnal cortisol in adolescence. Psychoneuroendocrinology. 2013;38:1630–1638. doi: 10.1016/j.psyneuen.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BPR. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Biobehav Rev. 2005;29:457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Oosterholt BG, Maes JHR, Van der Linden D, Verbraak MJPM, Kompier MAJ. Burnout and cortisol: evidence for a lower cortisol awakening response in both clinical and non-clinical burnout. J Psychosom Res. 2015;78:445–451. doi: 10.1016/j.jpsychores.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain. 2007;23:812–820. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev. 2007;31:218–231. [Google Scholar]
- Powell DJ, Schlotz W. Daily life stress and the cortisol awakening response: testing the anticipation hypothesis. PLoS One. 2012;7:e52067. doi: 10.1371/journal.pone.0052067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Matthews KA, Pesonen AK, Pyhala R, Paavonen EJ, Feldt K, Jones A, Phillips DIW, Seckl JR, Heinonen K. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab. 2010;95:2254–2261. doi: 10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Rodríguez-Colón SM, He F, Bixler EO, Fernandez-Mendoza J, Vgontzas AN, Calhoun S, Zheng ZJ, Liao D. Sleep variability and cardiac autonomic modulation in adolescents—Penn State Child Cohort (PSCC) study. Sleep Med. 2015;16:67–72. doi: 10.1016/j.sleep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Murphy MLM, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ, Roy-Gagnon MH, Tu MT. Stability of the diurnal cortisol profile in children and adolescents. Psychoneuroendocrinology. 2012;37:1981–1989. doi: 10.1016/j.psyneuen.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Boggero IA, Smith GT, Sephton SE. Variability and reliability of diurnal cortisol in younger and older adults: implications for design decisions. Psychoneuroendocrinology. 2014;49:299–309. doi: 10.1016/j.psyneuen.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Vanderbilt-Adriance E, Shaw DS, Forbes EE, Whalen DJ, Ryan ND, Dahl RE. Resilience among children and adolescents at risk for depression: mediation and moderation across social and neurobiological contexts. Dev Psychopathol. 2007;19:841–865. doi: 10.1017/S0954579407000417. [DOI] [PubMed] [Google Scholar]
- Smetana JG, Campione-Barr N, Metzger A. Adolescent development in interpersonal and societal contexts. Annu Rev Psychol. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Smollar J, Youniss J. Transformations in adolescents’ perceptions of parents. Int J Behav Dev. 1989;12:71–84. [Google Scholar]
- Soderstrom M, Ekstedt M, Akerstedt T, Nilsson J, Axelsson J. Sleep and sleepiness in young individuals with high burnout scores. Sleep. 2004;27:1369–1378. doi: 10.1093/sleep/27.7.1369. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockray S, Smyth N, Evans P, Hellhammer DH. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Straus MA. Measuring intrafamily conflict and violence: the conflict tactics (CT) scales. J Marriage Fam. 1979;41:75–88. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Tiba PA, Machado RB. REM sleep rebound as an adaptive response to stressful situations. Front Neurol. 2012;3:1–12. doi: 10.3389/fneur.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG. Effects of cognitive arousal and physiological arousal on sleep perception. Sleep. 2004;27:69–78. doi: 10.1093/sleep/27.1.69. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ. Daily family assistance and the psychological well-being of adolescents from Latin American, Asian, and European backgrounds. Dev Psychol. 2009a;45:1177–1189. doi: 10.1037/a0014728. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ. A longitudinal daily diary study of family assistance and academic achievement among adolescents from Mexican, Chinese, and European backgrounds. J Youth Adolesc. 2009b;38:560–571. doi: 10.1007/s10964-008-9391-7. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Gonzales N, Fuligni AJ. Family obligation values and family assistance behaviors: protective and risk factors for Mexican–American adolescents’ substance use. J Youth Adolesc. 2014;43:270–283. doi: 10.1007/s10964-013-9941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu KM, Erath SA, El-Sheikh M. Peer victimization and adolescent adjustment: the Moderating role of sleep. J Abnorm Child Psych. 2015;43:1447–1457. doi: 10.1007/s10802-015-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin P, Wand GS. Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Vargas I, Lopez-Duran N. Dissecting th eimpact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology. 2014;40:10–16. doi: 10.1016/j.psyneuen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Villegas ALR, Cruz JS. Executive functioning and adaptive coping in healthy adults. Appl Neuropsychol Adult. 2015;22:124–131. doi: 10.1080/23279095.2013.864972. [DOI] [PubMed] [Google Scholar]
- Wang X, Sánchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, Seeman TE, Roux AVD. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49:310–320. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–693. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–217. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- Wright KP, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Wuyts J, De Valck E, Vandekerckhove M, Pattyn N, Bulckaert A, Berckmans D, Haex B, Verbraecken J, Cluydts R. The influence of pre-sleep cognitive arousal on sleep onset processes. Int J Psychophysiol. 2012;83:8–15. doi: 10.1016/j.ijpsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Yip T. The effects of ethnic/racial discrimination and sleep quality on depressive symptoms and self-esteem trajectories among diverse adolescents. J Youth Adolesc. 2015;44:419–430. doi: 10.1007/s10964-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescence. J Adolesc Health. 2011;48:566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Roosa MW. Perceived discrimination and diurnal cortisol: examining relations among Mexican American adolescents. Horm Behav. 2012;61:541–548. doi: 10.1016/j.yhbeh.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: the moderating role of racial–ethnic minority status. Psychoneuroendocrinology. 2014;50:280–288. doi: 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: a review. J Psychosom Res. 2012;73:1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med. 2009;71:771–775. doi: 10.1097/PSY.0b013e3181ae58e8. [DOI] [PubMed] [Google Scholar]