Abstract
Both early adversity and depression are associated with heightened inflammation. However, few studies have focused on inflammatory reactivity to psychosocial stress and examined adiposity as a potential moderator. Yet, repeated heightened inflammatory reactivity over time is thought to contribute to low-grade chronic inflammation and adipose tissue is a key source of pro-inflammatory cytokines. The purpose of the present study was to examine whether early adversity and depressive symptoms were related to stress-induced inflammation and whether these associations varied by total body and abdominal adiposity as measured by body mass index (BMI) and waist circumference (WC) in a sample of late adolescents. Participants reported on their early family environment and current depressive symptoms, had their height, weight, and WC assessed for adiposity markers, and provided blood samples for IL-6 assessment before and after a standardized laboratory stress task. No main effect of early adversity on IL-6 reactivity to acute stress was observed. However, significant interactions between early adversity and BMI and WC emerged. Greater exposure to early adversity was associated with greater IL-6 responses only among adolescents with higher BMI or WC. The same pattern of findings was observed for depressive symptoms. Additionally, moderated mediation analyses indicated that among adolescents with greater adiposity, early adversity indirectly influenced IL-6 reactivity via current depressive symptoms. These findings contribute to our understanding of vulnerability factors that may amplify the associations between early adversity and depressive symptoms and inflammation during relatively early stages of life.
Keywords: early life stress, risky families, depressive symptoms, body mass index, waist circumference, IL-6, adolescents
1. Introduction
Exposure to early adversity and depression increase risk for developing a wide array of physical health problems in adulthood. These include some of the most debilitating chronic conditions, such as cardiovascular disease, diabetes, and selected cancers, as well as premature all-cause mortality (Brown et al., 2009; Cuijpers et al., 2014; Evans et al., 2005; Felitti et al., 1998; Miller et al., 2011; Norman et al., 2012; Rotella and Mannucci, 2013). These effects are thought to be driven in part by slow-progressing changes in physiological systems, particularly inflammatory processes. Indeed, cross-sectional studies show that pro-inflammatory cytokines and C-reactive protein (CRP) are elevated among depressed individuals and among those who experienced early adversity (Dowlati et al., 2010; Haapakoski et al., 2015; Howren et al., 2009; Lacey et al., 2014; Liu et al., 2012; Matthews et al., 2014; Taylor et al., 2006). Prospective studies have similarly shown that exposure to early adversity or to clinical and subclinical levels of depression predict higher levels of subsequent inflammation (Copeland et al., 2012, 2014; Danese et al., 2007; Deverts et al., 2010; Matthews et al., 2010; Slopen et al., 2013).
Most studies examining the effects of early adversity or depression on inflammatory biology have focused on inflammation under tonic conditions or in response to biological challenges, such as vaccination, in vivo administration of endotoxin, and in vitro incubation with pathogens. Empirical examination of how early adversity and depression relate to inflammatory reactivity to psychosocial threat has been less common. To our knowledge, only a handful of studies to date have examined this. In these studies, early adversity, depressive symptoms, and clinical depression were related to heightened inflammatory reactivity to an acute stressor among healthy adults (Carpenter et al., 2010; Fagundes et al., 2013; Janusek et al., 2017; Pace et al., 2006). The dearth of studies focusing on inflammatory reactivity to psychosocial threat is particularly surprising in light of past theoretical work suggesting that early adversity and depression may increase biological and psychological sensitivity to stress, and that repeated exaggerated inflammatory reactivity to stress over time contributes to low-grade chronic inflammation and increases risk for poor health-related outcomes (Brydon and Steptoe, 2005; Chiang et al., 2015b; Danese and Lewis, 2017; Fagundes and Way, 2014; McEwen and Seeman, 1999; Nusslock and Miller, 2016; O’Hara et al., 2014).
Also not yet fully understood are the individual difference factors that may help explain observed variations in the associations between early adversity and depression and inflammation. One factor of particular importance may be adiposity given its central role in inflammation. Adipocytes secrete pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β, and nutrient excess increases their secretion of pro-inflammatory cytokines (Ferrante, 2007; McNelis and Olefsky, 2014). Increased adiposity also activates extant macrophages in adipose tissue to become more pro-inflammatory and increases recruitment of monocytes to adipose tissue, ultimately increasing the number of macrophages and further promoting a state of inflammation (Ferrante, 2007; Weisberg et al., 2003; Xu et al., 2003). Approximately a quarter of circulating inflammatory markers are from adipose tissue (Black, 2003), and greater adiposity, as measured by BMI and WC, has been consistently and strongly linked to heightened inflammation in numerous correlational studies (e.g., Galcheva et al., 2011; Himmerich et al., 2006; Panagiotakos et al., 2005; Steene-Johannessen et al., 2010). Experimental studies similarly have shown that higher BMI is associated with enhanced levels of pro-inflammatory cytokines in response to stress (McInnis et al., 2014; Wirtz et al., 2008). Despite the fact that increased adiposity has been linked to heightened inflammation and is a known risk factor for chronic diseases of aging, it has mostly been studied separately from early adversity and depression in relation to inflammation; or, it has been treated as a potential confounding or mediating variable. The potential modulating role of adiposity has rarely been considered.
Adolescence may an especially important developmental period to examine the influence of early adversity and depression on stress-induced inflammation and the potential moderating role of adiposity given that sensitivity to social stress may be heightened during this time (Blakemore and Mills, 2014; Somerville, 2013; Spear, 2009). Compared to children and adults, adolescents display greater neuroendocrine and negative emotional responses to social stressors, and heightened neural reactivity to negative interpersonal stimuli (Gunnar et al., 2009; Sebastian et al., 2010; Stroud et al., 2009). Adolescence is also when depression frequently emerges, with prevalence rates increasing from very low childhood rates to comparable adult rates (Avenevoli et al., 2015; Kessler et al., 2001). Furthermore, adolescence is a key developmental period that sets the stage for adult health. Many inflammation-related chronic conditions are life-course diseases that begin to develop early in life. For example, signs of metabolic syndrome and atherosclerosis can be observed in adolescence (Cook et al., 2003; Strong et al., 1999). Despite this, the majority of prior work on inflammatory consequences of early adversity and depression have focused on adults.
The overarching goal of the present investigation was to deepen our understanding of how early adversity and depression relate to inflammatory processes during adolescence. Specifically, we examined whether early adversity and current depressive symptoms were each associated with heightened inflammatory reactivity to stress and whether these relations varied as a function of adiposity in a sample of late adolescents. Because early adversity and symptoms of depression often co-vary, and early adversity has been shown to precipitate depression (Felitti et al., 1998; Hazel et al., 2008; MacMillan et al., 2001; Spence et al., 2002; St Clair et al., 2015), in subsequent exploratory analyses, we also examined whether any observed associations between early adversity and inflammatory reactivity could be explained by current depressive symptoms. Additionally, we explored whether early adversity and depressive symptoms and their interactions with adiposity had similar effects on hypothalamic-pituitary-adrenal (HPA) axis functioning. Both early adversity and depression have been associated with alterations in the HPA axis, which regulates inflammatory processes (Burke et al., 2005; Chiang et al., 2015b; Repetti et al., 2011; Rohleder, 2014). Furthermore, there is some evidence suggesting HPA functioning is dysregulated in the presence of greater adiposity (Rodriguez et al., 2015). As such, interaction effects between early adversity and depressive symptoms and adiposity may extend to the HPA axis.
2. Methods
2.1. Participants
Participants (n = 91; 57.14% female) were recruited from an ongoing three-wave longitudinal study examining the role of psychosocial factors and daily socio-emotional experiences in the development of early risk for poor health. The original sample of the larger study comprised of 316 adolescent-parent dyads recruited from the 10th and 11th grades of four Los Angeles high schools. Youth and their parents completed a series of questionnaires, provided measures for several biomarkers, and completed a daily diary protocol at each wave. More details on the parent study can be found elsewhere (Chiang et al., 2015a, 2016).
The present laboratory-based study focused on the youth participants and was added during the second wave of the study when adolescents were in their last year of high school or first year of college. Individuals were eligible to participate in the present investigation if they were at least 18 years of age and self-identified as Latino or European-American. Other ethnicities were excluded due to insufficient numbers in the larger study. Individuals were also excluded if they were currently pregnant, or breast-fed within the last six months. All participants provided written informed consent and all procedures were approved by the UCLA Institutional Review Board.
Participants were between the ages of 18 and 20 (M = 18.37, SD = .51) and were mostly of Latino descent (European-American: 37.36%; Latino: 62.64%). Caregiver reports of annual household income and their own and spouse’s highest level of education completed (11-point scale: 1 = some elementary school, 11 = graduated from medical law, or graduate school) indicated that participants were mostly from middle class backgrounds. Median household income was $79,000 (range = $11,000–$350,000). Averaging education across parents indicated that approximately 14.4% of participants had parents with less than a high school education, 7.8% had at least one parent who completed high school, 38.9% had at least one parent who completed vocational trade school or some college, 23.3% had at least one parent who completed college, and 15.6% had at least one parent who completed some or all of graduate or professional school.
2.2. Procedure
Laboratory sessions were conducted at the Clinical and Translational Research Center (CTRC) at the University of California, Los Angeles between the hours of 12 pm and 6 pm, with the majority of visits (89%) beginning at 12 or 1 pm and the remaining visits beginning between 2 and 4 pm. Participants arrived without their parents, and a nurse assessed vital signs, collected anthropometric measures, and inserted an indwelling intravenous catheter in the antecubital vein of the non-dominant arm for blood sample collection. Use of an indwelling catheter allows for repeated blood draws without multiple needle insertions. To facilitate acclimation to the testing environment and indwelling catheter, participants then watched a neutral-content video quietly for 20 minutes. After this baseline period, the first blood and saliva samples were collected.
Next, participants completed the Trier Social Stress Task (TSST), a well-established laboratory stressor (Kirschbaum et al., 1993) that reliably increases inflammation (Steptoe et al., 2007). Participants were instructed to prepare and deliver a speech on why they were the best candidates for their ideal jobs in front of an evaluative panel of expert interviewers. In actuality, the panel consisted of research assistants trained to provide nonverbal negative feedback. Participants were also informed that they would be given a second task that was to be explained by the judging panel. They were given five minutes to prepare for the upcoming task, after which they delivered their speech in front of the evaluative panel. Immediately after, participants performed a five-minute mental arithmetic task that involved subtracting by 13’s from 2935 as quickly and accurately as possible. During this task, the judging panel urged participants to go more quickly if they got three consecutive answers correct and to start from the beginning each time they made a mistake. Following the TSST, participants provided three additional blood samples at 30, 60, and 90 min post-TSST onset. Additional saliva samples were also collected immediately after the TSST, and 30, 45, 60, and 75 min after TSST onset. Participants were debriefed and compensated before dismissed.
2.3. Measures
2.3.1. Early adversity
The Risky Families (RF) questionnaire (Taylor et al., 2004) was used to assess early adversity. Participants indicated the frequency of conflict, violence, harsh discipline, affectionate behaviors, neglect, and chaos/disorganization in their family environment from ages 5 to 15 years using a scale from 1 = not at all to 5 = very often. Positive-meaning items were reverse scored, and responses were averaged across items such that higher scores indicate greater exposure to early adversity. This scale has been shown to have high agreement with clinical interviews assessing early life stress (Taylor et al., 2004), and in the current sample, the measure demonstrated good reliability (α = .84).
2.3.2. Depressive symptoms
Depressive symptomatology was assessed using the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D) (Radloff, 1977). Using a 4-point scale (1 = rarely or none of the time, 4 = most or all of the time), participants indicated how often they experienced cognitive, affective, and somatic symptoms of depression during the past week. Example items include, “I had trouble keeping my mind on what I was doing,” “I felt sad,” “I felt people disliked me,” and “I could not get going.” Cronbach’s α was .91, indicating good internal consistency.
2.3.3. Inflammation
Four blood samples were collected throughout the laboratory visit at baseline, and 30, 60, and 90 min after TSST onset. At each time point, six mL of blood was collected into EDTA lavender-top tubes, placed on ice immediately after collection, centrifuged for acquisition of plasma, separated into three plasma aliquots, and stored at −80 °C. Upon data collection completion, samples were assayed for IL-6. We focused on IL-6 because it increases in response to acute stress and has been associated with early adversity and long-term chronic health conditions during adulthood (Carpenter et al., 2010; Slopen et al., 2013; Steptoe et al., 2007; Yudkin et al., 2000).
Samples were assayed in duplicate using the Quantikine high sensitivity human IL-6 ELISA kits (R&D Systems, Inc., Minneapolis, MN) by the UCLA Inflammatory Biology Core Laboratory. Each subject’s samples were assayed in the same run, and intra- and inter-assay coefficients of variability were below 7%. No sample was below the lower limit detection of .2 pg/mL. Consistent with past research (Carpenter et al., 2010; Carroll et al., 2011; Pace et al., 2006; Slavich et al., 2010), change scores were calculated from baseline to peak IL-6 (i.e., 90 min post-stressor) to index absolute magnitude of IL-6 increases in response to the TSST.
2.3.4. HPA axis function
Participants provided six saliva samples throughout the laboratory visit immediately after the neutral-content video (baseline), after the TSST, and 30, 45, 60, and 75 min after TSST onset. Saliva samples were collected using oral swabs (Salimetrics). Samples were stored at −80 °C until data collection was complete, after which samples were shipped on ice to the Laboratory of Biological Psychology at the Technical University of Dresden, Germany. Saliva samples were assayed for cortisol in duplicate using chemiluminescence-immunoassays with high sensitivity (IBL, International, Hamburg, Germany). Intra- and inter-assay coefficients of variations were below 10%. Following previous research, area under the curve with respect to ground (AUCg) and increase (AUCi) were computed to index total cortisol output across the laboratory visit and cortisol increase in response to the TSST, respectively (Pruessner et al., 2003).
2.3.5. Adiposity
Two anthropometric indices were used to index adiposity: BMI and WC, which index total or general adiposity and central or abdominal adiposity, respectively. Height and weight without shoes were assessed using an electronic scale and stadiometer. BMI was computed as weight in kilograms divided by height in meters squared. WC was assessed at the middle point between iliac crest and lower rib using measuring tape. Measures were taken in duplicate and averaged. BMI and WC have been validated as measures of adiposity, with BMI being highly correlated with total body fat and percent of body weight as fat in adolescents and WC accurately identifying youth with high trunk fat mass (Pietrobelli et al., 1998; Taylor et al., 2000). Moreover, BMI and WC have consistently been associated with heightened inflammation and been shown to be robust predictors of cardio-metabolic risk in youth, with some evidence suggesting that WC may be a better predictor (Bauer et al., 2015; Garnett et al., 2007; Herder et al., 2007; Janssen et al., 2005; Katzmarzyk et al., 2004).
2.3.6. Potential confounds
Potential confounding variables included sociodemographic characteristics (i.e., age, gender, ethnicity, and family socioeconomic status), as well as health behaviors, potential current illness, and time of laboratory visit. As part of the larger parent study, participants reported on their own ethnicity and gender while their parents reported on participants’ date of birth, from which age was calculated. Participants’ parents also reported on the highest level of education that they and their spouse completed, which was subsequently averaged across the two parents to index family socioeconomic status.
Experimenters recorded participants’ time of arrival to the laboratory visit. During the visit, participants reported on the number of days they engaged in physical activity or smoked cigarettes during the past week, and whether they had a medical history of any major physical or psychiatric condition. Six adolescents reported smoking in the past week and 14 reported a medical history of allergies or asthma; autoimmune, blood, metabolic or cardiovascular condition; depression, anxiety, or PTSD; or other major physical or mental health condition.
2.4. Statistical approach
All analyses were performed using Stata 14 (StataCorp LP, USA). Before analyses, inflammatory and HPA indices were natural log-transformed to correct for non-normality and continuous variables were mean-centered. Repeated measures ANOVA were first conducted to determine whether the TSST was effective in increasing IL-6 and cortisol. A series of hierarchical regression models were then estimated to examine links from early adversity and depressive symptoms to inflammation and HPA activity, and whether adiposity moderated these associations. Early adversity or depressive symptoms, markers of adiposity, and sociodemographic characteristics (i.e., age, gender, ethnicity, and parent education) were entered in the first step, followed by the interaction terms in the second step. Any significant interactions were followed up with tests of simple slopes at ±1SD of the moderating variable. Additionally, we conducted regions of significance analyses to more precisely probe interactions.
Given results from primary analyses focusing on IL-6 reactivity, and that early adversity is a known precursor to depression (Hazel et al., 2008; Spence et al., 2002), we conducted moderated mediation analyses to explore whether current depressive symptoms acted as a pathway linking early adversity to heightened inflammatory reactivity among adolescents with greater adiposity. Moderated mediation models were tested using Model 15 in the PROCESS SPSS macro (Hayes, 2013) with early adversity as the predictor, current depressive symptoms as the mediator, IL-6 reactivity as the outcome, and adiposity as the moderator of the links from early adversity and depressive symptoms to IL-6 reactivity (Fig. 1). Following guidelines for conducting moderated mediation analyses (Hayes, 2015), we examined the main effect of early adversity on depressive symptoms and the independent interaction effects between early adversity and adiposity and between depressive symptoms and adiposity on IL-6 reactivity. All models controlled for sociodemographic variables. A significant main effect of early adversity on depressive symptoms and an interaction effect between depressive symptoms and adiposity on IL-6 reactivity would suggest moderated mediation. Bootstrapping (5000 samples) was conducted for significance testing of moderated indirect effects.
Fig. 1.
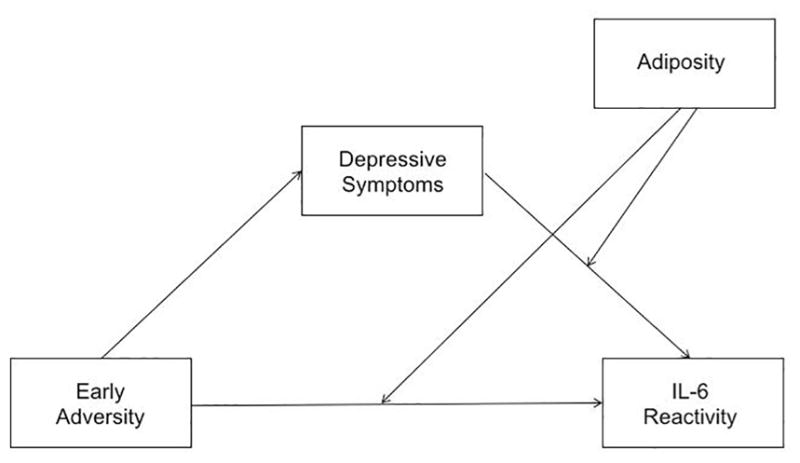
Conceptual moderated mediation model of adiposity (i.e., BMI or WC) moderating the extent to which depressive symptoms mediates the association between early adversity and IL-6 reactivity.
3. Results
Descriptive statistics are displayed in Table 1. Overall, average RF scores were fairly low, though comparable to those observed in previous research with adolescents that found that RF scores were related to greater stimulated production of IL-6 over time (Miller and Chen, 2010). Mean CESD scores were relatively close to the threshold suggesting clinically significant depressive symptoms (i.e., 16) and were also comparable to those observed in past work on youth (Kubzansky et al., 2012; McKeown et al., 1997; Parent et al., 2014). Nearly 30% of participants had CESD scores in the clinical range. Mean BMI was relatively high according to adult weight status categories. According to adolescent weight status categories based on BMI percentiles, 2.2% of adolescents were underweight, 65.2% were of healthy weight, 20.2% were overweight, and 11.2% were obese.
Table 1.
Descriptive Data and Bivariate Correlations Among Study Variables.
| Variable | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 18.37 (.51) | −.29** | .14 | .11 | .09 | .11 | .17 | .24* | .18 | −.08 | −.06 | .17 | .18 | |
| 2. Parent education | 7.44 (2.01) | −.13 | −.04 | −.22* | −.22* | −.25* | .20 | −.08 | .08 | −.19† | −.24 | .16 | ||
| 3. Risky families | 1.92 (.60) | .45*** | .00 | .00 | −.04 | −.03 | .05 | −.29** | −.15 | .18 | .22* | |||
| 4. Depressive symptoms | 13.38 (9.69) | −.17 | −.13 | −.11 | −.08 | .10 | −.13 | .12 | −.04 | .01 | ||||
| 5. IL-6 baseline | .10 (.72) | .95*** | .90*** | .81*** | −.12 | .12 | .19† | .60*** | .35*** | |||||
| 6. IL-6 +30 min | .10 (.69) | .95*** | .86*** | .02 | .12 | .26* | .59*** | .36*** | ||||||
| 7. IL-6 +60 min | .27 (.69) | .92*** | .19 | .09 | .30** | .54*** | .31** | |||||||
| 8. IL-6 +90 min | .42 (.69) | .40*** | .02 | .29** | .51*** | .29** | ||||||||
| 9. IL-6 reactivity | .86 (.32) | −.20* | .13 | −.03 | −.01 | |||||||||
| 10. Cortisol AUCg | 224.79 (40.64) | .06 | .01 | .04 | ||||||||||
| 11. Cortisol AUCi | 13.03 (39.06) | .14 | .18 | |||||||||||
| 12. BMI | 25.08 (5.93) | .84*** | ||||||||||||
| 13. WC (cm) | 81.90 (14.94) |
Note.
p < .08
p < .05,
p < .01,
p < .001.
Parent education reflects the average of highest level of education completed across both parents. IL-6 (pg/mL) and cortisol (nmol/L) values are natural log-transformed. IL-6 reactivity = 90 min post TSST – baseline.
Bivariate correlations also displayed in Table 1 show that higher RF scores were related to more depressive symptoms, greater WC, and lower cortisol AUCg, but not to cortisol AUCi or IL-6. BMI was fairly strongly related to IL-6 at each time point, but was not correlated with IL-6 change scores. WC was highly correlated with BMI but was only moderately correlated with IL-6 at each time point. Like BMI, WC was unrelated to IL-6 reactivity. IL-6 levels at each time point were correlated with one another and with cortisol AUCi, though they were not associated with magnitude of reactivity to the TSST. Greater parent education was associated with lower levels of IL-6 at baseline and 30 and 60 min post-TSST, but not to IL-6 at 90 min post-TSST and to IL-6 change scores.
3.1. TSST-induced inflammatory and HPA reactivity
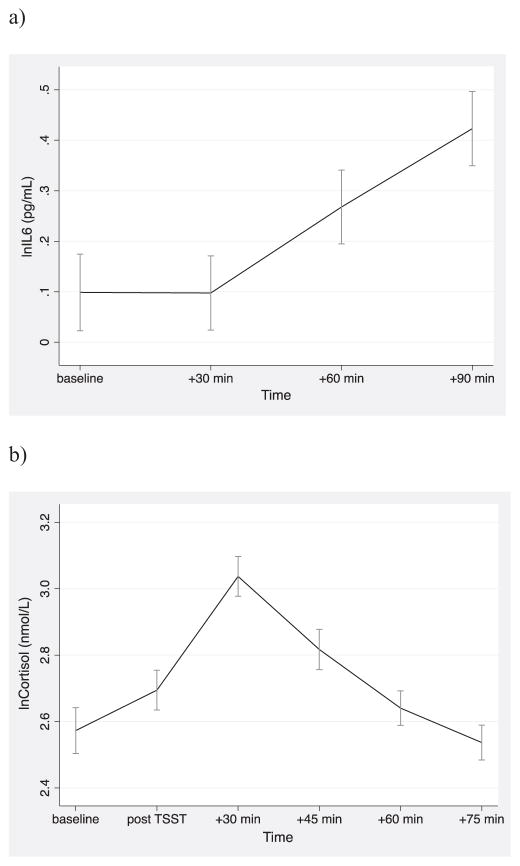
Results from repeated measures ANOVA showed a significant effect of time on IL-6 and cortisol, indicating that the TSST elicited significant increases in IL-6 (F(1.78, 156.30) = 44.18, p < .001) and activated the HPA axis (F(2.49, 224.14) = 27.96, p < .001). Bonferroni-corrected pairwise comparisons indicated that IL-6 increased significantly from baseline to +60 and +90 min post-TSST and from +60 to +90 min post task (ps < .001); baseline and +30 min post-TSST onset did not differ from each other (p = .99; Fig. 2a). Cortisol increased significantly from baseline to +30 and +45 min after the TSST (ps < .001) and returned to baseline levels by +60 min post task (ps = .99; Fig. 2b).
Fig. 2.
Mean (±SEM) natural log-transformed (a) IL-6 and (b) cortisol at baseline and following the TSST.
3.2. Early adversity, depressive symptoms, and inflammatory reactivity
3.2.1. Early adversity
For baseline IL-6, there was no main effect of early adversity (b (SE) = −.13 (.10), p = .19), and no interaction effects between early adversity and measures of adiposity (RF × BMI: b(SE) =−.02 (.02), p = .32; RF × WC (b(SE) = −1.78 (1.29), p = .17).
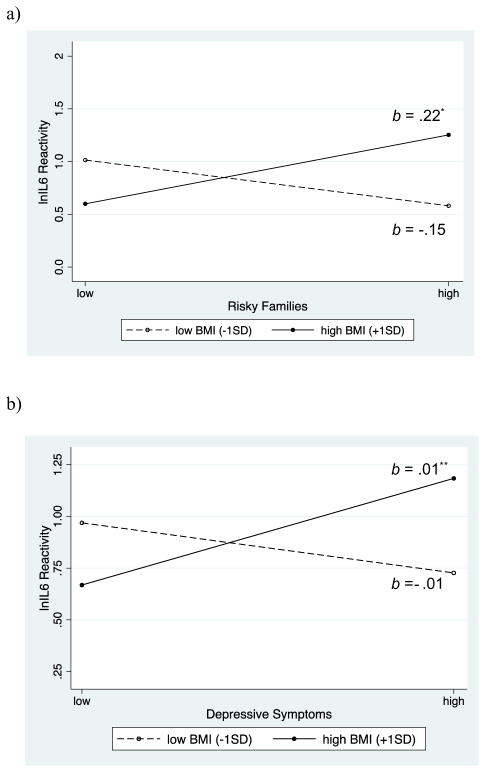
As shown in Table 2 (columns 1 and 3), there was also no main effect of early adversity on IL-6 reactivity (ps > .51). However, early adversity interacted with BMI to predict IL-6 reactivity (p = .03; column 2). Follow-up tests of simple slopes indicated that early adversity was related to greater inflammatory reactivity among adolescents with higher BMI (b(SE) = .22 (.10), p = .03; Fig. 3a). This association was not observed among those with lower BMI (b(SE) =−.15 (.10), p = .16). Regions of significance analyses revealed that the link between early adversity and IL-6 responses were significant for adolescents with a BMI of at least 28.99 (i.e., +.66 SD).
Table 2.
Stepwise hierarchical regression models predicting natural log-transformed IL-6 reactivity from early adversity, indices of adiposity, and their interactions.
| BMI
|
WC
|
|||
|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | |
| b (SE) | b (SE) | b (SE) | b (SE) | |
| Intercept | .89 (.08)*** | .85 (.08)*** | .89 (.08)*** | .87 (.08)*** |
| Age | .11 (.07) | .06 (.07) | .11 (.07) | .09 (.07) |
| Gender | .07 (.07) | .10 (.07) | .06 (.07) | .08 (.07) |
| Latino | −.10 (.08) | −.09 (.08) | −.10 (.08) | −.10 (.08) |
| Parent education | −.01 (.02) | −.02 (.02) | −.01 (.02) | −.02 (.02) |
| Risky families | .04 (.06) | .04 (.06) | .04 (.06) | .03 (.06) |
| Adiposity | −.005 (.01) | −.01 (.01) | −.001 (.003) | −.003 (.003) |
| Risky families x Adiposity | .03 (.01)* | .01 (.004)* | ||
p < .05,
p < .01,
p < .001.
Fig. 3.
(a) Early adversity and (b) depressive symptoms were related to greater natural log-transformed IL-6 reactivity among individuals with higher BMI but not among those with lower BMI.
A similar pattern emerged for central adiposity as assessed by WC. There was a significant interaction between early adversity and WC (p = .03; column 4), and tests of simple slopes revealed that greater exposure to early family adversity was associated with heightened IL-6 responses among those with higher WC (b(SE) = .16 (.08), p = .05), but not lower WC (b(SE) =−.11 (.09), p = .23). As identified by regions of significance analyses, the early adversity-IL-6 reactivity association was significant at WC values of at least 97.19cm (i.e., +1.01 SDs).
3.2.2. Depressive symptoms
Depressive symptoms were not related to baseline levels of IL-6 (b(SE) = −.01 (.01), p = .20) and no interactions with BMI or WC emerged (BMI: b(SE) = −.0004 (.001), p = .63; WC: b(SE) = −.0003 (.0004), p = .41).
Paralleling results for early adversity, there was no main effect of depressive symptoms on IL-6 reactivity (ps > .19), as displayed in Table 3 (columns 1 & 3). However, depressive symptoms interacted with adiposity indices to predict reactivity (BMI: p = .01; column 2; WC: p = .01; column 4). Greater depressive symptoms were associated with larger IL-6 responses among adolescents with higher BMI (b(SE) = .01 (.004), p = .01), but not among those with lower BMI (b(SE) = −.01 (.01), p = .26; Fig. 3b). Region of significance analyses indicated that the association between greater depressive symptoms and heightened IL-6 responses was significant for adolescents with a BMI of at least 27.75 (i.e., +.45 SD).
Table 3.
Stepwise hierarchical regression models predicting natural log-transformed IL-6 reactivity from depressive symptoms, indices of adiposity, and their interactions.
| BMI
|
WC
|
|||
|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | |
| b (SE) | b (SE) | b (SE) | b (SE) | |
| Intercept | .88 (.08)*** | .87 (.07)*** | .88 (.08)*** | .89 (.07)*** |
| Age | .10 (.07) | .06 (.07) | .10 (.07) | .06 (.07) |
| Gender | .07 (.07) | .11 (.07) | .06 (.07) | .08 (.07) |
| Latino | −.09 (.08) | −.12 (.08) | −.09 (.08) | −.12 (.08) |
| Parent education | −.01 (.02) | −.01 (.02) | −.01 (.02) | −.02 (.02) |
| Depressive symptoms | .005 (.004) | .003 (.004) | .005 (.004) | .004 (.004) |
| Adiposity | −.004 (.01) | −.004 (.01) | −.001 (.002) | −.002 (.002) |
| Depressive symptoms x Adiposity | .001 (.001)** | .001 (.0001)** | ||
p < .05,
p < .01,
p < .001.
Similarly, greater depressive symptoms were linked to greater IL-6 responses only among those with higher WC (b(SE) = .01 (.004), p = .01) but not among those with lower WC (b(SE) = −.004 (.004), p = .31). Regions of significance analyses more precisely identified the depressive symptoms-inflammatory reactivity link as being significant at WC values of at least 87.90cm (i.e., +.39 SD).
3.2.3. Exploratory analyses: moderated mediation
Based on the above results, we next tested a moderated mediation scenario in which early adversity increased depressive symptoms to heighten inflammatory reactivity among adolescents with greater adiposity. Results indicated that greater exposure to early adversity was associated with greater depressive symptoms (b(SE) = 7.34 (1.64), p < .001). In addition, depressive symptoms continued to interact with adiposity to predict IL-6 reactivity independent of the early adversity by adiposity interaction. Specifically, the interaction effect between depressive symptoms and BMI marginally predicted IL-6 responses (b(SE) = .001 (.001), p = .06) while the early adversity by BMI interaction was not significant (b(SE) = .01 (.02), p = .58). The interaction between depressive symptoms and WC also remained significant (b(SE) = .001 (.0002), p = .03) while the interaction between early adversity and WC was reduced to non-significance (b(SE) = .005 (.004), p = .29).
These findings suggest that the extent to which depressive symptoms mediate the link between early adversity and IL-6 reactivity varies according to adiposity. Bootstrapping analyses indicated that BMI did not moderate the extent to which depressive symptoms acted as a mediator (b(SE) = .01 (.01), 95% CI [−.002–.02]). However, the indirect effect of depressive symptoms was moderated by WC (b(SE) = .004 (.002), 95% CI [.001–.01]). Among adolescents with a WC at +1SD above the mean, greater depressive symptoms mediated the association between early adversity and heightened IL-6 reactivity (b(SE) = .08(.04), 95% CI [.01–.18]). Mediation of the link between early adversity and IL-6 reactivity by depressive symptoms was not observed among those with a WC at -1SD below the mean (b(SE) = −.03(.04); 95% CI [−.15–.03]).
3.3. Early adversity, depressive symptoms, and HPA axis functioning
3.3.1. Early adversity
As shown in Table 4, exposure to greater levels of early adversity was associated with decreased AUCg (ps < .004) and AUCi (ps < .08), indicating that adolescents with more adversity tended to have lower total cortisol output and cortisol reactivity. These relations did not vary by BMI or WC (ps > .10).
Table 4.
Stepwise hierarchical regression models predicting cortisol AUCg and AUCi from early adversity, indices of adiposity, and their interactions.
| AUCg
|
AUCi
|
|||||||
|---|---|---|---|---|---|---|---|---|
| BMI
|
WC
|
BMI
|
WC
|
|||||
| Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | |
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| Intercept | 231.90 (9.14)*** | 231.93 (9.45)*** | 229.68 (9.34)*** | 230.67 (9.52)*** | 1.04 (9.02) | .94 (9.33) | −2.47 (8.82) | −4.97 (8.85) |
| Age | −2.30 (8.62) | −2.26 (9.02) | −1.73 (8.81) | −1.16 (8.89) | −8.46 (8.51) | −8.58 (8.91) | −10.07 (8.31) | −11.50 (8.27) |
| Gender | −14.56 (8.49) | −14.58 (8.65) | −14.20 (8.79) | −14.70 (8.86) | 7.26 (8.39) | 7.32 (8.54) | 12.62 (8.30) | 13.90 (8.24) |
| Latino | 4.20 (9.62) | 4.19 (9.70) | 5.97 (9.75) | 5.80 (9.79) | 11.30 (9.49) | 11.33 (9.58) | 11.96 (9.20) | 12.39 (9.10) |
| Parent education | 1.48 (2.39) | 1.48 (2.42) | 1.31 (2.41) | 1.54 (2.45) | −2.99 (2.36) | −3.01 (2.39) | −2.93 (2.27) | −3.50 (2.28) |
| Risky families | −24.39 (7.28)*** | −24.38 (7.34)*** | −22.79 (7.38)** | −22.26 (7.46)** | −12.90 (7.19)† | −12.92 (7.25)† | −14.22 (6.97)* | −15.57 (6.94)* |
| Adiposity | .47 (.74) | .47 (.74) | .19 (.30) | .25 (.31) | 1.03 (.73) | 1.03 (.73) | .68 (.28)* | .54 (.29)† |
| Risky families x Adiposity | −.02 (1.66)** | −.31 (.11) | .08 (1.64) | .78 (.47) | ||||
p < .08
p < .05,
p < .01,
p < .001.
3.3.2. Depressive symptoms
Depressive symptoms were unrelated to AUCg (b(SE) = −.49 (.46), p = .28) and AUCi (b(SE) = .46 (.42), p = .28). Additionally, there were no interaction effects between depressive symptoms and measures of adiposity on AUCg (BMI: b(SE) = −.02 (.07), p = .68; WC: b(SE) = −.01 (.03), p = .60) and on AUCi (BMI: b(SE) = .03 (.07), p = .67; WC: b(SE) = .01 (.02), p = .67).
3.4. Sensitivity analyses
To rule out the confounding roles of time of laboratory visit, physical activity, smoking behavior, and potential current medical conditions, we repeated analyses while adjusting for these variables. Controlling for these factors did not alter findings. We also examined whether any outliers influenced results by winsorizing values exceeding three standard deviations from the mean on primary study variables, including early adversity (n = 1), BMI (n = 3), WC (n = 2), and IL-6 reactivity (n = 1). Results remained unchanged, indicating that extreme values did not influence findings.
4. Discussion
The present study aimed to deepen our understanding of the relation between early adversity and depressive symptoms and inflammatory biology by focusing on inflammatory reactivity to acute stress and by examining the moderating role of adiposity during adolescence. We found that the links from early adversity and depressive symptoms to IL-6 reactivity depended on youths’ BMI and WC, such that growing up in harsh family environment or having higher levels of current depressive symptoms was related to greater inflammatory reactivity to an acute stressor only among adolescents with higher BMI or WC. Moderated mediation analyses suggested that among adolescents with greater central adiposity, higher levels of current depressive symptoms mediated the link between early adversity and greater inflammatory reactivity. These findings were specific to inflammatory reactivity and were not apparent for HPA axis functioning. Results suggest that adiposity is an important vulnerability factor that may amplify the inflammatory effects of early adversity and depressive symptoms relatively early in life. They also highlight the importance of examining inflammatory reactivity to stress in relation to early adversity among young individuals.
The relations between early adversity and depressive symptoms and heightened inflammation emerged only in the context of higher adiposity, and this was evident only for IL-6 responses to psychosocial stress, but not for baseline levels of IL-6. A potential explanation for the observed findings is that adipose tissue may be a major source of stress-induced inflammation. Psychosocial stress upregulates sympathetic activity, increasing norepinephrine which can stimulate adipocytes and macrophages in adipose tissue, other organs, and in circulation to release pro-inflammatory cytokines (Black, 2006; Irwin and Cole, 2011; Wohleb et al., 2011). As described above, greater adiposity dramatically increases the number of adipose tissue macrophages and biases them towards a more pro-inflammatory phenotype. Adipocytes also become more inflammatory when adiposity increases. Greater adiposity, then, may foster an underlying tendency towards heightened inflammatory reactivity, and early adversity or depressive symptoms in conjunction with adiposity may lead to earlier emergence of higher IL-6 responses to stress during late adolescence.
Consistent with past research, there was some evidence that the association between early adversity and inflammatory reactivity among adolescents with greater adiposity was mediated by current depressive symptoms. Early adversity has been shown to be a potent risk factor for current and future depression. For instance, childhood and adolescent experiences of less supportive and more conflictual family environments predict symptoms and clinical diagnosis of depression concurrently and prospectively (Felitti et al., 1998; Sheeber et al., 1997; St Clair et al., 2015). In turn, a substantial body of work has elucidated an intimate bi-directional relation between depression and inflammation, with prior studies showing that symptoms of depression can subsequently increase risk for heightened inflammation (Copeland et al., 2012; Dowlati et al., 2010; Haapakoski et al., 2015; Matthews et al., 2010; Miller and Cole, 2012). Together with these past studies, the present study supports a mediation scenario whereby early adversity increases inflammation by increasing depressive symptoms, at least among adolescents with greater adiposity.
Main effects of early adversity and depressive symptoms as well as modulation of their links to inflammation by adiposity were not observed for baseline levels of IL-6. This may be due to the fact that we focused on adolescents, and levels of inflammation remain low early in life. Younger individuals tend to have relatively intact immune systems that keep inflammatory activity from overshooting and fostering a chronic inflammatory state (Miller and Chen, 2010). Consequently, effects of negative experiences such as early adversity and higher levels of depressive symptoms on baseline circulating levels of inflammation, even in the presence of other risk factors like greater adiposity, may not manifest until later in life. Our findings show that among younger individuals, the connections from early adversity and depressive symptoms to inflammation are evident when multiple risk factors are present and when challenging the inflammatory system. These results may help explain why early adversity has been more consistently associated with higher levels of inflammation under tonic conditions among adults (Danese et al., 2007; Lacey et al., 2014) compared to younger individuals (Miller and Chen, 2010; Miller et al., 2009; Schreier et al., 2014).
We explored whether results for inflammatory reactivity would extend to the HPA axis given cortisol’s inhibitory effects on inflammation and previously observed HPA axis alterations among individuals with a history of early adversity, depression, or obesity. Measures of adiposity did not interact with early adversity or depressive symptoms to influence total HPA output and reactivity to the TSST. This may be due to potential opposing effects of early adversity and adiposity on HPA axis functioning. In past studies, early adversity has been associated with decreased HPA activity whereas adiposity has been associated with enhanced HPA activity, though not all studies have observed these relations (Chiang et al., 2015b; Repetti et al., 2011; Rodriguez et al., 2015). Similarly, in the present study, greater exposure to early adversity was associated with decreased total cortisol output and reactivity whereas greater WC was associated with greater HPA reactivity. Rather than having synergistic effects, early adversity and abdominal adiposity may have independent additive effects on HPA axis functioning.
Several limitations of the study should be acknowledged. First, we relied on retrospective reports of early family stress. Responses may have been influenced by affective states or subject to recall biases. However, responses to the RF questionnaire concur with responses to clinical interviews assessing early adversity (Taylor et al., 2004), and similar scales retrospectively assessing early experiences have reliably been linked to adverse mental and physical health outcomes (Felitti et al., 1998). Second, adiposity was assessed indirectly using anthropometric measures BMI and WC. Although correlated with more direct measures of adiposity and associated with inflammation and cardio-metabolic risk (Bauer et al., 2015; Garnett et al., 2007; Herder et al., 2007; Janssen et al., 2005; Katzmarzyk et al., 2004; Pietrobelli et al., 1998; Taylor et al., 2000), these measures are not always precise; for instance, muscular individuals with less adipose tissue can have a high BMI. More accurate measures of adiposity, such as those based on dual energy X-ray absorptiometry, should be used in future work. Third, the correlational design precludes any inferences of causality, and other unmeasured factors may have influenced results. Relatedly, because the present study is cross-sectional, persistence of heightened inflammatory reactivity and long-term health consequences cannot be determined. Lastly, the artificiality of the laboratory-controlled setting makes unclear whether the present findings would translate to naturalistic settings. Participants were in a clinic setting with an indwelling catheter in their arms, which may not reflect typical experiences of psychosocial stress in everyday life. However, neuroendocrine responses to laboratory-based stressors have been related to neuroendocrine functioning in everyday life, pointing to the ecological validity of laboratory-based stress paradigms (Kidd et al., 2014). Furthermore, greater daily stress has been related to higher levels of IL-6 among individuals who were abused in early life but not among non-abused individuals (Gouin et al., 2012). This suggests that the present findings may apply to inflammatory responses to naturalistic stressors that occur in everyday life.
Despite these limitations, the present study points to the utility of considering vulnerability factors and going beyond baseline measures of inflammation to assess stress-induced inflammatory reactivity when studying the inflammatory consequences of early adversity or depressive symptoms during earlier life stages. Extending past studies showing adiposity to be a pathway linking early adversity to heightened inflammation (Matthews et al., 2014), we demonstrate that adiposity may also operate as an important modulator that may lead to earlier emergence of increased inflammation related to stressful experiences early in life during adolescence. To the extent that adolescents with greater adiposity and exposure to early adversity or depressive symptoms continue to mount an exaggerated inflammatory response to psychosocial threat over time, they may be more prone to developing inflammatory-related conditions including insulin resistance and atherosclerosis (Libby, 2012; Pradhan et al., 2001). It will be important for future work to ascertain the endurance and clinical significance of the observed findings.
Acknowledgments
This research was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD062547), UCLA California Center for Population Research funded by the National Institute of Child Health and Human Development (R24-HD041022), UCLA Older Americans Independence Center funded by the National Institute of Aging (P30-AG028748), UCLA Cousins Center for Psychoneuroimmunology, University of California Institute for Mexico and the US, American Psychological Association, and Division 38 of the American Psychological Association. The content does not necessarily represent the official views of the National Institute of Child Health and Human Development, National Institute of Aging, or the National Institutes of Health.
Footnotes
Conflicts of interest
None.
References
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the National Comorbidity Survey-Adolescent Supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37–44. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer KW, Marcus MD, Ogden CL, Foster GD. Cardio-metabolic risk screening among adolescents: understanding the utility of body mass index, waist circumference and waist to height ratio. Pediatr Obesity. 2015;10(5):329–337. doi: 10.1111/ijpo.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17(5):350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67(4):879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Brydon L, Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. J Hypertens. 2005;23(5):1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun. 2011;25(2):232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Almeida DM, Irwin MR, Seeman T, Fuligni AJ. Socioeconomic status, daily affective and social experiences, and inflammation during adolescence. Psychosom Med. 2015a;77(3):256–266. doi: 10.1097/PSY.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Taylor SE. Early adversity, neural development, and inflammation. Dev Psychobiol. 2015b;57(8):887–907. doi: 10.1002/dev.21329. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Tsai KM, Park H, Bower JE, Almeida DM, Dahl RE, Fuligni AJ. Daily family stress and HPA axis functioning during adolescence: the moderating role of sleep. Psychoneuroendocrinology. 2016;71:43–53. doi: 10.1016/j.psyneuen.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analyses. Biol Psychiatry. 2012;71(1):15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Lereya ST, Shanahan L, Worthman CM, Costello JE. Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. Proc Natl Acad Sci. 2014;111(21):7570–7575. doi: 10.1073/pnas.1323641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- Danese A, Lewis SJ. Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma? Neuropsychopharmacology. 2017;42:99–114. doi: 10.1038/npp.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. http://dx.doi.org/10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverts DJ, Cohen S, DiLillo VG, Lewis CE, Kiefe C, Whooley M, Matthews KA. Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2010;72(8):734–741. doi: 10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Coyne JC. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Way BM. Early-life stress and adult inflammation. Curr Directions Psychol Sci. 2014;23(4):277–283. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ferrante AW. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Galcheva SV, Iotova VM, Yotov YT, Bernasconi S, Street ME. Circulating proinflammatory peptides related to adiposity and cardiometabolic risk factors in healthy prepubertal children. Eur J Endocrinol. 2011;164:553–558. doi: 10.1530/EJE-10-1124. [DOI] [PubMed] [Google Scholar]
- Garnett SP, Baur LA, Srinivasan SR, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr. 2007;86(3):549–555. doi: 10.1093/ajcn/86.3.549. [DOI] [PubMed] [Google Scholar]
- Gouin J, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med. 2012;44(2):287–292. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Guilford Press; 2013. [Google Scholar]
- Hayes AF. An index and test of linear moderated mediation. Multivar Behav Res. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- Hazel NA, Hammen C, Brennan PA, Najman J. Early childhood adversity and adolescent depression: the mediating role of continued stress. Psychol Med. 2008;38(04):581–589. doi: 10.1017/S0033291708002857. [DOI] [PubMed] [Google Scholar]
- Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, Martin S. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metabol. 2007;92(12):4569–4574. doi: 10.1210/jc.2007-0955. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Pollmächer T. TNF-α, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw. 2006;17(3):196–201. [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. http://dx.doi.org/10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics. 2005;115(6):1623–1630. doi: 10.1542/peds.2004-2588. [DOI] [PubMed] [Google Scholar]
- Janusek LW, Tell D, Gaylord-Harden N, Mathews HL. Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: an epigenetic link. Brain Behav Immun. 2017;60:126–135. doi: 10.1016/j.bbi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114(2):e198–e205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas KR. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kidd T, Carvalho LA, Steptoe A. The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biol Psychol. 2014;99:34–40. doi: 10.1016/j.biopsycho.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Gilthorpe MS, Goodman E. A prospective study of psychological distress and weight status in adolescents/young adults. Ann Behav Med. 2012;43(2):219–228. doi: 10.1007/s12160-011-9323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey RE, Kumari M, Bartley M. Social isolation in childhood and adult inflammation: evidence from the National Child Development Study. Psychoneuroendocrinology. 2014;50:85–94. doi: 10.1016/j.psyneuen.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Beardslee WR. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: Role of obesity. Brain Behav Immun. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers MF. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun. 2010;24(1):96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McInnis CM, Thoma MV, Gianferante D, Hanlin L, Chen X, Breines JG, Rohleder N. Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun. 2014;42:33–40. doi: 10.1016/j.bbi.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown RE, Garrison CZ, Jackson KL, Cuffe SP, Addy CL, Waller JL. Family structure and cohesion, and depressive symptoms in adolescents. J Res Adolesc. 1997;7(3):267–281. [Google Scholar]
- McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. doi: 10.1037/a0024768. http://dx.doi.org/10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro-and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71(1):57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Med. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara RE, Armeli S, Boynton MH, Tennen H. Emotional stress-reactivity and positive affect among college students: the role of depression history. Emotion. 2014;14(1):193. doi: 10.1037/a0034217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T, Mletzko T, Alagbe O, Musselman D, Nemeroff CB, Miller A, Heim C. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis. 2005;183(2):308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parent J, Forehand R, Dunbar JP, Watson KH, Reising MM, Seehuus M, Compas BE. Parent and adolescent reports of parenting when a parent has a history of depression: associations with observations of parenting. J Abnorm Child Psychol. 2014;42(2):173–183. doi: 10.1007/s10802-013-9777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132(2):204–210. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Am Med Assoc. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Dev Psychopathol. 2011;22:921–938. doi: 10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Rodriguez ACI, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology. 2015;62:301–318. doi: 10.1016/j.psyneuen.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76(3):181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. Journal of Clinical Psychiatry. 2013;74:31–37. doi: 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- Schreier HMC, Roy LB, Frimer LT, Chen E. Family chaos and adolescent inflammatory profiles: The moderating role of socioeconomic status. Psychosom Med. 2014;76(6):460–467. doi: 10.1097/PSY.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sheeber L, Hops H, Alpert A, Davis B, Andrews J. Family support and conflict: prospective relations to adolescent depression. J Abnorm Child Psychol. 1997;25(4):333–344. doi: 10.1023/a:1025768504415. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38(2):188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH. The teenage brain: sensitivity to social evaluation. Curr Directions Psychol Sci. 2013;22(2):121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21(01):87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH, Najman JM, Bor W, O’Callaghan MJ, Williams GM. Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. J Child Psychol Psychiatry. 2002;43(4):457–469. doi: 10.1111/1469-7610.00037. [DOI] [PubMed] [Google Scholar]
- St Clair MC, Croudace T, Dunn VJ, Jones PB, Herbert J, Goodyer IM. Childhood adversity subtypes and depressive symptoms in early and late adolescence. Dev Psychopathol. 2015;27(03):885–899. doi: 10.1017/S0954579414000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steene-Johannessen J, Kolie E, Reseland JE, Anderssen SA, Andersen LB. Waist circumference is related to low-grade inflammation in youth. Int J Pediatr Obes. 2010;5(4):313–319. doi: 10.3109/17477160903497035. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, III, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. J Am Med Assoc. 1999;281(8):727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21(01):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72(2):490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Pers. 2004;72(6):1365–1394. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann DC, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz PH, Ehlert U, Emini L, Suter T. Higher body mass index (BMI) is associated with reduced glucocorticoid inhibition of inflammatory cytokine production following acute psychosocial stress in men. Psychoneuroendocrinology. 2008;33(8):1102–1110. doi: 10.1016/j.psyneuen.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, La’Tonia MS, Bailey MT, Sheridan JF. B-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Tartaglia LA. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]