Abstract
Purpose of review
Treatment with trastuzumab is a cornerstone of human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer treatment, but carries an unfortunate risk of toxicity to the cardiovascular system. Here we review recent findings on trastuzumab-associated cardiotoxicity, focusing on its incidence, diagnosis, and treatment.
Recent findings
Screening with multigated acquisition scan (MUGA) or echocardiogram (ECHO) is recommended to assess cardiac function prior to and during trastuzumab therapy. Because trastuzumab-induced cardiotoxicity is typically reversible, cessation of trastuzumab and/or administration of first line heart failure agents effectively restores cardiac function in most cases. Severe trastuzumab-induced cardiotoxicity is rare enough that the risk-benefit ratio still weighs in favor of its use in the vast majority of patients with HER2+ breast cancer.
Summary
An improved understanding of the pathophysiology underlying trastuzumab-induced cardiotoxicity and the identification of patients at highest risk will allow us to continue to safely administer trastuzumab in patients with breast cancer.
Keywords: breast cancer, trastuzumab, cardiotoxicity, cardiomyopathy, HER2, cardio-oncology
Introduction
According to the American Cancer Society (ACS), there will be over 255,000 new cases of breast cancer diagnosed in 2017, increasing the estimated total number of breast cancer survivors to more than 3.1 million living with or through their disease in the United States (1). Improvements in survival rates have spurred a growing recognition of the importance of cancer survivorship and the management of toxicities. Cardiotoxicity during treatment and/or post-treatment is addressed by the growing field of cardio-oncology. Cardiovascular complications including heart failure and coronary artery disease have been a long-standing problem for patients treated for breast cancer due to the frequent use of anthracyclines (e.g., doxorubicin and epirubicin) and radiation to the chest as part of treatment (2, 3). Risk of reduced left ventricular ejection fraction (LVEF) has been noted in a dose-dependent manner secondary to free radical generation from anthracycline use, even up to 30 years after treatment (2). In addition, exposure of the heart to radiotherapy has been linked to a long-standing dose-dependent increased rate of ischemic heart disease, especially in women with preexisting cardiac risk factors (3).
Over the past two decades, the introduction and use of trastuzumab therapy to treat the ~20% of breast cancers that overexpress human epidermal growth factor receptor 2 (HER2) has brought unique challenges to the field of cardio-oncology (4). Some of the HER2-directed therapeutic agents (e.g. trastuzumab and lapatinib) that target and inhibit tyrosine kinase cellular signaling pathways have been shown to detrimentally impact the cardiovascular system in some patients (5). Initial studies of trastuzumab in combination with chemotherapy demonstrated substantial improvements in progression-free and overall survival in patients with metastatic HER2+ disease, who previously had a very poor prognosis (6–9). Subsequently, large adjuvant clinical trials noted reduced recurrence rates with the use of one year of trastuzumab in combination with chemotherapy as adjuvant therapy for early stage HER2+ breast cancer, and this treatment approach is now standard of care (10–12).
Continuous use of HER2-directed therapy (often including multiple years of trastuzumab) is recommended for treatment of patients with HER2+ metastatic breast cancer. Reductions in LVEF are relatively common during trastuzumab therapy, but usually these are reversible with an interruption in or cessation of trastuzumab therapy (+/− introduction of cardioprotective medications). This manuscript describes the current evidence regarding the incidence, diagnosis, and treatment of trastuzumab-related cardiotoxicity.
Pathophysiology of trastuzumab cardiotoxicity
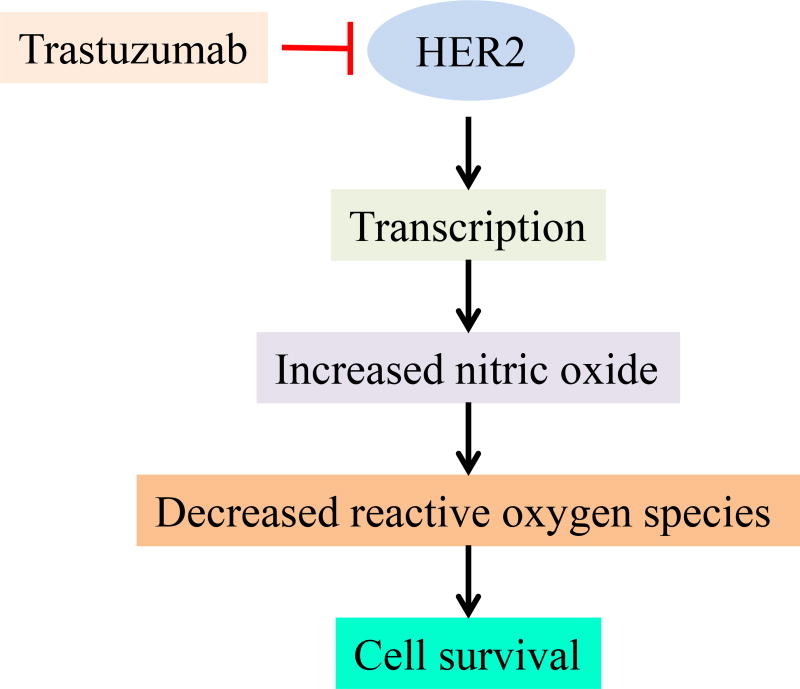
The pathophysiology underlying cardiac dysfunction in patients treated with HER2-targeted agents is not well understood. HER2 is one of the tyrosine kinases of the epidermal growth factor receptor family of proteins. Preclinical studies have demonstrated that HER2 signaling is important for cardiac development in the embryo, survival of myocytes, and protection from cardio-toxins. Neuregulin, a ligand to the epidermal growth factor receptor pathway, typically acts on cardiac cells via the HER2 receptor to protect cells from stress (13, 14). Blockade of HER2 receptors is thought to play a key role in mediating trastuzumab-induced cardiotoxicity by triggering a stress response (Figure 1). This makes the cardiac cells more susceptible to injury from other agents such as anthracyclines. Thus, trastuzumab is thought to interfere with the ligand binding-induced cardioprotective pathways that are needed for recovery from cardiac injury (15–17). It is believed that exposure to trastuzumab leads to loss of contractility due to cellular stunning rather than myocyte death (18). This theory is supported by the fact that the associated reductions in ejection fraction are frequently reversible.
Figure 1.
Mechanism of trastuzumab induced cardiotoxicity (Ref. 97)
Incidence, timing, and risk factors for trastuzumab-related cardiotoxicity
As we now have over two decades of experience administering trastuzumab, there is significant information available regarding the rates, timing, and long-term outcomes of trastuzumab-related cardiotoxicity (19). In addition, patient and treatment characteristics that increase risk are well characterized. Table 1 lists the factors associated with heightened risk of trastuzumab-induced cardiotoxicity.
Table 1.
Factors associated with cardiotoxicity
| Risk factors | Reference | |
|---|---|---|
| Baseline risk factors for trastuzumab-induced cardiotoxicity | Age>65 years | (60, 86–88) |
| BMI>30 kg/m2 | (60, 89–91) | |
| Hypertension | (60, 90, 91) | |
| Previous or concomitant anthracycline use | (60, 88, 92–94) | |
| Previous left ventricle dysfunction | (60, 86, 94) | |
| Previous radiation therapy | (60, 93, 95, 96) |
Rates and long-term outcomes of trastuzumab-related cardiotoxicity in patients treated with curative intent
Data from large adjuvant trastuzumab trials suggests that the proportion of patients who suffer asymptomatic LVEF declines during or after trastuzumab therapy in the curative-intent setting ranges from 4.1% to 30.1%, but rates of symptomatic CHF are much lower (0.6% to 3.8%) (20–25). In the Herceptin adjuvant (HERA) trial, a significant decrease in LVEF was defined as a drop in the ejection fraction of ≥10% points from baseline to an LVEF of less than 50% at any time. Compared to the observation arm, the 2-year and 1-year trastuzumab arms were associated with a higher incidence of severe congestive heart failure (CHF, 0.0%, 0.8%, and 0.8%, respectively) and significant decrease in LVEF (0.9%, 7.2%, and 4.1%, respectively) (26). Long term follow-up of 7–9 years for the adjuvant trastuzumab trials have indicated a low incidence of later development of cardiac events. Despite advancing patient age supported by up to 10 years of serial screening by LVEF, there was no evidence of delayed cardiotoxicity attributable to trastuzumab that emerged in the HERA trial (26).
Long-term follow up from NSABP B-31, a trial comparing adjuvant doxorubicin and cyclophosphamide (AC) followed by either weekly paclitaxel (AC-T, arm A); paclitaxel then trastuzumab sequentially (arm B); or paclitaxel plus trastuzumab concurrently followed by trastuzumab alone (arm C) in 1,944 women with operable HER2+ breast cancer, demonstrated that 2.6% of those who received trastuzumab and 0.9% of those in the control group (AC-T) developed CHF (22). Age ≥60, baseline LVEF between 50–55%, and antihypertensive use were associated with increased rates of cardiotoxicity. A decline in LVEF, defined as 16+% from any baseline or 10–15% from baseline to below the lower limit of normal, was noted at least once during follow-up in 15.4% patients in the AC-T group, 31.1% in the sequential group (AC-T followed by trastuzumab), and 27.1% in the concurrent group (AC-T with trastuzumab administered concurrent with the paclitaxel). The incidences of grade 2–4 CHF (with grade 2 defined as slight limitation of physical activity but comfortable at rest, grade 3 defined as marked limitation of physical activity but comfortable at rest, and grade 4 defined as inability to carry on any physical activity without discomfort), were 0.3%, 2.8%, and 3.3%, respectively, in these groups (27). However, in the APT trial, in which 406 patients received only trastuzumab and paclitaxel for 12 weeks followed by 9 months of trastuzumab (with no anthracycline administered), only 2 patients (0.5%) developed symptomatic CHF and came off study, and only 13 (3.2%) developed a significant LVEF decrease, 11 of whom went on to complete the treatment protocol (20). In BCIRG 006, comparing docetaxel-carboplatin-trastuzumab (TCH) to AC followed by docetaxel-trastuzumab (AC-DH) to AC followed by docetaxel (AC-D), the incidence of CHF was higher in the group receiving AC-DH (2.0%) than in the AC-D group (0.7%) or the TCH group (0.4%) (p<0.001). In addition, significant decline in LVEF was more common AC-DH group than the TCH group (18.6% vs. 9.4%, p<0.001), with a rate of 11.2% in the AC-D group (28). A subsequent meta-analysis of adjuvant treatment trials (n=18,111) confirmed that adjuvant trastuzumab is associated with an increased risk of grade 3 and 4 CHF [RR 3.04 (95 % CI 1.12 to 7.85; p<0.00001)], but absolute rates are still low (29). The majority of episodes of LVEF decline do not progress to clinical CHF, instead resolving quickly after cessation of therapy (often with medical intervention).
Rates and long-term outcomes of trastuzumab-related cardiotoxicity in the metastatic setting
The most common first line chemotherapy regimen used to treat HER2+ breast cancer is currently a taxane in combination with trastuzumab and pertuzumab. However, when trastuzumab was first introduced in 1997, its cardiotoxicity was less well understood, and anthracyclines were sometimes concurrently administered. In a seminal paper published by Slamon et al. in the New England Journal of Medicine in 2001, symptomatic or asymptomatic EF cardiac dysfunction occurred in 39 (27.2%) of 143 patients receiving doxorubicin and cyclophosphamide (AC) and trastuzumab concurrently, in 8.1% of patients receiving AC alone, in 13.2% of patients receiving paclitaxel and trastuzumab, and in 1.1% of patients receiving single-agent paclitaxel in a clinical trial (30).
In subsequent clinical trials of trastuzumab combined with non-anthracycline chemotherapy or endocrine therapy for metastatic disease, lower rates of cardiotoxicity were noted (0% to 1%) (31–34). Adding pertuzumab, another HER2-directed humanized monoclonal antibody, to trastuzumab does not appear to increase these risks in recent clinical trials (35, 36). Thus, the results from repeated echocardiographic monitoring on clinical trials demonstrate that the incidence of LVEF decline in patients receiving modern trastuzumab-based regimens without concurrent anthracycline is low, especially in patients without a low baseline LVEF (20, 28, 37–41). These data, along with the high rate of reversibility for trastuzumab-induced cardiotoxicity, favor continued trastuzumab use even in the face of asymptomatic LVEF declines (25, 42, 43).
Other data on rates and long-term outcomes of trastuzumab-related cardiotoxicity
A large meta-analysis (n=29,598) of randomized and cohort studies demonstrated that the risk of cardiac toxicity is likely very low if none is seen during the first year of treatment (44). Combining 58 studies, severe cardiotoxicity occurred in 3% of patients and the risk did not increase significantly over time. Age was a predictive factor for trastuzumab-induced cardiotoxicity (2.31% for <50 years of age, 3.46% for 50–59 years of age, and 4.91% in those >60 years of age). The study found the incidence of cardiotoxicity to be higher in patients with tobacco dependence (5.3%), dyslipidemia (3.9%), body-mass index (BMI) ≥25 (6.5%), diabetes (6.2%), hypertension (5.5%), and prior history of cardiac disease (19.1%). Different comorbidity burdens may partly account for the significant variability in the rates of trastuzumab-induced cardiotoxicity between different studies.
Retrospective cohort studies have sometimes noted a higher rate of LVEF decline with trastuzumab use than have clinical trials, possibly due to real world use in older patients with more cardiac comorbidities that are typically excluded from clinical trials (45–47). In a retrospective study of 12,500 women diagnosed with breast cancer (mean age = 60 years, range = 22–99 years), the risks of cardiotoxicity (defined as LVEF < 40% or a documented qualitative description of moderate or severely reduced systolic function) in the anthracycline, trastuzumab, and anthracycline + trastuzumab groups were 1.2%, 3.6% and 6.2%, respectively, but these risks were found to have increased to 4.3%, 12.1% and 20.1% after five years (45). A different study of over 9,500 breast cancer patients who were at least 66 years of age (with median age= 71 years) identified higher rates of CHF in trastuzumab users (29.4% in trastuzumab users vs 18.9% in non-trastuzumab users, p<0.001) (46). Finally, in another study of 45,537 older women (mean age = 76.2 years), the rates of cardiomyopathy [as defined by claims with HF or CM diagnoses according to the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes] were 32.1% and 41.9% in patients receiving trastuzumab and anthracycline + trastuzumab, respectively (p<0.001) (47). However, it is possible that using administrative codes to identify these endpoints may overestimate their prevalence (perhaps because providers use these diagnosis codes when they are ordering tests to assess cardiac function).
Monitoring trastuzumab-induced cardiotoxicity
Recommendations from the package insert
Due to the cardiotoxic effects of trastuzumab, the trastuzumab package insert recommends monitoring LVEF with an echocardiogram or multigated acquisition (MUGA) scan at baseline immediately prior to starting trastuzumab, every 3 months during treatment, upon completion of trastuzumab, and every 6 months for 2 years after completion (in the adjuvant setting) (48). There is no specific indication in the insert that monitoring should be stopped or made less frequent at a certain point for patients with metastatic disease. When LVEF decline ≥16% from baseline or a decrease in LVEF ≥10% from baseline to a value below institutional limits of normal is identified, it is suggested that trastuzumab be held for up to 8 weeks with repeat LVEF measures at 4 and 8 weeks. If LVEF returns to normal limits with an absolute decrease from baseline of ≤15%, it is recommended that trastuzumab be resumed. The insert recommends that trastuzumab be permanently discontinued in the setting of LVEF decline that lasts more than 8 weeks or if trastuzumab has been held on 3 or more occasions for cardiomyopathy.
Guideline recommendations
In contrast to the insert, the National Comprehensive Cancer Network (NCCN) specifies that optimal LVEF monitoring frequency during adjuvant therapy is not known (12). The 2012 European Society of Medical Oncology (ESMO) guidelines differ for patients with metastatic disease in that they recommend baseline assessment of LVEF and infrequent monitoring if asymptomatic cardiac dysfunction (49); whereas in the adjuvant setting, they are more specific, recommending monitoring of cardiac function at baseline, 3, 6, 9 months during treatment, and then 12 and 18 months following treatment (fewer tests than the package insert recommends) (50). The American Society for Clinical Oncology (ASCO) recommends LVEF monitoring at baseline and as deemed necessary by the physician during treatment for asymptomatic patients at risk for cardiac dysfunction with both localized and metastatic disease. The recommended testing frequency is said to be based on clinical judgment and patient circumstances. For individuals with clinical signs or symptoms concerning for cardiac dysfunction, a diagnostic assessment of LVEF is recommended during and/or after treatment. The ASCO guidelines also recommend the consideration of echocardiography between 6 and 12 months after completion of trastuzumab in asymptomatic patients considered to be at increased risk of cardiac dysfunction (based primarily on older age, comorbidities, and if anthracycline was also administered) (51).
Areas of controversy regarding frequency of cardiac monitoring
Several recent publications have called aspects of these cardiac monitoring recommendations into question (52–54). The intent of intensive monitoring is to identify asymptomatic cardiac dysfunction (stage B heart failure) to allow drug hiatus and medical management that will prevent the development of heart failure. However, the true value of this cardiac monitoring is uncertain, particularly in patients with metastatic disease. Since the implementation of guidelines, it has been noted that many of the declines in LVEF are asymptomatic, potentially reversible, and not necessarily predictive of future CHF. Frequent cardiac monitoring in all patients treated with trastuzumab may lead to withdrawal of essential HER2-directed therapy and suboptimal oncologic care in response to findings that may ultimately be of little clinical impact. If clinicians and patients decide that the value of trastuzumab for controlling breast cancer growth outweighs any recommendation to hold or stop treatment for asymptomatic LVEF decline, cardiac monitoring is not warranted or should at least be done less frequently. Even in the adjuvant setting, serial cardiac monitoring as currently recommended may be excessive and not cost-effective for the vast majority of patients with no exposure to anthracycline-based regimens and few baseline cardiac risk factors.
Additional monitoring modalities
Although current guidelines recommend echocardiogram or MUGA scans, there have been concerns regarding the limitations of echocardiography as it requires an adequate acoustic window, LVEF measurements have inherent variability, and LVEF decline only occurs after considerable cardiomyocyte dysfunction (55). A false-positive decline in LVEF could have the detrimental effect of leading the clinician to discontinue valuable cancer-directed therapy. The least temporal and inter-observer variability has been seen with use of three-dimensional echocardiography (56). A pilot study looking at the feasibility of cardiac MRI monitoring noted reduced longitudinal and circumferential strains at 6 months that reflected subclinical LV dysfunction and might assist with borderline detection of cardiotoxicity at an earlier stage. However, the predictive value of reduced strain for subsequent clinically significant heart failure was not determined and the results were not directly compared to echocardiography (57).
Myocardial strain, which measures myocardial deformation, has been considered as a surrogate marker for detecting early subclinical myocardial injury. However, currently there is no consensus on the threshold for change in global longitudinal strain due to factors such as variability in strain measurements, differences in strain values between vendors, and analysis software. As a result, serial measurements of global longitudinal strain appear to be more useful for predicting early cardiotoxicity. The American Society of Echocardiography suggests a threshold change in global longitudinal strain by >15% during cancer treatment to define cardiotoxicity (58). Results from the ongoing multicenter, randomized controlled trial, SUCCOUR [Strain Surveillance During Chemotherapy for Improving Cardiovascular Outcomes], will help identify whether strain is a better marker than LVEF for earlier detection of cardiotoxicity (59).
Current limitations of LVEF measurement as the standard of monitoring have also prompted research into the utility of blood-based biomarkers as an alternative monitoring strategy in hopes of detecting cardiotoxicity before significant myocardial damage occurs. One of the most studied biomarkers is cardiac troponin. The 2016 European Society of Cardiology (ESC) position paper on cancer treatments and cardiotoxicity heavily emphasizes the importance of identifying patients with pre-existing cardiovascular disease prior to initiating cardiotoxic oncologic treatments in order to decide who might benefit from serial troponin tests in addition to LVEF monitoring (60), but troponins have not yet been widely accepted in the United States as a routine blood-based biomarker of cardiac dysfunction during or after trastuzumab. A recent review of cardiac biomarker studies involving trastuzumab-treated patients noted that troponin elevation is generally only detected in patients who have been pretreated with anthracyclines. This is likely because trastuzumab usually causes impaired contractility rather than actual myocyte loss. Also, even in the setting of prior anthracyclines, it is difficult to measure troponins frequently enough to be useful, and when elevations occur, they are not always predictive of LVEF reduction. Furthermore, the optimal assay and the definition of positivity remain controversial (61). Another biomarker of interest is NT-proBNP, but this has not yet been found to be of use for detecting cardiotoxicity from trastuzumab (61, 62). Ongoing clinical trials are evaluating the use of cardiac MRI and biomarkers such as BNP and troponin I for detecting and treating asymptomatic chemotherapy-induced cardiotoxicity (63).
Adherence
Perhaps due in part to the controversies regarding optimal cardiac monitoring strategies, a large retrospective study noted poor adherence to guideline-directed cardiac monitoring. Adequate cardiac monitoring, defined as having a baseline cardiac evaluation (with echocardiogram or MUGA scan within 4 months before first trastuzumab dose) and subsequent follow-up cardiac evaluation at least every 4 months while receiving trastuzumab therapy, occurred in only 17.4% of 2,203 patients. Interestingly, patients with cardiac comorbidities were no more likely to receive adequate cardiac monitoring (64). It was noted that physician characteristics were more likely to influence adherence to cardiac monitoring than patient factors. As aspects of the current cardiac monitoring guidelines are further scrutinized and adjusted, it will be important to continue to assess compliance and cost effectiveness.
Preventing trastuzumab-induced cardiotoxicity
Optimal prevention strategies for cardiotoxicity from trastuzumab are unknown. However, one clearly effective approach is to minimize sequential or concurrent anthracycline use. A drop in the frequency of anthracycline administration for breast cancer has occurred over the last decade due to the availability of novel efficacious non-anthracycline-based regimens (e.g. TCH) (28, 65). One study showed that 51% of early stage breast cancer patients receiving chemotherapy now receive taxane-based chemotherapy, while only 32% receive anthracycline-based chemotherapy (though that study was not focused on patients with HER2+ disease) (66). This trend will lower the cardiac risks from trastuzumab for the average patient, though anthracyclines will likely continue to be administered to patients with higher risk of recurrence (including those treated on the currently enrolling large ISPY-2 trial).
Other potential prevention strategies involve cardio-protective drugs. Although observational studies and retrospective reviews of trial data from patients receiving trastuzumab therapy have indicated a possible decrease in incidence of new heart failure events with beta blocker (BB) use and complete or partial recovery of cardiac function with guideline-directed heart failure treatment (67, 68), randomized clinical trial data are inconclusive. The Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) 101 study randomized patients with HER2+ early stage breast cancer to receive perindopril (angiotensin converting enzyme inhibitor, ACEI), bisoprolol (BB), or placebo (1:1:1) for the duration of adjuvant trastuzumab therapy. Though declines in LVEF were reduced by 3% with prophylactic ACEI and 1% with prophylactic BB use, the primary outcome of left ventricular remodeling was not prevented post-cycle 17 of trastuzumab therapy. It was also noted that the population in the trial was young with few baseline cardiovascular risk factors. Upcoming 24-month cardiac MRI results will further clarify long-term outcomes in this study (69). The Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) study assessed the value of prophylactic angiotensin receptor blocker (ARB) and BB therapy in patients receiving anthracycline-based chemotherapy with or without trastuzumab. When compared to placebo in a 2×2 factorial design, less LVEF reduction from baseline (0.8%) was noted in the candesartan group (ARB) compared to placebo (2.6%, p=0.026). Metoprolol (BB) was not found to have an effect on LVEF decline either alone or with candesartan (ARB) (70). Another randomized clinical trial of candesartan (ARB) vs. placebo during trastuzumab therapy identified no statistically significant difference in the incidence of LVEF decline between the groups (71), suggesting that the benefit of using this drug preventively is likely modest at best in this setting. A recent review noted that these studies have all been small with a short average follow up of approximately 6 months. These trials have also not been powered to assess clinical outcomes, instead measuring LVEF as a primary endpoint. It has been questioned if the observed reduction in LVEF decline is of clinical relevance (72).
Ongoing research in this area includes a phase II randomized clinical trial evaluating lisinopril (ACEI) or carvedilol (BB) in breast cancer patients receiving adjuvant or neoadjuvant trastuzumab therapy. The target accrual is 468 participants and the primary outcome will be the incidence of cardiotoxicity and changes in LVEF (73). The SAFE trial is a phase III randomized clinical trial that will evaluate potential preventive effects of bisoprolol (BB) and ramipril (ACE) on cardiotoxicity in non-metastatic breast cancer patients exposed to neoadjuvant or adjuvant anthracyclines and/or trastuzumab in a four arm, placebo-controlled design. The primary outcome will be changes in LVEF based on serial cardiac monitoring as well as maximum LVEF change (74).
The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines suggest several lifestyle modifications and optimization of risk factors that might help minimize the risk of heart failure during and after trastuzumab therapy. Smoking cessation is encouraged as it is strongly associated with increased heart failure incidence in the general population. Heavy use of alcohol, defined as consuming 15 drinks or more per week for men and 8 drinks or more per week for women, has been associated with increased heart failure risk in the general population as well. Extrapolating observations in the general population to those on trastuzumab therapy, treatment of hypertension with diuretic-based therapy along with ACEIs, ARBs, and BBs may add benefit too. Hyperlipidemia should be managed aggressively with statin medications, and efforts to achieve a normal BMI via diet and exercise are encouraged. Exercise is known to improve functional status and reduce mortality in patients with CHF in the general population (75). The Mediterranean diet, high in fruits, vegetables, nuts, cereals, fish, and olive oil with moderate red wine consumption and minimal amounts of red meat and dairy products has been linked to beneficial effects on vascular disease, blood pressure, glycemic control, and lipids (76, 77). However, evidence is limited and variable regarding cardio-protection from diet in the general population (78), and almost nonexistent in patients receiving trastuzumab specifically. Aggressive glycemic control is also recommended in guidelines despite a paucity of evidence that this will decrease heart failure risk. Salt restriction is likely not appropriate as a preventive measure unless symptoms or signs of reduced LVEF develop. Treatment of sleep apnea with continuous positive airway pressure (CPAP) increases LVEF and improves functional status in heart failure patients (79).
Treatment for trastuzumab-induced cardiotoxicity
Expert consensus recommendations suggest treatment with first-line heart failure medications and early cardiology consultation if symptoms or signs of trastuzumab-induced cardiotoxicity develop (80). ACEIs/ARBs and BBs are appropriate first-line agents followed by loop diuretics, hydralazine-nitrates, and aldosterone antagonists per the ACC/AHA guidelines (79). Newer agents may also be of benefit such as the neprilysin-angiotensin receptor antagonist, sacubitril-valsartan, and the If channel inhibitor, ivabradine (72). For advanced heart failure, cardiac resynchronization therapy, mechanical circulatory support, and orthotopic heart transplant may be of benefit (81). However, data are scant pertaining to the impact of traditional heart failure treatments in patients who develop cardiotoxicity from trastuzumab (72).
Current guidelines recommend at least temporary discontinuation of trastuzumab therapy if significant decline in LVEF is detected via monitoring, symptomatic or asymptomatic. However, a retrospective study of 92 patients who experienced asymptomatic trastuzumab-induced LVEF declines found no development of heart failure at follow-up despite continued trastuzumab therapy in 34% of the patients (82). The SAFE-HEaRt study is a single-arm pilot study that is currently exploring the safety of continuing trastuzumab despite significant LVEF decline to 40–49% (83). These studies are important as they may allow for continuation of important oncologic therapy with close monitoring in cases of asymptomatic LVEF decline.
Referral to a cardiologist is recommended once an abnormal baseline LVEF or significant decrease in LVEF during monitoring is noted to ensure early treatment of myocardial injury (80). Additionally, referral to a cardio-oncology provider prior to initiation of trastuzumab based therapy in those at highest risk for developing trastuzumab-induced cardiac complications is recommended. A recent large study assessed the quality of cardiac care provided to cancer patients diagnosed with left ventricle dysfunction associated with chemotherapy. Poor physician (oncologist) adherence to guideline-recommended heart failure therapy was noted (78% for ACEI/ARB, 70% for BB, 65% combination). Cardiology consultation was associated with an improved rate of guideline adherence (100% vs 52% for ACEI and 94% vs 41% for BB) (84). The importance of cardiology consultation is further reinforced by a recent survey showing considerable disparity in the practices of oncologists in regards to cardiotoxicity (85).
Conclusion
The development of either asymptomatic or symptomatic cardiotoxicity with trastuzumab therapy can complicate the treatment of HER2+ breast cancer. In most patients, the risk of cardiotoxicity is justified given the substantial improvements in breast cancer-related outcomes provided by trastuzumab. Current guidelines include routine monitoring with serial echocardiography, but this is likely most important for patients without metastatic disease and with more risk factors for cardiotoxicity (e.g., older age, obesity, hypertension, diabetes, etc.). Ongoing research pertaining to optimal cardiac surveillance and prevention strategies that are tailored to individual cardiac risks and trastuzumab-related oncologic benefits will inform best practices going forward.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2017. [Internet] [cited June 7, 2017];2017 Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures.html.
- 2.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Breast Cancer Facts & Figures 2015–2016 [Internet] [cited June 3, 2017]; Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2015-2016.pdf.
- 5.Krause DS, Van Etten RA. Tyrosine Kinases as Targets for Cancer Therapy. New England Journal of Medicine. 2005;353(2):172–87. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 6.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Journal of Clinical Oncology. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Carbonell X, Castañeda-Soto N-J, Clemens M, Green M, Harvey V, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. Journal of Clinical Oncology. 2005;23(10):2162–71. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The M77001 study group. Journal of clinical oncology. 2005;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 10.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New England Journal of Medicine. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 11.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New England Journal of Medicine. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Breast Cancer Version 2.2017. 2017 https://wwwnccnorg/professionals/physician_gls/pdf/breastpdf. April 6, 2017.
- 13.Tocchetti CG, Ragone G, Coppola C, Rea D, Piscopo G, Scala S, et al. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. European journal of heart failure. 2012;14(2):130–7. doi: 10.1093/eurjhf/hfr165. [DOI] [PubMed] [Google Scholar]
- 14.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circulation research. 2012;111(10):1376–85. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crone SA, Zhao Y-Y, Fan L, Gu Y, Minamisawa S, Liu Y, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature medicine. 2002;8(5):459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 16.ElZarrad MK, Mukhopadhyay P, Mohan N, Hao E, Dokmanovic M, Hirsch DS, et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PloS one. 2013;8(11):e79543. doi: 10.1371/journal.pone.0079543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuppinger C, Timolati F, Suter TM. Pathophysiology and diagnosis of cancer drug induced cardiomyopathy. Cardiovascular toxicology. 2007;7(2):61–6. doi: 10.1007/s12012-007-0016-2. [DOI] [PubMed] [Google Scholar]
- 18.Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. Journal of cardiac failure. 2008;14(5):437–44. doi: 10.1016/j.cardfail.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Ponde NF, Lambertini M, de Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO open. 2016;1(4):e000073. doi: 10.1136/esmoopen-2016-000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K, et al. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA oncology. 2016;2(1):29–36. doi: 10.1001/jamaoncol.2015.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31):3792–9. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J Clin Oncol. 2016;34(6):581–7. doi: 10.1200/JCO.2015.61.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slamon DJEW, Robert NJ, Giermek J, Martin M, Jasiowka M, Mackey JR, Chan A, Liu M-C, Pinter T, Valero V, Falkson C, Fornander T, Shiftan TA, Bensfia S, Hitier S, Xu N, Bée-Munteanu V, Drevot P, Press MF, Crown J. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. In: On Behalf of the BCIRG-006 Investigators, editor. San Antonio Breast Cancer Symposium Abstract S5-04 Presented; Dec 11, 2015. 2015. [Google Scholar]
- 25.Romond EH, Jeong J-H, Rastogi P, Swain SM, Geyer CE, Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2–positive breast cancer. Journal of Clinical Oncology. 2012;30(31):3792–9. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32(20):2159–65. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 27.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 28.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. New England Journal of Medicine. 2011;365(14):1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long HD, Lin YE, Zhang JJ, Zhong WZ, Zheng RN. Risk of Congestive Heart Failure in Early Breast Cancer Patients Undergoing Adjuvant Treatment With Trastuzumab: A Meta-Analysis. Oncologist. 2016;21(5):547–54. doi: 10.1634/theoncologist.2015-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 31.Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, et al. Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat. 2007;101(3):355–65. doi: 10.1007/s10549-006-9306-9. [DOI] [PubMed] [Google Scholar]
- 32.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27(33):5529–37. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 34.von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47(15):2273–81. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. New England Journal of Medicine. 2015;372(8):724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianni L, Pienkowski T, Im Y-H, Tseng L-M, Liu M-C, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. The Lancet Oncology. 2016;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 37.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. New England Journal of Medicine. 2015;372(2):134–41. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore I, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. The Lancet Oncology. 2013;14(11):1121–8. doi: 10.1016/S1470-2045(13)70384-X. [DOI] [PubMed] [Google Scholar]
- 39.Swain SM, Ewer MS, Cortés J, Amadori D, Miles D, Knott A, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. The oncologist. 2013;18(3):257–64. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. Journal of Clinical Oncology. 2001;19(10):2587–95. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 41.Dang C, Iyengar N, Datko F, D'Andrea G, Theodoulou M, Dickler M, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. Journal of Clinical Oncology. 2014;33(5):442–7. doi: 10.1200/JCO.2014.57.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Azambuja E, Procter MJ, Van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01) Journal of clinical oncology. 2014;32(20):2159–65. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 43.Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. Journal of Clinical Oncology. 2015;34(6):581–7. doi: 10.1200/JCO.2015.61.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantarro S, Rossi M, Bonifazi M, D’Amico R, Blandizzi C, La Vecchia C, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Internal and emergency medicine. 2016;11(1):123–40. doi: 10.1007/s11739-015-1362-x. [DOI] [PubMed] [Google Scholar]
- 45.Bowles EJA, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. Journal of the National Cancer Institute. 2012;104(17):1293–305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. Journal of Clinical Oncology. 2013;31(33):4222–8. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. Journal of the American College of Cardiology. 2012;60(24):2504–12. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 48.Genentech: Herceptin (trastuzumab): highlights of prescribing information. http://wwwgenecom/download/pdf/herceptin_prescribingpdf. 4/2015 update.
- 49.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Annals of Oncology. 2012;23(suppl_7):vii155–vii66. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 50.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Annals of Oncology. 2012;23(suppl 7):vii155–vii66. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 51.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2017;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 52.Brann AM, Cobleigh MA, Okwuosa TM. Cardiovascular Monitoring With Trastuzumab Therapy: How Frequent Is Too Frequent? JAMA oncology. 2016;2(9):1123–4. doi: 10.1001/jamaoncol.2016.1288. [DOI] [PubMed] [Google Scholar]
- 53.Davis CC, Zelnak A, Eley JW, Goldstein DA, Switchenko JM, McKibbin T. Clinical utility of routine cardiac monitoring in breast cancer patients receiving trastuzumab. Annals of Pharmacotherapy. 2016 doi: 10.1177/1060028016654160. 1060028016654160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. Journal of Clinical Oncology. 2016;34(10):1030–3. doi: 10.1200/JCO.2015.64.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. Journal of the American Society of Echocardiography. 2013;26(5):493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes. Journal of the American College of Cardiology. 2013;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Nakano S, Takahashi M, Kimura F, Senoo T, Saeki T, Ueda S, et al. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: A pilot study to evaluate the feasibility of the method. Cardiol J. 2016;23(3):270–80. doi: 10.5603/CJ.a2016.0023. [DOI] [PubMed] [Google Scholar]
- 58.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Negishi T, Negishi K, Nolan M, Fukuda N, Yamada H, Lemieux J, et al. Persistence of Higher Concordance of Strain Over Ejection Fraction for Evaluation of Left Ventricular Function: Results from the Succour Trial. Journal of the American Society of Echocardiography. 2016;29(6):B66. [Google Scholar]
- 60.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) European heart journal. 2016;37(36):2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 61.Witteles RM. Biomarkers as predictors of cardiac toxicity from targeted cancer therapies. Journal of cardiac failure. 2016;22(6):459–64. doi: 10.1016/j.cardfail.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II–positive breast cancer treated with adjuvant trastuzumab therapy. Journal of the American College of Cardiology. 2011;57(22):2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 63. [June 2, 2017];2017 https://clinicaltrials.gov/
- 64.Chavez-MacGregor M, Niu J, Zhang N, Elting LS, Smith BD, Banchs J, et al. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. Journal of Clinical Oncology. 2015;33(19):2176–83. doi: 10.1200/JCO.2014.58.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Annals of oncology. 2013;24(9):2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 66.Giordano SH, Lin Y-L, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the Use of Anthracyclines for Breast Cancer. Journal of Clinical Oncology. 2012;30(18):2232–9. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of Beta-Adrenoceptor blockade in breast cancer patients undergoing chemotherapy: a follow-up study of heart failure. Circulation: Heart Failure. 2013 doi: 10.1161/CIRCHEARTFAILURE.112.000055. CIRCHEARTFAILURE. 112.000055. [DOI] [PubMed] [Google Scholar]
- 68.Russell SD, Blackwell KL, Lawrence J, Pippen JE, Jr, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. Journal of Clinical Oncology. 2010;28(21):3416–21. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 69.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101–Breast): A randomized trial for the prevention of trastuzumab-associated cardiotoxicity. Journal of Clinical Oncology. 2016 doi: 10.1200/JCO.2016.68.7830. JCO. 2016.68. 7830. [DOI] [PubMed] [Google Scholar]
- 70.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2× 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. European heart journal. 2016;37(21):1671–80. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boekhout AH, Gietema JA, Kerklaan BM, van Werkhoven ED, Altena R, Honkoop A, et al. Angiotensin II–receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA oncology. 2016;2(8):1030–7. doi: 10.1001/jamaoncol.2016.1726. [DOI] [PubMed] [Google Scholar]
- 72.Mukku RB, Fonarow GC, Watson KE, Ajijola OA, Depasquale EC, Nsair A, et al. Heart Failure Therapies for End-Stage Chemotherapy–Induced Cardiomyopathy. Journal of cardiac failure. 2016;22(6):439–48. doi: 10.1016/j.cardfail.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Guglin M, Munster P, Fink A, Krischer J. Lisinopril or Coreg CR in reducing cardiotoxicity in women with breast cancer receiving trastuzumab: A rationale and design of a randomized clinical trial. American Heart Journal. 2017;188:87–92. doi: 10.1016/j.ahj.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meattini I, Curigliano G, Terziani F, Becherini C, Airoldi M, Allegrini G, et al. SAFE trial: an ongoing randomized clinical study to assess the role of cardiotoxicity prevention in breast cancer patients treated with anthracyclines with or without trastuzumab. Medical Oncology. 2017;34(5):75. doi: 10.1007/s12032-017-0938-x. [DOI] [PubMed] [Google Scholar]
- 75.Collaborative E. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) Bmj. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martínez-González MÁ, De la Fuente-Arrillaga C, Nuñez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. Bmj. 2008;336(7657):1348–51. doi: 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rumawas ME, Meigs JB, Dwyer JT, McKeown NM, Jacques PF. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. The American journal of clinical nutrition. 2009;90(6):1608–14. doi: 10.3945/ajcn.2009.27908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liyanage T, Ninomiya T, Wang A, Neal B, Jun M, Wong MG, et al. Effects of the Mediterranean Diet on Cardiovascular Outcomes—A Systematic Review and Meta-Analysis. PLoS One. 2016;11(8):e0159252. doi: 10.1371/journal.pone.0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013 CIR. 0b013e31829e8776. [Google Scholar]
- 80.Cueva J, Antolín S, Calvo L, Fernández I, Ramos M, de Paz L, et al. Galician consensus on management of cardiotoxicity in breast cancer: risk factors, prevention, and early intervention. Clinical and Translational Oncology. 2017:1–12. doi: 10.1007/s12094-017-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bianco CM, Al-Kindi SG, Oliveira GH. Advanced Heart Failure Therapies for Cancer Therapeutics–Related Cardiac Dysfunction. Heart Failure Clinics. 2017;13(2):327–36. doi: 10.1016/j.hfc.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Yu AF, Yadav NU, Eaton AA, Lung BY, Thaler HT, Liu JE, et al. Continuous Trastuzumab Therapy in Breast Cancer Patients With Asymptomatic Left Ventricular Dysfunction. Oncologist. 2015;20(10):1105–10. doi: 10.1634/theoncologist.2015-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lynce F, Barac A, Tan MT, Asch FM, Smith KL, Dang C, et al. SAFE-HEaRt: Rationale and Design of a Pilot Study Investigating Cardiac Safety of HER2 Targeted Therapy in Patients with HER2-Positive Breast Cancer and Reduced Left Ventricular Function. The Oncologist. 2017;22(5):518–25. doi: 10.1634/theoncologist.2016-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ammon M, Arenja N, Leibundgut G, Buechel RR, Kuster GM, Kaufmann BA, et al. Cardiovascular management of cancer patients with chemotherapy-associated left ventricular systolic dysfunction in real-world clinical practice. Journal of cardiac failure. 2013;19(9):629–34. doi: 10.1016/j.cardfail.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Jovenaux L, Cautela J, Resseguier N, Pibarot M, Taouqi M, Orabona M, et al. Practices in management of cancer treatment-related cardiovascular toxicity: A cardio-oncology survey. International Journal of Cardiology. 2017 doi: 10.1016/j.ijcard.2017.02.154. [DOI] [PubMed] [Google Scholar]
- 86.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac Safety Analysis of Doxorubicin and Cyclophosphamide Followed by Paclitaxel With or Without Trastuzumab in the North Central Cancer Treatment Group N9831 Adjuvant Breast Cancer Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1231–8. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan-Chiu E, Yothers G, Romond E, Jr, C EG, Ewer M, Keefe D, et al. Assessment of Cardiac Dysfunction in a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel, With or Without Trastuzumab As Adjuvant Therapy in Node-Positive, Human Epidermal Growth Factor Receptor 2–Overexpressing Breast Cancer: NSABP B-31. Journal of Clinical Oncology. 2005;23(31):7811–9. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 88.Russo G, Cioffi G, Di Lenarda A, Tuccia F, Bovelli D, Di Tano G, et al. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Internal and Emergency Medicine. 2012;7(5):439–46. doi: 10.1007/s11739-012-0794-9. [DOI] [PubMed] [Google Scholar]
- 89.Guenancia C, Lefebvre A, Cardinale D, Yu AF, Ladoire S, Ghiringhelli F, et al. Obesity As a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Oncology. 2016;34(26):3157–65. doi: 10.1200/JCO.2016.67.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gunaldi M, Duman BB, Afsar CU, Paydas S, Erkisi M, Kara IO, et al. Risk factors for developing cardiotoxicity of trastuzumab in breast cancer patients: An observational single-centre study. Journal of Oncology Pharmacy Practice. 2016;22(2):242–7. doi: 10.1177/1078155214567162. [DOI] [PubMed] [Google Scholar]
- 91.Valicsek E, Kószó R, Dobi Á, Uhercsák G, Varga Z, Vass A, et al. Cardiac surveillance findings during adjuvant and palliative trastuzumab therapy in patients with breast cancer. Anticancer research. 2015;35(9):4967–73. [PubMed] [Google Scholar]
- 92.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac Dysfunction in the Trastuzumab Clinical Trials Experience. Journal of Clinical Oncology. 2002;20(5):1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 93.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99(9):634–9. doi: 10.1136/heartjnl-2012-303151. [DOI] [PubMed] [Google Scholar]
- 94.Cochet A, Quilichini G, Dygai-Cochet I, Touzery C, Toubeau M, Berriolo-Riedinger A, et al. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Research and Treatment. 2011;130(3):845–54. doi: 10.1007/s10549-011-1714-9. [DOI] [PubMed] [Google Scholar]
- 95.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95(7):1592–600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 96.Cao L, Cai G, Chang C, Yang Z-Z, Feng Y, Yu X-L, et al. Early cardiac toxicity following adjuvant radiotherapy of left-sided breast cancer with or without concurrent trastuzumab. Oncotarget. 2016;7(1):1042–54. doi: 10.18632/oncotarget.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: A 'dual-hit'. Exp Clin Cardiol. 2011;16(3):70–4. [PMC free article] [PubMed] [Google Scholar]