Abstract
Objectives
Lung cancer is the leading cause of cancer related deaths worldwide and mutation activating KRAS is one of the most frequent mutations found in lung adenocarcinoma. Identifying regulators of KRAS may aid in the development of therapies to treat this disease. The mitogen-induced gene 6 MIG-6 is a small adaptor protein modulating signaling in cells to regulate the growth and differentiation in multiple tissues. Here, we investigated the role of Mig-6 in regulating adenocarcinoma progression in the lungs of genetically engineered mice with activation of Kras.
Materials and Methods
Using the CCSPCre mouse to specifically activate expression of the oncogenic KrasG12D in Club cells, we investigated the expression of Mig-6 in CCSPCreKrasG12D-induced lung tumors. To determine the role of Mig-6 in KrasG12D-induced lung tumorigenesis, Mig-6 was conditionally ablated in the Club cells by breeding Mig6f/f mice to CCSPCreKrasG12D mice, yielding CCSPCreMig-6d/dKrasG12D mice (Mig-6d/dKrasG12D).
Results
We found that Mig-6 expression is decreased in CCSPCreKrasG12D-induced lung tumors. Ablation of Mig-6 in the KrasG12D background led to enhanced tumorigenesis and reduced life expectancy. During tumor progression, there was increased airway hyperplasia, a heightened inflammatory response, reduced apoptosis in KrasG12D mouse lungs, and an increase of total and phosphorylated ERBB4 protein levels. Mechanistically, Mig-6 deficiency attenuates the cell apoptosis of lung tumor expressing KRASG12D partially through activating the ErbB4 pathway.
Conclusions
In summary, Mig-6 deficiency promotes the development of KrasG12D-induced lung adenoma through reducing the cell apoptosis in KrasG12D mouse lungs partially by activating the ErbB4 pathway.
Keywords: Mig-6 (ERRFI1), Kras, ErbB4, Lung cancer/tumor, ErbB signaling, Apoptosis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide [1, 2]. Human lung cancers are categorized as non-small cell lung carcinoma (NSCLC) and small cell carcinoma (SCLC) [3, 4]. NSCLC accounts for about 80% of lung cancers and its adenocarcinoma (AD) subtype is found in 30–40% of all lung cancers [5–7]. Genomic analyses have identified somatic mutations in key signaling pathways in lung adenocarcinoma [8]. Activating Kras mutations, among the most frequently identified in human tumors, are found in 25–50% of human lung ADs and associated with poor prognosis [9, 10]. Activated Kras constitutively turns on PI3K/AKT and RAF/MEK/ERK signaling pathways, resulting in abnormal cell proliferation and apoptosis [11–15]. Kras mutants change the tumor growth microenvironment through the activation of tissue remodeling, inflammation, and angiogenesis [13, 15, 16]. For example, mice with the constitutively active KrasG12D mutant allele develop adenocarcinoma [17]. Identification of modifiers of Kras may present targets for treatment of patients with Kras-driven AD and one potential regulator of KRAS is MIG-6. Downregulation of MIG-6 has been demonstrated to enhance resistance of KRAS mutant human colorectal and gastric adenocarcinoma cells to MEK inhibitors [18], suggesting that MIG-6 inactivation regulates the properties of cancers having the KRAS mutation.
MIG-6, also known as ErbB receptor feedback inhibitor 1 (Errfil) [19], is a ubiquitously expressed scaffold protein that is induced by growth factors or other stress stimuli [20]. MIG-6 modulates growth factor activation by regulating signaling pathways through the alterations of auto-phosphorylations of protein kinases [19, 20]. Downregulated MIG-6 expression is observed in multiple human cancers, including lung cancer [21–25]. Germline ablation of Mig-6 in mice results in partial neonatal mortality due to abnormal lung development [26]. The surviving adult mice have joint defects and tumorigenesis in the lung, skin, and liver [27–29] as well as features that resemble chronic obstructive pulmonary disease (COPD) [26]. Mig-6 null mice also show accelerated lung AD when crossed with an Egfr mutant-driven transgenic mouse [30]. Moreover, airway ablation of Mig-6 in combination with loss of the tumor suppressor gene, Pten, shows activated ERBB2 phosphorylation and the development of AD [31]. To investigate the role of Mig-6 in Kras-driven lung cancer development in vivo, we crossed Mig-6f/f alleles into the CCSP-Cre/LSL-KrasG12D (KrasG12D) background to generate CCSPCre/LSL-KrasG12D/Mig-6f/f (Mig-6d/dKrasG12D) mice.
Results
Decreased MIG-6 expression in KrasG12D-induced Mouse Lung Hyperplasia and Adenomas
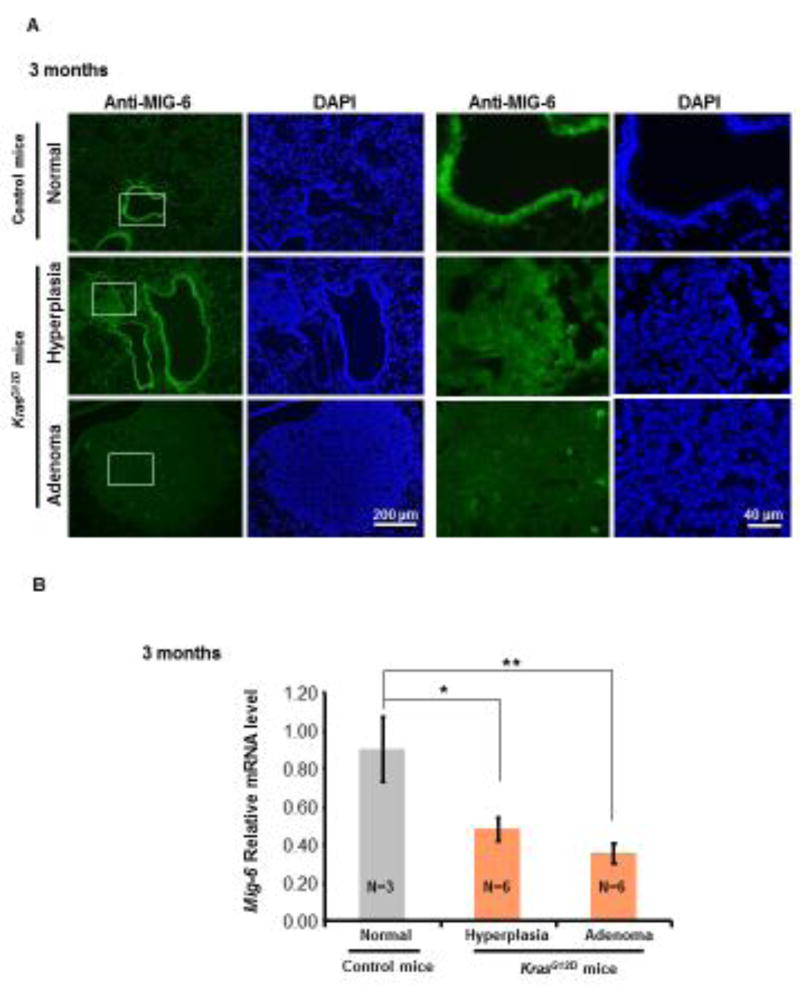
The expression of MIG-6 was investigated in the tumors of CCSPCre KrasG12D (KrasG12D) mouse lungs. Immunostaining revealed that expression of MIG-6 protein was prominent in epithelia of normal airways, slightly reduced in hyperplastic lesions, and almost lost completely in adenomatous lesions (Fig. 1A). Mig-6 mRNA levels were significantly reduced not only in the hyperplastic airways, but also in adenomatous lesions compared to normal airways (Fig.1B). The post-transcriptional modification may cause the differences between MIG-6 protein and its mRNA levels in hyperplastic airways. This discrepancy may also be due to the sensitivity of each assay. These results indicate that Mig-6 expression is negatively correlated with lung cancer progression.
Figure 1. Mig-6 expression was down-regulated in oncogenic Kras-induced (CCSPCreKrasG12D) mouse lung tumors.
(A) Representative immunofluorescent (IF) staining of Mig-6 in normal airways of LSL-KrasG12D mice and in hyperplastic lesions and adenomatous lesions of KrasG12D mice (3 months of age). Three control mice and six KrasG12D mice were used for IF staining and whole lungs were examined. One representative region for each group was chosen to present here. Regions in the white boxes were further magnified. (B) RT-qPCR analysis of Mig-6 mRNA levels in Laser Capture Micro-dissected normal airways from the control mice (LSL-KrasG12D) and hyperplastic lesions and adenomatous lesions from the lungs of KrasG12D mice. (*p<0.05 and **p<0.01 vs. normal airways) (3 months of age).
Mig-6 Deficiency Accelerated KrasG12D-induced Mouse Lung Tumor Phenotypes
To determine the role of Mig-6 in KrasG12D-induced lung tumorigenesis, Mig-6 was conditionally ablated in Club cells by breeding Mig6f/f mice to CCSPCreKrasG12D mice, yielding CCSPCreMig-6d/dKrasG12D mice (Mig-6d/dKrasG12D) [31]. Mig-6d/dKrasG12D mice had an average survival time of 18 weeks while KrasG12D mice survived for an average time of 33 weeks (Fig. 2A). No mortality was observed in the CCSPCreMig-6f/f (Mig-6d/d) mice during this period (Fig. 2A). These results indicate that compound mutations of Mig-6 deficiency and Kras activation imposed a severe negative impact on the health of the mice and shortened their life span.
Figure 2. Reduced life span and increased lung neoplasia in Mig-6d/dKrasG12D mice.
(A) Kaplan-Meier survival curves of control (Mig-6f/f or LSL-KrasG12D), Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (N=12 per each group). (B) H&E staining of lung tissues of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice at the age of 2 months. Regions in black boxes were further magnified and shown in the right panels.
Histological analysis of the lungs of 2-month-old Mig-6d/dKrasG12D mice revealed that Mig-6d/dKrasG12D mice developed severe airway hyperplasia and massive tumors throughout the lungs (Fig. 2B). Mig-6d/d mouse lungs exhibited no obvious morphological changes, while KrasG12D mice exhibited prevalent hyperplasia surrounding airways compared with normal control animals (Fig. 2B). These results demonstrated that concomitant inactivation of Mig-6 with KrasG12D expression led to an early onset and robust progression of lung tumors.
To confirm the deletion of Mig-6, we examined genomic DNA isolated from the lungs of experimental mice. The lungs of global Mig-6 knockout mice (Mig-6−/−, a positive control) showed a 433 bp recombined band, whereas wild-type control lungs showed a 145 bp band (Sup. Fig. 1) [32]. At 1 month of age, we failed to detect the recombined Mig-6 alleles in the Mig-6d/d and Mig-6d/dKrasG12D mouse lungs by PCR. By 4 months of age, the excised Mig-6 allele was detected in Mig-6d/dKrasG12D lungs by PCR, but not in KrasG12D lungs. These results suggest that the sensitivity of the PCR analysis was not able to detect the recombination in the 1-month old Mig-6d/d and Mig-6d/dKrasG12D mouse lungs due to contamination with the other lung cell types as well as Club cells without recombination at these loci. However, as tumor development progressed the number of cells with recombination at the Mig-6 allele increased to a detectable level.
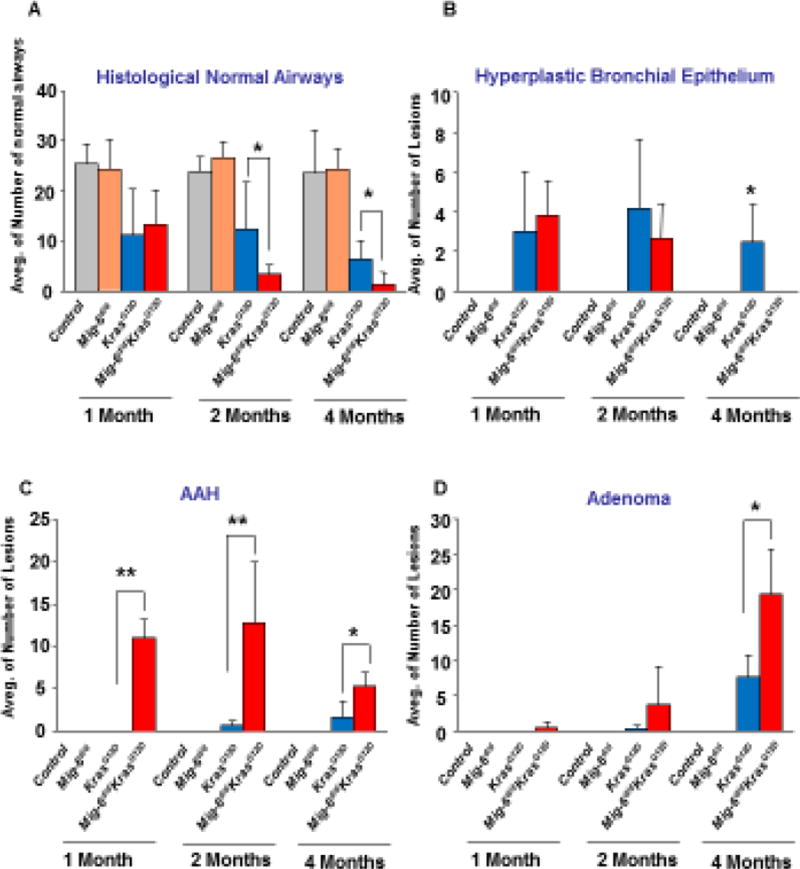
The pathology of Mig-6 inactivation on the progression of KrasG12D-induced lung tumors was quantified by scoring normal airways, hyperplastic lesions, atypical adenomatous hyperplasia (AAH), and adenomatous lesions in experimental mice up to 4 months of age. Across all ages, Mig-6d/d and control lungs showed only normal airway morphology (Fig. 3). With KrasG12D, animals exhibited less normal airways and more hyperplastic lesions, as early as, 1 month of age (Fig. 3A-B). Moreover, AAH and adenomatous lesions began to emerge at 2 months of age in KrasG12D animals (Fig. 3C-D), indicating that cancer development is in progress [33]. Compared with the KrasG12D animals, the Mig-6d/dKrasG12D mice had significantly more adenoma at 4 months of age (Fig. 3D) and increased presence of AAH across all ages (Fig. 3C) at the expense of normal airways (Fig. 3A). Mig-6 deficiency and Kras activation jointly facilitated cancer progression, advancing all the diseased foci to become AAH and adenoma instead of hyperplastic lesions (Fig. 3B-D). Collectively, these results demonstrate that Mig-6 inactivation greatly enhanced KrasG12D–induced lung tumor progression.
Figure 3. Mig-6 inactivation enhanced histopathological changes during KrasG12D–induced lung tumor progression.
Histopathological changes were scored in H&E stained sections of the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (at the age of 1, 2, and 4 months). Three mice were used for each group. Average numbers of normal airways (A), hyperplastic bronchial epithelium (B), Atypical Adenomatous Hyperplasia (AAH) (C), and adenomas (D) were calculated and shown in bar graphs. Independent pathologists counted and defined these histopathological patterns microscopically. *p<0.05 and **p<0.01 by One Way ANOVA followed by Tukey’s analysis.
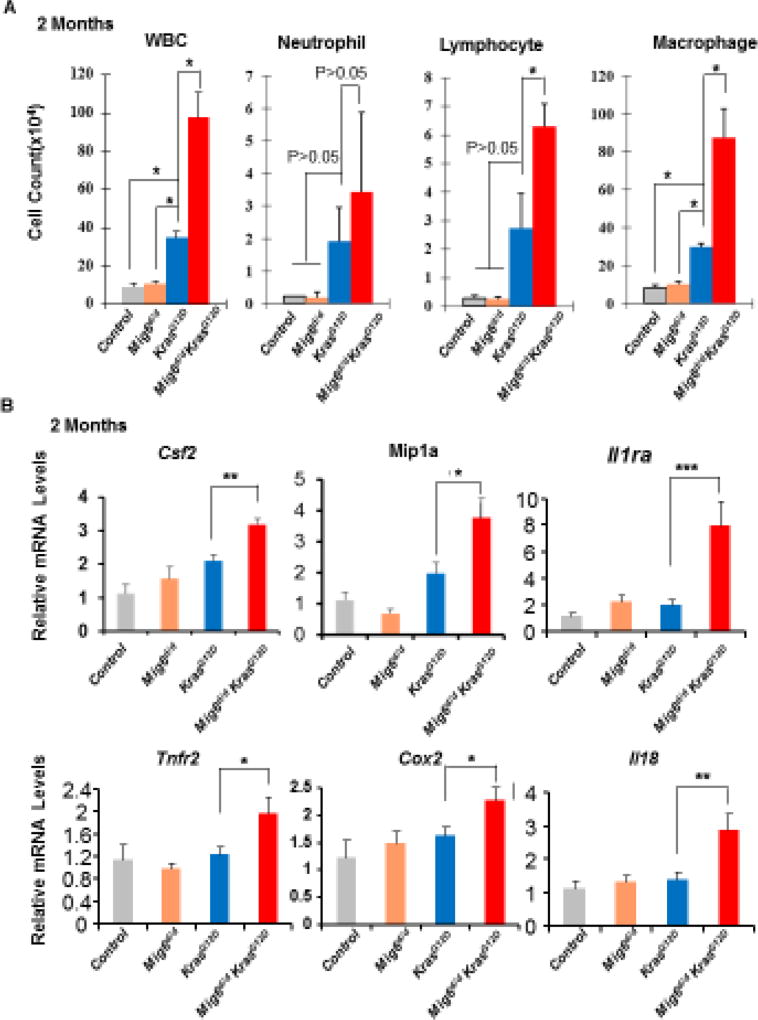
Inflammation Was Enhanced in Mig-6d/dKrasG12D Mouse Lungs Compared to KrasG12D Mouse Lungs
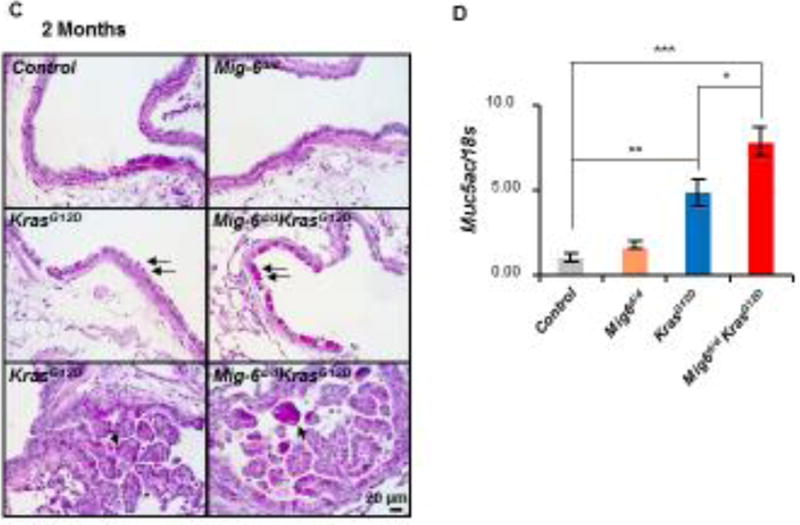
Inflammation has been shown to promote Kras mediated oncogenesis [34]. Our previous study showed that Mig-6 null mice developed a COPD-like phenotype caused by recurrent inflammation [26], suggesting the role of Mig-6 in inflammation. To determine whether Mig-6 inactivation alters inflammatory responses during lung tumorigenesis, we examined the immune cell composition in bronchoalveolar lavage fluid (BALF) collected by lavaging lungs of experimental mice at 2 months of age. Consistent with histological findings, Mig-6d/d and control mice were comparable in both white blood cell (WBC) counts and expression levels of inflammatory markers (Fig. 4A). As expected, the KrasG12D BALF exhibited statistically higher counts in total WBCs and lymphocytes and a trend of increase in neutrophils and macrophages compared with control and Mig-6d/d mice (Fig. 4A). Importantly, Mig-6 deficiency further stimulated the inflammatory response in KrasG12D mouse lungs, as demonstrated by the significantly increased numbers of WBCs, lymphocytes, and macrophages in Mig-6d/dKrasG12D BALF compared to KrasG12D mice (Fig. 4A). Moreover, elevated pro-inflammatory genes including Csf2, Mip1a, Il13a1, Tnfr2, Cox2, and Il18 were observed in Mig-6d/dKrasG12D lungs compared to those in KrasG12D lungs (Fig. 4B). Notably, all these genes were shown to be associated with cancer inflammation and increased cancer risk [35–40]. Meanwhile, mucous cell metaplasia is a common phenomenon in lung inflammation and is also a feature for subtypes of lung AD [41]. To determine whether mucous cell metaplasia developed in Mig-6d/dKrasG12D lungs, we performed PAS staining in the lungs of experimental mice at 2 months of age [42]. As shown in Fig. 4C, we found metaplastic mucous cells in KrasG12D lungs (black arrows), but not in control and Mig-6d/d mice. Mig-6 deficiency in the Kras active background further increased the number of metaplastic mucous cells in comparison with that in KrasG12D mice (Fig. 4C). To confirm the results from PAS staining, we examined the mRNA levels of Muc5ac, a marker gene in the lung mucous cell metaplasia [42]. Consistent with the PAS staining results, Mig-6 inactivation greatly increased the expression of Muc5ac in the KrasG12D background (Fig. 4D). Although those analyses don’t directly prove that this increased inflammation promoted tumor development in Mig-6d/dKrasG12D mice, these results showed inflammation was enhanced in Mig-6d/dKrasG12D lungs compared to KrasG12D lungs, suggesting that this is a potential mechanism to explain the enhanced tumorigenesis in Mig-6d/dKrasG12D lungs based on previous findings of inflammation promoting Kras mediated lung tumorigenesis [34].
Figure 4. Inflammation was enhanced in Mig-6d/dKrasG12D mouse lungs.
(A) Analysis of white blood cells (WBC), neutrophils, lymphocytes, and macrophages in the BALF of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice, *P<0.05. Three mice were used for each group. (B) RT-qPCR analysis of the expression of pro-inflammatory genes in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice. * p<0.05, ** p<0.01 and *** p<0.001. (C) PAS staining of lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (2 months of ages). Arrows indicate metaplastic mucus cells in the bronchial epithelium. (D) RT-qPCR analysis of mRNA levels of Muc5ac in the lungs of mice (2 months of age). * p<0.05, ** p<0.01 and *** p<0.001.
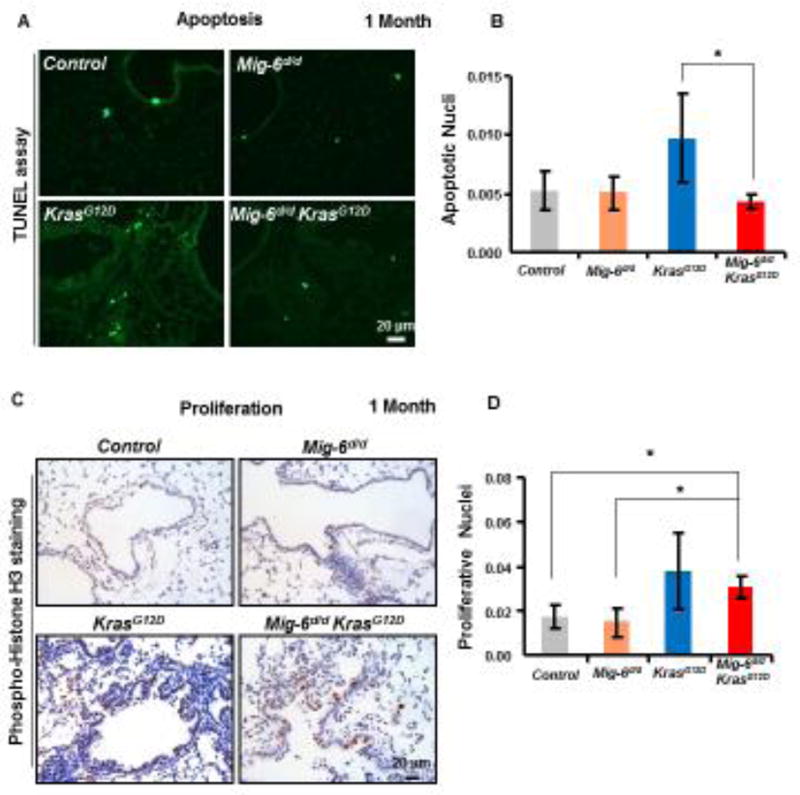
Ablation of Mig-6 Reduced Apoptosis in KrasG12D-driven Tumors
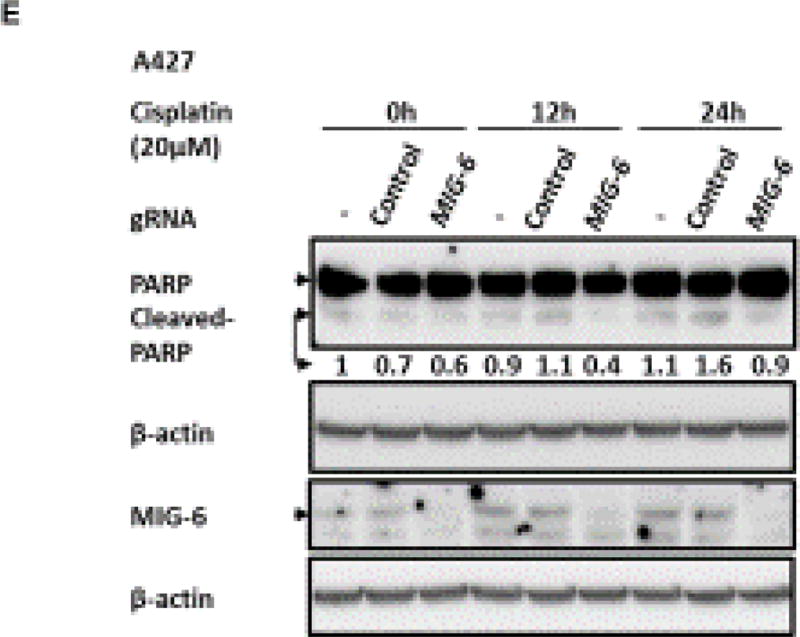
In order to determine if the accelerated tumorigenesis as a result of Mig6 deletion was due to an inhibition of apoptosis or an increase in cell proliferation, we assayed mouse lungs utilizing TUNEL staining and phospho-histone H3 staining, respectively, at 1 month of age. Figure 5 shows that while KrasG12D had an increase in apoptosis, Mig-6d/dKrasG12D lungs show similar apoptotic levels as control and Mig-6d/d mice (Fig. 5A-B). On the other hand, the number of proliferative cells in Mig-6d/dKrasG12D lungs was significantly more than that in the control and Mig-6d/d lungs (Fig. 5C-D), whereas there was no significant difference between KrasG12D and Mig-6d/dKrasG12D lungs (Fig. 5D). These results suggest that Mig-6 deficiency promotes cell survival by reducing apoptosis in KrasG12D mouse lungs, while it does not affect cell proliferation in this Kras dependent lung tumor mouse model. To further demonstrate that MIG-6 deficiency attenuates the apoptosis in pulmonary epithelial cells with KRASG12D expression, MIG-6 was ablated by the CRISPR/Cas9 technology in A427 cells, a human lung adenocarcinoma cell line expressing KRASG12D, followed by Cisplatin challenge to induce apoptosis. As shown in Figure 5E, cleaved-PARP, a marker of apoptosis, exhibited lower levels in the MIG-6 ablated cells compared with that in control groups, especially under Cisplatin challenge. This finding indicates that MIG-6 deficiency suppresses apoptosis in human KRAS-active AD cells, which is the consistent with the apoptosis-attenuating capacity of Mig-6 deficiency in mouse lung AD. Taken together, our data reveal that Mig-6 is an important positive regulator of apoptosis in lung KRAS-active AD cells (Fig. 5).
Figure 5. Ablation of Mig-6 Reduced Apoptosis in KrasG12D-driven Tumors.
(A) TUNEL assay in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (1 month of age). Apoptotic cells stained bright green. (B) Quantification of apoptotic cells in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice. *p<0.05. (C) Phospho-Histone H3 staining in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (1 month of age). (D) Quantification of proliferative cells (phospho-histone H3 positive) cells in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice. *p<0.05. At least 4 left lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice were used in the quantification and at least 10 random areas from each lung were measured. Fluorescein positive nuclei or phospho-Histone H3 positive nuclei were counted and the percentages of positively stained nuclei to total nuclei were calculated. (E) Western Blot (WB) analysis of apoptosis of A427 cells after knock-out of MIG-6 with or without treatment of Cisplatin. Cleaved-PARP is a typical indicator of apoptosis.
ErbB4 Pathway Was Activated in MIG-6 Deficient, KRAS Active Lung Tumor and Attenuated Apoptosis
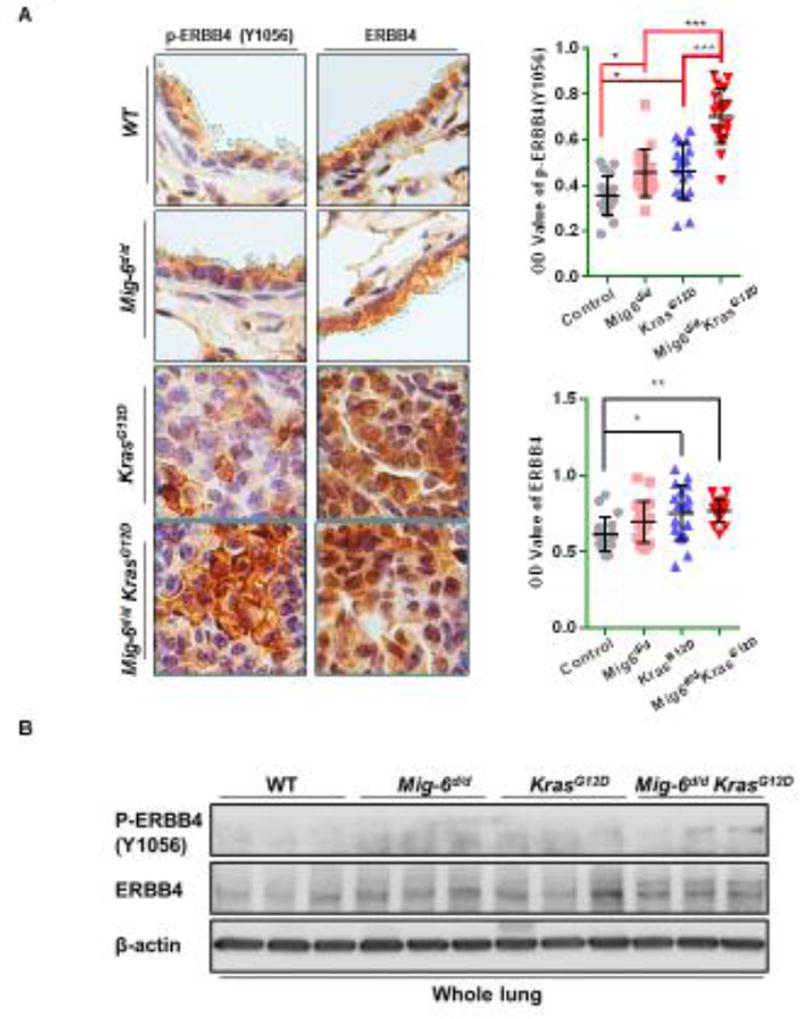
Based on the findings that Mig-6 suppresses EGFR, AKT and mTOR signaling pathways [26], we examined the total and phosphorylated protein levels of the EGFR family, AKT and mTOR in lungs and lung tumors of 2-month old mice using Western blot and immunohistochemical analysis. As shown in Fig. 6A-B and Sup. Fig. 2A-F, the phosphorylation of all ERBB proteins was increased after ablation of Mig-6 in wild type mouse lung. However, only the phosphorylation of ERBB4 was increased when Mig-6 was deleted in the KrasG12D mouse lungs (Fig. 6A-B). Additionally, ERBB4 protein was also increased in KrasG12D and Mig-6d/dKrasG12D mouse lungs compared to wild type mouse lungs (Fig. 6A-B), indicating that ErbB4 pathway was activated in the Mig-6d/d KrasG12D mice by the increase of its phosphorylation and total protein.
Figure 6. ErbB4 pathway was activated in lung tumor (Mig-6−/−KrasG12D) and attenuated apoptosis.
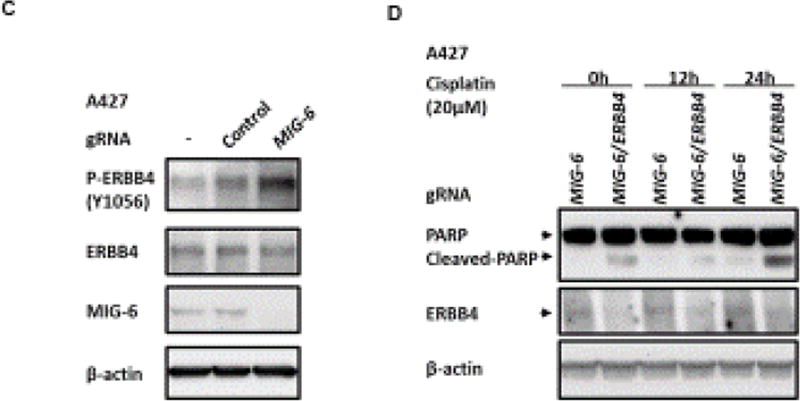
(A and B) Immunohistochemistry (A) and WB (B) analyses of total and phosphorylated (p-) ERBB4 proteins in lung and lung tumors of 2-month old mice. (A) Left panel: IHC staining results are the representative results for each group; Right panel: quantification results are calculated from three mice per group (six regions for each mouse) using Image J [60]. Optical Density (OD) = log (max intensity/Mean intensity). The blue dashes point the area for the quantification. (B) WB analyses of total and p-ERBB4 proteins in lung of 2-month old mice. (C) WB analysis of p-ERBB4 in A427 cells after knock-out of MIG-6. (D) WB analysis of apoptosis of A427 cells after knock-out of MIG-6 or MIG-6/ErbB4 with or without treatment of Cisplatin.
To assess whether the increased ERBB4 phosphorylation is due to Mig-6 ablation in KRAS active AD cells, we examined p-ERBB4 levels in MIG-6 knock-out A427 cells. As shown in Fig. 6C, p-ERBB4 levels were increased after knock-out of MIG-6, demonstrating a conserved role of MIG-6 in regulation of p-ERBB4 in lung tumors expressing KRASG12D between mouse and human. Next we examine whether ErbB4 pathway may alter apoptosis in lung adenoma of Mig-6−/−KrasG12D background. By ablating ERBB4 in A427 cells that have MIG-6 deficiency and constitutive KRASG12D expression, ERBB4 ablation showed increased cell apoptosis with or without the treatment of Cisplatin (Fig. 6D). This finding demonstrates the activation of the ErbB4 pathway suppressed apoptosis in MIG-6 deficient, KRAS active lung AD cells.
Interestingly, we did not detect differences on mTOR phosphorylation levels between KrasG12D and Mig-6d/dKrasG12D mouse lung tumors (Sup. Fig. 2G), and AKT phosphorylation levels were very low in both KrasG12D and Mig-6d/dKrasG12D mouse lung tumors (Sup. Fig. 2H) despite the fact that AKT is often activated by EGFR signaling [43]. Our results are consistent with a previous report that AKT phosphorylation is not increased in response to activated Kras-induced mouse lung tumors [44]. In summary, our results suggest that the ErbB4 pathway is activated in MIG-6 deficient, KRAS active lung AD cells to prevent apoptosis.
Discussion
In the present study, we showed that Mig-6 ablation in Club cells enhanced KrasG12D-driven mouse lung tumorigenesis through suppressing the cell apoptosis in KrasG12D mouse lungs partially by activation of the ErbB4 pathway.
ERBB4 is mutated in 5.4% of NSCLC patients [45]. ERBB4 overexpression is correlated with Tumor, Lymph node, and Metastasis (TNM) staging and decreased survival after operations in NSCLC patients [46]. It is well documented that Mig-6 is a negative regulator of ErbB signaling and Mig-6 expression is closely associated with ErbB signaling in lung cancers [30, 31, 47–49]. Although p-EGFR has been reported to be a major ERBB member regulated by MIG-6 [20], surprisingly only p-ErbB4 levels, instead of p-EGFR levels, were significantly increased after ablation of Mig-6 in KrasG12D mouse lungs and ERBB4 protein was also increased in KrasG12D and Mig-6d/dKrasG12D mouse lungs compared to wild type mouse lungs. Previous studies have demonstrated that ERBB4 mutants (Y285C, D595V, D931Y and K935I) increase both basal and ligand-induced ERBB4 phosphorylation and promote NIH 3T3 cell survival under serum starvation, probably by sustaining their own phosphorylation status [45]. Moreover, ERBB4 has been shown to promote human lung cancer cell proliferation [50] as well as exhibits oncogenic activities in both breast cancer cells [51, 52] and pancreatic tumor cell lines [53]. These results collectively suggest that the activated ErbB4 pathway could be a potential mechanism to explain how Mig-6 affects Kras-mutant-driven lung tumor development. Indeed, we observed a remarkable decrease of cell apoptosis in Mig-6d/dKrasG12D lungs, with the elevated phosphorylated ERBB4 levels, compared to those in KrasG12D lungs at the early stage of tumor formation. Functional assessment on A427 cells further showed the role of MIG-6 and ERBB4 in regulation of apoptosis. Given that inhibition of cell apoptosis may be the major advantage that tumor cells gained upon loss of MIG-6 [54], the increased ErbB4 signaling, in response to MIG-6 deficiency, likely serves as a major downstream effector for MIG-6 to regulate cell apoptosis. Further studies are needed to help understand how the ErbB4 pathway regulates apoptosis in lung cancer.
The inflammatory response was also impacted in lung tumor progression in Mig-6d/dKrasG12D mice. Our previous study showed that Mig-6 null mice developed a COPD-like phenotype that was accompanied by increased macrophage invasion and mucous cell metaplasia [26]. In agreement with the previous findings, we observed a significant increase in inflammatory cell number in the BALF, as well as, upregulated expression of tumor inflammation-associated genes in Mig-6d/dKrasG12D lungs compared to KrasG12D lungs. In addition, Mig-6d/dKrasG12D mice also exhibited increased mucous cell metaplasia in the airways, another indication of increased inflammation associated with Mig-6 inactivation in comparison with those of KrasG12D mice. In the present study, it is difficult to determine the causal relationship between the increased inflammatory responses and lung tumor development. However, inflammation has been shown to promote Kras mediated lung tumorigenesis [34], suggesting that heightened inflammation may contribute to enhanced tumor progression.
It is well documented that MIG-6 expression is decreased in several human cancers [48, 49, 55]. Our previous work shows that MIG-6 levels decrease during the development of lung adenosquamous induced by Pten and Smad4 ablation [31]. In the present study, we also found decreased Mig-6 expression in hyperplastic and adenomatous lesions in KrasG12D mouse lungs. Previous findings have demonstrated that the expression level of MIG-6 is reduced in human lung adenocarcinoma A427 cells having KRASG12D due to epigenetic silencing of MIG-6 through methylation and histone deacetylation without having a physical alteration in its promoter [56]. The relationship between MIG-6 expression and KRAS activation may be an indication of how the pro-oncogenic signaling caused by Kras mutation suppresses inhibitors of tumor progression and provides enhanced growth advantages for the transformed cells while the loss of Mig-6 provides a selective advantage for tumor cell growth. Therefore, it is important to understand how tumor suppressors, such as MIG-6, are inhibited during cancer development. This will provide new avenues for the treatment of lung cancer.
Besides Mig-6 preventing KrasG12D-driven lung tumor progression as reported in the present study, these two genes are also implicated in cancer cell sensitivity to drug treatment [18]. LOVO (colorectal AD) and SNU668 (gastric AD) cells carry mutant KRAS and are sensitive to MEK inhibition. Interestingly, down-regulation of Mig-6 confers LOVO and SNU668 cell resistance to MEK inhibition. In contrast, overexpression of Mig-6 re-sensitizes the cells to MEK inhibition while mutant Kras positive parental HCT116 (colorectal AD) and AGS (gastric AD) cells are resistant to MEK inhibition [18]. Meanwhile, Sun et al. showed that transcriptional induction of ERBB3 caused the resistance to MEK inhibition in KRAS mutant lung and colon cancer [57]. These findings collectively suggest that targeting the oncogenic pathway regulated by MIG-6, such as the ERBB4 pathway, might be key to effective treatment of KRAS-driven lung tumors since there is no FDA approved drug specific for treatment of lung cancer patients having KRAS mutations.
In summary, we developed genetically engineered mouse lung tumor models and revealed the role of Mig-6 inactivation in promoting KrasG12D-driven lung tumor development by attenuating apoptosis in KrasG12D mouse lungs, which is partially through elevated ErbB4 signaling. This discovery provides a new direction to develop novel preventive and/or therapeutic strategies (e.g., restoration of Mig-6 expression or inhibition of the ErbB4 pathway) for treating KRAS-mutant-driven lung cancer in patients.
Materials and Methods
Animals, BALF, and Histology
All the animal protocols are approved by the National Institute of Environmental Health Sciences and the Baylor College of Medicine. All experiments were conducted in accordance with relevant guidelines and regulations of both institutions. Bronchoalveolar lavage fluid (BALF) was collected by lavaging lungs of mice with 2 ml PBS. Total leukocytes were counted with a hemocytometer and differential inflammatory cells were counted from cytocentrifuged BALF (100 µl) followed with Wright-Giemsa staining [58]. Hematoxylin and eosin (H&E) and Periodic Acid Schiff (PAS) staining were performed according to previous protocols [31].
Laser Capture Microdissection
Laser capture was performed according to standard protocols by using the Arcturus PixCell II Microdissection system (www.arcturus.com) with the following parameters being utilized: laser spot size 7.5 um, pulse power 50 mW, pulse width 0.75 ms and a threshold voltage of 205 mV.
Cell line and culture
A427 cells (ATCC® HTB-53™) were purchased from ATCC and cultured in ATCC-formulated Eagle's Minimum Essential Medium (Cat. 30-2003) following the culture method of ATCC. 20 µM Cisplatin was used to treat A427 cells for 0h, 12h and 24h, at which time cells were collected for protein analysis.
Immunofluorescence (IF) staining, immunohistochemistry (IHC) and Western Blot (WB)
IF, IHC and WB were performed as previously described [31]. The following primary antibodies were used: anti-Mig-6 (MilliporeSigma, Billerica, MA), anti-p-Histone H3 (MilliporeSigma, Billerica, MA), anti-CCSP (DeMayo lab), anti-pro-SP-C (Seven Hills Bioreagents, Cincinnati, OH), anti-p-EGFR (CST3777), anti-p-EGFR (sc-12351), anti-EGFR (CST4267), anti-p-ERBB2 (sc-293110), anti-ERBB2 (sc-284), anti-p-ERBB3 (CST4791), anti-ERBB3 (sc-285), anti-p-ERBB4 (ab92782 and sc-33040), anti-ERBB4 (CST4795 and sc-283) (Santa Cruz Biotechnologies, Dallas, TX), anti-PARP (CST 9542), anti-MIG-6 (DeMayo lab), anti-AKT (CST4691), anti-p-AKT (CST4060), p-mTOR (CST 2971), and anti-mTOR (CST2983). Cisplatin (22-515-0) was purchased from ThermoFisher Scientific, Waltham, MA.
Cell proliferation and apoptosis analysis
Cell proliferation was assayed with phospho-Histone H3 immunohistochemistry as described above and apoptotic cells were detected with the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) following the instructions of the manufacturer.
Quantitative Real-time PCR
Quantitative Real-time PCR was performed as previously described [31]. All the primers and Taqman probes were purchased from Applied Biosystems/ThermoFisher Scientific (Waltham, MA).
Lentiviral CRISPR/Cas9-gRNA targeting human MIG-6 and ERBB4
The sequences of gRNA targeting MIG-6 and ERBB4 are “CTCGGTGTGCGCGAGTTACT” and “TTATGAGGATCGATATGCCT”, respectively. These gRNAs were cloned into LentiCRISPRv2 vector [59] by GenScript (Piscataway, NJ). CRISPR-Lenti non-targeting control plasmid was purchased from MilliporeSigma (Billerica, MA) (CRISPR12-1EA) and its sequences of gRNA is “CGCGATAGCGCGAATATATT”. Lentiviruses were made in the Viral Vector Core Laboratory of NIH/NIEHS and then were used to infect A427 cells for knocking out MIG-6 and ERBB4. The pooled cells infected by gRNA were collected for WB to confirm the knockout efficiency. Non-infected and gRNA-Control-infected cells were used as controls.
Statistics
Measurement values are expressed as mean ± SE (standard error). Student’s t test was used for comparison of two group averages. When there were more than two groups, One-way ANOVA followed by Tukey’s analysis was performed. All the Ns were ≥3 and statistically significance was considered when P values were ≤ 0.05.
Supplementary Material
Sup. Figure 1. Detection of recombined conditional alleles in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (1 month and 4 months of ages) by PCR. Genomic DNA from Mig-6−/− mouse lung was used as a positive control. The excised Mig-6 conditional allele generated a 433 bp band whereas wild-type Mig-6 allele generated a 145 bp band.
Sup. Figure 2. Immunohistochemistry and Western Blot analyses of total and phosphorylated EGFR/ERBB2/ERBB3/AKT/mTOR proteins in lung and lung tumors of 2-month old mice. (A-F) IHC analyses of total and phosphorylated EGFR (A), ERBB2 (C), ERBB3 (E) and WB analyses of EGFR (B), ERBB2 (D) and ERBB3 (F). Left panel of IHC results (A, C and E) are quantitative results in mouse lungs. The blue dashes point to the areas used for quantification. G. Representative results of phosphorylated p-mTOR (S2448) / mTOR (right panel) and the quantitative results (left panel) in mouse lungs. All quantifications are calculated from three mice per group (six regions for each mouse) using Image J [60]. Optical Density (OD) = log (max intensity/Mean intensity). H. Representative results of phosphorylated AKT (S473) and its total protein in mouse lungs.
Highlights.
Mig-6 deficiency promotes the development of KrasG12D-induced mouse lung adenoma.
MIG-6 deficiency attenuates apoptosis of lung adenoma expressing KRASG12D.
Total and phosphorylated ERBB4 is increased in adenoma of Mig-6d/dKrasG12D lung.
Ablation of ERBB4 increases apoptosis of lung adenocarcinoma cells (MIG-6−/−KRASG12D).
Acknowledgments
We thank Jinghua Li, Bryan Ngo, Jie Yang, and Janet DeMayo, M.S. for technical assistance; Janet DeMayo, M.S., Weiwen Long, Ph.D. and Heather Franco, Ph.D. for manuscript preparation. This research is supported by the Intramural Research Program of the National Institute of Environmental Health Sciences Project Z1AES103311-01 (F.J.D.) and by the National Cancer Institute Grant U01CA105352 (F.J.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions statement
J.L., S.-N.C. and F.J.D. designed the experiments. J.L. and S.-N.C. performed the experiments and data analysis. J.L., S.-N.C., S.-P.W. and F.J.D. wrote the manuscript. N.-l.J. helped analyze the mouse phenotypes. S.J.M., J.L.G. and I.W. characterized mouse lung tumor types and helped Laser Capture Microdissection. F.J.D. supervised the project.
Conflict of interest: The authors have no conflict of interest.
References
- 1.Landis SH, et al. Cancer statistics, 1999. CA: a cancer journal for clinicians. 1999;49(1):8–31. doi: 10.3322/canjclin.49.1.8. 1. [DOI] [PubMed] [Google Scholar]
- 2.Kwon MC, Berns A. Mouse models for lung cancer. Molecular oncology. 2013;7(2):165–77. doi: 10.1016/j.molonc.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Zandwijk N, Mooi WJ, Rodenhuis S. Prognostic factors in NSCLC. Recent experiences. Lung cancer. 1995;(12 Suppl 1):S27–33. doi: 10.1016/0169-5002(95)00418-z. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH. Current standards of care in small-cell and non-small-cell lung cancer. Oncology. 2001;(61 Suppl 1):3–13. doi: 10.1159/000055386. [DOI] [PubMed] [Google Scholar]
- 5.Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;12(4):587–96. doi: 10.1188/08.CJON.587-596. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD. Pathology of lung cancer. Clinics in chest medicine. 2002;23(1):65–81. doi: 10.1016/s0272-5231(03)00061-3. viii. [DOI] [PubMed] [Google Scholar]
- 8.Ding L, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slebos RJ, Rodenhuis S. The ras gene family in human non-small-cell lung cancer. Journal of the National Cancer Institute. Monographs. 1992;(13):23–9. [PubMed] [Google Scholar]
- 10.Sanders HR, Albitar M. Somatic mutations of signaling genes in non-small-cell lung cancer. Cancer genetics and cytogenetics. 2010;203(1):7–15. doi: 10.1016/j.cancergencyto.2010.07.134. [DOI] [PubMed] [Google Scholar]
- 11.Campbell SL, et al. Increasing complexity of Ras signaling. Oncogene. 1998;17(11 Reviews):1395–413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 12.Tsutsumi K, et al. Visualization of Ras-PI3K interaction in the endosome using BiFC. Cellular signalling. 2009;21(11):1672–9. doi: 10.1016/j.cellsig.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang XQ, et al. Oncogenic K-Ras regulates proliferation and cell junctions in lung epithelial cells through induction of cyclooxygenase-2 and activation of metalloproteinase-9. Molecular biology of the cell. 2009;20(3):791–800. doi: 10.1091/mbc.E08-07-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A, et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148(4):639–50. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji H, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25(14):2105–12. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 16.Okada F, et al. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):3609–14. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19(6):643–64. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- 18.Yoon YK, et al. Down-regulation of mitogen-inducible gene 6, a negative regulator of EGFR, enhances resistance to MEK inhibition in KRAS mutant cancer cells. Cancer letters. 2012;316(1):77–84. doi: 10.1016/j.canlet.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YW, Vande Woude GF. Mig-6, signal transduction, stress response and cancer. Cell cycle. 2007;6(5):507–13. doi: 10.4161/cc.6.5.3928. [DOI] [PubMed] [Google Scholar]
- 20.Anastasi S, et al. Regulation of the ErbB network by the MIG6 feedback loop in physiology, tumor suppression and responses to oncogene-targeted therapeutics. Semin Cell Dev Biol. 2016;50:115–24. doi: 10.1016/j.semcdb.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Anastasi S, et al. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene. 2005;24(28):4540–8. doi: 10.1038/sj.onc.1208658. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, et al. Downregulation of Mig-6 in nonsmall-cell lung cancer is associated with EGFR signaling. Molecular carcinogenesis. 2012;51(7):522–34. doi: 10.1002/mc.20815. [DOI] [PubMed] [Google Scholar]
- 23.Jeong JW, et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8677–82. doi: 10.1073/pnas.0903632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CI, et al. Mitogen-inducible gene-6 is a multifunctional adaptor protein with tumor suppressor-like activity in papillary thyroid cancer. The Journal of clinical endocrinology and metabolism. 2011;96(3):E554–65. doi: 10.1210/jc.2010-1800. [DOI] [PubMed] [Google Scholar]
- 25.Ying H, et al. Mig-6 controls EGFR trafficking and suppresses gliomagenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):6912–7. doi: 10.1073/pnas.0914930107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin N, et al. Mig-6 is required for appropriate lung development and to ensure normal adult lung homeostasis. Development. 2009;136(19):3347–56. doi: 10.1242/dev.032979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YW, et al. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene. 2007;26(2):269–76. doi: 10.1038/sj.onc.1209790. [DOI] [PubMed] [Google Scholar]
- 28.Ferby I, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nature medicine. 2006;12(5):568–73. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 29.Reschke M, et al. Mitogen-inducible gene-6 is a negative regulator of epidermal growth factor receptor signaling in hepatocytes and human hepatocellular carcinoma. Hepatology. 2010;51(4):1383–90. doi: 10.1002/hep.23428. [DOI] [PubMed] [Google Scholar]
- 30.Maity TK, et al. Loss of MIG6 Accelerates Initiation and Progression of Mutant Epidermal Growth Factor Receptor-Driven Lung Adenocarcinoma. Cancer Discov. 2015;5(5):534–49. doi: 10.1158/2159-8290.CD-14-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, et al. ErbB2 Pathway Activation upon Smad4 Loss Promotes Lung Tumor Growth and Metastasis. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin N, et al. Generation of a Mig-6 conditional null allele. Genesis. 2007;45(11):716–21. doi: 10.1002/dvg.20348. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam SJ, et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol. 2009;40(4):443–53. doi: 10.1165/rcmb.2008-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitajima S, Thummalapalli R, Barbie DA. Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. Semin Cell Dev Biol. 2016;58:127–35. doi: 10.1016/j.semcdb.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He JQ, et al. Association of genetic variations in the CSF2 and CSF3 genes with lung function in smoking-induced COPD. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2008;32(1):25–34. doi: 10.1183/09031936.00040307. [DOI] [PubMed] [Google Scholar]
- 36.Koch AE, et al. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. The Journal of clinical investigation. 1994;93(3):921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothenberg ME, et al. IL-13 receptor alpha1 differentially regulates aeroallergen-induced lung responses. Journal of immunology. 2011;187(9):4873–80. doi: 10.4049/jimmunol.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizoguchi E, et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122(1):134–44. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 39.Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4(1):17–23. [PubMed] [Google Scholar]
- 40.Rovina N, et al. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respiratory medicine. 2009;103(7):1056–62. doi: 10.1016/j.rmed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Nikitin AY, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer research. 2004;64(7):2307–16. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 42.Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. American journal of respiratory cell and molecular biology. 2010;42(3):268–75. doi: 10.1165/rcmb.2009-0151TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bliesath J, et al. Combined inhibition of EGFR and CK2 augments the attenuation of PI3KAkt- mTOR signaling and the killing of cancer cells. Cancer Lett. 2012;322(1):113–8. doi: 10.1016/j.canlet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 44.Xu C, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25(5):590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurppa KJ, et al. Activating ERBB4 mutations in non-small cell lung cancer. Oncogene. 2016;35(10):1283–91. doi: 10.1038/onc.2015.185. [DOI] [PubMed] [Google Scholar]
- 46.Deng Z, et al. [A study on the expression of erbB4/HER4 in non-small cell lung cancer] Zhongguo Fei Ai Za Zhi. 2002;5(3):177–9. doi: 10.3779/j.issn.1009-3419.2002.03.06. [DOI] [PubMed] [Google Scholar]
- 47.Hackel PO, Gishizky M, Ullrich A. Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol Chem. 2001;382(12):1649–62. doi: 10.1515/BC.2001.200. [DOI] [PubMed] [Google Scholar]
- 48.Ferby I, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006;12(5):568–73. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, et al. Downregulation of Mig-6 in nonsmall-cell lung cancer is associated with EGFR signaling. Mol Carcinog. 2012;2011(7):20815. doi: 10.1002/mc.20815. [DOI] [PubMed] [Google Scholar]
- 50.Starr A, et al. ErbB4 increases the proliferation potential of human lung cancer cells and its blockage can be used as a target for anti-cancer therapy. Int J Cancer. 2006;119(2):269–74. doi: 10.1002/ijc.21818. [DOI] [PubMed] [Google Scholar]
- 51.Mill CP, et al. ErbB2 Is Necessary for ErbB4 Ligands to Stimulate Oncogenic Activities in Models of Human Breast Cancer. Genes Cancer. 2011;2(8):792–804. doi: 10.1177/1947601911431080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haskins JW, Nguyen DX, Stern DF. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci Signal. 2014;7(355):ra116. doi: 10.1126/scisignal.2005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mill CP, Gettinger KL, Riese DJ., 2nd Ligand stimulation of ErbB4 and a constitutively-active ErbB4 mutant result in different biological responses in human pancreatic tumor cell lines. Exp Cell Res. 2011;317(4):392–404. doi: 10.1016/j.yexcr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim TH, et al. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene. 2010;29(26):3770–80. doi: 10.1038/onc.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amatschek S, et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res. 2004;64(3):844–56. doi: 10.1158/0008-5472.can-03-2361. [DOI] [PubMed] [Google Scholar]
- 56.Zhang YW, et al. Cancer-type regulation of MIG-6 expression by inhibitors of methylation and histone deacetylation. PLoS One. 2012;7(6):e38955. doi: 10.1371/journal.pone.0038955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun C, et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep. 2014;7(1):86–93. doi: 10.1016/j.celrep.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 58.Strober W. Wright-Giemsa and nonspecific esterase staining of cells. In: Coligan John E, et al., editors. Current protocols in immunology. 2001. Appendix 3: p. Appendix 3C. [DOI] [PubMed] [Google Scholar]
- 59.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–4. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatterjee S, et al. Quantitative immunohistochemical analysis reveals association between sodium iodide symporter and estrogen receptor expression in breast cancer. PLoS One. 2013;8(1):e54055. doi: 10.1371/journal.pone.0054055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sup. Figure 1. Detection of recombined conditional alleles in the lungs of control, Mig-6d/d, KrasG12D, and Mig-6d/dKrasG12D mice (1 month and 4 months of ages) by PCR. Genomic DNA from Mig-6−/− mouse lung was used as a positive control. The excised Mig-6 conditional allele generated a 433 bp band whereas wild-type Mig-6 allele generated a 145 bp band.
Sup. Figure 2. Immunohistochemistry and Western Blot analyses of total and phosphorylated EGFR/ERBB2/ERBB3/AKT/mTOR proteins in lung and lung tumors of 2-month old mice. (A-F) IHC analyses of total and phosphorylated EGFR (A), ERBB2 (C), ERBB3 (E) and WB analyses of EGFR (B), ERBB2 (D) and ERBB3 (F). Left panel of IHC results (A, C and E) are quantitative results in mouse lungs. The blue dashes point to the areas used for quantification. G. Representative results of phosphorylated p-mTOR (S2448) / mTOR (right panel) and the quantitative results (left panel) in mouse lungs. All quantifications are calculated from three mice per group (six regions for each mouse) using Image J [60]. Optical Density (OD) = log (max intensity/Mean intensity). H. Representative results of phosphorylated AKT (S473) and its total protein in mouse lungs.