Abstract
Background
The value of subjective global assessment (SGA) as nutritional assessor of protein-energy wasting (PEWSGA) in chronic kidney disease (CKD) patients depends on its mortality predictive capacity. We investigated associations of PEWSGA with markers of nutritional status and all-cause mortality in CKD patients.
Methods
In 1031 (732 CKD1-5 non-dialysis and 299 dialysis) patients, SGA and body (BMI), lean (LBMI) and fat (FBMI) body mass indices, % handgrip strength (% HGS), serum albumin, and high sensitivity C-reactive protein (hsCRP) were examined at baseline. The five-year all-cause mortality predictive strength of baseline PEWSGA and during follow-up were investigated.
Results
PEWSGA was present in 2% of CKD1-2, 16% of CKD3-4, 31% of CKD5 non-dialysis and 44% of dialysis patients. Patients with PEWSGA (n = 320; 31%) had higher hsCRP and lower BMI, LBMI, FBMI, %HGS and serum albumin. But, using receiver operating characteristics-derived cutoffs, these markers could not classify (by kappa statistic) or explain variations of (by multinomial logistic regression analysis) presence of PEWSGA. In generalized linear models, SGA independently predicted mortality after adjustments of multiple confounders (RR: 1.17; 95% CI: 1.11–1.23). Among 323 CKD5 patients who were re-assessed after median 12.6 months, 222 (69%) remained well-nourished, 37 (11%) developed PEWSGA de novo, 40 (12%) improved while 24 (8%) remained with PEWSGA. The latter independently predicted mortality (RR: 1.29; 95% CI: 1.13–1.46).
Conclusions
SGA, a valid assessor of nutritional status, is an independent predictor of all-cause mortality both in CKD non-dialysis and dialysis patients that outperforms non-composite nutritional markers as prognosticator.
Introduction
Poor nutritional status due to protein-energy wasting (PEW) is a common complication [1–3] associated with increased mortality in patients with chronic kidney disease (CKD) [4–7]. Most nutritional markers used in clinical practice are influenced by CKD, co-morbidities [8, 9] or non-nutritional factors such as inflammation, overhydration, and protein losses [10–13]. Furthermore, their mortality predictive capacity may be skewed; for example, higher levels of BMI [14–16] and serum lipids [17] that associate with poor outcomes in the general population may predict improved survival in CKD, a phenomenon referred to as reverse epidemiology.
Subjective global nutritional assessment (SGA) is a practical, non-invasive and inexpensive composite tool that is widely used in clinical practice [18]. The concurrent and mortality predictive validity of the SGA score system has been established in conservatively treated CKD patients and incident and prevalent dialysis patients [8, 19, 20]. However, SGA of non-dialysis-dependent CKD patients [21] and the mortality predictive role of temporal changes in SGA [22, 23] have with some exceptions been less thoroughly investigated.
SGA is thought to give a valid composite measure of nutritional status in CKD patients; however, its value as a nutritional assessor depends on its mortality predictive capacity. Therefore, we evaluated SGA in patients with different stages of CKD and different dialysis modalities, explored factors classifying presence of PEW as assessed by SGA (PEWSGA), and analyzed the association of PEWSGA with all-cause mortality.
Methods
Study patients
In this post hoc analysis we used SGA data from 1031 CKD patients including 83 CKD stage 1–2, 101 CKD stage 3–4, and 548 non-dialyzed CKD stage 5 (CKD5-ND) patients, and 299 prevalent dialysis (CKD5-D) patients, 212 hemodialysis (HD) and 87 peritoneal dialysis(PD) patients, from six cohorts, the details of which were described previously [8, 24–27]. We determined the prevalence of PEWSGA and analyzed associations of PEWSGA with nutritional markers at baseline and with subsequent 5 years all-cause mortality. Analyses were repeated for 323 CKD 5 non-dialysis (CKD 5-ND) patients who were re-assessed with SGA after median 12.6 months. The flow of the study subjects is described in S1 Fig.
Informed written consent was obtained from each individual. The Ethics Committee of the Karolinska Institute (EPN) at the Karolinska University Hospital Huddinge, Stockholm, Sweden, approved study protocols. The studies were conducted in adherence to the Declaration of Helsinki.
Included cohorts are described briefly, as follows:
CKD stage 1–2. 83 individuals from PRIMA controls cohort, a population-based sample randomly selected by Statistics Sweden (a government agency) from the Stockholm region, and recruited from February 2003 until May 2013, and who were found to have signs of mild CKD (macro- or microalbuminuria or reduced glomerular filtration rate, GFR). This cohort was created to provide an appropriate control group for the PRIMA cohort with similar age and gender distribution, see below. The median age was 61 years, 70% were males, 8% had diabetes and 8% had cardiovascular disease (CVD). Their median (10th to 90th percentile) estimated GFR (eGFR) was 85.5 (68.5–109.0) ml/min/1.732.
CKD stage 3–4. 101 CKD stage 3–4 patients from the PRIMA cohort [28] recruited from December 1996 until November 2014. Their median age was 59 years, 72% were males, 39% had diabetes and 35% had CVD. The most common causes of CKD were glomerulonephritis (26%), diabetic nephropathy (19%), hypertension/renal vascular disease (4%) and others (51%). Their median eGFR was 27.9 (16.7–46.5) ml/min/1.732.
CKD5-ND. 548 CKD5-ND patients (501 CKD5-ND patients initiating dialysis therapy from MIA cohort [29] and 47 patients undergoing living donor renal transplantation, LD-Rtx cohort [30]) were included in the study, and recruited from June 1994 until June 2016. Their median age was 55 years, 63% were males, 30% were diabetics, 25% had CVD and 31% were malnourished (SGA>1), and median eGFR was 6.3 (4.0–10.3) ml/min/1.732. The common causes of CKD were glomerulonephritis (26%), diabetic nephropathy (26%), hypertension/renal vascular disease (21%) and others (28%).
CKD5-D. 299 prevalent dialysis patients were recruited from MIMICK1 (24), (Mapping of Inflammation Markers in Chronic Kidney Disease 1), MIMICK2 [31] (Mapping of Inflammation Markers in Chronic Kidney Disease 2) and LD-Rtx [32] cohort from October 2003 to June 2016. Altogether 212 patients (71%) were treated by HD (174 from MIMICK1, 38 from LD-Rtx) and 87 patients (29%) were on PD (51 from MIMICK2, 36 from LD-Rtx). Their median age was 62 years, 60% were males, 19% had diabetes, 45% had CVD, 44% were malnourished (SGA score >1), and median eGFR was 0 (0–5.3) ml/min/1.732. Causes of CKD included glomerulonephritis (18%), diabetic nephropathy (11%), hypertension/renal vascular disease (18%) and others (53%).
Collection of clinical data
Each patient’s medical chart was reviewed to extract data pertaining to underlying etiology of CKD and co-morbidities as described previously [8, 28, 30, 32, 33].
Assessment of nutritional status by SGA
Nutritional status was assessed using the 4-point SGA scale consisting of six components: three based on the patient’s history of weight loss, incidence of anorexia and vomiting, and three based on the physician’s grading of muscle wasting, presence of edema and loss of subcutaneous fat as described previously [1]. PEWSGA was defined as SGA score >1 while a score of 1 indicated normal nutritional status. The 323 CKD 5 patients who were re-assessed with SGA after median 12.6 months were classified into four groups according to changes in nutritional status: Group 1 WN-WN, patients with a stable status of being well-nourished; Group 2 MN-WN, patients who improved their nutritional status; Group 3 WN-MN, patients with worsening nutritional status; Group 4 MN-MN, patients with PEWSGA at baseline and at follow-up.
Anthropometric evaluation
At the time of recruitment, body weight, BMI (kg/m2), and other anthropometric measurements were obtained. Skinfold thickness was measured with a Harpenden caliper at four sites on the non-dominant arm of the controls and in the fistula-free arm of the CKD patients. Lean body mass and fat mass were calculated by anthropometry with measurements of biceps, triceps, sub-scapular and supra-iliac skinfold thickness using the Durnin and Womersley caliper method [34], and by equations proposed by Siri [35]. Lean (LBMI) and fat (FBMI) body mass indices were calculated according to the method of Kyle et al [36] and expressed as kg/m2. Handgrip strength (HGS) was measured both in the dominant and non-dominant hands by using a Harpenden Handgrip Dynamometer (Yamar, Jackson, MI, USA). Each measurement was repeated three times for each arm, and the highest value for each arm was noted. Individuals in the CKD 1–2 cohort served as controls, and HGS values of CKD 3–5 patients were converted into percentage of the controls (% HGS).
Laboratory analysis
Blood samples were collected after an overnight fast (except for HD patients). The plasma was separated within 30 min, and samples were kept frozen at -70°C if not analyzed immediately. Plasma concentrations of insulin-like growth factor-1 (IGF-1), interleukin -6 (IL-6) and tumor necrosis factor alpha (TNF-α) were measured on an Immulite TM Automatic Analyzer (Siemens Healthcare; Diagnostics Products Ltd.) according to the manufacturer’s instructions. Concentrations of serum creatinine, serum albumin (bromcresol purple), calcium, phosphate, intact parathyroid hormone (iPTH), cholesterol, triglyceride (TG), hemoglobin, hsCRP (high-sensitivity nephelometry assay) were measured by routine methods at the Department of Laboratory Medicine, Karolinska University Hospital at Huddinge.
GFR was assessed in CKD 5-ND (n = 548) and PD (n = 87) patients by the mean of renal urea and creatinine clearances from a 24-hour urine collection, in CKD stage 1–2 and CKD stage 3–4 patients GFR by iohexol clearance, while in HD patients who in general had no or minimal renal function GFR was assumed to be zero. For comparative reasons, GFR in all patients (except HD patients) were also estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [37].
Statistical analyses
All variables are expressed as median (10th and 90th percentile) or percentage, or relative risk ratio (95% CI, confidence intervals), as appropriate. Statistical significance was set at the level of P<0.05. Comparisons between two groups were assessed with the non-parametric Wilcoxon test for continuous variables and Fischer exact test for nominal variables. Differences between three or more groups were analyzed by Kruskal-Wallis test. Univariate Spearman’s rank correlation was used to determine correlations between PEWSGA and other variables. The agreement between PEWSGA and other nutritional markers was evaluated by Kappa coefficient test. Receiver operating characteristics (ROC) derived area under the curve (AUC) values were used as cutoffs for analyses by multinomial logistic regression and generalized linear model (GENMOD procedure). A multinomial logistic regression model was used to assess the strength, expressed as pseudo-r, by which various factors could ascertain the presence of baseline PEWSGA. We used Kaplan-Meier and Tukey-Kramer test for multiple comparison between groups. Multivariable GENMOD regression was used to analyze all-cause mortality risk at baseline and during follow-up following adjustments for age, gender, diabetes mellitus, cardiovascular disease (CVD), % HGS, LBMI, albumin, hsCRP, calendar year and treatment modality. A multiple imputation of missing values was performed using the function PROC MI, with all variables in the covariate section used to produce the values for imputation. The original n for each variable is given throughout. The results for each imputation were generated using PROC GENMOD and PHREG and then, combined using PROC MIANALYZE. We used five imputed datasets for this study to ensure that our effect estimates were not overly inaccurate because of Monte Carlo variability. All statistical analyses were performed using statistical software SAS version 9.4 (SAS Campus Drive, Cary, NC, USA).
Results
Clinical and biochemical characteristics
Characteristics of the 1031 patients are shown in Table 1. According to SGA, PEWSGA (SGA>1) was present in 320 patients (31%) while 711 (69%) patients were well-nourished. PEWSGA patients were older, more prone to be women (PEWSGA 37% vs 28% among men), more often smokers, on dialysis, diabetic and with CVD, and had higher hsCRP, IL-6, TNF and iPTH, while eGFR, %HGS, BMI, LBMI, FBMI, serum albumin, hemoglobin and IGF-1 were lower.
Table 1. Baseline demographic and biochemical characteristics of 1031 CKD patients according to presence of malnutrition defined as SGA score >1.
| Variables | Well-nourished (n = 711) | Malnourished (n = 320) | P value |
|---|---|---|---|
| Demography | |||
| Age (years) | 56 (33–73) | 61 (38–76) | 0.0001 |
| Gender, male (%) | 474(67) | 183(57) | 0.004 |
| Diabetes mellitus, n (%) | 170(24) | 99(31) | 0.02 |
| CVD, n (%) | 202(28) | 168(53) | <0.0001 |
| Smoking, n (%) (n = 590/281) | 284(48) | 178(63) | <0.0001 |
| Cause of kidney disease, n (%): | <0.0001 | ||
| Glomerulonephritis | 172(26) | 53(17) | 0.0007 |
| Diabetic nephropathy | 115(18) | 77(24) | 0.02 |
| Hypertension/Renal vascular disease | 105(16) | 66(21) | 0.07 |
| Unknown or other etiology | 243(37) | 122(38) | 0.69 |
| eGFR (ml/min/1.732) a | 6.1 (0–68.8) | 5.6 (0–11.4) | <0.0001 |
| Mean BP (mmHg; n = 644/268) | 106 (88–124) | 102 (83–128) | 0.02 |
| Dialysis, n (%) | 168(24) | 131(41) | <0.0001 |
| Anthropometric measurements | |||
| % HGS (n = 688/297) | 93 (58–119) | 67 (37–102) | <0.0001 |
| BMI (kg/m2) | 25.4 (20.9–30.9) | 22.8 (18.4–29.5) | <0.0001 |
| LBMI (kg/m2; n = 620/270) | 17.6 (14.3–20.7) | 16.0 (13.4–19.9) | <0.0001 |
| FBMI (kg/m2; n = 620/270) | 7.6 (4.5–11.7) | 6.1 (3.4–10.5) | <0.0001 |
| Biochemical parameters | |||
| Creatinine (μmol/L) | 664 (95–1017) | 627 (403–917) | 0.79 |
| S-Albumin (g/L) | 36 (29–41) | 33 (25–39) | <0.0001 |
| Calcium(mmol/L; n = 690/301) | 2.4(2.1–2.5) | 2.4(2.1–2.8) | 0.02 |
| Phosphate (mmol/L; n = 690/301) | 1.6(0.9–2.5) | 1.7(1.2–2.6) | 0.03 |
| Ca×PO4 (mmol2/L2; n = 690/301) | 3.9 (2.2–6.1) | 4.2 (2.7–6.4) | 0.007 |
| iPTH (ng/l; n = 632/281) | 171 (34–541) | 210 (41–607) | 0.03 |
| Cholesterol (mmol/L; n = 707/318) | 4.7 (3.3–6.5) | 4.6 (3.3–7.1) | 0.42 |
| TG (mmol/L; n = 703/319) | 1.6 (0.8–3.3) | 1.6 (0.8–2.9) | 0.64 |
| IGF-1 (μg/ml;n = 562/251) | 171 (88–320) | 150 (66–297) | 0.0008 |
| Hemoglobin (g/L) | 115 (93–143) | 110 (91–128) | <0.0001 |
| hsCRP (mg/L) | 2.5 (0.5–17) | 7.3 (0.7–45.8) | <0.0001 |
| IL-6 (pg/ml; n = 626/290) | 4.2 (1.0–12.3) | 7.8 (2.0–22.8) | <0.0001 |
| TNF (pg/ml; n = 588/275) | 11.2 (5.7–19.1) | 13.6 (7.7–17.4) | <0.0001 |
Data presented as median (10th–90th percentile), number or percentage. Abbreviations: SGA, subjective global assessment; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; BP, blood pressure; % HGS, handgrip strength as percentage of the controls; BMI, body mass index; LBMI, lean body mass index; FBMI, fat body mass index; S-Albumin, serum albumin; Ca×PO4, calcium phosphate product; iPTH, intact parathyroid hormone; TG, triglyceride; IGF-1, insulin-growth like factor-1; hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6; TNF, tumor necrosis factor.
aIn hemodialysis patients (HD) who in general had no or minimal renal function, eGFR was assumed to be zero; eGFR in all patients (except HD patients) were estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
The prevalence of PEWSGA increased with decline of renal residual function; from 2% in CKD 1–2 to 16% in CKD 3–4 and 31% in CKD 5-ND, and was 44% in prevalent dialysis patients (S1 Table). To investigate associations of PEWSGA with age (S2 Table), gender (S3 Table), co-morbidities (CVD (S4 Table) diabetes (S5 Table)), renal replacement therapy (RRT) (S6 Table), anthropometry (%HGS (S7 Table), LBMI (S8 Table), FBMI (S9 Table) BMI (S10 Table)), serum albumin (S11 Table), and inflammatory status (S12 Table), we divided the patients according to these factors. The prevalence of PEWSGA increased with higher age; for age ≤ 45 years, 45–65 years and >65 years, 22%, 31% and 38%, respectively (p = 0.0003) in parallel with increased co-morbidity and inflammation, and lower %HGS and serum albumin. Females had higher prevalence of PEWSGA and their %HGS, LBMI, FBMI and serum albumin levels were lower. Patients with co-morbidities had higher prevalence of PEWSGA, higher hsCRP, and lower %HGS and serum albumin levels. Dialysis patients had worse SGA scores, and also lower levels of %HGS, serum albumin and FBMI. Patients with lower values of anthropometric measurements (%HGS, LBMI, FBMI, BMI), all tended to have higher frequency of PEWSGA.
Univariate correlations, kappa analysis and multinomial logistic regression analysis of factors associated with PEWSGA
In both dialysis (n = 299) and non-dialysis (n = 732) patients, PEWSGA was negatively associated with % HGS, BMI, LBMI, FBMI, albumin and IGF-1, and positively associated with hsCRP and IL-6 (Table 2). In addition, PEWSGA correlated with age, DM, CVD, eGFR, mean BP, iPTH and TNF in non-dialysis patients, and, in dialysis patients, with female gender. The strongest correlations in non-dialysis patients was for %HGS (rho = -0.38; p<0.001) and in dialysis patients for IL-6 (rho = 0.27; p<0.001).
Table 2. Univariate Spearman’s Rho correlations of SGA with other parameters in 1031 CKD patients.
| Rho correlations with SGA>1 | ||
|---|---|---|
| Variables | CKD- non dialysis (n = 732) |
CKD 5- dialysis (n = 299) |
| Age (years) | 0.10 b | 0.08 |
| Gender (male/female) | -0.05 | -0.16 b |
| Diabetes mellitus, % | 0.12b | 0.03 |
| CVD, % | 0.28c | 0.09 |
| Smoking, % (n = 582/258) | 0.12 b | 0.18 b |
| eGFR (ml/min/1.732)d | -0.08 a | |
| Mean BP (mmHg, n = 582/224) | -0.08a | 0.03 |
| % HGS (n = 696/289) | -0.38 c | -0.22 b |
| BMI (kg/m2) | -0.26c | -0.26c |
| LBMI (kg/m2, n = 691/280) | -0.23 c | -0.23 c |
| FBMI (kg/m2, n = 691/280) | -0.18 c | -0.18 b |
| Creatinine (μmol/L) | 0.06 | -0.23 c |
| Cholesterol (mmol/L, n = 730/295) | 0.01 | 0.01 |
| Triglyceride (mmol/L, n = 729/296) | 0.02 | -0.05 |
| IGF1 (μg/ml, n = 535/278) | -0.13 b | -0.13a |
| iPTH (ng/l, n = 698/215) | 0.11 b | -0.10 |
| Ca×PO4 (mmol2/L2; n = 690/301) | 0.07 | 0.08 |
| Hemoglobin (g/L,n = 729/293) | -0.23 c | -0.04 |
| S-Albumin (g/L) | -0.26 c | -0.20 b |
| hsCRP (mmol/L) | 0.27c | 0.21 b |
| IL-6 (pg/ml,n = 631/285) | 0.24 c | 0.27 c |
| TNF (pg/ml, n = 578/285) | 0.18 c | 0.11 |
Abbreviations: SGA, subjective global assessment; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; BP, blood pressure; % HGS, handgrip strength as percentage of the controls; BMI, body mass index; LBMI, lean body mass index; FBMI, fat body mass index; IGF-1, insulin-growth like factor -1; i PTH, intact parathyroid hormone; Ca×PO4, calcium phosphate product; S-Albumin, serum albumin; hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6; TNF, tumor necrosis factor. Significant correlations are marked:
a P < 0.05,
b P < 0.01,
c P < 0.001
dIn hemodialysis patients (HD) who in general had no or minimal renal function, eGFR was assumed to be zero; eGFR in all patients (except HD patients) were estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Kappa analysis did not show good agreement of presence of PEWSGA with nutritional markers indicating that these markers were inadequate markers of nutritional status as assessed by SGA (S13 Table).
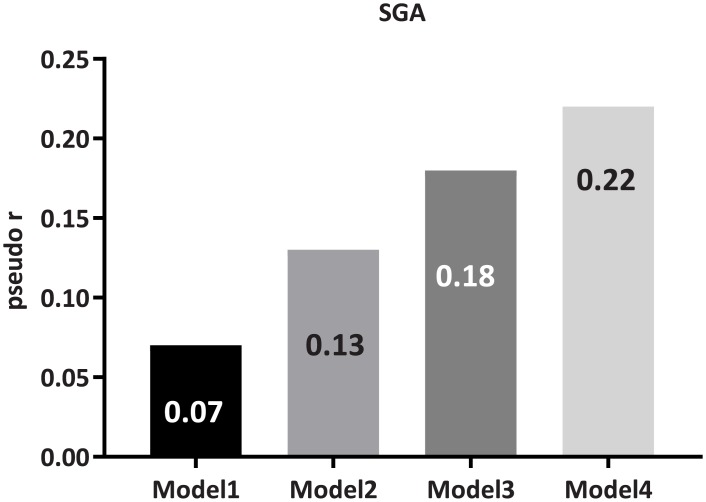
In multinomial logistic regression analysis of the strength, expressed as pseudo-r, by which various factors explained the variation of presence of PEWSGA, age, gender, DM and CVD together only predicted 7% of the variation of presence of PEWSGA (Model 1; Fig 1). With addition of BMI and LBMI in Model 2 this increased to 13%, and to 18% after adding albumin and hsCRP in Model 3. With further addition of % HGS and RRT in Model 4, still only 22% of the variation of presence of PEWSGA could be explained.
Fig 1. The predictive strength, expressed as pseudo-r, by which clinical and nutrition-related parameters could explain variation of presence of malnutrition by SGA.
Model 1: Age, gender, diabetes mellitus and cardiovascular disease; Model 2: Model 1 + body mass index and lean body mass index; Model 3: Model 2 + high-sensitivity C-reactive protein and serum albumin; Model 4: Model 3 + handgrip strength (percentage of the controls) and treatment modality (dialysis/non-dialysis). The analysis was performed by multinomial logistic regression using cut-off values derived from receiver operating characteristics curves.
PEWSGA as independent predictor of all-cause mortality
During 5 years follow up there were 268 deaths, 8 in CKD1-2, 16 in CKD3-4, 136 in CKD 5-ND and 108 in dialysis (86 in HD and 22 in PD) patients. The relative risk of death, during the 5 years follow up, was independently associated with baseline presence of PEWSGA (RR = 1.17; 95% CI, 1.11–1.23, p<0.0001) after adjustments for age, gender, DM, CVD, %HGS, LBMI, albumin, hsCRP, calendar year and RRT in 1031 patients (Table 3). In a separate analysis of the mortality predictive role of PEWSGA in different strata (S14 Table) and (S15 Table), PEWSGA was a predictor of all-cause mortality irrespective of whether patients were treated by dialysis (RR = 1.15; 95% CI, 1.07–1.23, p<0.0001) or not (RR = 1.19; 95% CI, 1.08–1.31, p = 0.0003).
Table 3. All-cause mortality risk for death occurring within 60 months based on imputed baseline data in 1031 patients, adjusted for all confounders, and expressed as relative risk ratio (95% CI).
| Relative Risk Ratio (95% CI) |
P value | |
|---|---|---|
| SGA>1, malnourished versus well nourished | 1.17 (1.11–1.23) | <0.0001 |
| Age, > 61 versus <61 years a | 1.14 (1.09–1.20) | <0.0001 |
| Gender, male versus female | 1.08 (1.02–1.14) | 0.01 |
| Diabetes mellitus, presence versus absence | 1.11 (1.05–1.17) | 0.0002 |
| CVD (yes/no),presence versus absence | 1.12 (1.06–1.18) | <0.0001 |
| % HGS, >74.07 versus <74.07a | 1.19 (1.13–1.25) | <0.0001 |
| LBMI, >17 versus < 17 kg/m2 a | 1.06 (1.01–1.12) | 0.03 |
| Albumin, >34 versus <34 g/La | 1.06 (1.01–1.11) | 0.02 |
| hsCRP, > 4.7 versus < 4.7 mg/La | 1.07 (1.02–1.12) | 0.01 |
| Calendar year, 1994–1999 vs 2010–2016 | 1.16 (1.07–1.25) | 0.0003 |
| Calendar year, 2000–2004 vs 2010–2016 | 1.17 (1.10–1.24) | <0.0001 |
| Calendar year, 2005–2009 vs 2010–2016 | 1.16 (1.08–1.25) | <0.0001 |
| Dialysis vs Non dialysis | 1.06 (1.01–1.12) | 0.03 |
Abbreviations: 95% CI, 95% confidence interval; SGA, subjective global assessment; CVD, cardiovascular disease; % HGS, handgrip strength as percentage of the controls; LBMI, lean body mass index; hsCRP, high sensitivity C-reactive protein.
a Cut-offs defined by ROC curve analysis.
Mortality risk of PEWSGA stratified by inflammation status
When patients were stratified into four groups according to presence or absence of inflammation (hsCRP≥ 4.7 mg/L; cutoff derived from ROC) and PEWSGA respectively, the survival rates by Kaplan-Meier estimates differed significantly (p<0.0001; Log-Rank test), S2 Fig. Inflamed patients with PEWSGA had the worst clinical outcome (p<0.0001). For both well-nourished and PEWSGA patients, those with an inflamed status had higher mortality risk (both P<0.0001). On the other hand, well-nourished patients tended to have survival advantage over PEWSGA patients irrespective of inflammation.
Changes in PEWSGA variation and nutritional parameters during follow up
After median 12.6 months follow up of 323 CKD 5-ND patients investigated prior to dialysis initiation, 222 (69%; Group 1 WN-WN) patients remained well nourished, 40 (12%; Group 2 MN-WN) improved, 37 (11%; Group 3 WN-MN) developed PEWSGA de novo, and 24 (8%; Group 4 MN-MN) remained with PEWSGA (Table 4). Serum albumin rose and hsCPR decreased in those with improving nutritional status (Group 2 MN-WN) while no significant changes in BMI, %HGS, LBMI or FBMI were observed during the follow up among any of the four groups of patients.
Table 4. Nutritional markers at baseline and after a median follow-up of 12.6 months in four groupsa defined by changes in SGA.
| Group WN-WN (n = 222) |
Group MN-WN (n = 40) |
Group WN-MN (n = 24) |
Group MN-MN (n = 37) |
P value | |
|---|---|---|---|---|---|
| S-Albumin, baseline, g/L | 36(29.0–41.0) | 33(26.1–38.9) | 33.5(25.0–40.5) | 33(24.8–41.0) | <0.0001 |
| S-Albumin, after follow-up, g/L (n = 213/38/22/34) | 37(31.4–43.0) b | 35(30.6–41.1)c | 36(26.0–41.1) | 34(26.0–42.0) | 0.005 |
| hsCRP, baseline, mg/L | 3.4(0.6–15.7) | 11.5(1.4–50.8) | 6.4(0.4–20) | 6.2(0.8–31.8) | 0.0002 |
| hsCRP, after follow-up, mg/L (n = 210/38/20/32) | 2.8(0.5–15.3) | 4.5(0.8–23.7) c | 2.6(0.6–126.2) | 5.8(0.2–47.3) | 0.06 |
| Cholesterol, baseline, mmol/L (n = 221/40/24/37) | 4.9(3.5–6.9) | 5.3(3.4–7.7) | 5.3(3.1–6.4) | 4.9(3.3–7.7) | 0.54 |
| Cholesterol, after follow-up, mmol/L (n = 214/38/22/34) | 5.3(3.6–7.4) | 5.5(3.8–8.5) | 4.9(2.9–7.8) | 5.2(3.8–7.2) | 0.20 |
| %HGS baseline (n = 215/39/22/36) | 93.0(66.0–126.6) | 74.1(55.6–103.7) | 74.4 (50.3–108.7) | 69.8(46.5–89.4) | <0.0001 |
| %HGS, after follow-up, (n = 221/40/24/36) | 95.3(66.8–125.9) | 79.1(64.1–113.4) | 74.8(51.2–115.1) | 67.1(38.8–96.7) | <0.0001 |
| BMI, baseline, kg/m2 | 25.7(21.3–31.4) | 23.4(19.5–32.4) | 24.9(18.7–31.0) | 21.4(17.4–27.3) | <0.0001 |
| BMI, after follow-up, kg/m2 (n = 222/40/24/36) | 25.4(21.5–31.6) | 24.7(21.0–31.9) | 23.2(17.8–27.4) | 21.5(16.7–27.4) | <0.0001 |
| LBMI, baseline, kg/m2 (n = 193/31/18/29) | 17.4(14.4–20.5) | 16.9(13.8–21.0) | 16.5(13.1–19.0) | 14.9(12.0–17.4) | <0.0001 |
| LBMI, after follow-up, kg/m2 (n = 179/29/19/29) | 17.1(14.5–20.4) | 16.4(14.0–18.8) | 15.8(12.1–17.6) | 14.2(11.4–16.6) | <0.0001 |
| FBMI, baseline, kg/m2 (n = 193/31/18/29) | 8.0(4.7–12.0) | 7.5(4.2–14.3) | 7.3(3.6–13.7) | 6.0(3.8–9.8) | 0.005 |
| FBMI, after follow-up, kg/m2 (n = 179/29/19/29) | 7.9(5.0–11.0) | 7.8(5.5–12.7) | 7.3(3.4–10.0) | 6.5(3.3–11.5) | 0.05 |
Data are presented as medians (range of 10th–90th percentiles). Abbreviations: S-Albumin, serum albumin; hsCRP, high sensitivity C-reactive protein; % HGS, handgrip strength as percentage of controls; BMI, body mass index; LBMI, lean body mass index; FBMI, fat body mass index.
a Groups were defined as: Group WN-WN, patients who remained well-nourished during follow up; Group MN-WN, patients who were improved with nutritional status during follow up; Group WN-MN, patients who developed PEW SGA during the follow-up; Group MN-MN, patients who remained with PEW SGA at baseline and at follow-up.
b compared with baseline level, p<0.001;
c compared with baseline level, p<0.05
All-cause mortality associated with presence of PEWSGA during follow-up
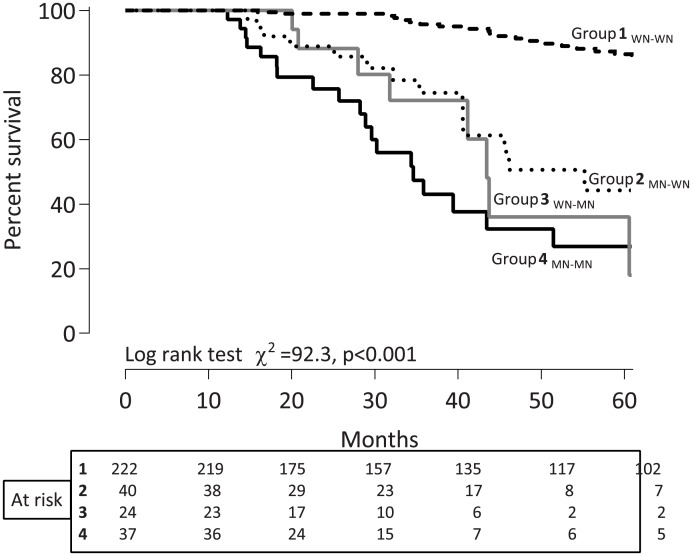
When investigating the association of nutritional changes with subsequent 5 years all-cause mortality, Kaplan-Meier curves showed marked differences (P<0.0001) in 5-year survival rates between the four groups: 91% (Group 1 WN-WN), 65% (Group 2 MN-WN), 67% (Group 3 WN-MN), and 49% (Group 4 MN-MN), respectively (Fig 2). All-cause mortality of patients with persistent PEWSGA (Group 1 MN-MN) was significantly higher compared with the other groups (P<0.0001) and also patients who developed PEW (Group 3 WN-MN) had better survival than those with PEWSGA at both occasions (Group 4 MN-MN) (P = 0.002). In a separate analysis using multivariable GENMOD regression (Table 5), relative risk ratios for all-cause mortality adjusted for all investigated confounders showed that presence of PEWSGA at baseline and at follow-up was an independent risk factor for 5-year death (RR = 1.29, 95% CI, 1.13–1.46, P = 0.0001).
Fig 2. Kaplan—Meier plot for all-cause mortality of the four groups of patients according to the nutritional status change assessed by SGA.
Abbreviations: Group 1 WN-WN, patients who remained well-nourished during follow up; Group 2 MN-WN, patients who were improved with nutritional status during follow up; Group 3 WN-MN, patients who developed PEWSGA during the follow-up; Group 4 MN-MN, patients who remained with PEWSGA at baseline and at follow-up.
Table 5. All-cause mortality risk for death occurring within 60 months based on imputed follow-upa data in 323 incident dialysis patients, adjusted for all confounders, and expressed as relative risk ratio (95% CI).
| Relative Risk Ratio (95% CI) | P value | |
|---|---|---|
| Group 2 MN-WN versus Group 1 WN-WN | 1.13(1.00–1.27) | 0.05 |
| Group 3 WN-MN versus Group 1 WN-WN | 1.15(1.00–1.33) | 0.05 |
| Group 4 MN-MN versus Group 1 WN-WN | 1.29(1.13–1.46) | 0.0001 |
| Age> 60 versus <60 years b | 1.08(0.99–1.17) | 0.08 |
| Gender, male versus female | 0.98(0.90–1.07) | 0.68 |
| Diabetes mellitus, presence versus absence | 1.11(1.02–1.21) | 0.02 |
| CVD, presence versus absence | 1.09 (1.00–1.19) | 0.06 |
| % HGS, >77.78 versus <77.78b | 1.16(1.07–1.27) | 0.0006 |
| LBMI >16.7 versus < 16.7 kg/m2 b | 0.98(0.89–1.07) | 0.62 |
| Albumin >33 versus <33 g/Lb | 1.10(1.02–1.19) | 0.02 |
| hsCRP > 6.1 versus < 6.1 mg/Lb | 1.06(0.98–1.15) | 0.16 |
| Recruitment year, 2003–2014 versus 1994–2002 | 0.91(0.84–0.98) | 0.02 |
| Hemodialysis versus peritoneal dialysis | 1.02(0.95–1.10) | 0.64 |
Abbreviations: 95% CI, 95% confidence interval; Group 1 WN-WN, patients who remained well-nourished during follow up; Group 2 MN-WN, patients who were improved with nutritional status during follow up; Group 3 WN-MN, patients who developed PEWSGA during the follow-up; Group 4 MN-MN, patients who remained with PEWSGA at baseline and at follow-up; CVD, cardiovascular disease; % HGS, handgrip strength as percentage of the controls; LBMI, lean body mass index; hsCRP, high sensitivity C-reactive protein
a The median follow-up time of the 323 incident dialysis patients was 12.6 months.
b Cut-offs defined by ROC curve analysis.
Discussion
We found that nutritional markers (Tables 1 and 2) were associated with SGA status, but none of them nor combinations of several markers could adequately classify PEW (S13 Table) or explain variation of presence of PEW according to SGA (Fig 1), indicating that the investigated nutritional markers cannot be relied upon to ascertain presence of PEWSGA. On the other hand, PEWSGA was an independent predictor of mortality supporting the clinical relevance and value of SGA as assessor of nutritional status in CKD patients.
SGA was found in several studies to be a reliable tool for evaluating nutritional status [19, 20, 38] and to be associated with clinical characteristics, anthropometrics and nutritional biomarkers [19, 39–42] as shown also in the present study. However, the validity of SGA as a nutritional marker has been questioned due to its subjective nature. Cooper et al [43] who compared SGA with total body nitrogen as the gold standard for protein stores in 76 dialysis patients reported that while SGA could differentiate severely malnourished patients from those with normal nutrition, SGA was not a reliable predictor of the degree of malnutrition. Jones et al [44] using a composite nutritional score derived from SGA, body mass index, percent of reference weight, triceps skinfold, mid-arm muscle circumference, and serum albumin found that SGA may not reliably identify HD patients with abnormal nutrition. On the other hand, Steiber et al [19] found that the 7-point scale SGA is a reliable and valid tool for nutritional assessment in adults on HD, and Cuppari et al [21] comparing SGA with anthropometric parameters found 7-point SGA to be a valid tool to assess PEW in nondialysis-dependent CKD patients. We concur with these more positive views on SGA but would argue that the diagnosis of malnutrition should rather be based on clinical assessment such as in the form of SGA against which the validity of proxy markers of nutritional status should be tested.
From this point of view it is interesting that PEWSGA could not—based on kappa coefficient analysis—be classified adequately by several investigated single proxy markers of nutritional status including serum albumin, inflammatory biomarkers, body composition and HGS. Furthermore, the predictive strength, expressed as pseudo-r, of these markers even when used concomitantly—together with age, gender and comorbidities (CVD and DM)–to ascertain SGA status was low; collectively they could explain no more than a small fraction (pseudo-r 0.22) of the variation of presence of PEWSGA. Given that PEW is a complex consequence of numerous interrelated factors it is not unexpected that no single parameter can unequivocally ascertain presence of PEW. The underlying premise in the current study is thus that the diagnosis of poor nutritional status should be based on clinical assessment and that PEWSGA should be regarded as a holistic clinical diagnosis. Particular features of PEW such as low muscle mass or strength are on the other hand best defined by specific markers such as LBM and HGS respectively.
In agreement with previous studies demonstrating that SGA is an independent predictor of all-cause mortality in dialysis patients [11, 39, 45], we found that PEWSGA was an independent predictor of mortality not only in dialysis patients (RR = 1.15; 95% CI, 1.07–1.23, p<0.0001) but also in nondialysis-dependent patients (RR = 1.19; 95% CI, 1.08–1.31, p = 0.0003). Furthermore, in the follow-up study of 323 CKD 5 patients, PEWSGA persisting during one year remained as an independent predictor for subsequent 5 year mortality risk together with only DM, %HGS, serum albumin, and recruitment period.
The strength—in spite of the subjective nature of SGA—of the association of SGA with adverse health outcomes suggests that SGA is clinically relevant. However, the link between malnutrition and increased mortality is not clear. Mutsert et al [46] found that the mortality risk of low serum albumin was partly explained by its links with inflammation, but not by malnutrition (assessed by 7-point SGA scale) in dialysis patients. In contrast, we show that PEWSGA predicted all-cause mortality independent of serum albumin and inflammation (in both dialysis patients and non-dialysis patients). In addition, we also found that among patients with or without inflammation, those with normal nutrition status had lower risk of mortality than those with poor nutritional status. These results suggest that presence of PEWSGA predisposes CKD patients to a worse clinical outcome, and that the mortality predictive capacity of PEWSGA is not much modified by inflammation.
Furthermore, in the analyses of follow-up of changes in nutritional status, we showed that despite the variation in nutritional status by SGA, there were no significant changes of BMI, % HGS, LBMI, and FBMI among the four groups. Thus, these proxy markers of nutritional markers were not influenced by the changes in nutritional status and moreover did not—with the exception for %HGS—associate independently with the subsequent clinical outcome. In addition, although there was a significant increase of serum albumin and decrease of hsCRP concentrations in the group where nutritional status improved (Group 2 MN-WN), they could not fully explain the change of nutritional status since no significant variation was observed in other groups.
As expected the prevalence of PEWSGA rose with the decline in GFR (from 2% in CKD 1–2, 16% in CKD 3–4, 31% in CKD 5 non-dialyzed to 44% in the dialysis patients) supplementing data from previous studies [10, 20, 41, 47]. This implies that routine monitoring of nutritional status by SGA from early stages of CKD is warranted, because not only the incidence but also severity of PEW in patients progressing into end-stage renal disease increases, and it is more difficult to treat PEW when it is severe.
The results should be interpreted considering some limitations. Firstly, because of the observational study design no conclusion can be made regarding causality and despite few missing values of nutrition-related anthropometric and biochemical parameters, we cannot rule out the impact of residual confounders. Secondly, we did not study the concurrent validity of SGA by comparing SGA with a reference method of nutritional status such as total body nitrogen and the premise of our study is that the diagnosis of PEW should be based on clinical assessment. However, the predictive validity of SGA was confirmed by the proven independent association of SGA with the risk of mortality due to poor nutritional status independent of co-morbid conditions or inflammation. Thirdly, although we evaluated nutritional status by SGA on two occasions, the stratified group sample size during follow-up was small, which may affect the association of SGA and other nutritional parameters with clinical outcome. Further studies on sequential recordings of SGA scores are warranted to verify the dynamic role of nutritional status in CKD patients.
In summary, PEW assessed by SGA was found to be an independent predictor of mortality. Our results showing that a range of non-composite nutritional markers could not adequately classify presence of clinically defined poor nutritional status, or explain the variation of SGA status, together with the finding that SGA is a robust prognosticator of clinical outcome, support the value of SGA as assessor of nutritional status in patients with CKD.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TXT)
Acknowledgments
We thank all patients and healthy subjects who participated in present study, and those who carried out the extensive clinical and laboratory work at the clinical investigational unit and the Renal Laboratory at Department of Renal Medicine, Karolinska University Hospital Huddinge. Lu Dai had a scholarship from the China Scholarship Council. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. The study also benefited from generous support from Amgen Inc., Karolinska Institutet Diabetes Theme Center, Swedish Research Council, Martin Rind Foundation, Njurfonden, and Westmans Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from Baxter Healthcare Corporation and Amgen Inc to Karolinska Institutet. The grant from Baxter Healthcare Corporation was a general grant to Renal Medicine and Baxter Novum, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, to support research activities at Karolinska Institutet to promote the understanding and treatment of renal disease which made it possible to carry out this and other studies. The grant from Amgen Inc was given to Renal Medicine, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, to support two observational studies on inflammation in dialysis patients treated by hemodialysis and peritoneal dialysis respectively. Lu Dai received a scholarship from the China Scholarship Council. The study also benefited from generous support from Swedish Research council (Peter Stenvinkel), Martin Rind Foundation (Peter Stenvinkel), Njurfonden (Peter Stenvinkel), and Westmans Foundation. Bengt Lindholm is employed by Baxter Healthcare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Qureshi AR, Alvestrand A, Danielsson A, Divino JC, Gutierrez A, Lindholm B, et al. Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int. 1998;53(3):773–82. doi: 10.1046/j.1523-1755.1998.00812.x [DOI] [PubMed] [Google Scholar]
- 2.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8. doi: 10.1038/sj.ki.5002585 . [DOI] [PubMed] [Google Scholar]
- 3.Cianciaruso B, Brunori G, Kopple JD, Traverso G, Panarello G, Enia G, et al. Cross-sectional comparison of malnutrition in continuous ambulatory peritoneal dialysis and hemodialysis patients. Am J Kidney Dis. 1995;26(3):475–86. . [DOI] [PubMed] [Google Scholar]
- 4.Acchiardo SR, Moore LW, Latour PA. Malnutrition as the main factor in morbidity and mortality of hemodialysis patients. Kidney Int Suppl. 1983;16:S199–203. . [PubMed] [Google Scholar]
- 5.de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW, et al. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23(9):2957–64. doi: 10.1093/ndt/gfn167 . [DOI] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29(1):3–14. doi: 10.1016/j.semnephrol.2008.10.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leinig CE, Moraes T, Ribeiro S, Riella MC, Olandoski M, Martins C, et al. Predictive value of malnutrition markers for mortality in peritoneal dialysis patients. J Ren Nutr. 2011;21(2):176–83. doi: 10.1053/j.jrn.2010.06.026 . [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–911. doi: 10.1046/j.1523-1755.1999.00422.x . [DOI] [PubMed] [Google Scholar]
- 9.Dong J, Wang T, Wang HY. The impact of new comorbidities on nutritional status in continuous ambulatory peritoneal dialysis patients. Blood Purif. 2006;24(5–6):517–23. doi: 10.1159/000096472 . [DOI] [PubMed] [Google Scholar]
- 10.Jones CH, Newstead CG, Will EJ, Smye SW, Davison AM. Assessment of nutritional status in CAPD patients: serum albumin is not a useful measure. Nephrol Dial Transplant. 1997;12(7):1406–13. . [DOI] [PubMed] [Google Scholar]
- 11.Fiedler R, Jehle PM, Osten B, Dorligschaw O, Girndt M. Clinical nutrition scores are superior for the prognosis of haemodialysis patients compared to lab markers and bioelectrical impedance. Nephrol Dial Transplant. 2009;24(12):3812–7. doi: 10.1093/ndt/gfp346 . [DOI] [PubMed] [Google Scholar]
- 12.Chan M, Kelly J, Batterham M, Tapsell L. Malnutrition (subjective global assessment) scores and serum albumin levels, but not body mass index values, at initiation of dialysis are independent predictors of mortality: a 10-year clinical cohort study. J Ren Nutr. 2012;22(6):547–57. doi: 10.1053/j.jrn.2011.11.002 . [DOI] [PubMed] [Google Scholar]
- 13.Gama-Axelsson T, Heimburger O, Stenvinkel P, Barany P, Lindholm B, Qureshi AR. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol. 2012;7(9):1446–53. doi: 10.2215/CJN.10251011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenvinkel P, Gillespie IA, Tunks J, Addison J, Kronenberg F, Drueke TB, et al. Inflammation Modifies the Paradoxical Association between Body Mass Index and Mortality in Hemodialysis Patients. J Am Soc Nephrol. 2016;27(5):1479–86. doi: 10.1681/ASN.2015030252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib Nephrol. 2009;163:124–31. doi: 10.1159/000223790 . [DOI] [PubMed] [Google Scholar]
- 16.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2007;49(5):581–91. doi: 10.1053/j.ajkd.2007.02.277 . [DOI] [PubMed] [Google Scholar]
- 17.Moradi H, Abhari P, Streja E, Kashyap ML, Shah G, Gillen D, et al. Association of serum lipids with outcomes in Hispanic hemodialysis patients of the West versus East Coasts of the United States. American journal of nephrology. 2015;41(4–5):284–95. doi: 10.1159/000381991 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8–13. doi: 10.1177/014860718701100108 . [DOI] [PubMed] [Google Scholar]
- 19.Steiber A, Leon JB, Secker D, McCarthy M, McCann L, Serra M, et al. Multicenter study of the validity and reliability of subjective global assessment in the hemodialysis population. J Ren Nutr. 2007;17(5):336–42. doi: 10.1053/j.jrn.2007.05.004 . [DOI] [PubMed] [Google Scholar]
- 20.Group C-UCPDS. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Journal of the American Society of Nephrology: JASN. 1996;7(2):198–207. . [DOI] [PubMed] [Google Scholar]
- 21.Cuppari L, Meireles MS, Ramos CI, Kamimura MA. Subjective global assessment for the diagnosis of protein-energy wasting in nondialysis-dependent chronic kidney disease patients. J Ren Nutr. 2014;24(6):385–9. doi: 10.1053/j.jrn.2014.05.004 . [DOI] [PubMed] [Google Scholar]
- 22.Prasad N, Gupta A, Sinha A, Sharma RK, Kumar A, Kumar R. Changes in nutritional status on follow-up of an incident cohort of continuous ambulatory peritoneal dialysis patients. J Ren Nutr. 2008;18(2):195–201. doi: 10.1053/j.jrn.2007.08.002 . [DOI] [PubMed] [Google Scholar]
- 23.Kwon YE, Kee YK, Yoon CY, Han IM, Han SG, Park KS, et al. Change of Nutritional Status Assessed Using Subjective Global Assessment Is Associated With All-Cause Mortality in Incident Dialysis Patients. Medicine (Baltimore). 2016;95(7):e2714 doi: 10.1097/MD.0000000000002714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snaedal S, Heimburger O, Qureshi AR, Danielsson A, Wikstrom B, Fellstrom B, et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis. 2009;53(6):1024–33. doi: 10.1053/j.ajkd.2009.02.008 . [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AR, Olauson H, Witasp A, Haarhaus M, Brandenburg V, Wernerson A, et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney international. 2015;88(6):1356–64. doi: 10.1038/ki.2015.194 . [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Cabezas-Rodriguez I, Qureshi AR, Heimburger O, Barany P, Snaedal S, et al. Increased Levels of Modified Advanced Oxidation Protein Products Are Associated with Central and Peripheral Blood Pressure in Peritoneal Dialysis Patients. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis. 2015;35(4):460–70. doi: 10.3747/pdi.2013.00064 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isoyama N, Leurs P, Qureshi AR, Bruchfeld A, Anderstam B, Heimburger O, et al. Plasma S100A12 and soluble receptor of advanced glycation end product levels and mortality in chronic kidney disease Stage 5 patients. Nephrol Dial Transplant. 2015;30(1):84–91. doi: 10.1093/ndt/gfu259 . [DOI] [PubMed] [Google Scholar]
- 28.Ghanavatian S, Diep LM, Barany P, Heimburger O, Seeberger A, Stenvinkel P, et al. Subclinical atherosclerosis, endothelial function, and serum inflammatory markers in chronic kidney disease stages 3 to 4. Angiology. 2014;65(5):443–9. doi: 10.1177/0003319713483000 . [DOI] [PubMed] [Google Scholar]
- 29.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–911. doi: 10.1046/j.1523-1755.1999.00422.x . [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AR, Olauson H, Witasp A, Haarhaus M, Brandenburg V, Wernerson A, et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015;88(6):1356–64. doi: 10.1038/ki.2015.194 [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Watanabe M, Qureshi AR, Heimburger O, Barany P, Anderstam B, et al. Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit Dial Int. 2015;35(2):206–15. doi: 10.3747/pdi.2013.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snaedal S, Heimbürger O, Qureshi A, Danielsson A, Wikström B, Fellström B, et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis. 2009;53(6):1024–33. doi: 10.1053/j.ajkd.2009.02.008 . [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Watanabe M, Qureshi AR, Heimbürger O, Bárány P, Anderstam B, et al. Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit Dial Int. 2015;35(2):206–15. doi: 10.3747/pdi.2013.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97. . [DOI] [PubMed] [Google Scholar]
- 35.Siri WE. Body composition from fluid space and density In: Brozek J, Hanschel A, (). Washington DNAoS, editors. Techniques for measuring body composition Washington, DC: National Academy of Science; 1961. p. 223–44. [Google Scholar]
- 36.Kyle UG, Schutz Y, Dupertuis YM, Pichard C. Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition. 2003;19(7–8):597–604. . [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enia G, Sicuso C, Alati G, Zoccali C. Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant. 1993;8(10):1094–8. . [PubMed] [Google Scholar]
- 39.de Mutsert R, Grootendorst DC, Boeschoten EW, Brandts H, van Manen JG, Krediet RT, et al. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr. 2009;89(3):787–93. doi: 10.3945/ajcn.2008.26970 . [DOI] [PubMed] [Google Scholar]
- 40.Pifer TB, McCullogh KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–45. doi: 10.1046/j.1523-1755.2002.00658.x [DOI] [PubMed] [Google Scholar]
- 41.Young GA, Kopple JD, Lindholm B, Vonesh EF, De Vecchi A, Scalamogna A, et al. Nutritional assessment of continuous ambulatory peritoneal dialysis patients: an international study. Am J Kidney Dis. 1991;17(4):462–71. . [DOI] [PubMed] [Google Scholar]
- 42.Paudel K, Visser A, Burke S, Samad N, Fan SL. Can Bioimpedance Measurements of Lean and Fat Tissue Mass Replace Subjective Global Assessments in Peritoneal Dialysis Patients? J Ren Nutr. 2015;25(6):480–7. doi: 10.1053/j.jrn.2015.05.003 . [DOI] [PubMed] [Google Scholar]
- 43.Cooper BA, Bartlett LH, Aslani A, Allen BJ, Ibels LS, Pollock CA. Validity of subjective global assessment as a nutritional marker in end-stage renal disease. Am J Kidney Dis. 2002;40(1):126–32. doi: 10.1053/ajkd.2002.33921 . [DOI] [PubMed] [Google Scholar]
- 44.Jones CH, Wolfenden RC, Wells LM. Is subjective global assessment a reliable measure of nutritional status in hemodialysis? J Ren Nutr. 2004;14(1):26–30. . [DOI] [PubMed] [Google Scholar]
- 45.Santin FG, Bigogno FG, Dias Rodrigues JC, Cuppari L, Avesani CM. Concurrent and Predictive Validity of Composite Methods to Assess Nutritional Status in Older Adults on Hemodialysis. J Ren Nutr. 2016;26(1):18–25. doi: 10.1053/j.jrn.2015.07.002 . [DOI] [PubMed] [Google Scholar]
- 46.de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW, et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127–35. doi: 10.1053/j.jrn.2008.08.003 . [DOI] [PubMed] [Google Scholar]
- 47.Stenvinkel P, Barany P, Chung SH, Lindholm B, Heimburger O. A comparative analysis of nutritional parameters as predictors of outcome in male and female ESRD patients. Nephrol Dial Transplant. 2002;17(7):1266–74. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TXT)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.