Abstract
Background:
Leptomeningeal disease (LMD) is a complication that results from solid tumor metastasis. Prognosis is extremely poor. As therapeutic options for solid tumors improve, the rate of LMD continues to increase. Until recently, treatment has been limited to radiation therapy, intrathecal chemotherapy, and systemic chemotherapy, with an overall survival of 2-3 months. Targeted molecular therapy and immunotherapies are promising new options for increasing overall survival and clinical improvement; however, optimal clinical management remains unknown.
Methods:
In this review, we discuss targeted molecular therapy and immunotherapy treatment options for LMD resulting from primary lung, breast, and melanoma tumors. In addition, we summarize dosing strategies, overall survival, clinical outcomes, and novel approaches to treatment.
Results:
Our review indicates a deficiency in the current literature. Presently, intrathecal trastuzumab administration may be an effective option for patients with HER2-positive breast cancer. BRAF inhibitors and cytotoxic T lymphocyte-associated antigen-4 targets have shown promising results in LMD resulting from melanoma. Finally, tyrosine kinase inhibitors may increase overall survival in epidermal growth factor receptor (EGFR)-mutant non–small cell lung cancer. Pulsatile drug administration or dual therapy may be beneficial for patients who progress to LMD while being treated with EGFR targets for their primary malignancy.
Conclusion:
Targeted molecular therapy and immunotherapy in LMD may provide favorable treatment options. Current literature is lacking in safety, efficacy, and overall response rates from the use of targeted therapy. Research is needed to draw significant conclusions about the most appropriate therapy for patients with LMD.
Keywords: Immunotherapy, meningeal neoplasms, molecular targeted therapy, mutation, radiotherapy
INTRODUCTION
Leptomeningeal disease (LMD) is a deadly complication of solid tumors and has a poor prognosis. Although the incidence of LMD is 5%-8% in patients with any malignant disease, the occurrence inevitably denotes a poor outcome, with a median survival rate of a few months.1-4
LMD is diagnosed in approximately 5% of patients with solid tumors.3 The most common primary cancers are melanoma, lung, and breast, with adenocarcinoma being the most frequent histology seen in LMD. Malignant melanoma has the highest rate of spread to the meninges (20%),5 followed by lung and then breast cancer (11% and 5%, respectively)6,7; however, the higher incidence of breast cancer leads to a greater proportion of LMD cases.
Malignant cells may spread via hematogenous spread (through venous or arterial flow), perineural migration along peripheral nerves, or via direct invasion from adjacent tumors.1 Once in the cerebrospinal fluid (CSF), the malignant cells can be transported to any location of the neurospinal axis and result in meningeal seeding, with preference for the basilar cisterns and the cauda equina.8-10 The incidence of leptomeningeal metastasis is increasing from all primary tumor types, likely because of improved technology that enhances detection, better cancer therapies that lead to longer survival, and the ability of the CSF space to restrict penetration of pharmaceutical agents.11
Therapy for LMD may include a combination of radiation therapy and intrathecal and systemic chemotherapy to improve symptoms and prolong survival, albeit by marginal time frames of 2-3 months.8,12,13 The treatment of LMD has proven to be difficult; systemic cytotoxic therapy is not effective because of its inability to cross the blood–brain barrier in adequate concentrations, while intrathecal tumors can damage the CSF, thus diminishing the efficacy of intrathecal chemotherapy. Whole-brain radiotherapy and methotrexate have been shown to have limited advantage in the treatment of LMD.14,15
Treatment focus is shifting to the use of targeted molecular therapy and immunotherapies for the treatment of malignancy. As treatments improve for patients with targetable molecular mutations, these patients are living longer, and thus higher rates of LMD are being reported in this population.16 For non–small cell lung cancer (NSCLC) with leptomeningeal metastasis, current therapies target epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangement via tyrosine kinase inhibitors (TKIs) in select patients. In breast cancer, HER2 (also known as HER2/neu, ERBB2, and CD340) malignant cells have a high affinity for the central nervous system (CNS).17 Targeting this receptor via intrathecal injection has shown promising results with respect to overall survival.15,18-20 Additionally, research from 2011 and 2013 indicates that LMD originating from melanoma cells harboring a BRAF V600E mutation may benefit from targeted therapies.21,22
The target mutation must be present for the patient to benefit from molecular therapy. Because mutation presence and prevalence vary between cancer types and populations, specimen testing is important. For instance, only approximately 10%-15% of NSCLC specimens from patients in the United States and Western Europe have an EGFR mutation, while 30%-50% of specimens from Asia harbor this somatic mutation.23,24 ALK rearrangement is present in approximately 4%-5% of NSCLC patients.25 The estimated range of tumors that overexpress HER2 is 18%-25%.26 Additionally, tumors with BRAF V600E mutations compose 33%-55% of melanoma.27-29
This review discusses targeted molecular therapy and immunotherapy treatment options for LMD resulting from lung, breast, and melanoma solid tumors.
METHODS
In February 2017, we conducted a comprehensive search using PubMed/MEDLINE, Embase, and Cochrane Library. We included all studies in which molecular therapy or immunotherapy was used to treat patients with LMD metastasized from solid tumors originating from melanoma, lung cancer, or breast cancer. Tumor types consisted of HER2-positive breast cancer, the BRAF V600E mutation in melanoma, or the EGFR mutation or ALK rearrangement in lung cancer. Therapies that targeted vascular endothelial growth factor (VEGF) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) receptor were also included in this study.
Case reports, clinical series, and randomized controlled trials were included. Search criteria were limited to English studies conducted in human subjects from 2006 to the present. All studies were searched using various combinations of the following medical subject heading terms: leptomeningeal disease, leptomeningeal cancer, treatment, immunotherapy, immuno*, and molecular target*. Titles and abstracts of all papers gathered in the electronic search were inspected for inclusion. References were also manually searched for any studies that did not populate via the electronic search. The first author (K.H.T.) cross-referenced poster and conference abstracts, letters to the editor, and other articles that did not meet inclusion criteria for relevance. Assessment for study eligibility was unblinded. Data extraction included the following: study design, age of subject, previous radiotherapy treatment, overall survival, side effects, therapy duration, molecular target, therapy used, and outcomes.
RESULTS
The initial search of the relevant databases (PubMed, Embase, Cochrane Library) in February 2017 found 1,215 and 72 studies of interest via electronic and manual searching, respectively. Of these, 82 titles passed screening and were forwarded for abstract review. After abstract review, 31 articles were included for full-text review. Of these remaining articles, exclusions were made on the basis of chemotherapy being the sole treatment (2), the addition of a vaccination to standard therapy (1), and brain metastasis being the malignancy treated (1). The remaining 27 studies were included in the literature review. The primary tumor was breast in 7 studies, melanoma in 5 studies, and NSCLC in 15 studies (Figure).
Figure.

QUOROM diagram of study selection. QUOROM, quality of reporting of metaanalyses.
Breast Cancer
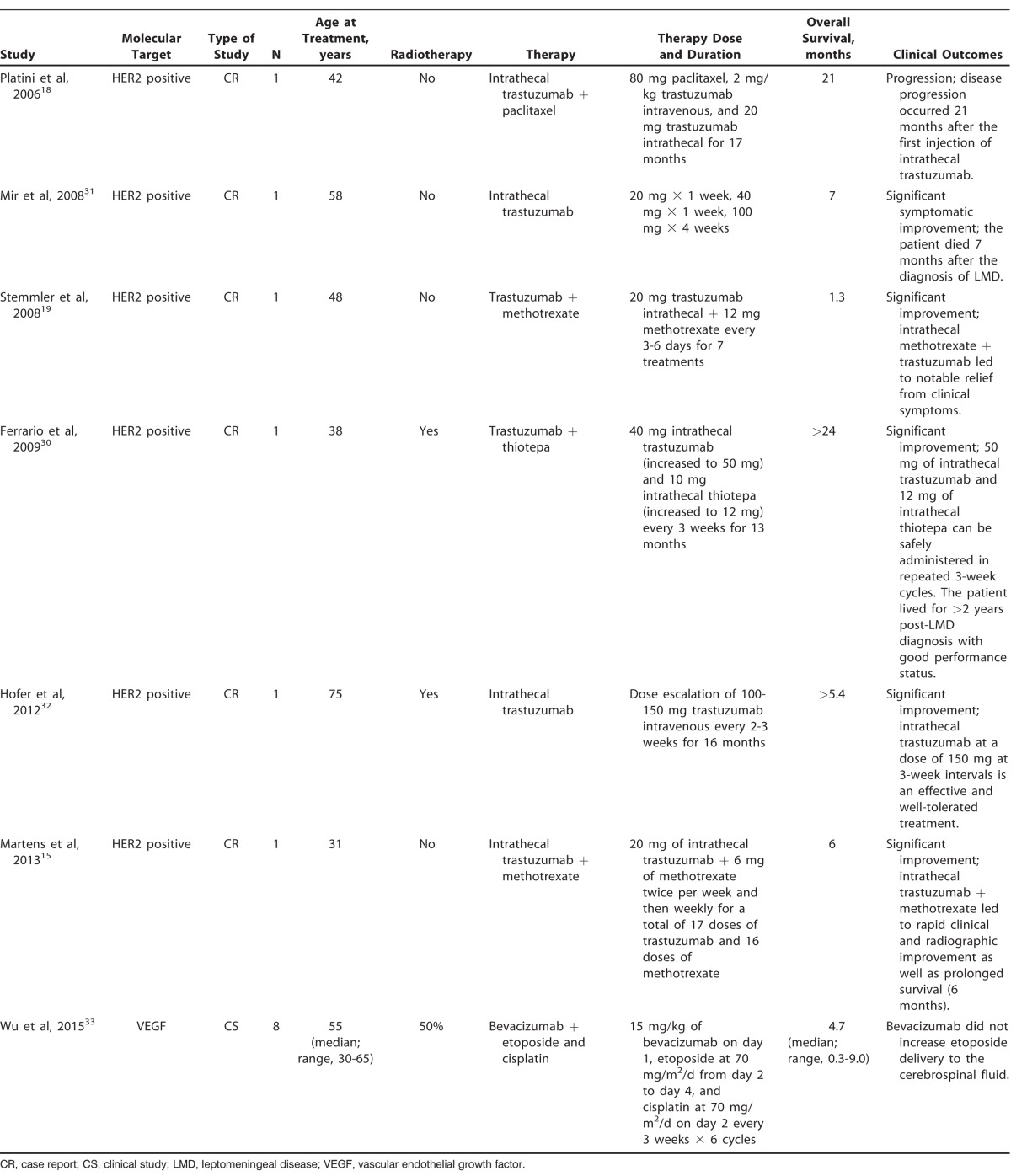
Seven of the 27 studies included in this review examined the effects of immunotherapy on LMD originating from breast cancer (Table 1). Six studies were case reports,15,18,19,30-32 and one was a prospective, multicenter pilot study.33 In all 6 of the case reports, patients had HER2-positive disease, and intrathecal trastuzumab in varying doses and combinations was used in the treatment. Two cases reported the effect of 20 mg of intrathecal trastuzumab in combination with either 12 mg of methotrexate given every 3-6 days19 or 6 mg of methotrexate given initially twice per week and then weekly.15 The combination of intrathecal trastuzumab and thiotepa administered in 3-week cycles was the treatment in one case report,30 and intrathecal trastuzumab in combination with paclitaxel weekly was used in another case.18 Escalating doses of intrathecal trastuzumab were examined at weekly31 and 3-week intervals.32 The pilot study targeted VEGF with bevacizumab with the aim of increasing etoposide penetration into the CSF.33
Table 1.
Literature Reports of Treatment With Immunotherapy or Molecular Targeted Therapy for Leptomeningeal Disease Resulting From Breast Cancer (n=7)
The average age of the women who were the subjects of the case reports was 48.7 years, and the participants in the pilot study had a median age of 55 years, with a range of 30-65 years. In the case reports, intrathecal trastuzumab was administered as second-, third-, or sixth-line therapy in 16.7%, 67%, and 16.7% of patients, respectively, and all patients presented with refractory disease. In the pilot study, 50% of patients had previously received radiotherapy,33 and 2 of the 6 (33.3%) patients in the case reports had previously received radiotherapy.30,32
Intrathecal trastuzumab was generally well tolerated; 83.3% of patients in the case reports had no significant side effects and tolerated trastuzumab well.15,18,19,31,32 The patient in one case reported grade 3 anemia and neutropenia.30 Among the case report patients, significant clinical and/or radiographic improvement was reported for 5 patients,15,19,30-32 and stabilization occurred in 1 patient.18 The average overall survival after initiation of therapy in the case reports was 10.73 months (range, 1.3 months to >2 years). The pilot study reported a median overall survival of 4.7 months (range, 0.3-9 months) after treatment with bevacizumab.33
Melanoma
Five of the 27 studies included in this review examined the effects of immunotherapy on LMD originating from melanoma (Table 2). Four studies were case reports21,34-36 and the fifth was a retrospective study with 39 patients.37 Malignant cells with the BRAF V600E mutation were the focus in 3 cases,21,34,36 the CTLA-4 receptor was the target in the fourth case,35 and the retrospective study included both molecular moieties.37 Vemurafenib was the treatment of choice in one case,21 vemurafenib plus dabrafenib and trametinib in another case,36 dabrafenib alone in one case,34 and ipilimumab alone in another.35 Geukes Foppen et al used BRAF inhibitors and CTLA-4 treatments, consisting of ipilimumab in 10 patients and varying treatments and combinations of vemurafenib or dabrafenib with or without trametinib in 14 patients.37
Table 2.
Literature Reports of Treatment With Immunotherapy or Molecular Targeted Therapy for Leptomeningeal Disease Resulting From Melanoma (n=5)
The average age of patients who were the subjects of case reports was 58.5 years, and the median age of patients in the retrospective study was 52.9 years. All of the studies incorporated radiotherapy into the treatment.34 Whole-brain radiotherapy was administered to the patients in 3 of the case reports.21,35,36 Both BRAF inhibitors and the CTLA-4 receptor targets were well tolerated in the studies that reported side effects.21,34-36 The majority of complaints were diarrhea, local injection site reactions, and dermatitis. The patient in one case reported grade 1 keratosis pilaris.34
Significant clinical improvement was reported in all studies.21,34-37 In the 2 case reports that used vemurafenib as therapy, both patients demonstrated significant improvement in clinical symptoms and long-term stabilization of LMD, with survival times >18 months.21,36 Average overall survival after initiation of therapy in all 4 of the case reports surpassed the expected overall survival, ranging from >15 months to >19 months. All 4 patients who were the subjects of the case studies were alive when the cases were written; no data are available for how long the patients survived thereafter. Geukes Foppen et al found that the median survival of patients treated with a BRAF inhibitor and CTLA-4 target was 21.7 weeks, with a range of 2-235 weeks.37 The 14 patients who were treated with BRAF inhibitors had a median survival rate of 24.9 weeks (range, 3-62 weeks). Of those, patients who had radiotherapy survived 25 weeks, while those who did not lived 16 weeks. Median survival of the 10 patients whose treatment included a CTLA-4 target was 15.8 weeks (range, 2-235 weeks). Of those, patients who had radiotherapy survived 47 weeks, while those who did not survived 6 weeks. Four patients were treated with radiotherapy only, and their median survival was 4.3 weeks (range, 2-16 weeks).37
Non–Small Cell Lung Cancer
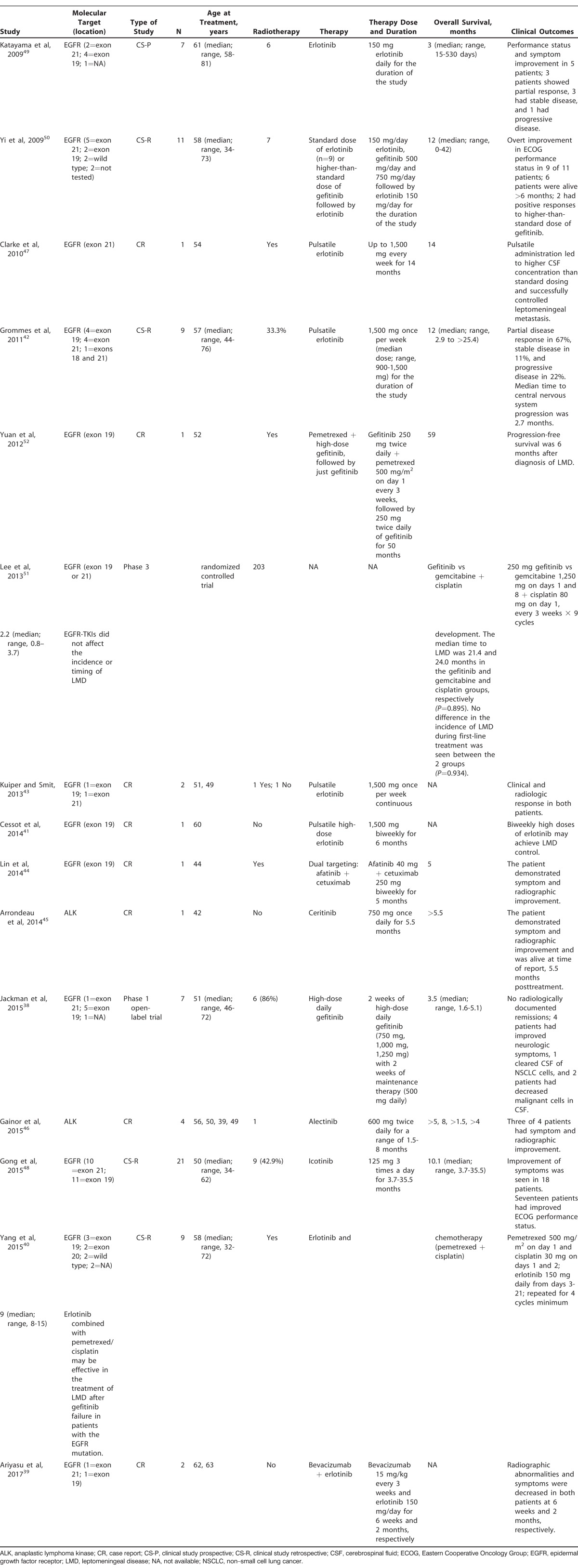
Fifteen38-52 of the 27 studies included in this review examined the effects of targeted therapy on LMD originating from NSCLC (Table 3): 8 case reports,39,41,43-47,52 a phase 1 open-label trial,38 a phase 3 randomized controlled trial,51 4 retrospective clinical studies,40,42,48,50 and a prospective clinical study.49 The number of subjects included in the clinical studies varied from 7-203 patients. Thirteen studies used EGFR-TKI therapies,38-44,47-52 and treatment with ALK inhibitors was the focus of 2 studies.45,46 Gefitinib was given in 3 studies: the phase 1 open-label trial studied the effect of high-dose daily gefitinib38; the phase 3 randomized controlled trial examined gefitinib vs gemcitabine plus cisplatin51; and in one case report, pemetrexed in combination with high-dose gefitinib was administered.52 Eight studies used erlotinib in varying doses and schedules, as well as in combination with other therapies.39-43,47,49,50 Pulsatile erlotinib was administered in 4 studies.41-43,47 High-dose erlotinib was used in one study.41 Erlotinib was also used in combination with bevacizumab39 or after the use of gefitinib.50 Another-first generation EGFR-TKI, icotinib, was used in a study involving 21 subjects.48 Lin et al studied the effect of dual targeting using cetuximab (an EGFR monoclonal antibody) and the second-generation EGFR-TKI afatinib.44 Alectinib was used in one case report focusing on ALK inhibitors,46 while ceritinib was used in another case report.45
Table 3.
Literature Reports of Treatment With Immunotherapy or Molecular Targeted Therapy for Leptomeningeal Disease Resulting From Non–Small Cell Lung Cancer (n=15)
The mean age of the patients who were the subjects of the case reports was 51.6 years, the median age of the 7 patients enrolled in the phase I open-label trial was 51 years (range, 46-72 years), and the ages of patients enrolled in the clinical studies ranged from 50-61 years. Side effects of EGFR-TKI and ALK inhibitors were tolerated well across all studies. One episode of toxic epidermal necrolysis was reported for a patient receiving gefitinib at a dose of 1,000 mg.38 One patient experienced grade 4 neutropenia and thrombocytopenia during the first cycle of pemetrexed at the standard dosage of 500 mg; however, the patient was able to complete 5 more cycles with the addition of granulocyte colony-stimulating factor and a 20% reduction in pemetrexed.52 The most common side effects reported were diarrhea, rash, and nausea.
In one patient, pulsatile administration of erlotinib led to increased levels of the drug in the CSF when compared to standard dosing.47 Jackman et al demonstrated that high-dose daily gefitinib led to no radiologically documented remissions; however, neurologic symptoms improved for 4 patients.38 One patient cleared the CSF of malignant cells, while 2 patients demonstrated a decrease in the presence of NSCLC cells on CSF analysis. The median overall survival for these patients was 3.5 months (range, 1.6-5.1 months).38 With regard to EGFR inhibitors, the case reports demonstrated an average overall survival of 26 months44,47,52; however, overall survival was not documented in 2 cases that reported on 3 patients.41,43 Both case reports that examined ALK inhibitors reported that subjects were alive at the time of publication45,46; the patient whose LMD was treated with ceritinib was alive for >5.5 months.45 Averaging the median overall survival reported for the clinical studies yields 9.22 months, with a wide range of 15 days to 42 months.40,42,48-50 Clinical trials that used gefitinib and gefitinib vs gemcitabine plus cisplatin reported a median overall survival of 3.5 months and 2.2 months, respectively.38,51 Clinical studies that used erlotinib showed an overall survival of 9 months (range, 3-12 months).40,42,49,50 Finally, Gong et al demonstrated that the use of icotinib in LMD resulted in a median overall survival of 10.1 months.48
DISCUSSION
The best management of LMD from solid tumors has not been established. Current available treatment with immunotherapy and molecular target has increased overall survival, but outcomes remain bleak.
Breast Cancer
The current review demonstrates that intrathecal trastuzumab and bevacizumab are generally well-tolerated treatments that prolong the lives of patients with LMD from breast cancer. Interestingly, our review demonstrated that the use of intrathecal trastuzumab resulted in an overall survival of 10.73 months, a marked improvement when compared to the 5.9 months reported among a group of controls, combinations of surgery, whole-brain radiotherapy, and local or systemic chemotherapy (or a combination of both).53 Although this discrepancy is striking, it is important to note that the 10.73-month result was calculated from case reports and must be interpreted carefully. Analysis of randomized controlled trials would be ideal; however, patients with LMD are frequently excluded from participating in clinical trials because of their poor prognosis.16 Consequently, our review was limited to case reports and one small clinical study.
Nevertheless, this review supports intrathecal trastuzumab as a treatment with a favorable toxicity profile. This finding is in line with other studies that demonstrate that monoclonal antibodies are well tolerated in both humans and animals.20,54,55 Recommended dosing with intrathecal trastuzumab, however, has not been established. Previous studies demonstrate that 20-100 mg weekly or 100-150 mg biweekly is a safe dose to use without any major toxicities.20,55 Results from our review are in line with these findings, as all but one study administered treatment within this range and demonstrated tolerable safety profiles. One study demonstrated that intrathecal trastuzumab at a dose of 150 mg administered at intervals of 3 weeks is an effective and well-tolerated treatment.32
Intrathecal trastuzumab has been used as a monotherapy or in combination with various treatments (methotrexate or thiotepa) with significant improvement in clinical symptoms in 4 of the 5 case reports.15,19,30,31 Of the case reports included in this review, the longest overall survival (>24 months) was achieved when combination therapy consisting of 50 mg of intrathecal trastuzumab and 12 mg of intrathecal thiotepa was administered in repeated 3-week cycles.30 Combining intrathecal monoclonal antibodies (such as trastuzumab and bevacizumab) in conjunction with chemotherapy regimens may prolong survival rates, but research in a clinical trials setting is needed.
The addition of radiotherapy may also play a beneficial role in prolonging overall survival. Previous treatment with radiotherapy has been hypothesized to impair the blood–brain barrier, thus allowing molecules that are normally prevented from accessing the CSF to enter and target malignant cells.56,57 Although other studies have reported that patients treated with radiotherapy prior to intrathecal trastuzumab have a longer overall survival attributable to the mechanism previously discussed, our analysis does not support those findings. Of the 2 patients in case reports who had previous radiotherapy,30,32 one patient (reported by Ferrario et al) had an overall survival greater than the average reported in historic controls.53,58 The clinical study conducted by Wu et al examining the effect of bevacizumab combined with etoposide and cisplatin reported previous radiotherapy use in 50% of subjects without recording individual overall survival rates.33 Consequently, we cannot infer if previous radiotherapy augmented the therapy in question. More data are needed to draw conclusions and make recommendations.
Melanoma
LMD from melanoma metastasis has been notoriously difficult to treat. Evidence supports that host cells (such as macrophages) in the CSF protect the melanoma cells from BRAF inhibitors. Additionally, cells that metastasize to the leptomeninges have been hypothesized to be genetically different from other metastases.59,60 The literature supports this theory because malignant cells in the CSF have higher AKT signaling61,62 and more extensive genomic variations compared to cells in the primary tumor site.63
The discovery in 2002 that approximately half of all melanoma cells held a mutation in the serine/threonine kinase BRAF resulted in the development of BRAF kinase inhibitors such as vemurafenib and dabrafenib.61,62,64 Since that discovery, very few studies examining the effectiveness of BRAF inhibitors on LMD have been generated. In the current review, we found that treatment with dabrafenib and/or vemurafenib led to improvement in clinical symptoms and overall survival.21,36
Multiple studies are currently investigating the effect of immune checkpoint inhibitors, such as anti-CTLA-4, programmed cell death 1 (PD-1), and anti–programmed cell death ligand 1 (PD-L1) antibodies in the setting of brain metastasis.65 The literature on such therapy in LMD is sparse, with only 2 case reports published on the effect of ipilimumab in melanoma-associated LMD.35,37 Our review supports the potential benefit of ipilimumab in treating LMD. Both studies that investigated this therapy showed significant improvement in clinical symptoms. One study reported complete clinical and radiologic response, with the patient living 1.5 years after treatment.35 It is important to note that the patient received whole-brain radiotherapy before initiation of ipilimumab without magnetic resonance imaging between treatment modalities. Therefore, we cannot say that treatment with ipilimumab alone caused this remarkable response; the effect of whole-brain radiotherapy should be considered. Nevertheless, these results are intriguing, and research should be conducted on ipilimumab and whole-brain radiotherapy. As of early 2017, no report has been published on the use of anti-PD-1/PD-L1 antibodies in this circumstance; thus, research in this area is also needed.
Disease progression can occur in patients treated with molecular-targeted therapy. A 2012 study by Simeone et al established progression to leptomeningeal metastasis while the patient was receiving dabrafenib.66 The patient died several days after the discovery of LMD. This outcome lends support to the idea that acquired resistance to BRAF inhibition may occur during treatment. Upregulation of the tyrosine kinase effectors, activation of alternative genes, or transformations in the downstream gene for methyl ethyl ketone (MEK) have been demonstrated to lead to therapeutic resistance.67,68 Whether current therapy should be stopped or new agents added when confirmed LMD occurs in a patient with melanoma is unclear. A study conducted by Kim et al demonstrated that continuing the BRAF inhibitor may be beneficial.36 It has also been proposed that the therapy be continued, with the addition of an MEK inhibitor or anti-CTLA-4 antibody. 36 In light of emerging mechanisms of resistance and the discovery of new therapies, postprogression treatment should also be the focus of future research.
Non–Small Cell Lung Cancer
Our review showed that erlotinib, gefitinib, and icotinib may have CNS activity in LMD. These TKIs target EGFR mutations. This mutation can occur in 7%-76% of patients with NSCLC depending on the geographic region or individual country surveyed.69 Patients with this mutation have been shown to have a better clinical course than those without the EGFR mutation.70
The discovery of EGFR mutations has proven to be a positive advancement in the treatment of LMD secondary to NSCLC because these patients show elevated response rates to TKIs, leading to improved progression-free survival rates.71 Nevertheless, CSF concentrations of EGFR-TKI are <1% of plasma concentrations, thus leading to CNS disease progression in approximately 25%-28% of EGFR-positive patients.72,73 As previously stated, adaptive molecular mutations may contribute to disease progression. Additionally, the pharmacokinetics of TKIs in the CSF may also play a contributing role in LMD.74 This review demonstrates that disease progression while on erlotinib may be treated successfully with pulsatile erlotinib, with reported survival ranging from 2.9 months to continued survival past the study report (>25.4 months).42,47 Additionally, pulsatile dosing of erlotinib at a rate of 1,500 mg once weekly has been shown to reach therapeutic levels within the CSF.47 Early trials showed that pulsatile dosing can result in large enough concentrations of erlotinib in the CSF to potentially avert drug resistance.74 Furthermore, dual targeting of EGFR has shown promising results for patients who develop LMD while on a TKI.44,50 Treatment with afatinib 40 mg and cetuximab 250 mg biweekly resulted in clinical and radiographic improvement.44 Other studies reported the benefit of combined therapy. Erlotinib in combination with pemetrexed cisplatin may be effective in treatment in those who have failed gefitinib,40 and bevacizumab plus erlotinib has been shown to reduce radiographic abnormalities and control symptoms.39 Bevacizumab is a VEGF inhibitor that has been shown to reduce tumor vasculature remodeling and enhance drug delivery in xenografts.39 Although not directly studied in vivo or in LMD, bevacizumab may exert the same effect and be useful in leptomeningeal metastasis.
None of the studies in this review used third-generation TKIs; however, these therapies showed promising results in an abstract presented at the 2016 World Conference on Lung Cancer because of their increased CNS permeability and ability to reach high concentrations in the CSF.75
ALK gene translocation occurs in approximately 4%-5% of NSCLC.25 Targeting this rearrangement offers another molecular target for the treatment of NSCLC. One patient with crizotinib-resistant LMD had clinical and radiographic improvements when treated with ceritinib.45 Alectinib, a second-generation ALK-TKI that is 5 times as potent as crizotinib, was shown to generate a significant clinical and radiographic improvement in 3 of 4 patients who had previously failed crizotinib or ceritinib.46 Future studies may focus on a highly potent ALK/ROS1 inhibitor that has shown substantial regression of ALK-propelled brain metastasis in animal models.76
Our review also demonstrates no significant benefit to whole-brain radiotherapy in respect to overall survival for patients with LMD treated with a TKI. The literature supports this finding; unlike in patients with EGFR-positive brain metastasis from NSCLC, whole-brain radiotherapy shows no significant increase in overall survival in patients with EGFR-positive LMD.2,77
Questions remain as to which population would benefit from high-dose vs pulsatile EGFR treatment. Furthermore, ideal scheduling and dosing of these immunotherapies remain unknown. Extensive research in this area is warranted.
CONCLUSION
This review demonstrates that new treatment approaches are critical to improve morbidity and mortality in LMD resulting from solid tumors. The incidence of LMD is likely to continue to increase in light of improved systemic control of primary solid tumor sites secondary to poor therapeutic penetration of the CNS.
Presently, no definite treatment tactic has been proposed for the treatment of leptomeningeal spread in patients with primary solid tumors. The use of targeted molecular therapy and immunotherapy in LMD may provide a promising treatment avenue. It is important to note that not all patients are eligible for molecular therapy; if the mutation is not present, the therapy is futile. Thus, molecular testing must be performed to understand the patient's therapeutic options.
Our review indicates that intrathecal trastuzumab administration seems to represent a safe and effective option for the treatment of patients with HER2-positive breast cancer. BRAF inhibitors and CTLA-4 targets have shown promising results in leptomeningeal metastasis from melanoma. Last, the use of high-dose TKIs may increase overall survival in EGFR-mutant NSCLC, and patients who progress to LMD on EGFR targets may benefit from targeting malignant cells with pulsatile drug administration or dual therapy.
Questions about optimal therapy, dosing, scheduling, and treatment plan remain unanswered. Patients with LMD are generally excluded from clinical trials because of their poor prognosis. Consequently, most of the information about LMD is from case reports and retrospective clinical studies, and significant conclusions about safety, efficacy, and overall response rates cannot be drawn from the contemporary literature. Ideally, future studies should focus on randomized clinical trials of new therapies for patients with LMD.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. . Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010. November; 136 11: 1729- 1735. 10.1007/s00432-010-0831-x. [DOI] [PubMed] [Google Scholar]
- 2. Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012. June; 76 3: 387- 392. 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 3. Le Rhun E, Taillibert S, Chamberlain MC. . Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013. May; 4 Suppl 4: S265- S288. 10.4103/2152-7806.111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. . Leptomeningeal metastases in the MRI era. Neurology. 2010. May 4; 74 18: 1449- 1454. 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. . Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978. August; 42 2: 660- 668. [DOI] [PubMed] [Google Scholar]
- 6. Rosen ST, Aisner J, Makuch RW, et al. Carcinomatous leptomeningitis in small cell lung cancer: a clinicopathologic review of the National Cancer Institute experience. Medicine (Baltimore). 1982. January; 61 1: 45- 53. [DOI] [PubMed] [Google Scholar]
- 7. Yap HY, Yap BS, Tashima CK, DiStefano A, Blumenschein GR. . Meningeal carcinomatosis in breast cancer. Cancer. 1978. July; 42 1: 283- 286. [DOI] [PubMed] [Google Scholar]
- 8. Taillibert S, Hildebrand J. . Treatment of central nervous system metastases: parenchymal, epidural, and leptomeningeal. Curr Opin Oncol. 2006. November; 18 6: 637- 643. [DOI] [PubMed] [Google Scholar]
- 9. Boyle R, Thomas M, Adams JH. . Diffuse involvement of the leptomeninges by tumour--a clinical and pathological study of 63 cases. Postgrad Med J. 1980. March; 56 653: 149- 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain MC. . Carcinomatous meningitis. Arch Neurol. 1997. January; 54 1: 16- 17. [DOI] [PubMed] [Google Scholar]
- 11. Smalley KS, Fedorenko IV, Kenchappa RS, Sahebjam S, Forsyth PA. . Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy. Int J Cancer. 2016. September 15; 139 6: 1195- 1201. 10.1002/ijc.30147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004. August 30; 223 2: 167- 178. [DOI] [PubMed] [Google Scholar]
- 13. Remon J, Le Rhun E, Besse B. . Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017. February; 53: 128- 137. 10.1016/j.ctrv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14. Dumitrescu C, Lossignol D. . Intrathecal trastuzumab treatment of the neoplastic meningitis due to breast cancer: a case report and review of the literature. Case Rep Oncol Med. 2013; 2013: 154674 10.1155/2013/154674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martens J, Venuturumilli P, Corbets L, Bestul D. . Rapid clinical and radiographic improvement after intrathecal trastuzumab and methotrexate in a patient with HER-2 positive leptomeningeal metastases. Acta Oncol. 2013. January; 52 1: 175- 178. 10.3109/0284186X.2012.689857. [DOI] [PubMed] [Google Scholar]
- 16. Sahebjam S, Forsyth PA, Smalley KS, Tran ND. . Experimental treatments for leptomeningeal metastases from solid malignancies. Cancer Control. 2017. January; 24 1: 42- 46. [DOI] [PubMed] [Google Scholar]
- 17. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003. June 15; 97 12: 2972- 2977. [DOI] [PubMed] [Google Scholar]
- 18. Platini C, Long J, Walter S. . Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006. September; 7 9: 778- 780. [DOI] [PubMed] [Google Scholar]
- 19. Stemmler HJ, Mengele K, Schmitt M, et al. Intrathecal trastuzumab (Herceptin) and methotrexate for meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer: a case report. Anticancer Drugs. 2008. September; 19 8: 832- 836. 10.1097/CAD.0b013e32830b58b0. [DOI] [PubMed] [Google Scholar]
- 20. Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013. May; 139 1: 13- 22. 10.1007/s10549-013-2525-y. [DOI] [PubMed] [Google Scholar]
- 21. Lee JM, Mehta UN, Dsouza LH, Guadagnolo BA, Sanders DL, Kim KB. . Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: a case report. Melanoma Res. 2013. April; 23 2: 175- 178. 10.1097/CMR.0b013e32835e589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaefer N, Rasch K, Moehlenbrunch M, et al. Leptomeningeal melanomatosis: stabilization of disease due to radiation, temozolomide and intrathecal liposomal cytarabine. Acta Oncol. 2011. November; 50 8: 1260- 1262. 10.3109/0284186X.2011.586001. [DOI] [PubMed] [Google Scholar]
- 23. Sequist LV, Bell DW, Lynch TJ, Haber DA. . Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007. February 10; 25 5: 587- 595. [DOI] [PubMed] [Google Scholar]
- 24. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. May 20; 350 21: 2129- 2139. [DOI] [PubMed] [Google Scholar]
- 25. Chia PL, Mitchell P, Dobrovic A, John T.. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014. November 20; 6: 423- 432. 10.2147/CLEP.S69718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan YO, Han S, Lu YS, et al. The prevalence and assessment of ErbB2-positive breast cancer in Asia: a literature survey. Cancer. 2010. December 1; 116 23: 5348- 5357. 10.1002/cncr.25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004. March 26; 3: 6 10.1186/1477-3163-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005. November 17; 353 20: 2135- 2147. [DOI] [PubMed] [Google Scholar]
- 29. Ugurel S, Thirumaran RK, Bloethner S, et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PLoS One. 2007. February 21; 2 2: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrario C, Davidson A, Bouganim N, Aloyz R, Panasci LC. . Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Ann Oncol. 2009. April; 20 4: 792- 795. 10.1093/annonc/mdp019. [DOI] [PubMed] [Google Scholar]
- 31. Mir O, Ropert S, Alexandre J, Lemare F, Goldwasser F. . High-dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2 overexpressing breast cancer. Ann Oncol. 2008. November; 19 11: 1978- 1980. 10.1093/annonc/mdn654. [DOI] [PubMed] [Google Scholar]
- 32. Hofer S, Mengele K, Stemmler HJ, Schmitt M, Pestalozzi B. . Intrathecal trastuzumab: dose matters. Acta Oncol. 2012. September; 51 7: 955- 956. 10.3109/0284186X.2012.673736. [DOI] [PubMed] [Google Scholar]
- 33. Wu PF, Lin CH, Kuo CH, et al. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer. 2015. April 17; 15: 299 10.1186/s12885-015-1290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilgenhof S, Neyns B. . Complete cytologic remission of V600E BRAF-mutant melanoma-associated leptomeningeal carcinomatosis upon treatment with dabrafenib. J Clin Oncol. 2015. October 1; 33 28: e109- e111. 10.1200/JCO.2013.48.7298. [DOI] [PubMed] [Google Scholar]
- 35. Bot I, Blank CU, Brandsma D. . Clinical and radiological response of leptomeningeal melanoma after whole brain radiotherapy and ipilimumab. J Neurol. 2012. September; 259 9: 1976- 1978. 10.1007/s00415-012-6488-4. [DOI] [PubMed] [Google Scholar]
- 36. Kim DW, Barcena E, Mehta UN, et al. Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: a case report. BMC Cancer. 2015. May 13; 15: 400 10.1186/s12885-015-1391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geukes Foppen MH, Brandsma D, Blank CU, van Thienen JV, Haanen JB, Boogerd W. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann Oncol. 2016. June; 27 6: 1138- 1142. 10.1093/annonc/mdw134. [DOI] [PubMed] [Google Scholar]
- 38. Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget. 2015. February 28; 6 6: 4527- 4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ariyasu R, Horiike A, Koyama J, et al. Efficacy of bevacizumab and erlotinib combination for leptomeningeal carcinomatosis after failure of erlotinib. Anticancer Drugs. 2017. June; 28 5: 565- 567. 10.1097/CAD.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 40. Yang H, Yang X, Zhang Y, et al. Erlotinib in combination with pemetrexed/cisplatin for leptomeningeal metastases and cerebrospinal fluid drug concentrations in lung adenocarcinoma patients after gefitinib failure. Target Oncol. 2015. March; 10 1: 135- 140. 10.1007/s11523-014-0326-9. [DOI] [PubMed] [Google Scholar]
- 41. Cessot A, Blanchet B, Goldwasser F. . Erlotinib treatment of meningeal carcinomatosis in lung cancer: more is better. Ann Oncol. 2014. October; 25 10: 2093- 2094. 10.1093/annonc/mdu261. [DOI] [PubMed] [Google Scholar]
- 42. Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011. December; 13 12: 1364- 1369. 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuiper JL, Smit EF. . High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases--one with a remarkable thoracic response as well. Lung Cancer. 2013. April; 80 1: 102- 105. 10.1016/j.lungcan.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 44. Lin CH, Lin MT, Kuo WY, Ho CC. . Afatinib combined with cetuximab for lung adenocarcinoma with leptomeningeal carcinomatosis. Lung Cancer. 2014. September; 85 3: 479- 480. 10.1016/j.lungcan.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 45. Arrondeau J, Ammari S, Besse B, Soria JC. . LDK378 compassionate use for treating carcinomatous meningitis in an ALK translocated non-small-cell lung cancer. J Thorac Oncol. 2014. August; 9 8: e62- e63. 10.1097/JTO.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 46. Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015. February; 10 2: 232- 236. 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. . High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010. September; 99 2: 283- 286. 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gong L, Xiong M, Huang Z, Miao L, Fan Y. . Icotinib might be effective for the treatment of leptomeningeal carcinomatosis in non-small cell lung cancer with sensitive EGFR mutations. Lung Cancer. 2015. September; 89 3: 268- 273. 10.1016/j.lungcan.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 49. Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009. November; 4 11: 1415- 1419. 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- 50. Yi HG, Kim HJ, Kim YJ, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009. July; 65 1: 80- 84. 10.1016/j.lungcan.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 51. Lee Y, Han J, Kim HT, et al. Impact of EGFR tyrosine kinase inhibitors versus chemotherapy on the development of leptomeningeal metastasis in never smokers with advanced adenocarcinoma of the lung. J Neurooncol. 2013. October; 115 1: 95- 101. 10.1007/s11060-013-1199-y. [DOI] [PubMed] [Google Scholar]
- 52. Yuan Y, Tan C, Li M, et al. Activity of pemetrexed and high-dose gefitinib in an EGFR-mutated lung adenocarcinoma with brain and leptomeningeal metastasis after response to gefitinib. World J Surg Oncol. 2012. November 7; 10: 235 10.1186/1477-7819-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee S, Ahn HK, Park YH, et al. Leptomeningeal metastases from breast cancer: intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat. 2011. October; 129 3: 809- 817. 10.1007/s10549-011-1682-0. [DOI] [PubMed] [Google Scholar]
- 54. Brastianos PK, Brastianos HC, Hsu W, et al. The toxicity of intrathecal bevacizumab in a rabbit model of leptomeningeal carcinomatosis. J Neurooncol. 2012. January; 106 1: 81- 88. 10.1007/s11060-011-0655-9. [DOI] [PubMed] [Google Scholar]
- 55. Gabay MP, Thakkar JP, Stachnik JM, Woelich SK, Villano JL. . Intra-CSF administration of chemotherapy medications. Cancer Chemother Pharmacol. 2012. July; 70 1: 1- 15. 10.1007/s00280-012-1893-z. [DOI] [PubMed] [Google Scholar]
- 56. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. . Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007. January; 18 1: 23- 28. [DOI] [PubMed] [Google Scholar]
- 57. Pieńkowski T, Zielinski CC. . Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol. 2010. May; 21 5: 917- 924. 10.1093/annonc/mdp353. [DOI] [PubMed] [Google Scholar]
- 58. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012. February 1; 30 4: 419- 425. 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fedorenko IV, Wargo JA, Flaherty KT, Messina JL, Smalley KS. . BRAF inhibition generates a host-tumor niche that mediates therapeutic escape. J Invest Dermatol. 2015. December; 135 12: 3115- 3124. 10.1038/jid.2015.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirata E, Girotti MR, Viros A, et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell. 2015. April 13; 27 4: 574- 588. 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davies MA, Stemke-Hale K, Lin E, et al. Integrated molecular and clinical analysis of AKT activation in metastatic melanoma. Clin Cancer Res. 2009. December 15; 15 24: 7538- 7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Niessner H, Forschner A, Klumpp B, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013. February; 2 1: 76- 85. 10.1002/cam4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Magbanua MJ, Melisko M, Roy R, et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 2013. December 1; 73 23: 7134- 7143. 10.1158/0008-5472.CAN-13-2051. [DOI] [PubMed] [Google Scholar]
- 64. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002. June 27; 417 6892: 949- 954. [DOI] [PubMed] [Google Scholar]
- 65. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012. May; 13 5: 459- 465. 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 66. Simeone E, De Maio E, Sandomenico F, et al. Neoplastic leptomeningitis presenting in a melanoma patient treated with dabrafenib (a V600EBRAF inhibitor): a case report. J Med Case Rep. 2012. May 17; 6: 131 10.1186/1752-1947-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011. August 1; 29 22: 3085- 3096. 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010. December 16; 468 7326: 973- 977. 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Midha A, Dearden S, McCormack R. . EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015. August 15; 5 9: 2892- 2911. [PMC free article] [PubMed] [Google Scholar]
- 70. Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012. March; 13 3: 239- 246. 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 71. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011. August; 12 8: 735- 742. 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 72. Ma X, Zhu H, Guo H, et al. Risk factors of brain metastasis during the course of EGFR-TKIs therapy for patients with EGFR-mutated advanced lung adenocarcinoma. Oncotarget. 2016. December 6; 7 49: 81906- 81917. 10.18632/oncotarget.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010. December 1; 16 23: 5873- 5882. 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. How J, Mann J, Laczniak AN, Baggstrom MQ. . Pulsatile erlotinib in EGFR-positive non-small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin Lung Cancer. 2017. July; 18 4: 354- 363. 10.1016/j.cllc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 75. Ahn MJ, Kim DW, Kim TM, et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol. 2016; 34 15 suppl: 9003- 9003. [Google Scholar]
- 76. Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015. July 13; 28 1: 70- 81. 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016. November; 11 11: 1962- 1969. 10.1016/j.jtho.2016.06.029. [DOI] [PubMed] [Google Scholar]