Abstract
Background:
The development of immune checkpoint inhibitors has revolutionized the treatment of cancer. Their use in non–small cell lung cancer (NSCLC) remains in its infancy, but rapid progress has been made in treating metastatic NSCLC.
Methods:
This article outlines the role of immune checkpoint inhibitors in the treatment of malignancy and reviews clinical trials of novel immunotherapies in the setting of metastatic NSCLC.
Results:
Traditional chemotherapy with a platinum-based doublet has long been the backbone in the treatment of metastatic NSCLC. While the treatment of NSCLC can be targeted to specific mutations such as epidermal growth factor receptor, these subgroups are rare. The development of immunotherapy has expanded the treatment options for patients who have failed initial chemotherapy. Additionally, new studies have shown positive results for the use of immunotherapy in the first-line setting under certain conditions, allowing pembrolizumab to become the first immunotherapy to be approved in the first-line setting.
Conclusion:
Treatment of NSCLC is constantly changing, and new immune checkpoint inhibitors have shown promising results. Clinical trials are examining their use in the adjuvant setting and in combination with other therapies, and these combination therapies have the potential to show even greater benefits and broader applications than the individual drugs themselves.
Keywords: Atezolizumab, carcinoma–non-small-cell lung, immunotherapy, lung neoplasms, nivolumab, pembrolizumab
INTRODUCTION
Lung cancer is one of the most common malignancies in the United States, with >200,000 patients diagnosed each year.1 Non–small cell lung cancer (NSCLC) is the most common form, comprising approximately 85% of all lung cancers.2 Lung cancer is also the most common cause of cancer-related death in the United States in both men and women.3 Moreover, lung cancer is projected to remain the leading cause of cancer-related death until 2030 with >150,000 expected deaths in the United States annually.1 While survival has modestly improved since 1975 among all patients with lung cancer, the 5-year survival rate remains poor at 19.5%.4 However, 5-year survival varies widely depending on the stage at the time of diagnosis, ranging from 49% for local disease to 2% for distant stage disease.5 Up to 40% of patients with NSCLC initially present with metastatic disease.2
While targeted therapy may be an option for patients with genetic mutations such as epidermal growth factor receptor (EGFR) mutations, these mutations are relatively uncommon.6 The treatment for advanced stage NSCLC has traditionally been centered on platinum-based 2-drug combination chemotherapy in patients with an appropriate performance status.7-9 However, immune checkpoint inhibitors are novel therapies that have had success in the treatment of patients who have failed traditional therapies and are now finding a role in first-line treatment under certain situations.10 These new immunotherapies have quickly become a promising approach in treating several different solid organ and hematologic malignancies.11,12
Neoplastic cells have conventionally been able to escape immunologic destruction through various mechanisms. Tumors change their microenvironment through cytokines, chemokines, and other soluble factors to evade immunologic detection and destruction.12 As a result, the mechanisms by which cancer cells escape the immune system have been an area of great interest and research to discover means by which to modulate the antitumor immune reaction.10,13
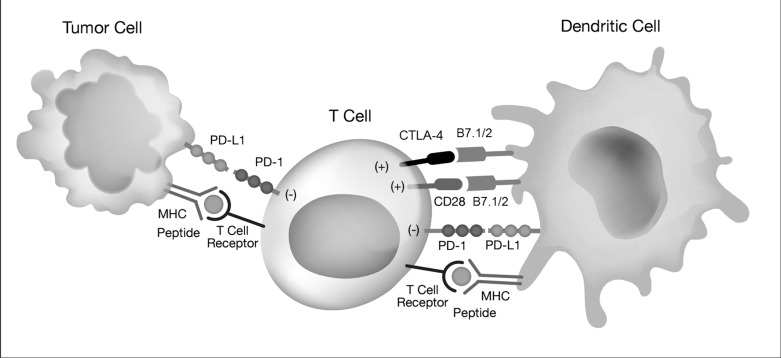
Cytotoxic T lymphocytes (CTLs) play a major role in the immune response to cancer cells through the recognition of tumor-associated antigens and subsequent activation of specific antitumor immune reactions.13 The CTL-associated antigen-4 (CTLA-4) is a molecule expressed by activated T cells that competes with CD28 signaling on the T cells.10 Activation of this pathway leads to decreased T-cell activation and proliferation to prevent autoimmunity and to allow for tolerance to self-antigens.10,12,14 Anti-CTLA-4 antibodies block the CTLA-4 pathway, resulting in prolonged T-cell activation and proliferation, thus leading to the amplification of antitumor immune response.12 Ipilimumab, one such anti-CTLA-4 antibody, was the first drug to show improved overall survival in patients with advanced melanoma.10,15 The success of ipilimumab showed that the blockage of immune checkpoints is a promising target for cancer therapy. The Figure depicts the mechanism of CTLA-4 and programmed cell death-1 (PD-1) receptor interaction.
Figure.
The mechanism of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) interaction allows the adaptive immune system to recognize and target tumor cells through cell-mediated T cell activity. MHC, major histocompatability complex; PD-L1, programmed death-ligand 1.
Another immune checkpoint that has been the focus of significant research is the PD-1 receptor, a T-cell inhibitory receptor similar to CTLA-4.10,12 The PD-1 receptor is found on activated T cells, B cells, and monocytes and has been linked to anergy and tumor immune escape.12 Two ligands exist for PD-1: PD-L1 and PD-L2. PD-L1 is found on T cells, B cells, macrophages, and dendritic cells, whereas PD-L2 is found principally on activated macrophages and dendritic cells.12 The presence of PD-L1 and PD-L2 on antigen-presenting cells has been shown to provoke anergy or apoptosis of T cells, and the expression of PD-L1 on peripheral tissues has been shown to repress self-reactive lymphocytes.12
PD-L1 expression testing is performed through a diagnostic immunohistochemistry assay. Traditionally, PD-L1 expression has required histologic samples, as the specimen needs to be fixed in formalin with a minimum of 100 viable tumor cells.16,17 However, 2016-2017 evidence suggests that cytology samples taken via endobronchial ultrasound with transbronchial needle aspiration should provide adequate material for PD-L1 testing.17-19 Ultimately, the means by which tissue sampling is pursued should be individualized to the patient and clinical scenario. When PD-L1 and PD-L2 are found on tumors, the result is immune suppression involving the inhibition of CD8-positive (CD8+) T cells.12 An inverse association between PD-L1 expression and intraepithelial CD8+ T-cell count has been identified, suggesting that PD-L1 expression on tumor cells leads to the suppression of antitumor CD8+ T cells.12 In addition, the levels of PD-L1 expression in tumors have been demonstrated to correlate with worse clinical outcomes in patients with various types of malignancies.12 As a result, important advances in cancer treatment have been developed focusing on these immune checkpoints and targeting the molecules used by tumors to escape immune surveillance.20 At the time of this article's publication, the Food and Drug Administration (FDA) had approved 3 immune checkpoint antibodies for use as monotherapy in the setting of NSCLC.
NIVOLUMAB
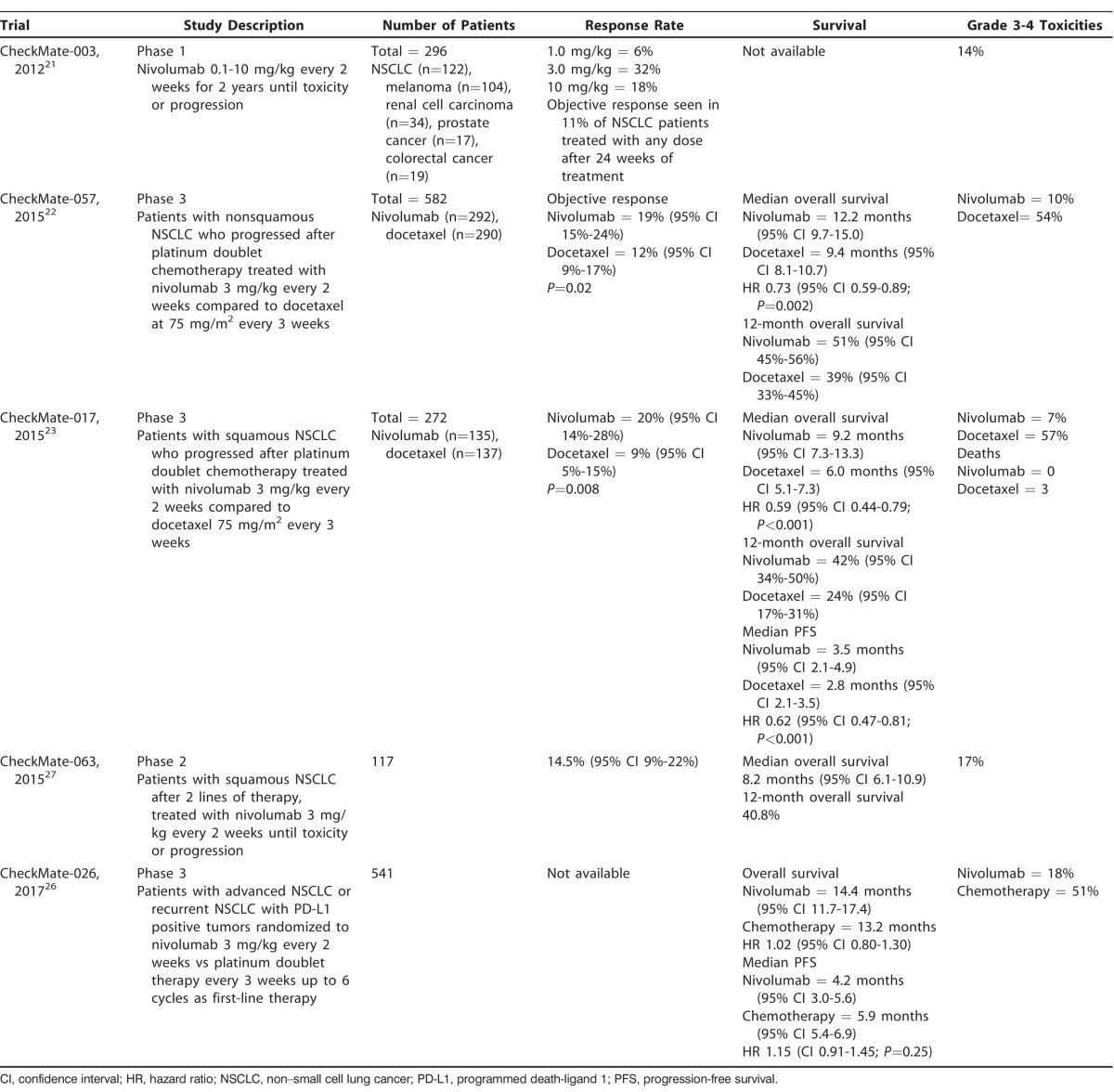
Nivolumab is a human immune checkpoint inhibitor antibody that targets the PD-1 receptor to overcome the immune resistance of cancer cells.21 The initial phase 1 study of nivolumab involved 296 patients with a variety of advanced stage malignancies, including NSCLC, melanoma, prostate cancer, renal cell carcinoma, and colorectal cancer.21 Objective responses (complete or partial responses) were seen in patients with NSCLC, melanoma, and renal cell carcinoma.21 Specifically with regard to NSCLC, the study found an 18% cumulative response rate (all doses) and a large percentage of durable responses lasting >1 year.21 Additionally, nivolumab had relatively manageable adverse events, with grade 3 or 4 toxicities experienced by 14% of patients.21 Subsequent phase 3 randomized trials treated both nonsquamous NSCLC (CheckMate-057)22 and squamous NSCLC (CheckMate-017)23 with promising results. The CheckMate-057 trial was a randomized, open-label, international study that compared nivolumab at 3 mg/kg every 2 weeks to docetaxel at 75 mg/m2 every 3 weeks.22 The primary endpoint of the study was overall survival, which was significantly longer in the nivolumab arm compared to the control arm.22 The median overall survival was 12.2 months in the 292 patients receiving nivolumab compared to 9.4 months in the 290 patients receiving docetaxel with a hazard ratio (HR) of 0.73.22 The overall survival rate was 51% at 1 year for patients receiving nivolumab compared to 39% at 1 year for patients receiving docetaxel.22 In addition, the progression-free survival (PFS) at 1 year was 19% and 8% in the nivolumab and docetaxel arms, respectively.22 Moreover, nivolumab use was associated with a lower grade 3 or 4 treatment-related adverse event rate (10%) compared to docetaxel (54%).22 As a result of these findings, nivolumab received FDA approval for treating NSCLC in the setting of metastatic disease that has progressed on platinum-based chemotherapy.24,25 Table 1 summarizes several other large trials that demonstrated similar results.21-23,26,27 Given the success of nivolumab in the second-line setting, the CheckMate-026 trial evaluated nivolumab in the first-line setting compared to platinum-based combination chemotherapy.26 The study examined 541 patients and showed that nivolumab did not improve PFS, with a median of 4.2 months in the nivolumab group compared to 5.9 months in the platinum-based combination chemotherapy group.26 As a result, nivolumab remains indicated only in the second-line setting after progression on traditional chemotherapy.28
Table 1.
Summary of CheckMate Trials Involving Nivolumab
PEMBROLIZUMAB
Pembrolizumab is a PD-1 receptor inhibitor that is FDA approved for the treatment of NSCLC.29 The phase 1 study of pembrolizumab examined 495 patients with advanced NSCLC.30 Regardless of PD-L1 expression, the overall response rate was 19.4%, and the median duration of response was 12.5 months.30 Furthermore, improved efficacy of pembrolizumab was seen in patients whose tumor cells had PD-L1 expressions ≥50%.30 The randomized, open-label, phase 2/3 clinical trial for pembrolizumab (KEYNOTE-010) enrolled 1,034 patients with previously treated NSCLC with a PD-L1 expression of at least 1% of tumor cells.31 Patients were divided into 3 study arms comprised of treatment with pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks. The primary endpoints for the study were overall survival and PFS.31 The overall survival was significantly longer for patients receiving either dose of pembrolizumab; the median overall survival was 10.4 months for patients receiving pembrolizumab 2 mg/kg, 12.7 months for patients receiving pembrolizumab 10 mg/kg, and 8.5 months for patients receiving docetaxel.31 The HRs for median overall survival were 0.71 when comparing pembrolizumab 2 mg/kg with docetaxel and 0.61 when comparing pembrolizumab 10 mg/kg with docetaxel. Looking specifically at the subgroup of patients with tumor cells that had PD-L1 expression ≥50%, the overall survival was even greater at 14.9 months and 17.3 months for patients receiving pembrolizumab 2 mg/kg and 10 mg/kg, respectively.31 Even though no significant difference was found in median PFS between all patients given pembrolizumab compared to those receiving docetaxel, the subgroup of patients with tumor cells expressing at least 50% PD-L1 had significantly longer PFS with either dose of pembrolizumab compared to docetaxel.31 Furthermore, pembrolizumab was much better tolerated than docetaxel with a grade 3-4 adverse event rate of 13% at a dose of 2 mg/kg and 16% at 10 mg/kg.31 Docetaxel had a much higher adverse event rate at 35%.31
In addition to receiving approval for the second-line setting of advanced NSCLC, pembrolizumab was also the first immune checkpoint inhibitor to be approved by the FDA for use in the first-line setting because of the results of the KEYNOTE-024 randomized phase 3 trial comparing 200-mg pembrolizumab every 3 weeks to platinum-based chemotherapy that was at the discretion of the investigator.32,33 The study included 305 patients with untreated advanced NSCLC who also had tumors with PD-L1 expressions of at least 50% or greater; the primary endpoint was PFS, and the secondary endpoints were overall survival, objective response rate, and safety.32 Median PFS was 10.3 months in the pembrolizumab arm compared to 6.0 months in the chemotherapy arm (HR of 0.50).32 Additionally, 6-month overall survival was 80.2% in the pembrolizumab arm and 72.4% in the chemotherapy group, with (HR of 0.60). The response rate was also higher in the pembrolizumab arm at 44.8% compared to 27.8% in the chemotherapy arm.32 Similar to previous studies, grade 3-5 treatment-related adverse events were much less frequently seen among patients receiving pembrolizumab compared to those receiving chemotherapy, 26.6% vs 53.3%, respectively.32 As a result, the FDA approved pembrolizumab as first-line therapy for patients with advanced NSCLC and with tumor PD-L1 expression levels ≥50%.
In May 2017, the FDA approved pembrolizumab for use in the first-line setting in conjunction with carboplatin and pemetrexed, irrespective of PD-L1 expression.34 The approval was based on KEYNOTE-021, a randomized phase 2 trial that used 4 cycles of 200-mg pembrolizumab in combination with carboplatin dosed at the area under curve 5 mg/mL per min and pemetrexed 500 mg/m2 every 3 weeks followed by pembrolizumab for 24 months and an optional indefinite pemetrexed maintenance compared to 4 cycles of carboplatin and pemetrexed alone followed by an optional indefinite pemetrexed maintenance35 The KEYNOTE-021 investigators randomized 123 patients with untreated advanced NSCLC into the aforementioned 2 groups with 60 and 63 patients, respectively; the primary endpoint was the objective response rate, with secondary endpoints being PFS, duration of response, overall survival, and the correlation between PD-L1 expression and antitumor activity. The study found that the objective response rate with pembrolizumab, carboplatin, and pemetrexed was 55% compared to 29% in the carboplatin and pemetrexed group.35 Additionally, PFS was greater in the cohort receiving pembrolizumab with a median PFS of 13.0 months vs 8.9 months in the cohort treated with carboplatin and pemetrexed alone.35 Moreover, 93% of the patients treated with pembrolizumab plus chemotherapy experienced treatment-related adverse events compared to 90% of the patients treated with chemotherapy only. Although a high number of adverse events were reported, only 39% and 26% of patients in the above groups, respectively, had events of grade 3 or worse severity.35 The landmark KEYNOTE trials for pembrolizumab are summarized in Table 2.
Table 2.
Summary of KEYNOTE Trials Involving Pembrolizumab
ATEZOLIZUMAB
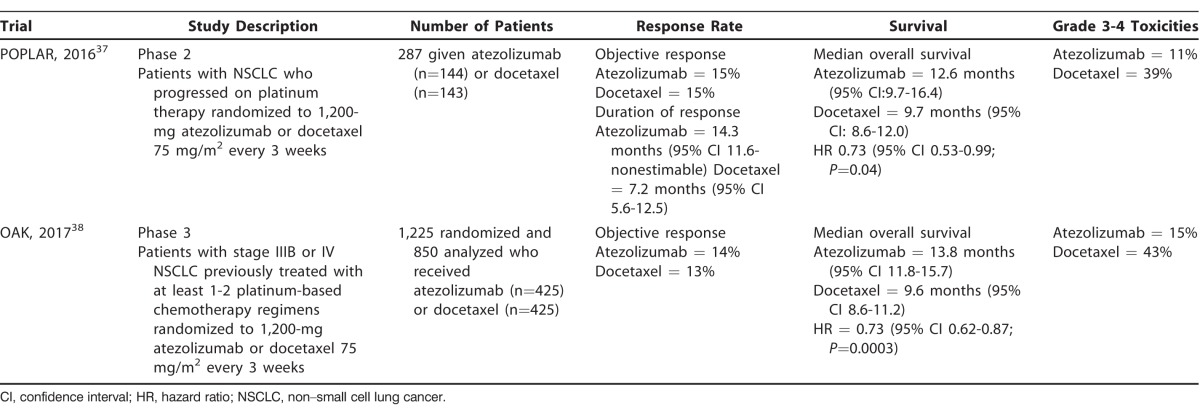
Atezolizumab, a PD-L1 inhibitor, is the third FDA-approved immune checkpoint inhibitor for use in patients with NSCLC.36 The early phase 2 multicenter, randomized controlled POPLAR trial divided 287 previously treated patients with advanced NSCLC into 2 treatment arms: 1,200-mg atezolizumab or docetaxel 75 mg/m2 every 3 weeks.37 The primary endpoint was overall survival, and patients who received atezolizumab had a higher median overall survival of 12.6 months compared to 9.7 months in the docetaxel group (HR 0.73).37 Increasing PD-L1 expression in tumor cells correlated with increasing improvement in overall survival.37 With regard to treatment-related adverse events, 11% of patients in the atezolizumab group experienced grade 3-4 adverse events compared to 39% of patients in the docetaxel group.37 A subsequent randomized phase 3 trial (OAK) looked at 1,225 previously treated patients with advanced NSCLC who were randomized to receive either atezolizumab 1,200 mg or docetaxel 75 mg/m2 every 3 weeks.38 The OAK trial identified a significantly longer overall survival among patients treated with atezolizumab, with a median overall survival of 13.8 months compared to 9.6 months for those treated with docetaxel (HR of 0.73).38 Similar to other studies investigating immune checkpoint inhibitors, the rate of treatment-related grade 3-4 adverse events was lower in the atezolizumab group compared to the docetaxel group, 15% vs 43%, respectively.38 The OAK and the POPLAR trials are summarized in Table 3.
Table 3.
Summary of Clinical Trials Involving Atezolizumab
FUTURE DIRECTIONS
At the time of this article's publication, several clinical trials investigating combination immune checkpoint inhibitors are in progress, exploiting both CTLA-4 as well as PD-1/PD-L1 in the setting of NSCLC because of the promising results seen in the treatment of other malignancies such as melanomas.39 Nivolumab combined with ipilimumab is also being evaluated in patients with recurrent NSCLC under a phase 1/2 trial (CheckMate-032) with some preliminary promising results.40-42 Similarly, a phase 1 study looking at combination pembrolizumab with ipilimumab is in progress with some promising results.41 In addition to the combination of various immune checkpoint inhibitors, various studies are evaluating the role of immune checkpoint inhibitors given alongside other targeted therapies such as EGFR inhibitors in EGFR-positive patients.40,41 Moreover, 2 upcoming studies combine these novel immune checkpoint inhibitors with traditional chemotherapy or radiation therapy, both with preliminary results suggestive of benefit.40,41
CONCLUSION
Immunotherapy offers a novel approach to the treatment of advanced NSCLC in the absence of targetable genetic mutations. As time passes, the indications for these novel therapies continue to expand, including more patients and changing the course of disease. Ultimately, the management of NSCLC has undergone revolutionary changes, and as a result, further advances in treatment are anticipated.
ACKNOWLEDGMENTS
Robert A. Ramirez, DO, is a consultant for Ipsen Biopharmaceuticals, Inc., and Biotheranostics, as well as a speaker for Merck & Co. Inc., Genentech, Astra Zeneca, and Ipsen Biopharmaceuticals, Inc. Jonathan Lu, MBBS, has no conflicts of interest to disclose.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, Systems-Based Practice, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. . Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014. June 1; 74 11: 2913- 2921. 10.1158/0008-5472.CAN-14-0155. Erratum in: Cancer Res . 2014 Jul 15;74(14):4006. [DOI] [PubMed] [Google Scholar]
- 2. Du L, Herbst RS, Morgensztern D. . Immunotherapy in lung cancer. Hematol Oncol Clin North Am. 2017. February; 31 1: 131- 141. 10.1016/j.hoc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 3. Torre LA, Siegel RL, Jemal A. . Lung cancer statistics. Adv Exp Med Biol. 2016; 893: 1- 19. 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 4. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; Accessed August 30, 2017. https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- 5. Alberg AJ, Ford JG, Samet JM;. American College of Chest Physicians. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. September; 132 3 Suppl: 29S- 55S. [DOI] [PubMed] [Google Scholar]
- 6. El-Telbany A, Ma PC. . Cancer genes in lung cancer: racial disparities: are there any? Genes Cancer. 2012. July; 3 7-8: 467- 480. 10.1177/1947601912465177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azzoli CG, Baker S Jr, Temin S, et al. American Society of Clinical Oncology. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009. December 20; 27 36: 6251- 6266. 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Souquet PJ, Chauvin F, Boissel JP, et al. Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet. 1993. July 3; 342 8862: 19- 21. [DOI] [PubMed] [Google Scholar]
- 9. Pignon JP, Tribodet H, Scagliotti GV, et al. LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008. July 20; 26 21: 3552- 3559. 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 10. Hiniker SM, Maecker HT, Knox SJ. . Predictors of clinical response to immunotherapy with or without radiotherapy. J Radiat Oncol. 2015; 4: 339- 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo L, Zhang H, Chen B. . Nivolumab as programmed death-1 (PD-1) inhibitor for targeted immunotherapy in tumor. J Cancer. 2017. February 10; 8 3: 410- 416. 10.7150/jca.17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. . Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012. Sep-Oct; 62 5: 309- 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leite KR, Reis ST, Junior. JP, et al. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol. 2015. October 15; 10: 189 10.1186/s13000-015-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engelhardt JJ, Sullivan TJ, Allison JP. . CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006. July 15; 177 2: 1052- 1061. [DOI] [PubMed] [Google Scholar]
- 15. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. August 19; 363 8: 711- 723. 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grigg C, Rizvi NA. . PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016. August 16; 4: 48 10.1186/s40425-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skov BG, Skov T. . Paired comparison of PD-L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol. 2017. August; 25 7: 453- 459. 10.1097/PAI.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 18. Stoy S, Rosen L, Murgu S. . The use of endobronchial ultrasound-guided transbronchial needle aspiration cytology specimens for programmed death ligand 1 immunohistochemistry testing in non-small cell lung cancer. J Bronchology Interv Pulmonol. 2017. July; 24 3: 181- 183. 10.1097/LBR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 19. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016. July; 24 6: 392- 397. 10.1097/PAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teixidó C, González-Cao M, Karachaliou N, Rosell R. . Predictive factors for immunotherapy in melanoma. Ann Transl Med. 2015. September; 3 15: 208 10.3978/j.issn.2305-5839.2015.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012. June 28; 366 26: 2443- 2454. 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015. October 22; 373 17: 1627- 1639. 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmer J, Reckamp KL, Baas P, et al. N Engl J Med. 2015. July 9; 373 2: 123- 135. 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016. May; 21 5: 634- 642. 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. FDA Expands Approved Use of Opdivo in Advanced Lung Cancer. US Food and Drug Administration; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm466413.htm. Accessed August 30, 2017. [Google Scholar]
- 26. Carbone DP, Reck M, Paz-Ares L, et al. CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017. June 22; 376 25: 2415- 2426. 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015. March; 16 3: 257- 265. 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Non-Small Cell Lung Cancer (Version 5.2017). National Comprehensive Cancer Network; https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed August 30, 2017. [Google Scholar]
- 29. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. . FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016. May; 21 5: 643- 650. 10.1634/theoncologist.2015-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garon EB, Rizvi NA, Hui R, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015. May 21; 372 21: 2018- 2028. 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 31. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016. April 9; 387 10027: 1540- 1550. 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 32. Reck M, Rodríguez-Abreu D, Robinson AG, et al. KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016. November 10; 375 19: 1823- 1833. [DOI] [PubMed] [Google Scholar]
- 33. Pembrolizumab (Keytruda) Checkpoint Inhibitor. US Food and Drug Administration; https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm526430.htm. Accessed August 30, 2017. [Google Scholar]
- 34. Pembrolizumab (Keytruda) 5-10-2017. US Food and Drug Administration; https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm558048.htm. Accessed August 30, 2017. [Google Scholar]
- 35. Langer CJ, Gadgeel SM, Borghaei H, et al. KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016. November; 17 11: 1497- 1508. 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atezolizumab (TECENTRIQ). US Food and Drug Administration; https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm525780.htm. Accessed August 30, 2017. [Google Scholar]
- 37. Fehrenbacher L, Spira A, Ballinger M, et al. POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016. April 30; 387 10030: 1837- 1846. 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 38. Rittmeyer A, Barlesi F, Waterkamp D, et al. OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017. January 21; 389 10066: 255- 265. 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013. July 11; 369 2: 122- 133. 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carrizosa DR, Gold KA. . New strategies in immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. 2015. October; 4 5: 553- 559. 10.3978/j.issn.2218-6751.2015.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bustamante Alvarez JG, González-Cao M, Karachaliou N, et al. Advances in immunotherapy for treatment of lung cancer. Cancer Biol Med. 2015. September; 12 3: 209- 222. 10.7497/j.issn.2095-3941.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016. July; 17 7: 883- 895. 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]