Abstract
Background:
Even though acute myeloid leukemia (AML) occurs most commonly in adults ≥60 years, the treatment of AML in older patients remains a significant challenge.
Methods:
We reviewed the current literature regarding patient assessment tools, treatment options, and current therapies in clinical trial for patients with AML who are ≥60 years.
Results:
Our approach to the older patient with AML is evolving with better understanding of the unique disease epidemiology in this population and the development of tools to assess individual patient functional status, including grading systems for comorbidities, geriatric assessment tools, and measurements of frailty. Almost all older patients will benefit from therapy, whether intensive curative therapy, such as allogeneic stem cell transplant that should be considered whenever possible, or low-intensity therapy that should be offered with concurrent palliative care at diagnosis to improve patient survival and quality of life. To achieve the improved survival demonstrated in younger adults, older patients should also be considered for clinical trial enrollment as more studies are being designed to specifically target this unique patient population.
Conclusion:
Older patients with AML are candidates for and benefit from the entire spectrum of AML therapy, including intense chemotherapy, allogeneic stem cell transplant, and clinical trial participation after thorough patient assessment. Older patients with AML would benefit from increased clinical trial enrollment and early inclusion of palliative medicine.
Keywords: Aged, chemotherapy, leukemia–myeloid–acute, palliative medicine, transplantation–homologous
INTRODUCTION
Acute myeloid leukemia (AML) is a cancer predominately of the elderly with a median age of diagnosis at 68 years.1 AML in older adults (≥60 years for the purpose of general discussion) presents a variety of patient- and disease-related challenges compared to younger populations.2 Survival after diagnosis has an inverse relationship with age, and standard therapy remains undetermined in older adults.2 All leukemias as a group are the eighth most common cancers in women and ninth most common cancers in men, with 20,000 diagnoses yearly, and are the fifth most common cause of cancer-related death, with 10,000 deaths in the United States each year.3 Unlike with younger adults, we have seen little progress in improving overall survival (OS) in older adults that remains approximately (median) 4 months without AML-directed therapy and 7-12 months for intermediate- or high-risk disease treated with therapy that depends on patient- and disease-related factors.4 With the average life expectancy in industrialized society now approaching 80 years, the need to understand how to approach older patients with AML is growing.5 In this article, we discuss cytogenetic risk and treatment selection, selecting patients for treatment, and clinical trials for older patients with AML.
CYTOGENETIC RISK AND TREATMENT SELECTION
The epidemiology of AML is different in older adults compared to younger populations. We see an increased incidence of secondary AML after myelodysplastic syndrome (MDS) or other hematologic malignancy, an increased incidence of treatment-related AML after prior chemotherapy or radiation therapy, and an increased risk of MDS and AML after treatment with azathioprine for autoimmune diseases.6,7 A 2015 study from the Mayo Clinic evaluated epidemiologic exposures associated with AML development and demonstrated that specific disease risk factors may be associated with specific leukemia cytogenetic risk profiles and outcomes after therapy.8 Exposures such as obesity were associated with intermediate abnormal cytogenetics, and history of statin therapy was associated with increasing complete remission rates after induction chemotherapy.8 The Mayo Clinic also demonstrated significant differences in AML risk factors in patients ≥70 years, highlighting epidemiologic differences within the population.9 Older adults typically have an adverse cytogenetic risk profile, with poor-risk cytogenetics. Older patients with intermediate-risk cytogenetics also have poor outcomes.10-12 AML cytogenetic and molecular risk categories are listed in the Table.13
Table.
Acute Myeloid Leukemia Cytogenetic and Molecular Risk Categories
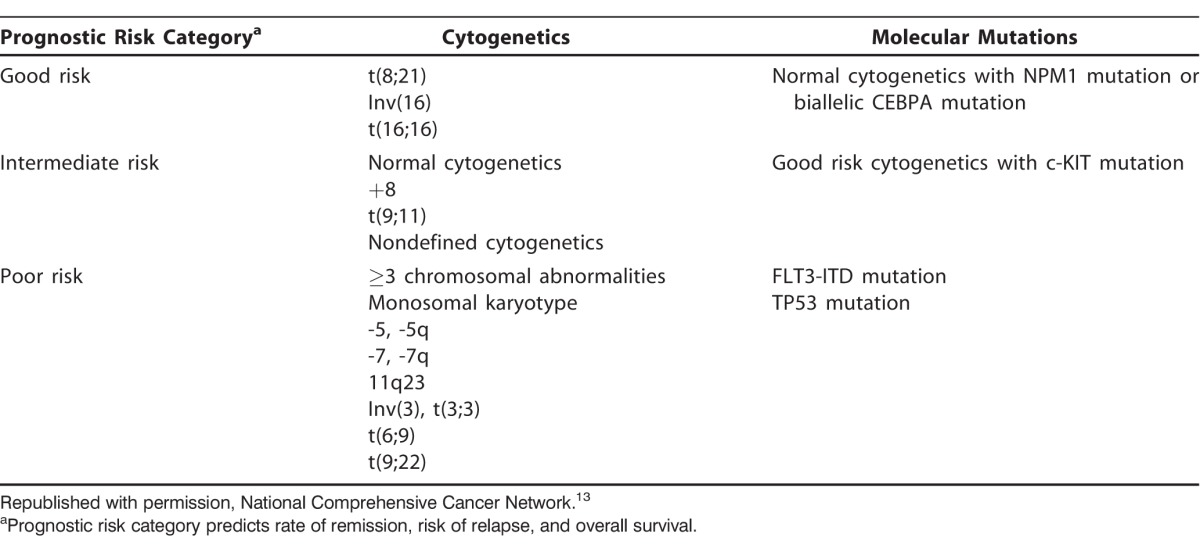
National registry data from Sweden inform us that all older AML patients should be considered for chemotherapy, and their outcomes are better with intensive chemotherapy rather than palliative or supportive care alone.14 Therapy for AML is considered intensive by intent to induce first complete remission (CR1).8 Intensive induction and consolidation chemotherapy may be considered curative for patients with good-risk cytogenetics, including core binding factor AML (t[8;21], inv[16], t[16;16]) without c-KIT mutations; however, core binding factor AML represents only 7%-12% of all patients with AML who are ≥60 years.10,15 Treatment of good-risk AML compares to the more common need of allogeneic stem cell transplant (ASCT) to potentially achieve a cure in patients with intermediate- or poor-risk cytogenetics and/or adverse molecular mutations (FLT3-ITD, c-KIT) or secondary AML.16 Traditional intensive chemotherapy for AML remains the 7+3 regimen with either daunorubicin or idarubicin anthracycline agent combined with cytarabine infusion. Induction chemotherapy can achieve a CR1 in 40%-60% of older patients with AML.14,17-19 Achieving a stringent CR1 without evidence of minimal residual disease (MRD) is an important prognostic factor in patient survival because MRD often indicates the presence of refractory disease or a high risk of relapse and may also impact outcomes after ASCT.20-22
Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) indicate that the use of ASCT in older patients with AML has increased during the past 15 years (2000-2015).23 With the development of reduced-intensity conditioning (RIC) regimens that rely on graft vs leukemia effects to eradicate leukemic cells (rather than the myeloablative antileukemic effect of conditioning) and the use of alternative donors (haploidentical and cord blood) when a matched donor is not available, the number of allogeneic transplants in older patients is increasing but still represents a minority of the actual transplant population.24-26 Patient age alone up to 75 years does not impact survival after RIC ASCT according to the Acute Leukemia Working Committee for the CIBMTR that reported a 2-year posttransplant OS of 35%.27 This analysis found no significant impact of age on nonrelapse mortality, disease-free survival, or OS, indicating that all older patients with AML should be referred to a bone marrow transplant center early in their diagnosis for appropriate patient assessment.27 Patients who receive ASCT as indicated for their disease appear to have improved OS compared to patients who receive chemotherapy alone. A Japanese registry study of adults aged 50-70 years in CR1 who received ASCT with a variety of conditioning regimens and alternative donor types showed improved 3-year OS with transplant compared to chemotherapy alone (62% vs 51%, respectively; P=0.12), demonstrating no disadvantage and in some high-risk patient populations a clear improvement in survival.28 Additional studies have demonstrated improved leukemia-free survival after ASCT compared to chemotherapy alone.29,30 The function of RIC ASCT in older patients with advanced disease or beyond CR1 is uncertain, as response to treatment and OS after ASCT decrease with refractory disease. However, outcomes in refractory or relapsed AML are still better after ASCT (dependent on disease risk categories and patient performance status), highlighting the need to carefully select patients for intense chemotherapy and ASCT.16,31,32
SELECTING PATIENTS FOR TREATMENT
A patient's numeric age does not accurately predict benefit from therapy and only relates to years of life, whereas physiologic age appears to influence the older patient's ability to tolerate traditional leukemia-directed treatments. Selecting patients for curative therapy is a multifactorial decision process in which both patient- and disease-related factors must be taken into consideration. These considerations include patient frailty and comorbidities, caregiver availability and social support dynamics, and the increased prevalence of high-risk disease features such as adverse cytogenetics and secondary AML. Patients must be able to tolerate the selected therapy, and treatment should not be futile in settings of highest risk or resistant AML.11,12,14
When considering intensive chemotherapy, the patient characteristics that must be considered include functional status, caregiver support, psychosocial issues, and the patient's own goals of care. The Hematopoietic Cell Transplantation-Specific Comorbidity Index score (HCT-CI) predicts nonrelapse mortality and OS after ASCT.33 The HCT-CI builds upon the Charlson Comorbidity Index but redistributes weights of comorbidities for the AML and MDS patient populations, including mental health, infection, and obesity, and has been validated prospectively.34 The HCT-CI also predicts early death and OS in patients ≥60 years who are receiving induction 7+3 chemotherapy with idarubicin and cytarabine for AML. Median OS for a score of 0 is 45 weeks, a score of 1-2 equates to a median of 31 weeks, and the median OS for a score of 3 is 19 weeks, demonstrating the importance of careful evaluation and attention to patients' active and past medical history prior to making treatment decisions.35 Nevertheless, retrospective data suggest a potential advantage for intensive therapy in older adults for all but those with the highest HCT-CI scores.36,37
In addition to patient comorbidities, patient frailty may be used to define potential tolerance to intensive therapy. Patient frailty is defined as a syndrome of unintentional weight loss, exhaustion, weakness, decreased walking speed, and decreased physical activity.38 At least 15% of patients ≥65 years are considered frail.39 Further expanding on measurements of frailty are comprehensive geriatric assessment tools to predict patient tolerance to chemotherapy and survival after ASCT. The University of Chicago prospectively incorporated a geriatric assessment prior to transplant and found that inclusion of the HCT-CI, frailty measurements, markers of inflammation, and mental health assessments predicted OS after ASCT.40 Wedding et al reported that performance of daily activities is the most important prognostic factor of survival in older adults.41 The success of intensive therapy and ASCT is largely determined by patient functional status.
Patients who are not candidates for intensive treatments may still be considered for nonintensive therapy that has a lower incidence of achieving CR1 but has a role in decreasing disease progression and improving patient quality of life. Nonintensive therapy includes the hypomethylating agents azacitidine and decitabine.42-44 Hypomethylating agents improve OS compared to conventional care regimens such as low-dose cytarabine and/or supportive care alone, although they have not shown an OS benefit in randomized phase 3 clinical trials.42,44,45
Referral to palliative medicine and supportive care services should be considered at the time of AML diagnosis for all patients, as palliative medicine services can be offered and administered at the same time as any treatment for AML, including curative induction chemotherapy and ASCT.46 Palliative care and hospice are both underutilized, even in patients with poor prognosis attributable to high-risk disease, comorbidities, and poor performance status, despite data showing that nearly 85% of older patients with AML are hospitalized within the month before their death.47 Decreased palliative medicine utilization may be partially related to limitations in blood product transfusion support, a common supportive care need of patients with AML that is not currently covered and provided in hospice treatment and reimbursement models. Nevertheless, early referral to palliative medicine improves both patient and caregiver quality of life and decreases healthcare utilization, including hospital readmissions.48-52
CLINICAL TRIALS FOR OLDER PATIENTS WITH ACUTE MYELOID LEUKEMIA
Just as palliative care is currently underutilized in older patients with AML, so were agents in clinical trial until recently. Few clinical trial agents were evaluated in the older patient population because of eligibility criteria or the lack of trials designed for this age group.53 Since 2014, more clinical trials have been designed exclusively for patients ≥60 years.
Challenging the long-time standard induction chemotherapy regimen of 7+3 is CPX-351, a liposomal formulation of cytarabine and daunorubicin.54 A randomized phase 2 study that enrolled patients ≥60 years with untreated AML demonstrated greater treatment response rates (66.7% vs 51.2%, respectively; P=0.07) and increased survival after ASCT in patients receiving CPX-351 compared to those treated with 7+3 chemotherapy.55,56 Older patients with high-risk disease and secondary AML were then enrolled in a phase 3 study of CPX-351 vs traditional 7+3 cytarabine/daunorubicin, and the patients treated with CPX-351 achieved the primary endpoint of improved OS (9.56 vs 5.95 months, respectively; P=0.005).57 With the expectation for Food and Drug Administration (FDA) approval, CPX-351 may become the new standard of care for induction therapy in older patients with AML.57
Older patients with AML may also be candidates for targeted therapy for high-risk molecular mutations. Midostaurin is an oral agent targeting the FLT3 mutation that the FDA approved in April 2017 for use during induction and consolidation chemotherapy in newly diagnosed adult patients with AML who harbor any FLT3 mutation.58 Patients achieving remission may also receive 12 months of single-agent midostaurin maintenance treatment.58 Of note, the phase 3 RATIFY trial that led to midostaurin approval did not enroll older patients with AML but limited enrollment to patients aged 18-59 years.59 The full prescribing instructions for midostaurin advise caution in patients ≥65 years, limiting use to patients who are also candidates for induction chemotherapy.58
An example of targeted therapy that is in active clinical trial is the isocitrate dehydrogenase enzyme (IDH1 and IDH2) inhibitors. Currently recruiting patients ≥60 years is a phase 3 study of the IDH2 inhibitor AG-221 compared to investigator choice of a conventional care regimen of best supportive care, azacitidine, low-dose cytarabine, or intermediate-dose cytarabine.60 Many additional novel agents are in clinical trial development, including the CD33 antibody conjugate actinium 225 lintuzumab, an alpha particle radiation conjugate in early-phase clinical trial enrolling patients ≥60 years.61-63
Studies from 2015-2016 suggest ongoing improvement in median survival with intensive therapy in older adults through enhanced patient selection and supportive care. An example is median survival of almost 14 months with standard (7+3 daunorubicin) therapy in the Eastern Cooperative Oncology Group (ECOG) E2906 trial, supporting the use of standard therapy whenever possible.64 However, low-intensity therapies may achieve similar results in select patients. For example, a 10-day decitabine regimen achieved complete responses in patients with adverse mutation profiles (TP53 mutation) in whom intensive therapy is less effective.65 Decitabine is being compared directly against intensive therapy in the ongoing European Organization for Research and Treatment of Cancer (EORTC) AML-21 trial. Decitabine is also being studied with azacitidine and, in some cases, low-dose cytarabine as potential backbone regimens to add to novel leukemia-targeted agents to improve remission rates and survival. Other agents in development for older patients with untreated AML include the hypomethylating agent guadecitabine (that is resistant to cytidine deaminase degradation), tosedostat (an aminopeptidase inhibitor), and volasertib (a polo-like kinase inhibitor) that all demonstrated complete remission rates of 30%-40% in early-phase clinical trials.66-68 Although volasertib has not demonstrated clear-cut improvement in survival in randomized trials, alternate treatment schedules are being considered. Venetoclax, a BCL-2 inhibitor, has shown response rates of 30%-40% in older patients with relapsed or refractory disease and very high response rates in combination with azacitidine in studies conducted at MD Anderson Cancer Center.69
Novel approaches to treating AML include research in cellular therapy, including vaccine trials enrolling patients up to 77 years, and novel trial designs such as the Leukemia & Lymphoma Society Beat AML Master Trial and the Southwest Oncology Group (SWOG) S1612 that are being developed to enroll newly diagnosed patients ≥60 years with the aim of matching patients with active therapy in an individualized and efficient manner.70,71
Beat AML is an innovative clinical trial that could potentially change the paradigm in older adults with AML by incorporating upfront molecular profiling at the time of diagnosis and assigning patients to novel low-intensity or high-intensity therapies based upon dominant leukemia-associated mutations at diagnosis. This important study will allow more rapid assessment of novel or targeted treatments in defined patient populations and has the potential to significantly advance the evaluation of new therapies in older adults.71 SWOG is developing a national intergroup rolling phase 2/3 randomized study (S1612) that will test concurrent and consecutive lower intensity novel therapies against standard regimen azacitidine in an expedited fashion, converting any early signal of improved survival rapidly into randomized phase 3 trials. S1612 is expected to open late 2017 and will allow efficient and systematic study of multiple regimens, significantly reducing the time it will take to study the impact of new treatments in older adults.
CONCLUSION
Age alone should not exclude older patients with AML from consideration for intensive chemotherapy, ASCT, or clinical trial participation, and, indeed, we must strongly support clinical trials whenever possible to improve outcomes in this difficult disease. As tools improve to assess patient frailty and functional status, the clinical ability to better select patients for varying degrees of treatment will also improve, enhancing patient outcomes. Early referral to palliative medicine and use of this subspecialty as a supportive care service can improve patient and caregiver quality of life.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. SEER Cancer Statistics Review 1975-2014. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/csr/1975_2014/. Updated June 28, 2017. Accessed July 18, 2017.
- 2. Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012. June; 18 6: 861- 873. 10.1016/j.bbmt.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. . Cancer statistics, 2016. CA Cancer J Clin. 2016. Jan-Feb; 66 1: 7- 30. 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4. Fröhling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006. November 15; 108 10: 3280- 3288. [DOI] [PubMed] [Google Scholar]
- 5. Central Intelligence Agency. The World Fact Book. https://www.cia.gov/library/publications/resources/the-world-factbook/index.html. Accessed July 18, 2017.
- 6. Ertz-Archambault N, Kosiorek H, Taylor GE, et al. Association of therapy for autoimmune disease with myelodysplastic syndromes and acute myeloid leukemia. JAMA Oncol. 2017. July 1; 3 7: 936- 943. 10.1001/jamaoncol.2016.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kayser S, Döhner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011. February 17; 117 7: 2137- 2145. 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 8. Finn L, Sproat L, Heckman MG, et al. Epidemiology of adult acute myeloid leukemia: impact of exposures on clinical phenotypes and outcomes after therapy. Cancer Epidemiol. 2015. December; 39 6: 1084- 1092. 10.1016/j.canep.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 9. Foran J, Heckman M, Sproat L, . et al. Distinct epidemiologic exposures and prognostic factors for survival in older adults age >70 years receiving low intensity therapy for acute myeloid leukemia (AML). European Hematology Association Abstract Book. 2015: 99803 https://learningcenter.ehaweb.org/eha/2015/20th/99803. Accessed September 14, 2017.
- 10. Mosna F, Papayannidis C, Martinelli G, et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am J Hematol. 2015. June; 90 6: 515- 523. 10.1002/ajh.24000. [DOI] [PubMed] [Google Scholar]
- 11. Juliusson G, Lazarevic V, Hörstedt AS, Hagberg O, Höglund M;. Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012. April 26; 119 17: 3890- 3899. 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006. May 1; 107 9: 3481- 3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017. July; 15 7: 926- 957. 10.6004/jnccn.2017.0116. [DOI] [PubMed] [Google Scholar]
- 14. Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009. April 30; 113 18: 4179- 4187. 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 15. Brunner AM, Blonquist TM, Sadrzadeh H, et al. Population-based disparities in survival among patients with core-binding factor acute myeloid leukemia: a SEER database analysis. Leuk Res. 2014. July; 38 7: 773- 780. 10.1016/j.leukres.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michelis FV, Gupta V, Zhang MJ, et al. Acute Leukemia Working Committee of the Center for International Blood and Marrow Transplant Research, a research collaboration between the National Marrow Donor Program/Be the Match Registry and the Medical College of Wisconsin. Cytogenetic risk determines outcomes after allogeneic transplantation in older patients with acute myeloid leukemia in their second complete remission: a Center for International Blood and Marrow Transplant Research cohort analysis. Cancer. 2017. June 1; 123 11: 2035- 2042. 10.1002/cncr.30567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reiffers J, Huguet F, Stoppa AM, et al. A prospective randomized trial of idarubicin vs daunorubicin in combination chemotherapy for acute myelogenous leukemia of the age group 55 to 75. Leukemia. 1996. March; 10 3: 389- 395. [PubMed] [Google Scholar]
- 18. Mandelli F, Petti MC, Ardia A, et al. A randomised clinical trial comparing idarubicin and cytarabine to daunorubicin and cytarabine in the treatment of acute non-lymphoid leukaemia. A multicentric study from the Italian Co-operative Group GIMEMA. Eur J Cancer. 1991; 27 6: 750- 755. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Yang YG, Zhou M, et al. Meta-analysis of randomised clinical trials comparing edarubicin + cytarabine with daunorubicin + cytarabine as the induction chemotherapy in patients with newly diagnosed acute myeloid leukaemia. PLoS One. 2013. April 5; 8 4: e60699 10.1371/journal.pone.0060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foran JM, Sun Z, Claxton DF, et al. Importance of achieving complete remission (CR) after intensive therapy for acute myeloid leukemia (AML) in older adults age ≥60 years: analysis of risk factors for early mortality and re-induction, and impact of quality of response on overall survival (OS) in the ECOG-ACRIN E2906 randomized trial. Blood. 2016. December 2; 128 22: 339- 339. [Google Scholar]
- 21. Shook D, Coustan-Smith E, Ribeiro RC, Rubnitz JE, Campana D. . Mininal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma. 2009; 9 Suppl 3: S281- S285. 10.3816/CLM.2009.s.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buccisano F, Maurillo L, Gattei V, et al. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia. 2006. October; 20 10: 1783- 1789. [DOI] [PubMed] [Google Scholar]
- 23. Center for International Blood and Marrow Transplant Research. Study Slides – HCT Trends and Survival Data. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Pages/index.aspx. Accessed July 18, 2017.
- 24. Hahn T, McCarthy PL Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013. July 1; 31 19: 2437- 2449. 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mawad R, Gooley TA, Sandhu V, et al. Frequency of allogeneic hematopoietic cell transplantation among patients with high- or intermediate-risk acute myeloid leukemia in first complete remission. J Clin Oncol. 2013. November 1; 31 31: 3883- 3888. 10.1200/JCO.2013.50.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. 2014. June; 20 6: 816- 822. 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010. April 10; 28 11: 1878- 1887. 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurosawa S, Yamaguchi T, Uchida N, et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. Biol Blood Marrow Transplant. 2011. March; 17 3: 401- 411. 10.1016/j.bbmt.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 29. Mohty M, de Lavallade H, Ladaique P, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005. June; 19 6: 916- 920. [DOI] [PubMed] [Google Scholar]
- 30. Farag SS, Maharry K, Zhang MJ, et al. Acute Leukemia Committee of the Center for International Blood and Marrow Transplant Research and Cancer and Leukemia Group B. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011. December; 17 12: 1796- 1803. 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weisdorf DJ, Millard HR, Horowitz MM, et al. Allogeneic transplantation for advanced acute myeloid leukemia: the value of complete remission. Cancer. 2017. June 1; 123 11: 2025- 2034. 10.1002/cncr.30536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gyurkocza B, Lazarus HM, Giralt S. . Allogeneic hematopoietic cell transplantation in patients with AML not achieving remission: potentially curative therapy. Bone Marrow Transplant. 2017. February 27 10.1038/bmt.2017.8. [Epub ahead of print]. [DOI] [PubMed]
- 33. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005. October 15; 106 8: 2912- 2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2015. August; 21 8: 1479- 1487. 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007. February; 136 4: 624- 627. [DOI] [PubMed] [Google Scholar]
- 36. Michelis FV, Messner HA, Alam N, et al. Analysis of impact of comorbidities constituting the HCT-CI score on the outcome of patients undergoing allogeneic hematopoietic cell transplant for acute myeloid leukemia. Blood. 2015; 126 23: 3201. [Google Scholar]
- 37. Sorror ML, Storer BE, Elsawy M, et al. Impact of comorbidities at diagnosis of acute myeloid leukemia on one-year mortality. Blood. 2015; 126 23: 532. [Google Scholar]
- 38. Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001. March; 56 3: M146- M156. [DOI] [PubMed] [Google Scholar]
- 39. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015. November; 70 11: 1427- 1434. 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014. August; 99 8: 1373- 1379. 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wedding U, Röhrig B, Klippstein A, Fricke HJ, Sayer HG, Höffken K. . Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006. October; 132 10: 665- 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. . Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010. February 1; 28 4: 556- 561. 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 43. Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010. February 1; 28 4: 562- 569. 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 44. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012. July 20; 30 21: 2670- 2677. 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015. July 16; 126 3: 291- 299. 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. LeBlanc TW, El-Jawahri A. . When and why should patients with hematologic malignancies see a palliative care specialist? Hematology Am Soc Hematol Educ Program. 2015; 2015: 471- 478. 10.1182/asheducation-2015.1.471. [DOI] [PubMed] [Google Scholar]
- 47. El-Jawahri AR, Abel GA, Steensma DP, et al. Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer. 2015. August 15; 121 16: 2840- 2848. 10.1002/cncr.29430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loggers ET, LeBlanc TW, El-Jawahri A, et al. Pretransplantation supportive and palliative care consultation for high-risk hematopoietic cell transplantation patients. Biol Blood Marrow Transplant. 2016. July; 22 7: 1299- 1305. 10.1016/j.bbmt.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 49. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014. May 17; 383 9930: 1721- 1730. 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 50. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010. August 19; 363 8: 733- 742. 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 51. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009. August 19; 302 7: 741- 749. 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finn L, Roche-Green A, Shannon R, et al. Early and systematic involvement of palliative medicine team during bone marrow transplant improved patient ccare, QOL, and patient and caregiver satisfaction. Biol Blood Marrow Transplant. 2016; 22 3: S289. [Google Scholar]
- 53. Dinmohamed AG, Visser O, van Norden Y, et al. Treatment, trial participation and survival in adult acute myeloid leukemia: a population-based study in the Netherlands, 1989-2012. Leukemia. 2016. January; 30 1: 24- 31. 10.1038/leu.2015.188. [DOI] [PubMed] [Google Scholar]
- 54. Feldman EJ, Lancet JE, Kolitz JE, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011. March 10; 29 8: 979- 985. 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014. May 22; 123 21: 3239- 3246. 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lancet JE, Hoering A, Uy GL, et al. Survival following allogeneic hematopoietic cell transplantation in older high-risk acute myeloid leukemia patients initially treated with CPX-351 liposome injection versus standard cytarabine and daunorubicin: subgroup analysis of a large phase III trial. Blood. 2016; 128 22: 906. [Google Scholar]
- 57. Sallman DA, Lancet JE. . What are the most promising new agents in acute myeloid leukemia? Curr Opin Hematol. 2017. March; 24 2: 99- 107. 10.1097/MOH.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 58. US Food and Drug Administration. Midostaurin. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555756.htm. Updated April 28, 2017. Accessed July 18, 2017.
- 59. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017. June 23 10.1056/NEJMoa1614359. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 60. An efficacy and safety study of AG-221 (CC-90007) versus conventional care regimens in older subjects with late stage acute myeloid leukemia harboring an isocitrate dehydrogenase 2 mutation (IDHENTIFY). https://clinicaltrials.gov/ct2/show/NCT02577406. Updated May 31, 2017. Accessed July 18, 2017.
- 61. Bixby DL, Stein AS, Fathi AT, et al. Vadastuximab talirine monotherapy in older patients with treatment naive CD33-positive acute myeloid leukemia (AML). Blood. 2016; 128 22: 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fathi AT, Erba HP, Lancet JE, et al. Vadastuximab talirine plus hypomethylating agents: a well-tolerated regimen with high remission rate in frontline older patients with acute myeloid leukemia (AML). Blood. 2016; 128 22: 591. [Google Scholar]
- 63. Jurcic JG, Levy MY, Park JH, et al. Phase I trial of targeted alpha-particle therapy with actinium-225 (225Ac)-lintuzumab and low-dose cytarabine (LDAC) in patients age 60 or older with untreated acute myeloid leukemia (AML). Blood. 2016; 128 22: 4050. [Google Scholar]
- 64. Foran JM, Sun Z, Claxton DF, et al. North American leukemia, intergroup phase III randomized trial of single agent clofarabine as induction and post-remission therapy, and decitabine as maintenance therapy in newly-diagnosed acute myeloid leukemia in older adults (age ≥60 years): a trial of the ECOG-ACRIN cancer research group (E2906). Blood. 2015; 126 23: 217. [Google Scholar]
- 65. Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016. November 24; 375 21: 2023- 2036. 10.1056/NEJMoa1605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kropf EJ P YK, O'Connell C, et al. Late responses and overall survival (OS) from long term follow up of a randomized phase 2 study of SGI-110 (guadecitabine) 5-day regimen in elderly AML who are not eligible for intensive chemotherapy. Haematologica. 2015; 100 S1: 218(P571). [Google Scholar]
- 67. Döhner H, Lübbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014. August 28; 124 9: 1426- 1433. 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Visani G, Loscocco F, Fuligni F, et al. Tosedostat plus low dose cytarabine induces a high rate of responses that can be predicted by genetic profiling in elderly AML. Blood. 2015; 126 23: 329. [Google Scholar]
- 69. Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016. October; 6 10: 1106- 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosenblatt J, Stone RM, Uhl L, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci Transl Med. 2016. December 7; 8 368: 368ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. The Beat AML Master Trial. Leukemia & Lymphoma Society. www.lls.org/beat-aml. Accessed April 14, 2017.


