Abstract
Background:
Although breast cancer is still the second most common cause of cancer-related deaths, breast cancer mortality has been declining because of advances in the use of adjuvant therapies.
Methods:
We summarize clinical trials involving endocrine therapies used to treat early breast cancer and discuss their inception and recent advances.
Results:
Endocrine therapies such as tamoxifen have revolutionized the treatment of breast cancer, resulting in significant decreases in cancer-related mortality. Aromatase inhibitors such as anastrozole and letrozole have further improved breast cancer survival.
Conclusion:
With the implementation of such therapies resulting in decreased mortality, patients with breast cancer are living longer than ever before. The focus of research is now directed toward the length of treatment and prediction models for recurrence.
Keywords: Aromatase inhibitors, breast cancer, estrogen receptor antagonists, tamoxifen
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer worldwide, with an incidence of 246,660 cases in 2016 in the United States alone, and is the second most common cause of cancer-related deaths among females, with 40,450 deaths from breast cancer in 2016 in the United States.1 Despite these numbers, breast cancer mortality rates have steadily declined since the 1970s. This decline is thought to be partly the result of advances in adjuvant therapy.2 In this review, we focus on the inception of and recent advances in endocrine therapies.
Estrogen and progesterone are the primary regulators of breast tissue growth and differentiation. Both steroid hormones are primarily produced in the ovaries. They exert their cellular effects through binding to and activating specific nuclear receptors, the estrogen receptors (ERs) and progesterone receptors (PRs). Once activated, the receptors exhibit transcriptional and membrane localized signaling activities. ERα and ERβ are the 2 major ERs. The majority of breast cancers express ERα (70%), while ERβ is less well characterized.3
The potential role of estrogen in breast tissue was first noted by George T. Beatson who noted that oophorectomy in rabbits resulted in a loss of lactation.4 Based on this result, Beatson performed an oophorectomy on June 15, 1895, on a premenopausal patient with unresectable breast cancer. She had complete remission and survived another 4 years.4 Beatson's early work provided the foundations on which hormonal therapy was built. Stanley N. Boyd confirmed the utility of this therapy in a series of 46 cases of unresectable breast cancer in premenopausal females treated with oophorectomy.5 In 1923, Edgar Allen and Edward Doisy discovered an ovarian hormone, estrogen, that regulates mammary tissue.6 During the next several decades, a full range of ablative hormonal therapies was researched and developed, leading to the discovery of tamoxifen in 1967 by Harper and Walpole.7
TAMOXIFEN TRIALS
Tamoxifen has been extensively researched to treat early breast cancer and has strong evidence supporting its use as an adjuvant endocrine therapy.8 Tamoxifen is a nonsteroidal antiestrogen that the US Food and Drug Administration (FDA) approved in the 1970s for the treatment of metastatic breast cancer in postmenopausal females.9 The role of tamoxifen expanded to the adjuvant setting with the treatment of postmenopausal females with positive nodes and ER-positive tumors under recommendations from the 1985 National Institutes of Health Consensus Conference on Breast Cancer Chemotherapy.10 Subsequently, the Nolvadex Adjuvant Trial Organization studies analyzed 1,285 patients who underwent total mastectomy with axillary node clearance or sampling followed by randomization to tamoxifen therapy for 2 years or no further treatment.11 The study demonstrated that 2 years of adjuvant tamoxifen was associated with a reduction in recurrence and death. Additionally, the improvement in overall survival was independent of menopausal, ER, or nodal status. Since 1958, the National Surgical Adjuvant Breast and Bowel Project (NSABP) has conducted numerous randomized controlled trials evaluating different aspects of adjuvant and surgical therapies.12 The NSABP B-14 trial evaluated 2,644 patients with receptor-positive, node-negative disease who were randomized to 5 years of tamoxifen or 5 years of placebo after surgery.13,14 The study found a significant benefit in disease-free survival (DFS) among females treated with tamoxifen compared to placebo.14 Extended follow-up of these patients after 10 years showed continued DFS benefit in the tamoxifen group compared to placebo, 69% vs 57%, respectively, as well as a significant survival advantage: 80% in the tamoxifen group vs 76% in the placebo group.15 Furthermore, the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) reviewed 194 randomized controlled trials and found that 5 years of adjuvant tamoxifen in patients with ER-positive breast cancer resulted in a reduction in the breast cancer mortality rate by 31% and was more effective than 1 or 2 years of tamoxifen therapy.16 In a follow-up metaanalysis, the EBCTCG noted that 5 years of adjuvant tamoxifen in patients with ER-positive breast cancer significantly reduced disease recurrence throughout the first 10 years and reduced breast cancer mortality by approximately one-third throughout the first 15 years.17 Because of the multitude of positive studies, treatment with tamoxifen for 5 years has been the backbone of adjuvant hormonal therapy, especially for premenopausal patients with breast cancer.8 However, duration of therapy has been debated extensively, specifically treatment extended beyond 5 years (we address extended therapy below).
AROMATASE INHIBITORS
In postmenopausal females, estrogen is no longer produced by ovarian tissue and is predominantly synthesized from nonglandular sources via the aromatase enzyme. Aromatase can be found in a number of tissues including subcutaneous fat, liver, and muscle; the enzyme has also been isolated in breast cancer cells.3 Because of the prior success with estrogen inhibition, inhibition of aromatase has been extensively explored as a treatment modality for breast cancer (Table 1).
Table 1.
Aromatase Inhibitor Trials
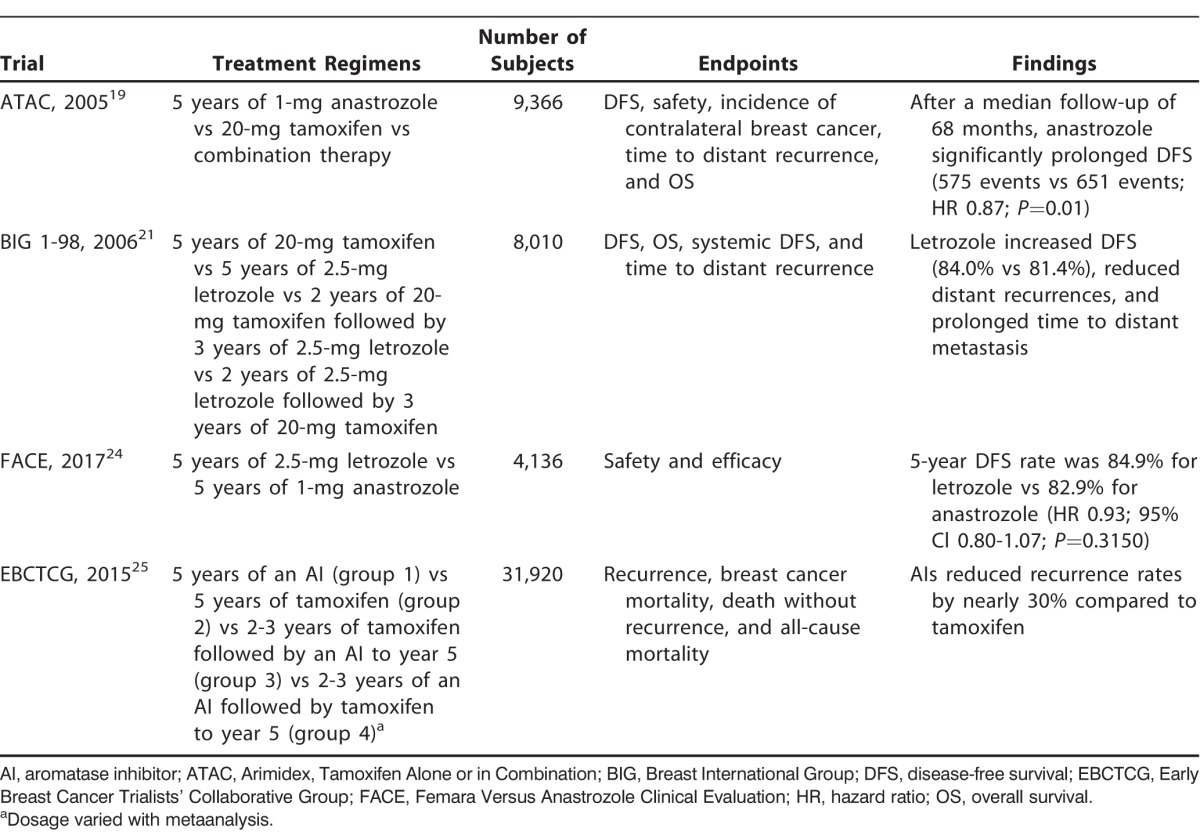
The first 2 generations of aromatase inhibitors (AIs) were effective in treating breast cancer but caused significant side effects because they inhibited other steroid hormones such as cortisol and aldosterone. Third-generation AIs have increased specificity for aromatase and are either categorized as steroidal (type I) or nonsteroidal (type II). Steroidal inhibitors lead to irreversible inhibition of enzymatic activity, while nonsteroidal inhibitors are reversible competitive inhibitors. Third-generation AIs were initially studied in patients with metastatic breast cancer. In 1998, Buzdar et al noted a statistically and clinically significant response with the use of anastrozole, a type II AI, instead of the standard treatment at the time, megestrol acetate, for postmenopausal females with advanced breast carcinoma that progressed on tamoxifen.18 Subsequent studies, discussed below, demonstrated the superiority of AIs over tamoxifen in postmenopausal females with advanced disease.
When the effectiveness of AI therapy was identified in metastatic breast cancer, the focus shifted to its use in the adjuvant setting. Many patients being treated with tamoxifen experienced recurrence because of drug resistance or developed side effects such as endometrial cancer and venous thromboembolic disease. The Arimidex, Tamoxifen Alone or in Combination (ATAC) trial compared anastrozole with tamoxifen for 5 years in 9,366 postmenopausal females with localized breast cancer.19 After a median follow-up of 68 months, anastrozole significantly prolonged DFS (575 vs 651 events; hazard ratio [HR] 0.87; 95% confidence interval [CI] 0.78-0.97; P=0.01), time to recurrence (402 vs 498 days; HR 0.79; 95% CI 0.70-0.90; P=0.0005), overall benefit in time to distant recurrences (324 vs 375 days; HR 0.86; 95% CI 0.78-0.97; P=0.04), and rate of contralateral breast cancer development (35 vs 59 cases; 42% relative risk [RR] reduction; P=0.01). The investigators reported fewer side effects and discontinuations of anastrozole. However, they reported significantly more fractures at 120 months of median follow-up with anastrozole compared to tamoxifen, (451 vs 351 events; odds ratio [OR] 1.33; 95% CI 1.15-1.55; P<0.0001). At the completion of treatment, the incidence of fractures was similar in the anastrozole and tamoxifen groups (66 vs 78 events; OR 0.84; 95% CI 0.60-1.19; P=0.3). The risk of venous thromboembolic events, endometrial cancer, and hot flashes was lower in patients treated with anastrozole.20
Letrozole, another type II AI, was compared to tamoxifen in a head-to-head trial in the Breast International Group (BIG) 1-98 Collaborative Group study. The phase 3, double-blind trial randomized 8,010 patients to tamoxifen for 5 years, letrozole for 5 years, tamoxifen for 2 years followed by letrozole, or letrozole for 2 years followed by tamoxifen.21 After a median follow-up of 25.8 months, the initial analysis compared the 2 groups assigned to receive letrozole initially with the 2 groups assigned to receive tamoxifen initially. The letrozole group had an improved 5-year survival rate compared to the tamoxifen group (84.0% and 81.4%, respectively). The study also found that thromboembolism, endometrial cancer, and vaginal bleeding were more common in the tamoxifen group. BIG 1-98 also found significantly increased rates of fractures with letrozole compared to tamoxifen (5.7% vs 4%; P<0.001).21 A companion study to the MA.17 trial evaluated the effect of letrozole on bone mineral density (BMD) prospectively and monitored BMD over time. At 24 months, patients taking letrozole had significant decreases in their BMD in the total hip and lumbar spine compared to patients receiving placebo.22
Based on these studies, along with the 51-month follow-up in the BIG 1-98 trial,23 the FDA approved anastrozole and letrozole for initial adjuvant therapy of hormone-sensitive early-stage breast cancer. The recent randomized phase 3 trial, Femara Versus Anastrozole Clinical Evaluation (FACE), compared the efficacy and safety of letrozole with anastrozole.24 The FACE trial evaluated 4,136 postmenopausal females who had hormone receptor–positive and node-positive breast cancer and found that letrozole was not significantly superior with regard to safety or efficacy compared to anastrozole, despite prior pharmacodynamic studies that demonstrated more effective estradiol suppression by letrozole.24
In addition, the EBCTCG performed a metaanalysis of the randomized trials for AIs vs tamoxifen in early breast cancer.25 They divided 31,920 postmenopausal females with ER-positive early breast cancer into multiple therapy subgroups and compared the groups. The subgroups were 5 years of an AI (group 1), 5 years of tamoxifen (group 2), 2-3 years of tamoxifen followed by an AI to complete 5 years (group 3), and 2-3 years of an AI followed by tamoxifen (group 4).25 The comparison of 5 years of an AI and the switching strategy of 2-3 years of tamoxifen followed by an AI to year 5 showed a recurrence reduction during the first year among the AI group, but that benefit was lost when both groups were taking an AI. When comparing the data from groups 1 and 2, groups 1 and 3, and groups 2 and 3, a greater recurrence reduction was noted in patients taking an AI during any point in the trial, even with varied treatment regimens. Overall, AIs reduced recurrence rates by nearly 30% compared to tamoxifen.
SWITCH TRIALS
AIs have also been studied as sequential therapy after 2-3 years of tamoxifen. The Austrian Breast and Colorectal Cancer Study Group (ABCSG) trial 8 conducted a combined analysis with the Arimidex-Nolvadex 95 (ARNO 95) trial in which they evaluated the efficacy of switching to anastrozole for 3 years after completing 2 years of adjuvant tamoxifen therapy.26 The researchers identified 3,224 postmenopausal hormone receptor–positive females who had received 2 years of tamoxifen and randomized them to receive 1 mg of anastrozole, 20 mg of tamoxifen, or 30 mg of tamoxifen. After a median follow-up of 28 months, a 40% decrease in risk for an event was noted with anastrozole (67 events with anastrozole vs 110 with tamoxifen; HR 0.60; 95% CI 0.44-0.81; P=0.0009).26 The Intergroup Exemestane Study (IES) evaluated exemestane, a type I AI, for sequential therapy after tamoxifen in the adjuvant setting.27 The investigators examined 4,742 postmenopausal females with ER-positive or ER-unknown breast cancer who had no evidence of disease after 2-3 years of adjuvant tamoxifen and who were randomized to either switching to exemestane or continuing tamoxifen therapy for a total of 5 years. After a median follow-up of 30.6 months, they found a 32% risk reduction, corresponding to an absolute benefit in terms of DFS of 4.7% at 3 years after randomization, in the exemestane group.27 These switch trials showed evidence that the sequential use of AIs and tamoxifen provided additional benefits. The correct sequence and duration of treatment need further clarification.
EXTENDED THERAPY TRIALS
As data from 5-year trials began to emerge, interest on extending the duration of adjuvant therapy and late recurrence increased (Table 2). The NSABP explored continuing tamoxifen therapy beyond 5 years. They evaluated the outcomes of patients in the B-14 trial through 10 years and the effects of 5 more years of tamoxifen vs placebo.15 A significant advantage was noted in DFS (69% vs 57%; P=0.001; RR 0.66; 95% CI 0.58-0.71), with 10 years of follow-up between the placebo and tamoxifen groups. However, no additional advantage was seen between the 5-year and 10-year groups of tamoxifen therapy (94% vs 96% 4-year survival; P=0.08).15 The Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial compared 12,894 females with early breast cancer who had completed 5 years of treatment and randomly assigned them to continue tamoxifen for 10 years or to stop at 5 years.28 The investigators knew 5 years of tamoxifen were more effective than 1-2 years, and they wanted to explore whether 10 years of treatment would have a greater effect. Greater protective effects against ER-positive breast cancer were noted with 10 years of tamoxifen, resulting in reductions in recurrence and mortality.28 These results were mirrored in the Adjuvant Tamoxifen–To Offer More? (aTTom) trial that compared 6,953 females who were randomized to stop adjuvant tamoxifen at 5 years or continue to year 10.29 This trial confirmed that the continuation of tamoxifen to year 10 resulted in reductions in recurrence and breast cancer deaths.29
Table 2.
Extended Trials
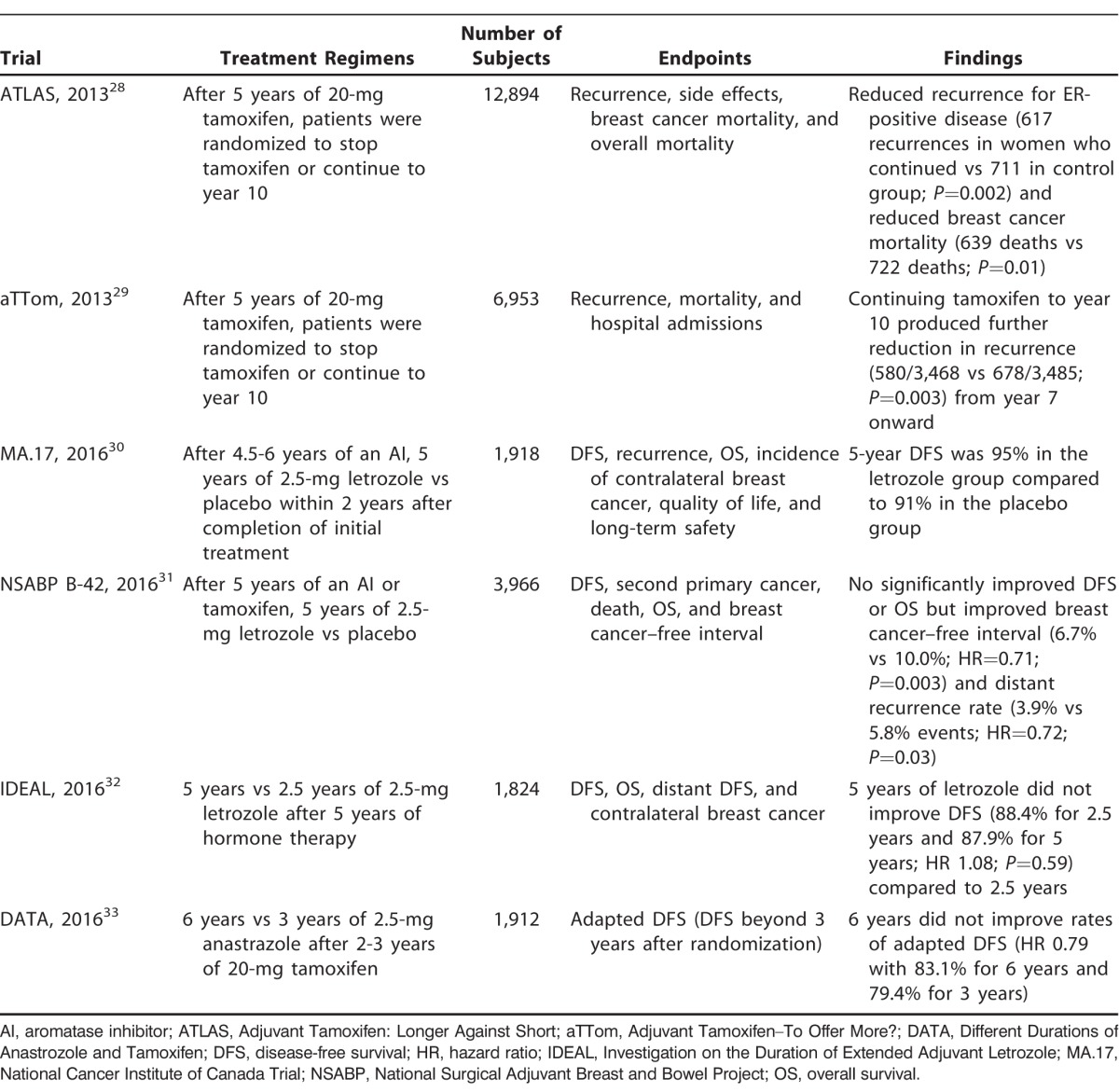
Extending adjuvant therapy in postmenopausal women with early ER-positive breast cancer using AIs was also examined in a hope to further reduce late recurrences. The MA.17 extended adjuvant phase 3, randomized, double-blind, placebo-controlled trial examined the effects of treatment with an AI for 10 years.30 The investigators randomized postmenopausal women with primary breast cancer who had already received 4.5-6 years of adjuvant therapy with an AI to either receive placebo or letrozole daily for another 5 years. They found that the 5-year DFS was 95% in the extended letrozole group compared to 91% in the placebo group (HR 0.66; P=0.01). Ultimately, the trial showed benefit in the prevention of disease recurrence that was independent of nodal status. However, 5-year overall survival was not higher in the extended letrozole group (93% vs 94%; HR 0.97; P=0.83).30
The NSABP B-42 trial evaluated the benefit of an additional 5 years of hormonal therapy with letrozole after an initial 5 years of an AI or tamoxifen.31 The study's endpoint was DFS with median follow-up from randomization at 6.9 years. Results showed a nonsignificant 15% reduction in DFS at approximately 7 years in the extended letrozole group. However, the study found improvement in the 7-year cumulative incidence of breast cancer–free intervals (6.7% vs 10.0% events; HR 0.71; P=0.003) and distant recurrence (3.9% vs 5.8% events; HR 0.72; P=0.03) in the extended letrozole group compared to placebo.31
The Investigation on the Duration of Extended Adjuvant Letrozole (IDEAL) trial from the Netherlands randomized patients to 2.5 or 5 years of letrozole after 5 years of hormone therapy.32 DFS was 88.4% with 2.5 years and 87.9% with 5 years. However, approximately 74% of patients completed 2.5 years of letrozole, and only 57% of patients completed 5 years of letrozole. Overall survival was 93% in both groups.32 The Different Durations of Anastrozole and Tamoxifen (DATA) trial from the Netherlands studied 3 vs 6 years of anastrozole after 2-3 years of tamoxifen in 1,912 patients.33 The trial was designed to detect an increase in adapted DFS (ADFS) after 6 years vs only 3 years, but instead the HR was 0.79 (P=0.07). ADFS is DFS after randomization (in this case 3 years). The 5-year ADFS was 83.1% in the 6-year group and 79.4% in the 3-year group. These findings did not support the use of extended adjuvant AI but suggested a benefit for node-negative disease and ER-positive/PR-positive disease with a 5-year HR for ADFS events of 1.01 (95% CI 0.62-1.63; P=0.9817) and 0.68 (95% CI 0.51-0.90; P=0.0072), respectively.33
THE BREAST CANCER INDEX
The Breast Cancer Index (BCI) is a computer algorithm that uses a gene expression assay–based signature to predict the early (defined as 0-5 years) and late (defined as >5 years) risk of distant recurrence in patients.34 The algorithm was developed using a combination of 2 independent gene expression profiles, the HOXB13/IL17BR (H:I) and the molecular grade index (MGI).35 While the MGI was a better prognostic predictor from 0-5 years and H:I was a better prognostic predictor beyond 5 years, the BCI was designed to be a continuous risk index.34 The BCI model was created using a cohort of patients from the Stockholm study, a randomized trial with 2,738 patients with early-stage breast cancer that compared 2 or 5 years of adjuvant tamoxifen to no endocrine therapy.36 Zhang et al provided validation for the BCI using tumors from 600 early-stage patients with ER-positive, node-negative breast cancer from the Stockholm study along with an additional 358 patients from a multiinstitutional cohort comprising ER-positive, node-negative, tamoxifen-treated patients from 2 other medical centers.34 They found the BCI to be a significant prognosticator of both early and late distant recurrence.34 The BCI has been further validated and compared to other prognostication tools. Sgroi et al compared the BCI to the Oncotype DX (Genomic Health, Inc.), a 21-gene recurrence score, and to IHC4, an immunohistochemical prognostic model, with regard to prognostic capabilities.37 The study assessed tumors from 665 ER-positive, node-negative breast cancer patients who were a part of the translational substudy of the ATAC trials (TransATAC) and tested the ability of the prognostic tools to predict risk of recurrence.37 Of the prognostic tools evaluated, only the BCI was able to significantly predict the risk of both early and late distant recurrence.37 The BCI has been adapted to assess patients with node-positive disease.38 The expansion of the BCI to node-positive patients was programmed based on 219 ER-positive tumor samples from patients with 1-3 positive nodes from the TransATAC trials.38 This BCI model was validated by Zhang et al who looked at 402 ER-positive, node-positive patients; the BCI detected a significant number of node-positive patients considered to be low risk for distant recurrence after chemotherapy and 5 years of endocrine therapy.39 The BCI is thus able to provide prognostic data on whether a patient will have a high or low likelihood of benefiting from extended endocrine therapy.34,37,40 As a result, patients and clinicians are able to use the BCI tool to help make challenging treatment decisions, leading to less anxiety and decisional conflict.41
CONCLUSION
The management of hormone receptor–positive breast cancer has changed significantly since the 1970s. After the adoption of adjuvant endocrine therapy with tamoxifen and AIs, investigators have attempted to determine the optimal duration of adjuvant therapy. Current efforts are focusing on how best to identify patients at risk for late recurrence and how best to manage them. As technology continues to advance and become readily available, treatment plans can become personalized to the individual patient and the particular characteristics of the tumor. The incorporation of these tools may improve patient outcomes.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, Systems-Based Practice, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. . Cancer statistics, 2016. CA Cancer J Clin. 2016. Jan-Feb; 66 1: 7- 30. 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2. Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015. March 30; 107(6: djv048 10.1093/jnci/djv048 . Erratum in: J Natl Cancer Inst. 2015. May; 107 5 Erratum in: J Natl Cancer Inst. 2015 Jul;107(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bland KI, Copeland EM. . The Breast E-Book: Comprehensive Management of Benign and Malignant Diseases. Elsevier; 2009. [Google Scholar]
- 4. Beatson GT. . On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896; 148 3803: 162- 165. [PMC free article] [PubMed] [Google Scholar]
- 5. Boyd S. . On oöphorectomy in the treatment of cancer. Br Med J. 1897. October 2; 2 1918: 890- 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen E, Doisy EA. . Landmark article Sept 8, 1923: an ovarian hormone: preliminary report on its localization, extraction and partial purification, and action in test animals. JAMA. 1983. November 18; 250 19: 2681- 2683. [DOI] [PubMed] [Google Scholar]
- 7. Harper MJ, Walpole AL. . A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967. February; 13 1: 101- 119. [DOI] [PubMed] [Google Scholar]
- 8. Jankowitz RC, Davidson NE. . Adjuvant endocrine therapy for breast cancer: how long is long enough? Oncology (Williston Park). 2013. December; 27 12: 1210- 1216, 1224. [PubMed] [Google Scholar]
- 9. Robert NJ. . Clinical efficacy of tamoxifen. Oncology (Williston Park). 1997. February; 11 2 Suppl 1: 15- 20. [PubMed] [Google Scholar]
- 10. Consensus conference. Adjuvant chemotherapy for breast cancer. JAMA. 1985. December 27; 254 24: 3461- 3463. [PubMed] [Google Scholar]
- 11. ‘Nolvadex' Adjuvant Trial Organisation. Controlled trial of tamoxifen as a single adjuvant agent in the management of early breast cancer. Br J Cancer. 1988. June; 57 6: 608- 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Band PR. . The birth of the subspecialty of medical oncology and examples of its early scientific foundations. J Clin Oncol. 2010. August 1; 28 22: 3653- 3658. 10.1200/JCO.2010.29.5261. [DOI] [PubMed] [Google Scholar]
- 13. Mamounas EP. . NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003. October; 1 4: 309- 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989. February 23; 320 8: 479- 484. [DOI] [PubMed] [Google Scholar]
- 15. Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996. November 6; 88 21: 1529- 1542. [DOI] [PubMed] [Google Scholar]
- 16. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. May 14-20; 365 9472: 1687- 1717. [DOI] [PubMed] [Google Scholar]
- 17. Davies C, Godwin J, Gray R, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011. August 27; 378 9793: 771- 784. 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buzdar AU, Jonat W, Howell A, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma. Cancer. 1998; 83: 1142- 1152. . [DOI] [PubMed] [Google Scholar]
- 19. Howell A, Cuzick J, Baum M, et al. ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005. January 1-7; 365 9453: 60- 62. [DOI] [PubMed] [Google Scholar]
- 20. Cuzick J, Sestak I, Baum M, et al. ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010. December; 11 12: 1135- 1141. 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 21. Thürlimann B, Keshaviah A, Coates AS, et al. Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005. December 29; 353 26: 2747- 2757. Erratum in: N Engl J Med. 2006 May 18;354(20):2200. [DOI] [PubMed] [Google Scholar]
- 22. Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006. August 1; 24 22: 3629- 3635. [DOI] [PubMed] [Google Scholar]
- 23. Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007. February 10; 25 5: 486- 492. [DOI] [PubMed] [Google Scholar]
- 24. Smith I, Yardley D, Burris H, et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized phase III femara versus anastrozole clinical evaluation (FACE) trial. J Clin Oncol. 2017. April 1; 35 10: 1041- 1048. 10.1200/JCO.2016.69.2871. [DOI] [PubMed] [Google Scholar]
- 25. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015. October 3; 386 10001: 1341- 1352. 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 26. Jakesz R, Jonat W, Gnant M, et al. ABCSG and the GABG. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005. August 6-12; 366 9484: 455- 462. [DOI] [PubMed] [Google Scholar]
- 27. Coombes RC, Hall E, Gibson LJ, et al. Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004. March 11; 350 11: 1081- 1092. Erratum in: N Engl J Med. 2004 Dec 2;351(23):2461. N Engl J Med. 2006 Oct 19;355(16):1746. [DOI] [PubMed] [Google Scholar]
- 28. Davies C, Pan H, Godwin J, et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013. March 9; 381 9869: 805- 816. Erratum in: Lancet. 2013 Mar 9;381(9869):804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gray RG, Rea D, Handley K, et al. aTTom Collaborative Group; aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6, 953 women with early breast cancer. J Clin Oncol. 2013. May; 31 15 suppl 5. 23169505 [Google Scholar]
- 30. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 Years. N Engl J Med. 2016. July 21; 375 3: 209- 219. 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mamounas EP, Bandos H, Lembersky BC, et al. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI): results from NRG Oncology/NSABP B-42. : Breast Cancer Symposium; 2016; San Antonio, TX: S1-05. [Google Scholar]
- 32. Blok EJ, van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al. Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy; results of the randomized phase III IDEAL trial (BOOG 2006-05). : Breast Cancer Symposium; 2016; San Antonio, TX: S1-04, P2-09-01. [Google Scholar]
- 33. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al. First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. : Breast Cancer Symposium; 2016; San Antonio, TX: S1-03, P2-09-04. [Google Scholar]
- 34. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013. August 1; 19 15: 4196- 4205. 10.1158/1078-0432.CCR-13-0804. [DOI] [PubMed] [Google Scholar]
- 35. Jankowitz RC, Cooper K, Erlander MG, et al. Prognostic utility of the breast cancer index and comparison to Adjuvant! Online in a clinical case series of early breast cancer. Breast Cancer Res. 2011. October 14; 13 5: R98 10.1186/bcr3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutqvist LE, Johansson H;. Stockholm Breast Cancer Study Group. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 2007; 46 2: 133- 145. [DOI] [PubMed] [Google Scholar]
- 37. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013. October; 14 11: 1067- 1076. 10.1016/S1470-2045(13)70387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sestak I, Zhang Y, Schroeder B, et al. Integration of tumor size and grade with the breast cancer index (BCI) for prediction of distant recurrence in hormone receptor-positive breast cancer with 1-3 positive lymph nodes. Cancer Res. 2016. February; 76 4 suppl: p2-08–12. [Google Scholar]
- 39. Zhang Y, Jerevall P, Schroeder B, et al. Validation of a prognostic model integrating Breast Cancer Index (BCI) with tumor size and grade for prediction of distant recurrence in hormone receptor-positive (HR+) breast cancer with 1-3 positive nodes. J Clin Oncol. 2016; 34 15 suppl: 541. [Google Scholar]
- 40. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst. 2013. July 17; 105 14: 1036- 1042. 10.1093/jnci/djt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat. 2015. December; 154 3: 533- 541. 10.1007/s10549-015-3631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


