Abstract
Background:
Dermatomyositis is an idiopathic inflammatory myopathy that has been established as one of the many paraneoplastic phenomena. Cardiac involvement can occur with dermatomyositis but has rarely been reported in the literature because symptoms are usually subclinical.
Case Report:
A 72-year-old female presented with generalized weakness for 1 month after a recent diagnosis of renal cell carcinoma. Her weakness was attributed to a myopathic process that was identified as dermatomyositis after muscle biopsy. Laboratory tests revealed persistently elevated cardiac troponin I despite the absence of cardiac symptoms and a subsequent negative ischemic workup. After administration of intravenous steroids and treatment of the underlying renal cell carcinoma, the patient's cardiac enzymes normalized, suggesting a paraneoplastic etiology of her cardiac manifestations.
Conclusion:
Cardiac involvement as a paraneoplastic process is a rare entity and can present with elevated troponin as shown in our case. Its underlying mechanism has not been clearly elucidated, but this case may shed some light on a new or unknown myocardial manifestation related to malignancy.
Keywords: Carcinoma–renal cell, cardiotoxicity, dermatomyositis, paraneoplastic syndromes, troponin I
INTRODUCTION
Paraneoplastic syndromes are a constellation of signs and symptoms not directly related to a primary neoplasm but that can occur as a consequence of hormone-like substances or proteins released by a tumor. In 1890, Auché described the first case of paraneoplastic syndrome associated with the peripheral nervous system.1 Paraneoplastic syndromes are known to be associated with various malignancies and can manifest in a variety of organ systems, including the endocrine, cutaneous, neurologic, hematologic, and rheumatologic systems.2 Dermatomyositis is an autoimmune inflammatory myopathy that has been established as one of the many paraneoplastic phenomena. Dermatomyositis is characterized by proximal muscle weakness and unique skin lesions including heliotrope rash, Gottron papules, and the V-neck and shawl signs.3-5 The overall incidence of dermatomyositis is 1:100,000 individuals, with an estimated 15%-30% of cases attributed to paraneoplastic syndromes.6 An estimated 12%-25% of dermatomyositis cases occur as a result of malignancy, and nasopharyngeal, breast, and lung cancer are the most frequently associated malignancies.7 Dermatomyositis usually occurs in patients between the ages of 50-59 years and is generally idiopathic.3,8 Cardiac involvement in dermatomyositis or as a paraneoplastic syndrome is rare. We present a case of paraneoplastic dermatomyositis coexisting with elevated troponin I in a patient with renal cell carcinoma (RCC).
CASE REPORT
A 72-year-old female presented with a 1-month history of generalized weakness and dysphagia. She had been diagnosed with clear cell RCC 2-3 months prior to the current illness that was progressive to the point that the patient was not able to ambulate without assistance. The weakness involved her neck and bilateral upper and lower extremity proximal muscles. Along with the weakness, the patient developed significant dysphagia. A complete review of systems was otherwise unremarkable. Her admission vital signs were blood pressure 103/65 mmHg, heart rate 79 bpm, and respiratory rate 15 breaths per min.
On examination, she was noted to be unable to swallow, to have generalized muscle weakness more pronounced in the proximal muscles, and to have difficulty flexing or extending her fingers. She had no skin rashes. Laboratory workup revealed markedly elevated muscle enzymes: creatinine phosphokinase (CPK) 3,222 U/L (normal, 22-198 U/L) and aldolase 31.7 U/L (normal, <8.1 U/L). Other laboratory values were hemoglobin 9.3 g/dL (normal, 12-15 g/dL), white blood cells (WBC) 3.9 K/mm3 (normal, 4-11 K/mm3), creatinine 0.8 mg/dL (normal, 0.6-1.2 mg/dL), and normal thyroid-stimulating hormone. Rheumatologic tests included nonreactive values for Sjögren syndrome antibodies (anti-Ro and anti-La), anti-Jo-1 antibody, and rheumatoid factor.
The patient had an initial elevated cardiac troponin I of 1.48 ng/mL (normal, <0.03 ng/mL) that peaked at 2.8 ng/mL on day 5 of hospitalization and elevated creatinine kinase of 369.7 IU/L (normal, 5-25 IU/L). Electrocardiogram showed normal sinus rhythm with no significant ST or T wave changes. Computed tomography (CT) angiogram of the chest with contrast showed no evidence of pulmonary embolism. Infection and renal injury were evaluated, revealing negative blood culture, normal WBC, and normal serum creatinine. Biceps muscle biopsy showed perifascicular myofiber atrophy with perivascular infiltrates of chronic inflammatory cells consistent with dermatomyositis (Figure 1).
Figure 1.
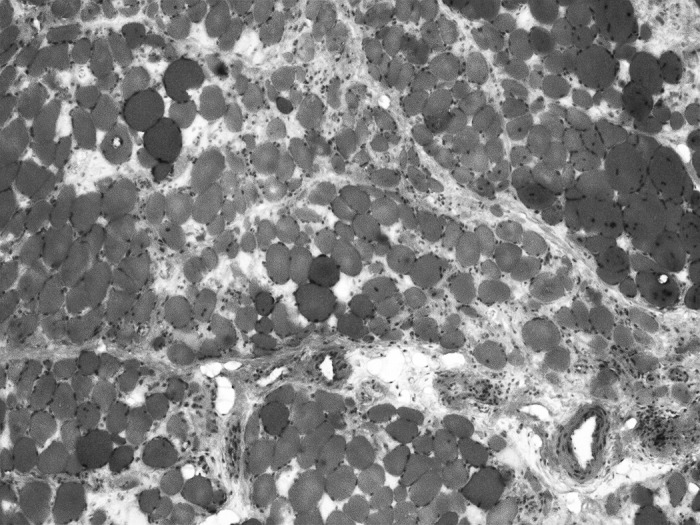
Skeletal muscle (bicep) biopsy demonstrating inflammatory myopathic process with a significant necrotizing component. Mild perivascular infiltrates of chronic inflammatory cells are present (hematoxylin and eosin stain, 200×).
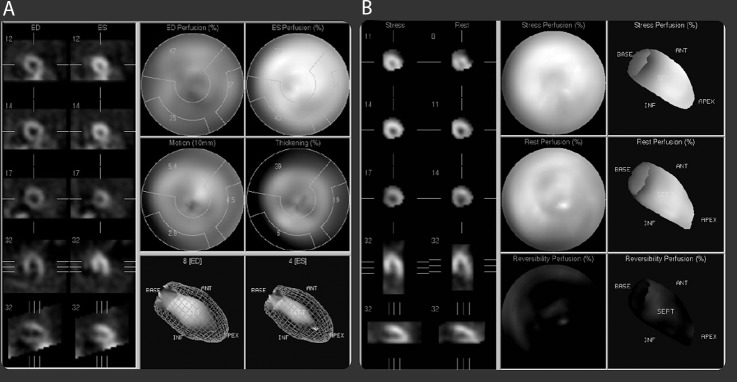
Transthoracic echocardiogram revealed a normal ejection fraction of 65% with no signs of ventricular hypertrophy or wall motion abnormalities (Figure 2). Regadenoson-induced myocardial perfusion imaging with single-photon emission CT demonstrated normal myocardial perfusion (Figure 3).
Figure 2.
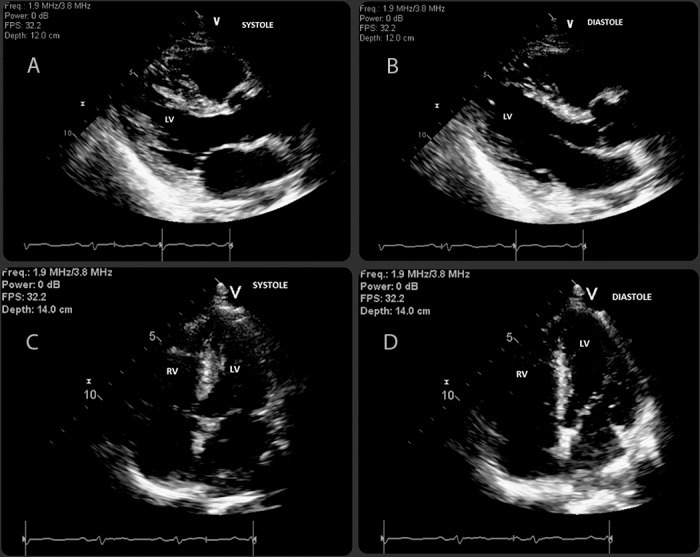
Transthoracic echocardiogram: left parasternal views in systole (A) and diastole (B) and apical 4-chamber views in systole (C) and diastole (D) demonstrating good ventricular contraction with an ejection fraction of 65% and normal-sized ventricles without regional wall motion abnormalities. LV, left ventricle; RV, right ventricle.
Figure 3.
Regadenoson-induced myocardial perfusion imaging with single-photon emission computerized tomography (Lexiscan test) demonstrating normal myocardial perfusion comparing rest and stress images (A and B).
A final diagnosis of paraneoplastic dermatomyositis sine dermatitis with paraneoplastic cardiac involvement was made based on clinical manifestations, lack of skin lesions, elevated muscle and cardiac enzymes, typical muscle biopsy findings, normal renal function, and negative ischemic workup.
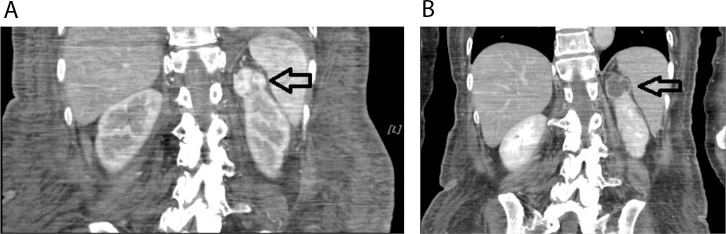
Because of the patient's poor functional status, she underwent interventional radiology–guided renal artery chemoembolization. Follow-up CT scan showed diminished vascularity around her renal mass (Figure 4). Azathioprine 50 mg daily and intravenous methylprednisolone 80 mg were started postembolization. After 3 days of treatment, the intravenous steroid was discontinued and the patient was given oral prednisone 50 mg with a tapering dose of 5 mg/d for a period of 10 days. By week 4 of medical therapy, the patient's CPK and cardiac troponin I levels had gradually decreased to 699 U/L and 0.03 ng/mL, respectively, along with steady improvement of her weakness. She was started on and tolerated a liquid diet before being discharged to a rehabilitation facility. She was continued on azathioprine 50 mg daily.
Figure 4.
Computed tomography of the abdomen pre and post chemoembolization (A and B) showing successful blood supply blockage of the renal mass, located at the upper pole of the right kidney. Note the absence of the contrast in the postembolization photo (B) compared to the preembolization image (A) (black arrows).
DISCUSSION
In 1899, cardiac involvement in dermatomyositis was first reported by Oppenheim.9 The incidence of cardiac involvement in myositis and dermatomyositis significantly varies (from 3%-75%) based on the type and definition of heart involvement, patient selection criteria, and clinically manifested and subclinical cardiac involvement.10 The most frequently reported cardiac manifestation of dermatomyositis is acute onset of congestive heart failure, with ischemic heart disease and myocardial ischemia being observed in 4%-18% and 5.6% of patients with dermatomyositis, respectively, but elevated cardiac troponin I in dermatomyositis has rarely been described in the literature.11 However, some cardiac pathologies such as conduction abnormalities, ventricular hypertrophy with valvular involvement, and pulmonary hypertension have been documented as subclinical entities.11
While the proposed pathogenesis of malignancy-related dermatomyositis is complex, recent studies have found a new antibody, novel myositis-specific autoantibody (anti-p155 or p155/140), that is suspected as the inciting agent.12-14 Antibody-related stimulation of complement-dependent humoral pathways with complement deposition in the endomysial vasculature and activation of T and B cells is believed to lead a florid inflammatory process with the capillary necrosis, endothelial injury, muscle ischemia, and damage seen in dermatomyositis.15 Tissue-specific autoantigen expression in both the tumor cells and newly regenerating muscles is believed to lead to an immunogenic stimulation against newly formed muscle cells and the subsequent development of autoimmunity.16
In our case report, we described a patient who presented with proximal muscle weakness that was diagnosed as dermatomyositis sine dermatitis based on the biopsy finding. She had elevated cardiac markers but a negative cardiac workup. Elevated cardiac troponin I could have been from underlying muscle damage, but Erlacher et al stated that troponin T has closer correlations with the severity of skeletal damage than cardiac troponin I which is a specific marker of cardiac origin.17 To the best of our knowledge, there has never been a single study of elevated cardiac troponin I in malignancy-related dermatomyositis without underlying cardiovascular disease, and in our extensive literature search, we found only 2 patients with dermatomyositis who had an elevated cardiac troponin I (explained by their underlying cardiac conditions).17,18 While remaining a diagnosis of exclusion, our patient's elevated cardiac troponin I was eventually attributed to cardiac involvement secondary to the paraneoplastic process.
Pavo et al studied cardiovascular biomarkers in patients with malignancies and found high levels of N-terminal pro-BNP and high-sensitive troponin T irrespective of underlying cardiac comorbidities.19 In December 2015, in a presentation at the annual meeting of the European Association of Cardiovascular Imaging, Venneri et al showed the presence of myocardial dysfunction, detected by strain imaging, that could occur in patients recently diagnosed with cancer regardless of underlying cardiac disease.20 Based on the Pavo et al and Venneri et al studies, cardiac biomarkers and strain imaging could be the tools to detect malignancy-related cardiac involvement, but the usage of cardiac troponin I has not yet been studied.
Jo-1, Mi-2, and PL-7 markers are myositis-specific autoantibodies seen in dermatomyositis, whereas myositis-associated antibodies include Scl and Ro. Muscle biopsy is the gold standard for diagnosis of dermatomyositis.21
Dermatomyositis has 2 types. The absence of rash with histologic evidence of dermatomyositis is known as atypical dermatomyositis or dermatomyositis sine dermatitis as seen in our patient. Amyopathic dermatomyositis is defined in the presence of the classic dermatomyositis rash without muscle involvement.
The therapeutic approach for RCC with dermatomyositis is the surgical removal of RCC followed by standard treatment for the dermatomyositis.22 For patients who have contraindications to surgery, radiofrequency ablation or cryoablation has been recommended. However, treatment of a cardiac manifestation in dermatomyositis remains controversial, with the current suggested treatment including corticosteroids, immunotherapy, and heart transplantation.11 Although the role of corticosteroids in cardiac events secondary to dermatomyositis is still debatable, there has been evidence of partial improvement of heart failure after initiation of therapy.10 Our patient showed a dramatic improvement in her cardiac troponin I level after intravenous steroid and azathioprine administration and chemoembolization of her RCC.
CONCLUSION
Our case illustrates that paraneoplastic cardiac involvement in dermatomyositis can present with elevated cardiac troponin I. Clinicians usually think of acute coronary syndrome in the setting of abnormal cardiac enzymes, but this case highlights the importance of considering other conditions, especially for patients with malignancies. Cardio-oncology is an emerging field with some understanding of cancer-related cardiac involvement. The implication of cardiac troponin I in patients with cancer and dermatomyositis has not been studied, and its significance is not entirely understood. Further research in these focused areas is needed to shed light on the importance of elevated cardiac troponin I in malignancy and paraneoplastic cardiac involvement.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1. Auché M. . Des névrites périphériques chez les cancéreux. Rev Med (Paris). 1890; 10: 785- 807. [Google Scholar]
- 2. Bilynsky BT, Dzhus MB, Litvinyak RI. . The conceptual and clinical problems of paraneoplastic syndrome in oncology and internal medicine. Exp Oncol. 2015. June; 37 2: 82- 88. [PubMed] [Google Scholar]
- 3. Callen JP, Hyla JF, Bole GG Jr, Kay DR. . The relationship of dermatomyositis and polymyositis to internal malignancy. Arch Dermatol. 1980. March; 116 3: 295- 298. [PubMed] [Google Scholar]
- 4. Bendewald MJ, Wetter DA, Li X, Davis MD. . Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010. January; 146 1: 26- 30. 10.1001/archdermatol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iaccarino L, Ghirardello A, Bettio S, et al. The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun. 2014. Feb-Mar; 48-49: 122- 127. 10.1016/j.jaut.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6. Dimachkie MM, Barohn RJ, Amato AA. . Idiopathic inflammatory myopathies. Neurol Clinc. 2014. August; 32 3: 595- 628, vii. 10.1016/j.ncl.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang YL, Chen YJ, Lin MW, et al. Malignancies associated with dermatomyositis and polymyositis in Taiwan: a nationwide population-based study. Br J Dermatol. 2009. October; 161 4: 854- 860. 10.1111/j.1365-2133.2009.09274.x. [DOI] [PubMed] [Google Scholar]
- 8. Kuo CF, See LC, Yu KH, et al. Incidence, cancer risk and mortality of dermatomyositis and polymyositis in Taiwan: a nationwide population study. Br J Dermatol. 2011. December; 165 6: 1273- 1279. 10.1111/j.1365-2133.2011.10595.x. [DOI] [PubMed] [Google Scholar]
- 9. Oppenheim H. . Zur dermatomyositis. Berl Klin Wochenschrift. 1899; 36: 805- 807. [Google Scholar]
- 10. Lundberg IE. . The heart in dermatomyositis and polymyositis. Rheumatology (Oxford). 2006. October; 45 Suppl 4:iv18-iv21. [DOI] [PubMed]
- 11. Zhang L, Wang GC, Ma L, Zu N. . Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol. 2012. November; 35 11: 686- 691. 10.1002/clc.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Targoff IN, Mamyrova G, Trieu EP, et al. Childhood Myositis Heterogeneity Study Group; International Myositis Collaborative Study Group. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006. November; 54 11: 3682- 3689. [DOI] [PubMed] [Google Scholar]
- 13. Kaji K, Fujimoto M, Hasegawa M, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford). 2007. January; 46 1: 25- 28. [DOI] [PubMed] [Google Scholar]
- 14. Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012. February; 64 2: 523- 532. 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 15. Dalakas MC, Hohlfeld R. . Polymyositis and dermatomyositis. Lancet. 2003. September 20; 362 9388: 971- 982. [DOI] [PubMed] [Google Scholar]
- 16. Shimizu J. . Malignancy-associated myositis [in Japanese]. Brain Nerve. 2010. April; 62 4: 427- 432. [PubMed] [Google Scholar]
- 17. Erlacher P, Lercher A, Falkensammer J, et al. Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin Chim Acta. 2001. April; 306 1-2: 27- 33. [DOI] [PubMed] [Google Scholar]
- 18. Kiely PD, Bruckner FE, Nisbet JA, Daghir A. . Serum skeletal troponin I in inflammatory muscle disease: relation to creatine kinase, CKMB and cardiac troponin I. Ann Rheum Dis. 2000. September; 59 9: 750- 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pavo N, Raderer M, Hülsmann M, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015. December; 101 23: 1874- 1880. 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 20. Venneri L, Calichio F, Manivarmane R, et al. Subclinical myocardial dysfunction in cancer patients: is there a direct effect of tumour growth? Eur Heart J Cardiovasc Imaging. 2015. May; 16 (Suppl 1):ii127.
- 21. Mastaglia FL, Phillips BA. . Idiopathic inflammatory myopathies: epidemiology, classification, and diagnostic criteria. Rheum Dis Clin North Am. 2002. November; 28 4: 723- 741. [DOI] [PubMed] [Google Scholar]
- 22. Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009. June; 7 6: 618- 630. [DOI] [PubMed] [Google Scholar]