Abstract
Cold environment is the main constraint for tea plants (Camellia sinensis) distribution and tea farming. We identified two tea cultivars, called var. sinensis cv. Shuchazao (SCZ) with a high cold-tolerance and var. assamica cv. Yinghong9 (YH9) with low cold-tolerance. To better understand the response mechanism of tea plants under cold stress for improving breeding, we compared physiological and biochemical responses, and associated genes expression in response to 7-day and 14-day cold acclimation, followed by 7-day de-acclimation in these two tea cultivars. We found that the low EL50, low Fv/Fm, and high sucrose and raffinose accumulation are responsible for higher cold tolerance in SCZ comparing with YH9. We then measured the expression of 14 key homologous genes, known as involved in these responses in other plants, for each stages of treatment in both cultivars using RT-qPCR. Our results suggested that the increased expression of CsCBF1 and CsDHNs coupling with the accumulation of sucrose play key roles in conferring higher cold resistance in SCZ. Our findings have revealed key genes regulation responsible for cold resistance, which help to understand the cold-resistant mechanisms and guide breeding in tea plants.
Introduction
Low temperature is a major constraint on the growth, geographical distribution, and yield of some plants. Cold resistance of many plants[1–4], e.g. Eucalyptus nitens, Miscanthus, Medicago sativa and North American Rhododendron can be improved by prior exposure to a period of low, nonfreezing temperatures, which known as cold acclimation (CA) [5–7]. For instance, CA improves the tolerance of North American Rhododendron from -7°C to -53°C [4]. Up to date, CA is a key strategy to increase the physiological adaptation of tea plants to low temperatures [8]. During CA, many physiological and biochemical processes are altered in plants. Those processes include the cytoskeleton rearrangement as an integrating system perceiving the signals [9], accumulated membrane phospholipids and modifications in lipid composition of different organelles. For example, the proportion of MGDG (monogalactosyldiacylglycerols) was decreased and the proportion of DGDG (digalactosyldiacylglycerols) was increased in the chloroplast in Rye [10–14]. Moreover, plants introduce the accumulation of antifreeze proteins and cryoprotectants like soluble sugars and proline [15–16]. The increased synthesis of soluble sugars, including sucrose, glucose, raffinose, and fructose, contributes directly to membrane stabilization in Alcantarea imperiali [17], and Camellia sinensis [8]. The raised content of proline in Triticum aestivum [18], Arabidopsis [19] and Camellia sinensis [8] was also observed during CA. Antioxidant metabolism is known to improve the scavenging activity of reactive oxygen species (ROS) and maintain redox balance during CA [20]. During CA, a high ratio of abscisic acid (ABA) to gibberellin content has been shown to increase freezing tolerance in some woody taxa [21].
Upon cold stress, the expression of various cold-regulated (COR) genes are induced to protect plants [22]. The expression of COR genes is regulated by both the CBF (C-repeat-binding factor)-mediated ABA-independent pathway and the bZIP (basic region/leucine zipper)-mediated ABA-dependent pathway [23]. CBF transcription factors regulate ~12% of the cold-responsive transcriptome [24]. ICE1 (inducer of CBF expression 1)-CBF-COR cold-response pathway in plants is critical for configuring cold-induced transcriptomic changes [25–26]. Genes of the ICE1-CBF cold-response pathway have been reported in woody and herbaceous plants [27–29]. Studies have shown that the cascade regulation of ICE1, CBF, and COR is the main pathway for cold acclimation [30–31]. In Arabidopsis, ICE1 express constitutively and is not responsive to cold stress, whereas ICE1 undergoes sumoylation to become functionally active [32]. Three CBFs (CBF1-3) were found in Arabidopsis. CBF1 and -CBF3 positively regulates the downstream CBF-target genes, while CBF2 negatively regulates them [33]. Wang at al. [34] found that the ICE1-CBF-COR pathway was conserved in tea plants. To date, several COR genes have been discovered in tea plant including one CsICE1 (FE861156), two CsCBFs, designated as CsCBF1 (EU563238), CsCBF2 (KC702795), and three dehydrin homologs designated as CsDHN1 (GQ228834.1), CsDHN2 (FJ436978) and CsDHN3 (KY270880) [34–36].
Several studies revealed the key enzymes’ activities during sugar synthesis, and associated genes expression during CA in plants. Sucrose is synthesized in the cytosol by the sucrose-phosphate synthase (SPS) and degraded by either sucrose synthase or invertase (INV) into a monosaccharide or derivative [37]. Raffinose synthase (RS) for raffinose synthesis was also explored in recent researches upon cold stress [38]. Yue et al. [8] analyzed the expression patterns of 32 genes during the natural CA in tea plant (var. sinensis cv. Longjing43) and found that expression of CsSPS, CsINV5 and CsRS2 was significantly induced. To date, it is known that the proline biosynthesis is catalyzed by P5C synthase (P5CS) and P5C reductase (P5CR) in plants [39–40]. Another key enzyme in the proline synthesis pathway is Ornithine-D-aminotransferase (OAT) [41]. Degradation of proline is catalyzed by Pro-dehydrogenase (ProDH) and P5C-dehydrogenase (P5CD) [42]. In tea plants, the sequences of CsP5CS (KJ143742.1), CsOAT (KJ641844.1) and CsP5CR (KY368574), CsP5CDH(KY368572) and CsProDH (KY368573) are available at NCBI (https://www.ncbi.nlm.nih.gov/).
Tea plants (Camellia sinensis (L.) O. Kuntze), one of the important economic wooden plants in the world, are mainly grown in subtropical and tropical regions. Two basic classes of varieties can be classified as var. assamica, a quick-growing tree well suited to tropical climates, and var. sinensis, a slower-growing bush that can withstand colder climates than assamica [43–44]. Tea plants are vulnerable to cold injury during winter such as in East Asia (China, Japan), especially in northern China. Recent studies have explored the response of tea plants to cold stress and natural CA [45–48]. However, a comparative study on cold resistance between cold-resistant and cold-susceptible cultivars has not been reported yet. The present study was conducted to explore the molecular mechanism of cold resistance by treating the cold-resistant camellia var. sinensis CV. Shuchazao (SCZ) and cold-susceptible camellia var. assamica CV Yinghong9 (YH9) under CA and de-acclimation (DA). We found difference in biochemical changes, including EL50 (temperature leading to 50% tissue damages due to leakage of electrolyte), Fv/Fm (maximum quantum yield of PSII photosystems), sugars and proline. Then we examined the expression of 14 genes related to these biochemical changes. Comparison of gene expression and study of biochemical changes in the responses to cold in two tea cultivars led to our finding of the difference in cold tolerance. Our results indicated that the increased expression of CsCBF1 and CsDHNs coupling with the accumulation of sucrose has played a role in conferring higher cold resistance in tea cultivar SCZ. The results provide understanding in biochemical and gene regulatory mechanisms of cold resistance in tea plants.
Materials and methods
Plant material
The clone cuttings of Camellia sinensis cv. Shuchazao and Camellia sinensis var. assamica cv.Yinghong9 were obtained from the Dechang Tea Plantation in Anhui (116° 56' 24'' E, 31° 27' N) and the Tea Research Institute of Guangdong Academy of Agricultural Sciences (113° 22' 48'' E, 24° 10' 12'' N), China, respectively. One-year-old cutting-propagated plants were transferred to a growth chamber with temperature cycles of 25°C at day time and 20°C at night time, 12 h photoperiod, and 70% relative humidity for one month. Subsequently, they were subjected to varying degrees of cold acclimation and de-acclimation. Ten well-grown one-year-old tea plants were collected and used as non-acclimation (NA). The following cold acclimation (CA) treatments were applied in this study: CA1 was conducted by exposing SCZ and YH9 to low temperature (10/4°C, day/night temperature) for 7 days. Afterwards, CA2 was conducted by exposing SCZ and YH9 to lower temperatures (4/0°C, day/night temperature) for another 7 days. Lastly, the plants were exposed to normal temperature (25/20°C, day/night temperature) for 7 days for de-acclimation. At each time point, the leaves were collected, immediately frozen in liquid nitrogen and stored at −80°C until use. Three biological replicates were conducted.
Electrolyte leakage assay
Relative electrolyte leakage was measured to evaluate the cell membrane damage as described with some modifications [49]. Briefly, after washing with distilled deionized water, the leaf pieces were obtained using a puncher from leaves after each treatment. After subsequently exposed to -2°C, -4°C, -6°C, -8°C, and -10°C for 12 hours, samples were placed in glass bottles containing 20 mL of distilled deionized water. The electrical conductivity of the solution (L1) was determined using a conductivity meter STARTER 3100C (Ohaus; America) at 25°C. The solutions were then heated to 100°C for 30 min and the final electrical conductivity (L2) was determined after cooling to 25°C. The REC (relative electrical conductivity) was calculated as L1/L2×100%.
Fv/Fm
Mature leaves (from third to fifth leaves) of tea cultivar SCZ and YH9 were carefully clamped in the middle part of the leaves, avoiding the main vein and then dark-adapted in leaf clips for 20 min prior to measurement. Chlorophyll fluorescence parameters Fm and Fo were measured by OS-30P modulated fluorometer (Opti-Sciences, USA) and Fv was obtained using Fv = Fm-Fo [50]. Ten biological replicates were performed for the experiment.
Measurement of proline content
Proline contents in SCZ and YH9 were measured by the colorimetric assay according to Bates method with some modifications [51]. Briefly, approximately 0.5 g leaves of SCZ and YH9 were ground into fine powder in liquid nitrogen. The powder was immediately resuspended in 5 mL of 4% sulfonic acid and sonicated for 30 min. The mixture was subsequently centrifuged for 30 min at 12000 rpm and the supernatant was collected. 2 mL supernatant, 2 mL glacial acetic acid and 3 mL ninhydrin reagent (2.5% [w/v] ninhydrin, 60% [v/v] glacial acetic acid, and 40% 6 M phosphoric acid) were added, mixed and heated to 100°C for 40 min. After cooling down to room temperature, 5 mL toluene was added and the absorbance was measured at 520 nm using an UV Spectrophotometer (U-5100, Hitachi).
Measurements of soluble carbohydrates
Contents of soluble carbohydrates of fructose, sucrose, glucose, raffinose and trehalose in the leaves of tea cultivar SCZ and YH9 were measured by High Performance Liquid Chromatography (HPLC) (Agilent, America). The samples were prepared following the protocol as previously described with some modifications [52]. Briefly, approximately 0.5 g leaves of SCZ and YH9 were weighed and ground in liquid nitrogen, and 10 ml of distilled water was added immediately. After heating at 100°C for 1 h, the mixture was subsequently centrifuged for 10 min at 12000 rpm and the supernatant was collected. The aqueous phase was collected and dried on a rotary evaporator. It was then resuspended in distilled water and filtered through a 0.22 μm filter membrane prior to HPLC analysis. Standard of fructose, sucrose, glucose, raffinose and trehalose were purchased from Sangon Biotech. Co. (Shanghai, China)
RNA extraction and real-time quantitative PCR analysis
Total RNAs were extracted from leaves of tea cultivar SCZ and YH9 with RNA prep Pure Plant kit (Tiangen, Beijing, China). The total RNAs were reverse transcribed into first-strand cDNA with PrimeScript Reagent Kit (TaKaRa, Dalian, China) and the cDNAs obtained were used as templates for PCR amplification with specific primers. Gene-specific primers (Table 1) were used for real-time quantitative RT-PCR. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal reference gene [53] and the relative expression was calculated using the 2ΔCt method [54]. Each reaction contained 12.5 μL of SYBR®Premix Ex Taq™II (Tli RNaseH Plus; TaKaRa, Dalian, China), 2 μL cDNA, and 1 μL 10 μM gene-specific primers in a final volume of 25 μL. All reactions were carried out using the CFX96™ Real-Time System (Bio-Rad, USA) using a two-step method: 95°C for 3 min; 40 cycles of 95°C for 10 s, 62°C for 30 s.
Table 1. Genes and corresponding primers used for the RT-qPCR experiments.
| Gene name | GenBank Accession No. | Primer | Primer sequence (5'–3') |
|---|---|---|---|
| GAPDH | GE651107 | Forward | TTG GCA TCG TTG AGG GTC T |
| Reverse | CAG TGG GAA CAC GGA AAG C | ||
| CsICE1 | FE861156 | Forward | ATG TTT TGT AGC CGC AGA C |
| Reverse | GCT TTG ATT TGG TCA GGA TG | ||
| CsCBF1 | EU563238 | Forward | AGA AAT CGG ATG GCT TGT GT |
| Reverse | TTG TCG TCT CAG TCG CAG TT | ||
| CsCBF2 | KC702795 | Forward | CAC AGC CTG CTC ATC ACT |
| Reverse | ACC ACT GCC ACA ATC TG | ||
| CsDNH1 | GQ228834.1 | Forward | ACA CCG ATG AGG TGG AGG TA |
| Reverse | AAT CCT CGA ACT TGG GCT CT | ||
| CsDNH2 | FJ436978 | Forward | ACT TAT GGC ACC GGC ACT AC |
| Reverse | CTT CCT CCT CCC TCC TTG AC | ||
| CsDNH3 | KY270880 | Forward | TCC ACA TCG GAG GCC AAA AG |
| Reverse | AAC CCT CCT TCC TTG TGC TC | ||
| CsSPS | KF696388 | Forward | ACC TGG AGG CGA TTC TGG ATG |
| Reverse | TTC CAA ATC CGC CAG CAC ATA | ||
| CsRS2 | KP053395 | Forward | CGG TTT GGC GCT TAC TCT TC |
| Reverse | TCT CCT CTT CTG CAA CCG GA | ||
| CsINV5 | KP053402 | Forward | AGT CTT GCC CCT TGA TGT CG |
| Reverse | AAC CAA ACG GTC CAA GAG CA | ||
| CsP5CS | KJ143742.1 | Forward | AGG CTC ATT GGA CTT GTG ACT |
| Reverse | CAT CAG CAT GAC CCA GAA CAG | ||
| CsOAT | KJ641844.1 | Forward | GCG GTT AAT CAG GGA CAT |
| Reverse | ACA CCT TCG GCA CCA GTA | ||
| CsP5CR | KY368574 | Forward | TAG GGG AGG CGG CAT CAG TT |
| Reverse | ACC CCT CCA TCA GCC AAA GC | ||
| CsP5CDH1 | KY368572 | Forward | TGC TGA TGG GAA GAC GAT |
| Reverse | GCC GAG CAC TTT TGA CCA CT | ||
| CsProDH | KY368573 | Forward | CAA AAC CCA AAT CCA ACC G |
| Reverse | TCC TCC TCA CTA CCC CCA AC |
Primer design
The primers were designed against the sequence of genes which is retrieved from Genbank using the listed accession number (Table 1). The software Primer Premier 5 (Premier Biosoft International, Palo Alto, California, USA) was used to designed specific primers (Table 1) and the primers were then synthesized by Sangon Biotech Co. (Shanghai, China). We checked the specify of the primers and which produced one peak in melting curve, indicating a single amplicom of target gene. Then we used these primers for the level of transcript (Figures A-O in S1 Text). qPCR products have been sequenced and the results evaluated using the DNAman computer software version 5.0 (Lynnon Biosoft) (Figures A-O in S2 Text).
Statistical analysis
EL50 was calculated by logistic equations. Statistical analyses were performed using DPS and Prism5, GraphPad Software. The results were expressed as mean value ± standard error (SE). Different letters indicate significant differences to Duncan’s multiple range tests with P < 0.05.
Results
Cold acclimation induces difference freezing tolerance in tea cultivars
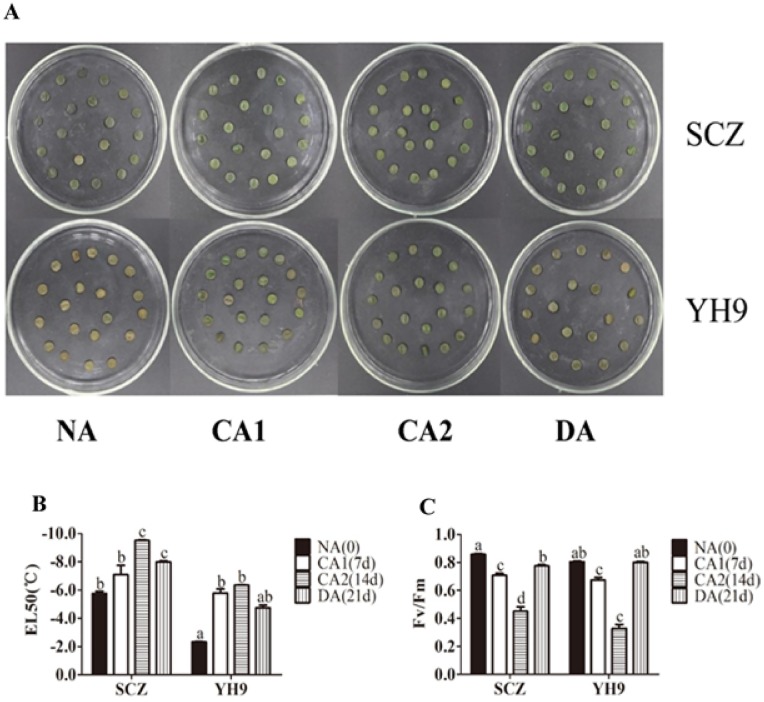
To investigate the cold tolerance, we selected two tea cultivars SCZ and YH9 which are known as with high and low cold tolerance, respectively (Fig 1). SCZ has been planted in cool areas in middle and warm areas in south China while cultivar YH9 in warm areas only in south China. Tea cultivar SCZ has smaller leaf than cultivar YH9 (Fig 1). We treated the one-year-old plants clonally propagated from cuttings of these two tea cultivars SCZ and YH9 with cold treatments CA1, CA2 and DA in growth camber to measure the physiological responses, membrane damage and chlorophyll content. We first treated the tea cultivar SCZ under 10/4°C (day/night) for 28 days and checked the change Fv/Fm and total sugar contents in a time course manner. We found that 7 days of cold treatment is enough to detect significant changes (S3 Text). Therefore, we used 7 days of treatment in this study. Our results showed that SCZ leaves remain green while those of YH9 became reddish brown after all treatments (Fig 2A). We further examined the electrolyte leakage which reflects cell membrane damage in cold by using EL50 analysis. As shown in Fig 2B, the EL50 had significant difference between SCZ and YH9, and the EL50 were -5.7°C and -2.3°C in SCZ and YH9 before cold treatment, respectively. Cold treatment CA1 treatment resulted in reduced EL50 values for both SCZ and YH9 (Fig 2B). Further cold treatment CA2 led to a more reduction of EL50 in SCZ to -9.4°C, while the EL50 value of YH9 remained unchanged compared with CA1. Lower EL50 represents less leakage. Thus, this result suggested higher cold tolerance in cultivar SCZ. The Fv/Fm value of both cultivars was relatively consistent in the range of 0.80~0.85 before treatment while both SCZ and YH9 displayed similar lower Fv/Fm values (P < 0.05) after CA1 treatment (Fig 2C). CA2 treatment further reduced the Fv/Fm value but the value of YH9 reduced more than SCZ (P < 0.05) (Fig 2C). The lower Fv/Fm suggests less chlorophyll, which explains the observed reddish color in YH9 after treatment. After DA treatment, the ratios of Fv/Fm in the two cultivars returned to the normal level (Fig 2C), which supported the Fv/Fm change in chlorophyll was caused by cold treatment and was reversible.
Fig 1. Comparison between Camellia sinensis cultivar YH9 and cultivar SCZ.
Images were taken from one-year-old plant clonally propagated from cuttings.
Fig 2. Effects of CA and DA on freezing tolerance of SCZ and YH9.
(A), The detached leaf discs of SCZ and YH9 exposed to -6°C for 12 h at different stages (NA, CA1, CA2, and DA). The values EL50 (B) and Fv/Fm (C) in SCZ and YH9 changed in response to CA and DA periods. Data were displayed as the mean of three replicates with standard error. Columns with different letters in (B) or (C) had significant differences according to Duncan’s multiple range tests with P < 0.05. NA: non-acclimation; CA1: cold acclimation of 7 days at 10/4°C, day/night temperature; CA2: cold acclimation of 7 days at 4/0°C, day/night temperature; DA: de-acclimation of 7 days at 25/20°C, day/night temperature.
Effect of CA and DA on soluble sugars accumulation in SCZ and YH9
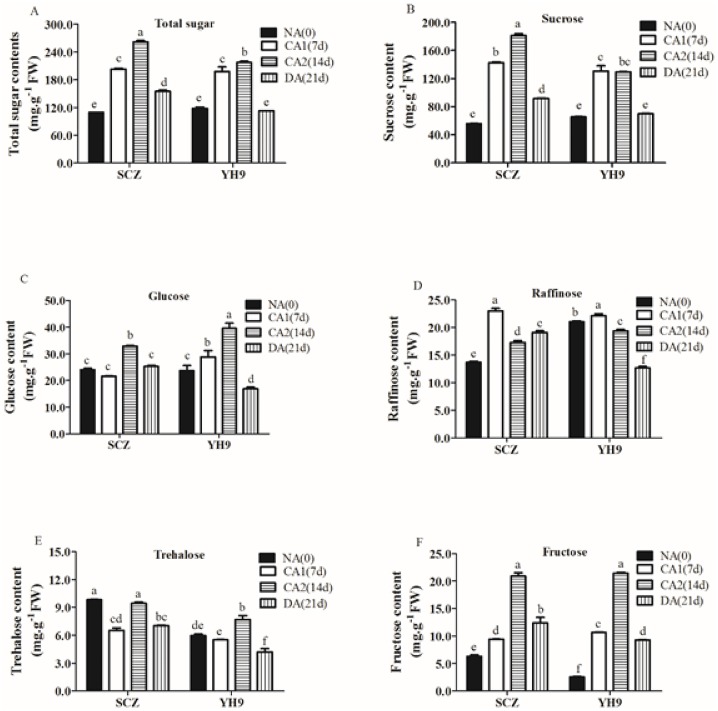
Sugar accumulation is known to have both osmotic and non-colligative functions, as it stabilizes cell membrane during cold acclimation and enhances freezing resistance in plants [7, 55]. As shown in Fig 3, the total sugar content and the sucrose level in both cultivars were significantly increased under CA condition, with a higher increase observed in SCZ. Relative to NA, the total sugar content in SCZ leaves was increased 2.39-fold after CA2, while the total sugar content in YH9 was increased 1.83-fold after CA2 (Fig 3A). Furthermore, sucrose content in SCZ was increased 2.56-fold after CA1 and reached 3.26-fold after CA2 relative to NA (P < 0.05). In contrast, the sucrose content in YH9 leaves was increased 2.0-fold after CA1 and remained constant after CA2 relative to CA1 (Fig 3B). In addition, CA1 and CA2 also induced a moderate increase in glucose and fructose contents in SCZ and YH9 (P < 0.05) (Fig 3C and 3F). Differently, CA1 and CA2 induced a little accumulation of raffinose in SCZ (P < 0.05), while only a small accumulation of trehalose was observed in YH9 under CA2 (P < 0.05) (Fig 3D and 3E). After DA, individual sugar content was decreased by a varying degree (Fig 3).
Fig 3. Effects of cold treatment on sugar contents in tea cultivars.
Data were displayed as the mean of three replicates and standard error. Columns with different letters had significant differences according to Duncan’s multiple range tests with P < 0.05. SCZ and YH9 represent tea cold resistant and cold susceptible tea varieties, respectively. NA: non-acclimation; CA1: cold acclimation of 7 days at 10/4°C, day/night temperature; CA2: cold acclimation of 7 days at 4/0°C, day/night temperature; DA: de-acclimation of 7 days at 25/20°C, day/night temperature.
Effect of CA and DA on proline accumulation between SCZ and YH9
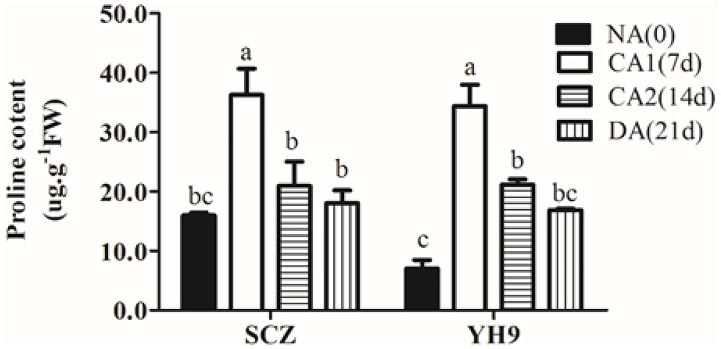
As the proline is a multi-functioned osmotic protective substance involved in cold tolerance [39], we measured the changes in proline content in SCZ and YH9 during CA and DA (Fig 4). As shown in Fig 4, the proline levels in both cultivar SCZ and YH9 were increased by 2.27-fold and 4.9-fold during CA1, respectively, and accumulated to similar contents. The study also revealed that the proline content reached the peak in CA1, and afterwards gradually decreased in both SCZ and YH9 (Fig 4).
Fig 4. Effects of cold treatment on proline accumulation in tea cultivars.
Data are displayed as the mean of three replicates and standard error. Columns with different letters had significant differences according to Duncan’s multiple range tests with P < 0.05. SCZ and YH9 represent tea cold resistant and cold susceptible tea varieties, respectively. NA: non-acclimation; CA1: cold acclimation of 7 days at 10/4°C, day/night temperature; CA2: cold acclimation of 7 days at 4/0°C, day/night temperature; DA: de-acclimation of 7 days at 25/20°C, day/night temperature.
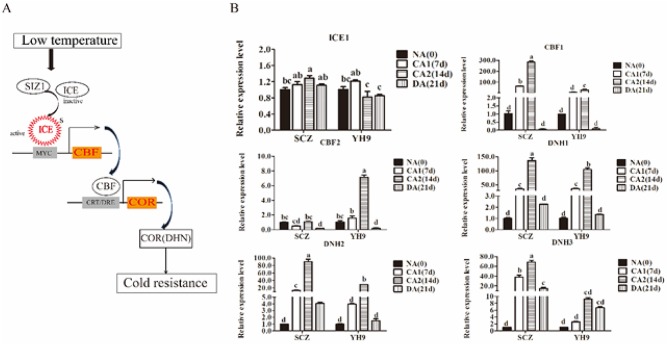
Effect of CA and DA on the gene expression of the ICE1-CBF pathway
The transcription of CBFs is regulated by ICE1 protein, which binds to the DRE/CRT cis-elements in the promoter regions of CORs (Fig 5A). CBFs play a central role in integrating the activation of multiple components of the CA respond to chilling and freezing stress in plants [31, 56]. This study did not observe significant changes in CsICE1 transcription in SCZ and YH9 during CA and DA (Fig 5B). The expression of CsCBF1 was significantly increased during CA, and reached approximately 282-fold and 29-fold in CA2, in SCZ and YH9, respectively. The transcript level of CsCBF1 during CA was 7.1–9.5 folds higher in SCZ than that of YH9 (P < 0.05) (Fig 5B). In contrast, the transcript level of CsCBF2 was increased in YH9 but remained unaffected in SCZ during CA (Fig 5B). For COR genes, the transcription of CsDNH1, CsDNH2 and CsDNH3 in both cultivars were increased significantly during CA and rapidly decreased following DA. Furthermore, the transcript levels of DHNs were higher in SCZ than in YH9 (Fig 5B). Specifically, CsDNH3 transcript level in SCZ was dramatically increased by 68.7-fold in CA2, while it was only increased by 9.2-fold in YH9 (Fig 5B).
Fig 5. Regulation of the CBF signaling pathway.
The pathway (A) was modified from Thomashow, 1999. Relative expression of the genes in ICE-CBF-COR pathway in SCZ and YH9 over CA and DA was shown in B. Gene transcript level was quantified using real-time quantitative RT-PCR approach. GAPDH was used as a control. Data are displayed as the mean of three replicates and standard error. Different letters indicate significant differences to Duncan’s multiple range tests with P < 0.05. SCZ and YH9 represent tea cold resistant and cold susceptible tea varieties, respectively. NA: non-acclimation; CA1: cold acclimation of 7 days at 10/4°C, day/night temperature; CA2: cold acclimation of 7 days at 4/0°C, day/night temperature; DA: de-acclimation of 7days at 25/20°C, day/night temperature.
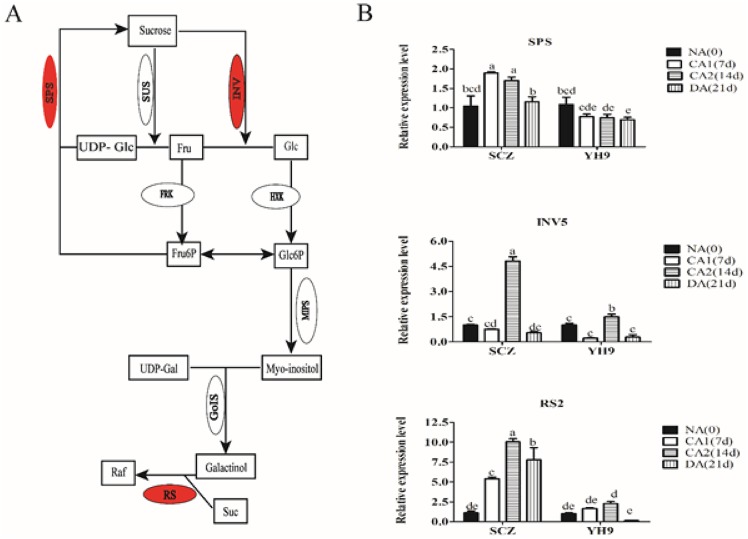
Effect of CA and DA on the transcription of sucrose- and raffinose-related genes
The CsSPS and CsINV5 are responsible for sucrose accumulation and converting to other saccharides (Fig 6A). The transcripts levels of these two genes were checked in both cultivars during CA and DA. We found that the transcript level of CsSPS increased in tea cultivar SCZ but reduced in YH9 through all CA stages, especially in stage CA1 after seven days of CA (Fig 6B). However, CsINV5 expression was decreased at CA1 after seven days of CA but increased by three folds (P < 0.05) at CA2 after 14 days in both cultivars. CsINV5 expression in cultivar SCZ increased by three folds at CA2 compared with less than one fold in YH9 (Fig 6B). Raffinose synthase gene CsRS2, responsible for synthesizing of raffinose, expression was also increased during CA in variety SCZ and then decreased following DA, while it had no distinct changes during CA in YH9 (Fig 6B). This suggested that the genes responsible for sucrose and raffinose accumulation acted positively to regulate the corresponding sugar accumulation in cold treatment.
Fig 6. Effect of cold treatment on gene expression of sugar metabolism (Image A, modified from Yue et al, 2015) in tea.
Gene transcript level (Image B) was quantified using real-time quantitative RT-PCR approach. GAPDH was used as a control. Data are displayed as the mean of three replicates and standard error. Different letters indicate significant differences to Duncan’s multiple range tests with P < 0.05. SCZ and YH9 represent tea cold resistant and cold susceptible tea varieties, respectively. NA: non-acclimation; CA1: cold acclimation of 7 days at 10/4°C, day/night temperature; CA2: cold acclimation of 7 days at 4/0°C, day/night temperature; DA: de-acclimation of 7 days at 25/20°C, day/night temperature.
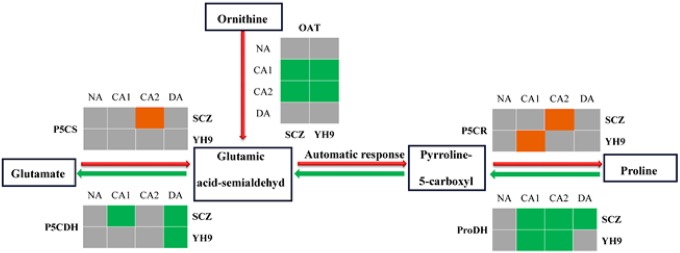
Effect of CA and DA on proline biosynthesis and degradation
We illustrated the proline synthesis and degradation in plants in schematic diagram (Fig 7). The expression patterns of these proline-associated enzyme encoding genes were studied in SCZ and YH9 over the treatments of CA and DA. CsP5CS and CsP5CR were up-regulated at CA2 in SCZ after 14-days cold treatment, and their levels were increased by 1.4-fold and 3.0-fold, respectively. By the contrary, no changes in transcription of CsP5CS were observed in YH9 over the treatments of CA and DA. While CsP5CR was up-regulated in CA1 in YH9. This suggested that long time induced CsP5CR in stage CA2 may be responsible for higher cold tolerance. In both cultivars, the transcription of CsOAT was decreased during CA treatments and returned to the normal level after DA treatment. In addition, the transcript level of CsOAT in SCZ was similar to that in YH9 (Fig 7). CsP5CDH was down-regulated in SCZ during CA1, whereas was unaffected by the CA treatments in YH9 (Fig 7). The transcription of CsProDH in both cultivars was decreased during CA treatments (Fig 7).
Fig 7. Effect of cold treatment on gene expression in proline-metabolism in tea cultivars.
Significant down-, up-regulation and statistically not up/down-regulation is indicated by green color, orange color, and gray color, respectively. Red arrow and green arrow indicate the flow of metabolites with biosynthetic and degradation, respectively. Gene transcript level was quantified using real-time quantitative RT-PCR approach. GAPDH was used as a control. Data are displayed as the mean of three replicates. Significant differences is based on Duncan’s multiple range tests with P < 0.05. SCZ and YH9 represent tea cold resistant and cold susceptible tea varieties, respectively. NA: non-acclimation; CA1: cold acclimation of 7 days at 10/4°C, day/night temperature; CA2: cold acclimation of 7 days at 4/0°C, day/night temperature; DA: de-acclimation of 7 days at 25/20°C, day/night temperature.
Discussion
Low temperature has been a major constraint for tea plantation [47]. During natural CA, tea plants can increase their tolerance to cold weather and survive the winter [8, 45]. In the present study, we demonstrated that plants of either cold-resistant or cold-sensitive tea cultivars can enhance their freezing tolerance due to the treatment of CA in experimental conditions, with a stronger freezing tolerance developed in SCZ than in YH9 (Fig 2B). To understand the mechanisms underlying such differential cold tolerance between SCZ and YH9, we investigated differences between the two cultivars in the physiological and molecular processes that were known to induce cold tolerance in other plants. Our results showed that SCZ exhibited a higher accumulation level of soluble sugars, particularly sucrose than YH9, during cold acclimation (Fig 3). The increased expression of both CBF1 and its targets DHNs could contribute to cold tolerance (Fig 5B). These findings may further elucidate how cold-resistant tea plants can induce strong freezing tolerance in winter.
Through the process of CA, cold resistance was steadily induced in SCZ, while it was induced in YH9 at a slower rate (Fig 2B). Similar to our results, both cold-resistant Medicago (M. falcate) and cold-susceptible Medicago (M. truncatula) could enhance their freezing tolerance by CA at 4°C [57]. However, Pennycooke et al. [58] found that the CA-induced freezing tolerance occurred only in cold-resistant plants, but not in cold-susceptible plants. Under cold stress, the inhibition of chlorophyll synthesis and chloroplast formation can lead to reduced Fv/Fm [59]. Bonnecarrère et al. [16] also used the Fv/Fm to identify cold-resistant rice between two japonica genotypes under identical cold stress. Our results indicated that Fv/Fm fell lower in YH9 than in SCZ during CA2 (Fig 2C), suggesting that Fv/Fm, combined with EL50, could be used to evaluate freezing tolerance in cold-resistant and cold-susceptible tea plants.
Soluble sugar accumulation during CA is positively correlated with freezing tolerance in plants [7]. Sucrose was found as a dominant component of enhanced soluble sugars in giant reed and Medicago during CA [57, 60]. But in Arabidopsis, the accumulation of glucose is largely responsible for the increased level of soluble sugars during cold acclimation and sucrose is the second most abundant sugar in CA [61]. In our study, the content of sucrose, glucose, raffinose, and fructose were increased during CA in SCZ and YH9, and the total sugar content was higher in SCZ than in YH9 (Fig 3). In other studies, greater accumulation of sucrose in cold resistant Medicago, wheat, and maize was found to be responsible for higher freezing tolerance [57, 62–63]. Trehalose accumulation conferred tolerance to cold stress serving as an osmolyte or protein/membrane protectant by acting as scavengers for ROS to alleviate oxidative damage to the membranes [64]. Therefore, the accumulation of sucrose, as the major sugar, in cold resistant tea plants could play an essential role in conferring higher freezing tolerance in tea plant. Trehalose accumulation was not observed in SCZ during the CA, although the trehalose content was induced slightly in YH9, yet lower than in SCZ (Fig 3E). Our data showed that raffinose contents in the two cultivars were very similar during CA (Fig 3D). However, raffinose was found to be not essential for basic freezing tolerance or for cold acclimation of A. thaliana [38]. Thus, the role of raffinose in cold resistance in tea plant may be not essential too. Yue et al. [8] reported the content of total sugars and several specific sugars including sucrose, glucose and fructose were constantly elevated in Longjing43 tea leaves during nature acclimation. While Shen et al. [47] reported the raffinose, maltose, glucose and fructose were all more abundant in HuangShanzhong tea leaves during nature acclimation.
During natural cold acclimation, a series of sugar-related genes, including CsSPS, CsRS2, and CsINV5, are up-regulated in the tea plant (cv. Longjing43) [8], which suggests that these genes might be responsible for sugar accumulation. Under the controlled cold treatment and DA in our study, the transcription of the CsSPS, CsINV5, and CsRS2 during CA was also up-regulated in SCZ but remained unchanged in YH9 (Fig 6). This demonstrated that the expression regulation of CsSPS, CsINV5, and CsRS2 during CA are cultivar specific. Therefore, these genes’ expression can be used as cultivar specific cold resistance indicator for tea breeding.
Free proline has been reported to accumulate in many plants in response to biotic and abiotic stresses, acting as a compatible solute against osmotic stress [39]. Free proline was one of the indicators used to identify dehydration ‘resistant’ wheat genotypes from ‘sensitive’ ones [65]. Kumar and Yadav [66] reported that enhanced proline could increase the tolerance of tea bud to cold stress. Similarly, tea cultivars ‘Zhuyeqi’ (drought-susceptible) and ‘Ningzhou 2’ (drought-tolerant) could be distinguished due to their differential proline contents under drought stress [67]. Our data showed that proline content was significantly increased in SCZ and YH9 during CA1, but no difference in the total proline content was found between the two cultivars (Fig 4). Therefore, we propose that proline content has effects on the abiotic resistance of tea plants and that the accumulated proline was not a key factor for conferring cold-resistant in tea plants. However, proline concentration is correlated with cold-resistance in giant reed and spring canola [60, 68]. According to Delauney [69], under abiotic stress, proline was accumulated by the glutamate biosynthesis pathway. Our result showed that the OAT, responsible for glutamie acid scmialdehyd, transcript level decreased in both cultivars (Fig 7). In accordance with Delauney [69], it was glutamate, not ornithine, which could likely be the main precursor for proline biosynthesis in tea plants during CA. The transcription of CsP5CS and CsP5CR for proline biosynthesis was higher in SCZ than in YH9, and the transcripts of CsProDH and CsP5CDH for proline degradation differed between two cultivars (Fig 7). It showed that the transcription level of the related genes was not consistent with metabolic changes and further enzymatic assays are required to elucidate the proline biosynthesis mechanisms.
To date, ICE and CBF genes are known to play key roles in cold tolerance. The transcription of CsCBF1, not CsICE1, was induced at 4°C [34]. With CA treatment, our results consistently showed that there was not change in expression of CsICE1 in both cold resistant and susceptible tea cultivars. We found that the transcription of CsCBF1 was significantly up-regulated by CA, and remained high level until DA (Fig 5B). A higher expression change of CsCBF1 was found in cold resistant cultivar SCZ than cold susceptible YH9 (Fig 5B), which may explain the difference in cold resistance in the two tea cultivars. A similar result was also found in Medicago and Jatropha [57, 60]. However, Pan et al. [70] reported a contradictory finding that the cold-susceptible rice had a much strong transcription of CBFs than cold-resistant rice. This might be caused by the species difference. Our data found a higher up-regulation of CsCBF2 transcription in YH9 than SCZ during CA (Fig 5B). AtCBF2 is a negative regulator of AtCBF1 in Arabidopsis. We hypothesized that that CsCBF2 in tea plant might also be a negative regulator of CsCBF1. In this case, the lowered transcription of CsCBF1 can be explained by the suppression of the increased transcription of CsCBF2 in YH9. The high level of CsCBF1 transcription in SCZ was a result of low transcription of CsCBF2. Further investigation of the suppression would be the priority in future study. In addition, CsCBF1 in SCZ and YH9 had the same ORF (Open Reading Frame) without Intron. We speculate that SNP and INDEL may be present in the promoter region of CsCBF1, and regulate the transcription of CsCBF1 between resistant and susceptible species. DHNs (COR genes), a subgroup of the late embryogenesis abundant protein family, can act as a cryoprotectant and molecular chaperone as well as an anti-oxidant. DHNs can be induced by cold stress, and their transcription is correlated with freezing tolerance [36, 71]. In this study, the transcripts of CsDHN1, CsDHN2, and CsDHN3 were accumulated at higher levels in SCZ than YH9 during CA (Fig 5B). Similar results were found in Loquat (Eriobotrya japonica), where seven dehydration genes were up-regulated under low-temperature stress, with significantly higher transcription observed in cold resistance than cold-susceptible cultivars [71]. The higher levels of CsDHN1, CsDHN2and CsDHN3 may result in higher amount of dehydrin proteins, thus protecting SCZ from dehydration under freezing stress.
Of course, other mechanisms may involve in the cold acclimation in tea plant. One of them is that the PLD (Phospholipase D) pathway, which responses to freezing and plays key roles in conferring higher cold resistance [11]. PLDs, lipid catabolism enzymes, are activated by a fall in temperature, and the expression levels are found to increase during cold stress [72, 73]. Phosphatidic acid (PA), a catalyzed production of phospholipase D (PLD), involves in many cellular processes, including cell signaling, vesicular trafficking and membrane remodeling [12, 72]. Cold acclimation also affects cell lipid composition, which in favor of the maintenance of plasma membrane functionality and fluidity [10, 74]. In particular, the proportion of unsaturated fatty acids making up the phospholipids is increased [74]. A substantial increase in linoleic acid (C18:2) has been reported for cold acclimated Solanum commersonii plants, a potato wild species able to increase freezing tolerance. While the freezing susceptible species, Solanum tuberosum, was an increase of C18:3 [75]. Fatty acids unsaturation is controlled by a transcriptional regulation of key desaturase genes. The cotyledons of cold acclimated plants produced a high-fold increase in delta 12 desaturase FAD2-3 (FAD2-3) expression compared with non-acclimated plants [13]. For tea plant (Cs var. sinensis), both CsFAD7 and CsFAD8 were cloned, and CsFAD8 genes has a high expression in cold resistant cultivar than susceptible cultivar [48]. Due to a fact that the cold acclimation may not apply to some plants such as crop wheat, it is worthy to investigate how the lipid metabolism is regulated under cold in tea and whether it is correlated with expression level of fatty acid related genes in cold in our future study using RNA-seq and metabolomics strategy.
In summary, the data presented here have demonstrated the difference of physiological, biochemical, and gene expression levels explained the difference in cold tolerance in cold-resistant tea cultivar SCZ and cold-susceptible tea cultivar YH9. These findings have contributed a better insight into the molecular mechanisms that underly cold tolerance in tea plants.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant number: 31270729], the Special Innovative Province Construction in Anhui Province (15czs08032), the Central Guiding the Science and Technology Development of the Local (2016080503B024).
Abbreviations
- CA
cold acclimation
- CBF
C-repeat/dehydration-responsive element binding factor
- CsINV5
Invertase gene
- CsOAT
Ornithine-D-aminotransferase
- CsP5CDH
P5Cdehydrogenase
- CsP5CR
1-pyrroline-5-carboxylate reductase
- CsP5CS
Δ -1-Pyrroline-5-carboxylate synthase
- CsProDH
Proline dehydrogenase
- CsRS2
Raffinose synthase gene
- CsSPS
Sucrose phosphate synthase
- DA
de-acclimation
- DHN
Dehydrin
- EL50
Temperature leading to 50% tissue damages due to leakage of electrolyte
- Fv/Fm
maximum quantum yield of PSII photosystems
- SCZ
Camellia sinensis cv. Shuchazao
- YH9
Camellia sinensis var. assamica cv.Yinghong9
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number: 31270729]. The URLs of funder's website http://www.nsfc.gov.cn/publish/portal1/. The funders had key role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gaete-Loyola J, Lagos C, Beltrán MF, Valenzuela S, Emhart V, Fernández M. Transcriptome profiling of eucalyptus nitens, reveals deeper insight into the molecular mechanism of cold acclimation and deacclimation process. Tree Genetics Genomes. 2017; 13: 37. [Google Scholar]
- 2.Le GH, Fontaine JX, Molinié R, Pelloux J, Mesnard F, Gillet F, et al. Nmr-based metabolomics to study the cold-acclimation strategy of two miscanthus genotypes. Phytochemical Analysis. 2017; 28: 58–67. doi: 10.1002/pca.2649 [DOI] [PubMed] [Google Scholar]
- 3.Bertrand A, Bipfubusa M, Claessens A, Rocher S, Castonguay Y. Effect of photoperiod prior to cold acclimation on freezing tolerance and carbohydrate metabolism in alfalfa (medicago sativa l.). Plant Sci. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Wei H, Dhanaraj AL, Rowland LJ, Fu Y, Krebs SL, Arora R. Comparative analysis of expressed sequence tags from cold-acclimated and non-acclimated leaves of Rhododendron catawbiense Michx. Planta. 2005; 221: 406–416. doi: 10.1007/s00425-004-1440-1 [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006; 57: 781 doi: 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- 6.Warren GJ. Cold stress: manipulating freezing tolerance in plants. Curr Biol Cb. 1998; 8: 514–516. [DOI] [PubMed] [Google Scholar]
- 7.Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Bio. 1999; 50: 571–599. [DOI] [PubMed] [Google Scholar]
- 8.Yue C, Cao HL, Wang L, Zhou YH, Huang YT, Hao XY, et al. Effects of CA on sugar metabolism and sugar-related gene expression in tea plant during the winter season. Plant Mol Biol. 2015; 88: 591–608. doi: 10.1007/s11103-015-0345-7 [DOI] [PubMed] [Google Scholar]
- 9.Khokhlova LP, Olinevich OV, Tarakanova NY, Timofeeva OA. Oryzalin-induced changes in water status and cytoskeleton proteins of winter wheat seedlings upon cold acclimation and aba treatment. Russ J Plant Physiol. 2004; 51: 684–696. [Google Scholar]
- 10.Li WQ, Wang RP, Li MY, Li LX, Wang CM, Welti R, et al. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in arabidopsis thaliana. J Biol Chem. 2008; 283: 461–468. doi: 10.1074/jbc.M706692200 [DOI] [PubMed] [Google Scholar]
- 11.Li WQ, Li MY, Zhang WH, Welti R, Wang XM. The plasma membrane-bound phospholipase ddelta enhances freezing tolerance in arabidopsis thaliana. Nat. Biotechnol. 2004; 22: 427–33. doi: 10.1038/nbt949 [DOI] [PubMed] [Google Scholar]
- 12.Kargiotidou A, Kappas I, Tsaftaris A, Galanopoulou D, Farmaki T. Cold acclimation and low temperature resistance in cotton: gossypium hirsutum phospholipase dalpha isoforms are differentially regulated by temperature and light. J Exp Bot. 2010; 61, 2991 doi: 10.1093/jxb/erq124 [DOI] [PubMed] [Google Scholar]
- 13.Kargiotidou A, Deli D, Galanopoulou D, Tsaftaris A, Farmaki T. Low temperature and light regulate delta 12 fatty acid desaturases (fad2) at a transcriptional level in cotton (gossypium hirsutum). J Exp Bot. 2008; 59: 2043–2056. doi: 10.1093/jxb/ern065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura M, Steponkus PL. Effect of cold acclimation on the lipid composition of the inner and outer membrane of the chloroplast envelope isolated from rye leaves. Plant Physiol. 1997; 114: 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John R, Anjum NA, Sopory SK, Akram NA, Ashraf M. Some key physiological and molecular processes of cold acclimation. Biol Plantrum. 2016; 60:603–618. [Google Scholar]
- 16.Bonnecarrère V, Borsani O, Díaz P, Capdevielle F, Blanco P, Monza J. Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Sci. 2011; 180: 726–732. doi: 10.1016/j.plantsci.2011.01.023 [DOI] [PubMed] [Google Scholar]
- 17.Mollo L, Martins MCM, Oliveira VF, Nievola CC, Cassia R, Figueiredo-Ribeiro L. Effects of low temperature on growth and non-structural carbohydrates of the imperial bromeliad Alcantarea imperialis cultured in vitro. Plant Cell Tissue Organ Cult. 2011; 107: 141–149. [Google Scholar]
- 18.Kamata T, Uemura M. Solute accumulation in heat seedlings during cold acclimation: contribution to increased freezing tolerance. Cryo Letters. 2004; 25: 311–322. [PubMed] [Google Scholar]
- 19.Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, et al. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007; 50: 967–981. doi: 10.1111/j.1365-313X.2007.03100.x [DOI] [PubMed] [Google Scholar]
- 20.Sanghera GS, Wani SH, Wasim H, Singh NB. Engineering Cold Stress Tolerance in Crop Plants. Curr Genomics. 2011; 12: 30–43. doi: 10.2174/138920211794520178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila O, Welling A, Li CY, Tsegay BA, Tapio Palva E. Physiological aspects of cold hardiness in northern deciduous tree species Plant Cold Hardiness: Molecular biology, Biochemistry, and physiology. 2002. pp. 65–76. [Google Scholar]
- 22.Yadav SK. Cold stress tolerance mechanisms in plants. A review. Agron Sustain Dev. 2010; 30: 515–527. [Google Scholar]
- 23.Nordin K, Heino P, Palva ET. Separate signal pathways regulate the expression of a low-temperature-induced gene in Arabidopsis thaliana, (L.) Heynh. Plant Mol Biol. 1991; 16:1061–1071. [DOI] [PubMed] [Google Scholar]
- 24.Fowler S, Thomashow M F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002; 14: 1675–1690. doi: 10.1105/tpc.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BH, Henderson DA, Zhu JK. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell. 2005; 17: 3155–3175. doi: 10.1105/tpc.105.035568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benedict C, Skinner JS, Meng R, Chang Y, Bhalerao R, Huner NP, et al. The cbf1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Env. 2006; 29: 1259–1272. [DOI] [PubMed] [Google Scholar]
- 27.Jaglo KR, Thomashow MF. Components of the Arabidopsis c-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in brassica napus and other plant species. Plant Physiol. 2001; 127: 910–917. [PMC free article] [PubMed] [Google Scholar]
- 28.Kitashiba H, Ishizaka T, Isuzugawa K, Nishimura K, Suzuki T. Expression of a sweet cherry DREB1/CBF ortholog in Arabidopsis confers salt and freezing tolerance. J Plant Physiol. 2004; 161: 1171–1176. doi: 10.1016/j.jplph.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 29.El KW, Navarro M, Marque G, Keller G, Marque C, Teulieres C. Expression profile of CBF-like transcriptional factor genes from eucalyptus in response to cold. J Exp Botany. 2006; 57: 2455–2469. [DOI] [PubMed] [Google Scholar]
- 30.Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene transcription in plants. Trends Plant Sci. 2007; 12: 444–451. doi: 10.1016/j.tplants.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 31.Thomashow MF. Molecular Basis of Plant CA: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010; 154: 571–577. doi: 10.1104/pp.110.161794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miura K, Jin JB, Lee J, Chan YY, Stirm V, Miura T, et al. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A transcription and freezing tolerance in Arabidopsis. Plant Cell. 2007; 19: 1403–1414. doi: 10.1105/tpc.106.048397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in CA and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA. 2007; 104: 21002–21007. doi: 10.1073/pnas.0705639105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Jiang CJ, Li YY, Wei CL, Deng WW. CsICE1 and CsCBF1: two transcription factors involved in cold responses in Camellia sinensis. Plant Cell Rep. 2012; 31: 27–34. doi: 10.1007/s00299-011-1136-5 [DOI] [PubMed] [Google Scholar]
- 35.Yuan HY, Zhu XP, Zeng W, Yang HM, Sun N, Xie SX, et al. Isolation and transcription activation analysis of the CsCBF1 gene from Camellia sinensis. Acta Botanica Boreali-Occidentalia Sinica. 2013; 110: 147–151. [Google Scholar]
- 36.Li YY, Zhou YQ, Xie XF, Shu XT, Deng WW, Jiang CJ. Cloning and transcription analysis of dehydrin gene (CsDHN) in tea plant (Camellia sinensis). J Agric Biotechnol. 2016; 24: 332–341. [Google Scholar]
- 37.Ruan Y L. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014; 65: 33–67. doi: 10.1146/annurev-arplant-050213-040251 [DOI] [PubMed] [Google Scholar]
- 38.Zuther E, Büchel K, Hundertmark M, Stitt M, Hincha DK, Heyer AG. The role of raffinose in the cold acclimation response of Arabidopsis thaliana. Febs Lett. 2004; 576: 169–173. doi: 10.1016/j.febslet.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 39.Hare PD, Cress WA, Staden JV. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J Exp Bot. 1999; 50: 413–434. [Google Scholar]
- 40.Székely G, Abrahám E, Cséplo A, Ga´bor R´, Laura Z, Jola´n C, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008; 53: 11–28. doi: 10.1111/j.1365-313X.2007.03318.x [DOI] [PubMed] [Google Scholar]
- 41.Roosens NH, Bitar FA, Loenders K, Angenon G, Jacobs M. Overexpression of ornithine-δ-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol Breeding. 2002; 9: 73–80. [Google Scholar]
- 42.Khavari-Nejad RA, Band RS, Najafi F, Nabiunia M, Gharari Z. The role of Pro-P5C Cycle in chs mutants of Arabidopsis under cold stress. Russ J Plant Physiol. 2013; 60: 375–382. [Google Scholar]
- 43.Banerjee B. Botanical classification of tea In: Wilson KC, Clifford MN, editors. Tea: cultivation to consumption. London: Chapman and Hall: 1992. pp. 25–51. [Google Scholar]
- 44.Kaundun S, Matsumoto S.Development of CAPS markers based on three key genes of the phenylpropanoid pathway in Tea, Camellia sinensis (L.) O. Kuntze and differentiation between assamica and sinensis varieties. Theor Appl Genet. 2003; 106: 375–383. doi: 10.1007/s00122-002-0999-9 [DOI] [PubMed] [Google Scholar]
- 45.Vyas D, Kumar S. Tea (Camellia sinensis (L.) O. Kuntze) clone with lower period of winter dormancy exhibits lesser cellular damage in response to low temperature. Plant Physiol Biochem. 2005; 43: 383–388. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Zhu X, Chen X, Song C, Zou Z, Wang Y, et al. Identification and characterization of cold-responsive microRNAs in tea plant (Camellia sinensis) and their targets using high-throughput sequencing and degradome analysis. BMC Plant Biol. 2014; 14: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen J, Wang Y, Chen C, Zhao TD, Jian HH, Chao Z, et al. Metabolite profiling of tea (Camellia sinensis, L.) leaves in winter. Sci Hort. 2015; 192: 1–9. [Google Scholar]
- 48.Ding ZT, Ma QP, Wang Y. The differences between two tea varieties in their response to natural cold conditions. J Hortic Sci Biotechnol. 2016; 91: 1–8. [Google Scholar]
- 49.Wang XC, Zhao QY, Ma CL, Zhang ZH, Cao HL, Kong YM, et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genomics. 2013; 14: 415 doi: 10.1186/1471-2164-14-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schreiber U, Bilger W, Neubauer C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of in Vivo Photosynthesis In: Schulze ED, Caldwell MM, editors. Ecophysiology of Photosynthesis. Berlin Heidelberg: Springer; 1995. pp. 49–70. [Google Scholar]
- 51.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973; 39: 205–207. [Google Scholar]
- 52.Castonguay Y, Nadeau P, Lechasseur P, Chouinard L. Differential accumulation of carbohydrates in alfalfa cultivars of contrasting winter hardiness. Crop Sci. 1995; 35: 509–516. [Google Scholar]
- 53.Velada I, Ragonezi C, Arnholdtschmitt B, Cardoso H. Reference genes selection and normalization of oxidative stress responsive genes upon different temperature stress conditions in hypericum perforatum l. Plos One. 2014; 9: e115206.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene transcription data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 55.Bolouri-moghaddam MR, Roy KL, Li XA, Rolland F, Ende WVD. Sugar signaling and antioxidant network connections in plant cells. Febs J. 2010; 277: 2022–2037. doi: 10.1111/j.1742-4658.2010.07633.x [DOI] [PubMed] [Google Scholar]
- 56.Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997; 94: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang LL, Zhao MG, Tian QY, Zhang WH. Comparative studies on tolerance of Medicago truncatula and Medicago falcata to freezing. Planta. 2011; 234: 445–457. doi: 10.1007/s00425-011-1416-x [DOI] [PubMed] [Google Scholar]
- 58.Pennycooke JC, Cheng H, Stockinger EJ. Comparative genomic sequence and expression analyses of Medicago truncatula and alfalfa subspecies falcata COLD-ACCLIMATION-SPECIFIC genes. Plant Physiol. 2008; 146: 1242–1254. doi: 10.1104/pp.107.108779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Chen Q, Wang S, Hong Y, Wang Z. Rice and cold stress: methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice. 2014; 7: 24 doi: 10.1186/s12284-014-0024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pompeiano A, Vita F, Miele S, Guglielminetti L. Freeze tolerance and physiological changes during cold acclimation of giant reed [Arundo donax (L.)]. Grass Forage Sci. 2013; 70: 168–175. [Google Scholar]
- 61.Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. Overtranscription of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2001; 124: 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tognetti JA, Salerno CL, Crespi MD, Pontis HG. Sucrose and fructan metabolism of different wheat cultivars at chilling temperatures. Physiol plantarum. 1990; 78: 554–559. [Google Scholar]
- 63.Sowiński P, Dalbiak A, Tadeusiak J, Ochodzki P. Relations between carbohydrate accumulation in leaves, sucrose phosphate synthase activity and photoassimilate transport in chilling treated maize seedlings. Acta Physiol Plant. 1999; 21: 375–381. [Google Scholar]
- 64.Iordachescu M, Imai R. Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol. 2008; 50: 1223–1229. doi: 10.1111/j.1744-7909.2008.00736.x [DOI] [PubMed] [Google Scholar]
- 65.Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C. Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Env. 2006; 29: 2143–2152. [DOI] [PubMed] [Google Scholar]
- 66.Kumar V, Yadav SK. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol Plant. 2009; 31: 261–269. [Google Scholar]
- 67.Liu SC, Yao MZ, Ma CL, Jin JQ, Ma JQ, Li CF, et al. Physiological changes and differential gene expression of tea plant under dehydration and rehydration conditions. Sci Hort. 2015; 184: 129–141. [Google Scholar]
- 68.Moieni-Korbekandi Z, Karimzadeh G, Sharifi M. Cold-induced changes of proline, malondialdehyde and chlorophyll in spring canola cultivars. J Plant Physiol Breeding 2014; 4: 1–11. [Google Scholar]
- 69.Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant J. 1993; 4: 215–223. [Google Scholar]
- 70.Pan X, Li Y, Li X. CBF regulon between Nipponbare (Japonica) and 93–11 (Indica) during cold acclimation. Rice Sci. 2013; 20: 165–172. [Google Scholar]
- 71.Paul A, Kumar S. Dehydrin2 is a stress-inducible, whereas Dehydrin1 is constitutively expressed but up-regulated gene under varied cues in tea [Camellia sinensis (L.) O. Kuntze]. Mol Biol Rep. 2013; 40: 3859–3863. doi: 10.1007/s11033-012-2466-2 [DOI] [PubMed] [Google Scholar]
- 72.Xu H, Yang Y, Li X, Feng C, Chen JW, Xu CJ. Involvement of multiple types of dehydrins in the freezing response in Loquat (Eriobotrya japonica). Plos One. 2014; 9:e87575 doi: 10.1371/journal.pone.0087575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meijer HJG, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003; 54, 265–306. doi: 10.1146/annurev.arplant.54.031902.134748 [DOI] [PubMed] [Google Scholar]
- 74.Rajashekar CB. Cold response and freezing tolerance in plants In: Wilkinson RE editor, Plant–environment interactions, 2nd ed New York: Marcel Dekker; 2000. pp. 321–341. [Google Scholar]
- 75.Palta JP, Whitaker BD, Weiss LS. Plasma membrane lipids associated with genetic variability in freezing tolerance and cold acclimation of solanum species. Plant Physiol. 1993; 103, 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.