Abstract
Scope
This study aimed to analyse the prevalence, antibiotic resistance and genetic relatedness of P. aeruginosa isolates obtained from potable and recreational water samples (n. 8,351) collected from different settings (swimming pools, n. 207; healthcare facilities, n 1,684; accommodation facilities, n. 1,518; municipal waterworks, n. 4,500; residential buildings, n. 235). Possible mechanisms underlying resistance to imipenem, with particular focus on those involving oprD-based uptake, were also explored.
Methods and results
Isolation and identification of Pseudomonas aeruginosa was performed according to the standardized procedure UNI EN ISO 16266:2008 followed by PCR confirmation. Antibiotic Susceptibility testing was conducted according to EUCAST standardized disk diffusion method. Genetic relatedness of strains was carried out by RAPD. The sequence of the oprD gene was analyzed by standard method. Fifty-three samples (0.63%) were positive for P. aeruginosa, of which 10/207 (4.83%) were from swimming pools. Five isolates (9.43%) were resistant to imipenem, one to Ticarcillin + Clavulanate, one to both Piperacillin and Ticarcillin + Clavulanate. The highest isolation rate of imipenem resistant P. aeruginosa was observed in swimming pool water. Identical RAPD profiles were found in isolates from the same location in the same year or even in different years.
Conclusions
Imipenem resistant strains were identified as carbapenemase-negative and resistance has been associated with inactivating mutations within the oprD gene, with a concomitant loss of porin. RAPD results proved that a water system can remain colonized by one strain for long periods and the contamination may be difficult to eradicate.
This study has revealed the presence of P. aeruginosa in different water samples, including resistant strains, especially in swimming pools, and confirmed the role of porins as a contributing factor in carbapenem resistance in Gram-negative bacteria.
Introduction
Pseudomonas aeruginosa is an opportunistic human pathogen implicated in a variety of acute and chronic infections such as respiratory, urinary tract and gastrointestinal infections as well as bacteremia. It is mainly found in subjects with compromised host defenses, e.g., cancer, HIV and cystic fibrosis (CF) patients [1], and is a significant cause of morbidity and mortality.
It is widespread in the environment, particularly in a variety of water sources such as hospital [2] and municipal drinking water systems [3], healthcare facilities [2,4], accommodation facilities [5], as well as in swimming pools and hot tubs [6,7], where P. aeruginosa is also a major cause of skin infections such as folliculitis and external otitis [8,9]. In these water systems, P. aeruginosa has the ability to grow [10–12] and form biofilms [13].
In Italy the quality of water intended for human consumption is ensured by Legislative Decree n.31/2001 [14] and its subsequent amendments, Legislative Decree n.27/2002 [15], while the microbiological criteria for swimming pool water are established by Italian Guidelines [16].
For P. aeruginosa in swimming pools, there is a maximum permissible limit of zero per 100 ml of inlet water and ≤1 cfu (colony-forming units) per 100 ml of pool water.
Because of its ubiquity, enormous versatility, intrinsic tolerance to many detergents, disinfectants and antimicrobial compounds, P. aeruginosa is difficult to control. Infections caused by this pathogen are often difficult to treat because of the bacterium’s drug and multidrug-resistant (MDR) phenotypes [17]. Indeed, in the last few years P. aeruginosa has shown increasing resistance to many antimicrobials, included carbapenems, a class of β-lactam antibiotics widely used in clinical settings [18]. Moreover, a recent study reported that 96% of P. aeruginosa isolates from swimming pools and hot tubs were found to be multidrug resistant [19], including resistance to front-line antipseudomonal drugs, with the highest percentage of isolates proving resistant to imipenem, β-lactam antibiotic of the carbapenem class.
In 2015, 30 European Union/ European Economic Area (EU/EEA) countries reported 12,689 P. aeruginosa isolates with antimicrobial susceptibility testing (AST) information for carbapenems (imipenem or meropenem). The number of carbapenem resistant isolates reported per country ranged from 12 to 1,925.
The national percentages of carbapenem-resistant P. aeruginosa isolates ranged from 0% (Iceland) to 66.3% (Romania), with a 23% prevalence in Italy [20].
Imipenem resistance can involve low permeability, the activity of an inducible β-lactamase [21], and multidrug efflux systems, but the most common mechanism underlying resistance involves the loss of OprD porins from the outer membrane [22], which can occur at the transcriptional or translational level or through the emergence of mutations in the oprD gene [23].
The aim of this study was to investigate the prevalence, antimicrobial susceptibility profiles and genetic relatedness of P. aeruginosa isolates obtained from water samples collected from different locations in the Marches Region, Central Italy.
Possible mechanisms underlying resistance to imipenem, with particular focus on those involving oprD-based uptake, were also explored.
Materials and methods
Collection of water samples
A total of 8,351 water samples were collected in a wide range of different settings in the Marches Region between 2012–2015: 207 samples in 41 public swimming pools; 1,684 samples in 7 healthcare facilities (hospitals, residential care homes); 1,518 samples in 72 accommodation facilities (campgrounds, hotels, residences, temporary guesthouses); 4,500 samples in 2 municipal waterworks; 235 samples in 30 residential buildings (households).
These samples were collected from Ancona, Ascoli Piceno, Fermo, Macerata, Pesaro-Urbino districts of Marches Region, except for swimming pool and municipal waterworks, which were from Pesaro-Urbino. This is a territorial area of about 9 365.86 km2 of Central-Eastern Italy. In the study facilities, water samples were taken from taps, showers and drinking fountains and collected in sterile bottles after 1–5 min of free flow, after disinfection of sample port by 70% ethanol (plastic tubes) or by flaming (metal tubes). For water samples a solution of 0.1 N of sodium thiosulphate was added to neutralize any residual chlorine. Samples were transported into the laboratory using a portable cooler (4–6°C) and analysed immediately.
The data reported in this study have been obtained through the official activity of the Azienda Sanitaria Unica Regionale (ASUR) and the Agenzia Regionale per la Protezione Ambientale delle Marche (ARPAM), which are the authorities responsible for water sampling and analysis. Further specific permissions are not required.
A complete list of sampling locations including isolated strains and facility identification is provided in S1 Table.
Isolation and identification of Pseudomonas aeruginosa
Isolation and identification of Pseudomonas aeruginosa was performed according to the standardized procedure UNI EN ISO 16266:2008 [24]. Briefly, 100 mL of each water sample was filtered with a cellulose ester membrane (0.45 μm porosity, 47 mm diameter; Millipore, Billerica, MA, USA), which was then placed onto a Pseudomonas Agar with Pseudomonas CN Supplement (PACN) (Oxoid, Basingstoke, UK) plate.
PACN plates were incubated at 35 ± 1°C for 44 ± 4 h before the counting of colonies. Blue/green pyocyanin-producing colonies were counted as confirmed P. aeruginosa according to UNI EN ISO 16266:2008 [24]. Fluorescent non-pyocyanin-producing or reddish brown colonies were recorded as presumptive P. aeruginosa and subjected to confirmation tests according to UNI EN ISO 16266:2008 [24].
Real-time PCR confirmation of Pseudomonas aeruginosa isolates
All stocked isolates were tested for species confirmation by amplification of a specific fragment of the ecfX gene. DNA was obtained from each isolate, subcultured on Tryptone Soya Agar (TSA, Thermo Scientific—Oxoid, Basingstoke, UK), by colony lysis by boiling. Lysates were then amplified with primers ecfXF-ecfXR (final concentration 0.4 μM each) and dual labeled probe ecfX-TM (0.16 μM), according to Amagliani et al. [25], with the Hot-Rescue Real-Time PCR Kit (Diatheva, Fano, Italy). Amplification reactions were conducted in a Rotor Gene 3000A (Corbett Research, Sydney, Australia) with the following thermal protocol: denaturation at 95°C for 10 min; 40 cycles at 95°C for 15 s and 60°C for 1 min. Negative (H2O) and positive (P. aeruginosa ATCC 10145 DNA) controls were used in each amplification run and analyzed in the Green channel along with the samples.
RAPD fingerprinting of Pseudomonas aeruginosa isolates
P. aeruginosa isolates were grown in 5 ml Pseudomonas Selective Broth (Biolife, Milan, Italy) with incubation at 35°C overnight. DNA was then extracted from bacterial pellets using the Bacterial Genomic DNA Isolation Kit (Norgen Biotek, Thorold, Canada) and its concentration estimated through the NanoDrop ND-1000 System (NanoDrop Technologies, Wilmington, DE). RAPD (Random Amplification of Polymorphic DNA) typing was carried out according to Lanotte et al. [26] with 272 primer (5’-AGCGGGCCAA-3’) and 40 ng DNA of each strain. PCR products (12 μl) were run on 1.5% agarose gel with GeneRuler 100 bp DNA Ladder (Thermo Scientific) and analyzed on a Gel Doc 2000 apparatus using Quantity One Quantitation Software (Bio-Rad, Hercules, CA, USA). The molecular size (bp) of each potential band position was determined across all RAPD-PCR profiles. At each band position, two possible alleles were considered either present (a score of 1) or absent (a score of 0). Different RAPD profiles were designated by different scores and classified as different genotypes.
A dendrogram was constructed through the MVSP software (ver. 3.22) (available at http://mvsp.software.informer.com/3.2/). After construction of a similarity matrix, data were cluster analyzed using the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method with similarity of distance according Jaccard’s coefficient.
Antibiotic Susceptibility testing (AST)
All P. aeruginosa isolates (n. 53) were tested for antibiotic sensitivity using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standardized disk diffusion method [27]. These tests were performed on Mueller-Hinton agar (Difco, Michigan, USA) using the disk diffusion technique by Kirby-Bauer. From the exponential bacterial growth (18–24 hours) in non-selective agar plates, colonies were suspended in 5 ml sterile saline (0.85% NaCl), adjusted to 0.5 McFarland scale turbidity (∼108CFU/ml) and then seeded in Mueller-Hinton agar with the aid of a sterile swab. Within 15 min, antimicrobial disks containing antibiotic were applied to the surface of the agar plates. All plates were incubated at 35°C ± 1° for 16–18 hours. Zones of inhibition around the disk were measured and interpreted as proposed by the EUCAST breakpoint criteria (http://www.eucast.org.).
Sixteen antimicrobial molecules, belonging to 6 different classes, were tested: Penicillins: Piperacillin 30 μg, Piperacillin + Tazobactam 30 + 6 μg, Ticarcillin + Clavulanate 75 + 10 μg, Ticarcillina 75 μg; Cephalosporins: Cefepime 30 μg, Ceftazidime 10 μg; Carbapenems: Doripenem 10 μg, Imipenem 10 μg, Meropenem 10 μg; Monobactams: Aztreonam 30 μg; Fluoroquinolones: Ciprofloxacin 5 μg, Levofloxacin 5 μg; Aminoglycosides: Amikacin 30 μg Gentamicin 10 μg Tobramycin 10 μg, Netilmicin 10 μg (Biolife, Milan, Italy)
The reference strain of P. aeruginosa ATCC 27853 was used as a quality control in all experiments. A control plate without antibiotics was included in each series.
Each experiment was performed three times.
PCR detection of β-lactamases genes
The Xpert Carba R assay (Cepheid, Sunnyvale, CA, USA), which detects and differentiates the most prevalent gene families associated with P. aeruginosa resistance to carbapenems (blaKPC, blaNDM, blaIMP, blaOXA-48-like, and blaVIM), was used on isolates showing resistance to carbapenems in disk diffusion tests according to the manufacturer’s instructions.
DNA sequencing and analyses of sequence data
The sequence of the oprD gene was analyzed in all isolates from swimming pools and one resistant strain from healthcare facilities. The entire coding sequence and its flanking regions, including the promoter, were amplified with specific primers: OprD_PCR F (5’-CGTCGCTTCGGAACCTCAACTA-3’) and OprD_PCR R (5’- GCCGTGACCTCGAACCTGA-3’). The PCR was performed in a final volume of 25 μl, using 200 nM of each primer and 2.5 U HotStarTaq DNA Polymerase (Qiagen), under the following conditions: 95°C for 15 min, 35 cycles at 94°C for 1 min, 63°C for 30 s and 72°C for 3 min. The PCR products were separated on 1% agarose gel, purified using MinElute Gel Extraction Kit (Qiagen), and directly sequenced. Specific primers for the target oprD sequence were designed and used for the DNA sequencing analyses (Table 1). Primers targeting the insertion sequences (IS) within the oprD gene were also designed and used in strains indicated in Table 1. Sequencing was performed with the BigDye Terminator Cycle Sequencing Kit v1.1 and run in the ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
Table 1. Primers used for sequencing.
| Name | 5’-3’ | Strainsa |
|---|---|---|
| Specific primers for the target oprD gene | ||
| oprD_PCR F | CGTCGCTTCGGAACCTCAACTA | All strains |
| oprD_PCR R | GCCGTGACCTCGAACCTGA | All strains |
| oprD_seq F | CCTGAAGCTCGACGGCACCTC | All strains |
| oprD_seq R | TCGCCGTAGCCGTAGTTCTTA | All strains |
| oprD_seq F2 | CAGCCGCCTGTTCCCGCAGACC | All strains |
| oprD_seq R2 | GGTAGGCCAAGGTGAAAGTGTG | All strains |
| oprD_seq F3 | GCTGCTCCGCAACTACTATTTC | All strains |
| Specific primers for the insertion sequence | ||
| oprD_seq R4 | GGCGTGCCGTCGTTCATCACTG | 16/PN/12, 53/PN/13, 482/PA/15 |
| oprD_seq F5 | AGAAGTTGGTCGAGCGTGCG | 482/PA/15 |
| oprD_seq R5 | TGCTTGCCTTCGGTGAAGTG | 482/PA/15 |
| oprD_seq F6 | CCCGCTCGATACGGTGTATCC | 482/PA/15 |
| oprD_seq R6 | GGCGTTGGTGGTGTAGATCACC | 482/PA/15 |
| oprD_seq F7 | TTGATCACCAACTCAGCATCCG | 16/PN/12, 53/PN/13 |
| oprD_seq R7 | TGATGTCGGACGAGTTCAATCG | 16/PN/12, 53/PN/13 |
| oprD_seq R8 | CAACGTCGTTCCTTTACGCCCG | 16/PN/12 |
aIsolates used for sequence analysis of oprD gene: 4/PN/12, 10/PN/12, 13/PN/12, 16/PN/12, 25/PN/12, 33/PN/12, 37/PN/13, 53/PN/13, 10/PN/14, 7/PN/15, and 482/PA/15.
Computer analyses of sequence data
The obtained nucleotide sequences were analyzed by BioEdit Sequence Alignment Editor version 7.2.5 software. Comparison of nucleotide sequences against sequence database was performed with BLAST. The translation of the nucleic acids sequences into amino acids was performed using ExPASy Bioinformatics Resource Portal (http://web.expasy.org/translate). The resulting protein sequences were then aligned and analyzed using Clustal Omega (Multiple Sequence Alignment) version 1.2.1 (https://www.ebi.ac.uk/Tools/msa/clustalo/). In every case, both the nucleotide and the amino acid sequences were compared with the PAO1 reference strain (GenBank accession no. AE004091.2).
Results
Prevalence and real-time PCR confirmation of Pseudomonas aeruginosa isolates
A total of 8,351 water samples were collected from 5 different locations (n = 152) and the prevalence of P. aeruginosa is shown in Table 2. The identity of all P. aeruginosa isolated strains was confirmed by real-time PCR. On the whole, 53 samples (53/8351, 0.63%) were positive for P. aeruginosa, of which 10/207 (4.83%) were from swimming pools, 10/1,684 (0.59%) from healthcare facilities, 19/1,518 (1.25%) from accommodation facilities, 9/4,500 (0.2%) from municipal waterworks and 5/235 (2.13%) from residential buildings.
Table 2. Prevalence of Pseudomonas aeruginosa in water samples from different locations.
| Location | N° | Tot. water samples | Positive samples | Prevalence (%) |
|---|---|---|---|---|
| Swimming pools | 41 | 207 | 10 | 4.83 |
| pool water | 153 | 5 | 3.26 | |
| inlet water | 54 | 5 | 9.2 | |
| Healthcare facilities | 7 | 1684 | 10 | 0.59 |
| Receptive facilities | 72 | 1518 | 19 | 1.25 |
| Municipal waterworks | 2 | 4500 | 9 | 0.2 |
| Residential buildings | 30 | 235 | 5 | 2.13 |
| Total | 152 | 8351 | 53 | 0.63% |
Antibiotic susceptibility profile
The antibiotic test was performed to establish susceptibility profiles to sixteen antimicrobial molecules belonging to 6 different classes. The results, summarized in Table 3, show that 46 P. aeruginosa isolates (86.8%) were susceptible to 13 tested antibiotics, while 5 isolates (9.43%) were resistant to imipenem, one isolate (1.88%) was resistant to Ticarcillin + Clavulanate, whereas one multi-resistant isolate (1.88%) was resistant to both Piperacillin and Ticarcillin + Clavulanate.
Table 3. Results of susceptibility testing of Pseudomonas aeruginosa (n = 53 isolates) of each antibiotic used.
| Disk content (μg) | Zone diameter breakpoint (mm) |
Susceptiblea | Resistanta | ||||
|---|---|---|---|---|---|---|---|
| S ≥ | R < | N. (%) | Inhibition zone Ø (average ± SD) |
N. (%) | Inhibition zone Ø (average ± SD) |
||
| Penicillins | |||||||
| Piperacillin | 30 | 18 | 18 | 52 (98.11) | 24.6 ± 2.13 | 1 (1.88) | 14 ± 1.00 |
| Piperacillin+Tazobactam | 30–6 | 18 | 18 | 53 (100) | 25.8 ± 1.65 | 0 | - |
| Ticarcillin + Clavulanate | 75–10 | 18 | 18 | 51 (96.22) | 25.8 ± 2.17 | 2 (3.77) | 12.8 ± 2.54 |
| Ticarcillin | 75 | 18 | 18 | 53 (100) | 24.8 ± 3.46 | 0 | - |
| Cephalosporins | |||||||
| Cefepime | 30 | 19 | 19 | 53 (100) | 28.0 ± 2.35 | 0 | - |
| Ceftazidime | 10 | 16 | 16 | 53 (100) | 25.2 ± 2.71 | 0 | - |
| Carbapenems | |||||||
| Doripenem | 10 | 25 | 22 | 53 (100) | 35.2 ± 4.52 | 0 | - |
| Imipenem | 10 | 20 | 17 | 48 (90.56) | 29.6 ± 2.02 | 5 (9.43) | 13.2 ± 1.40 |
| Meropenem | 10 | 24 | 18 | 53 (100) | 34.7 ± 6.15 | 0 | - |
| Monobactams | |||||||
| Aztreonam | 30 | 50 | 16 | 53 (100) | 29.5 ± 2.31 | 0 | - |
| Fluoroquinolones | |||||||
| Ciprofloxacin | 5 | 25 | 22 | 53 (100) | 36.6 ± 2.96 | 0 | - |
| Levofloxacin | 5 | 20 | 17 | 53 (100) | 30.0 ± 2.49 | 0 | - |
| Aminoglycosides | |||||||
| Amikacin | 30 | 18 | 15 | 53 (100) | 23.3 ± 2.40 | 0 | - |
| Gentamicin | 10 | 15 | 15 | 53 (100) | 18.6 ± 2.17 | 0 | - |
| Tobramycin | 10 | 16 | 16 | 53 (100) | 24.7 ± 1.95 | 0 | - |
| Netilmicin | 10 | 12 | 12 | 53 (100) | 15.2 ± 2.59 | 0 | - |
aData of susceptible (S) or resistant (R) isolates according to EUCAST clinical breakpoint (www.eucast.org). Average of three experiments. Isolates with intermediate susceptibility are included into the “susceptible” category.
Intermediate susceptibility to aztreonam was found for all 53 P. aeruginosa isolates. Three isolates from swimming pools showed intermediate susceptibility to meropenem, and 1 isolate from a swimming pool showed intermediate susceptibility to doripenem.
RAPD fingerprinting of Pseudomonas aeruginosa isolates
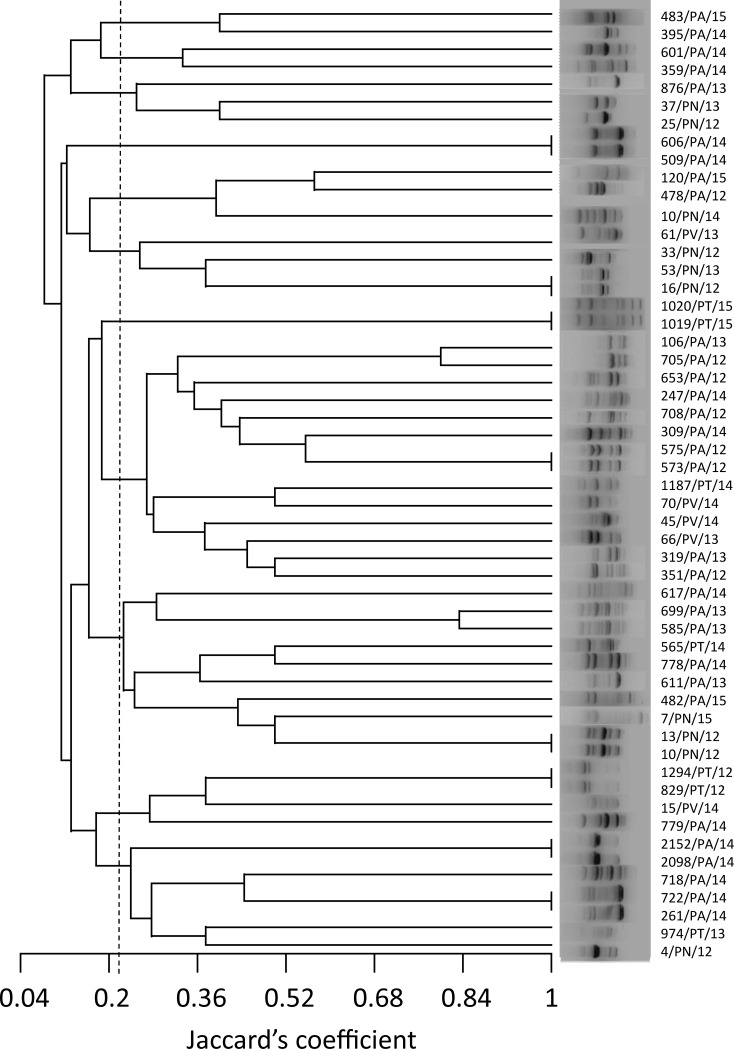
The RAPD-derived banding pattern for the 53 P. aeruginosa isolates is shown in Fig 1.
Fig 1. Dendrogram of random amplified polymorphic DNA (RAPD) analysis of 53 P. aeruginosa isolates.
UPGMA clustering produced 12 groups at an arbitrary similarity level of 22% (Jaccard’s coefficient, dotted line). The methodology, using primer 272, revealed great diversity in genotypes (n = 45), identifying 37 unique genotypes and 8 clones.
Primer 272 produced clear banding patterns. The number of amplified DNA fragments for each strain ranged from 3 to 11, in a panel of 29 possible fragments of different molecular weight. RAPD banding patterns showed high diversity of genotypes (n = 45) among isolates, identifying 37 unique genotypes and 8 clones.
Cluster analysis was performed on the basis of similarity co-efficient generated from RAPD profiles. UPGMA clustering of RAPD data produced a dendrogram that could separate the 53 isolates into 12 groups at an arbitrary similarity level of 22% (Jaccard’s coefficient) (Fig 1).
PCR detection of β-lactamases genes
Molecular screening tests carried out by the Xpert Carba R assay showed that Imipenem resistant P. aeruginosa isolates were β-lactamases-negative, at least for the main β-lactamase gene families.
OprD sequence
The OprD amino acid sequences of the 11 isolated strains, deduced from the analysis of the oprD gene, were compared with the sequence of the PAO1 OprD. The OprD sequences were clustered into two major classes based on their susceptibility to imipenem (Table 4). The ‘susceptible strains’ major class, with 6 strains, was further classified into a total of 3 types of ‘full length type’ strains, whose OprD proteins were fully encoded. These 3 groups were characterized by different polymorphisms, which involved specific amino acid substitutions compared with the PAO1 OprD sequence. The second major group included the 5 imipenem resistant strains, which were clustered into three specific subgroups. Two strains, 10/PN/12 and 13/PN/12, showed several polymorphisms, which determined 4 amino acidic substitutions, and a frameshift mutation due to nucleotide deletion of 37 bp determining a variation of the amino acid sequence from the S349 and a premature stop codon after the 421 amino acid.
Table 4. Different OprD types and mechanisms of imipenem resistance detected among the P. aeruginosa isolates.
| Imipenem resistance phenotype | Isolates | Alteration(s) of oprD nucleic sequence | Alteration(s) or amino acidic substitution(s) of OprD sequencea | Effect on OprD protein |
|---|---|---|---|---|
| Susceptible strains | 4/PN/12, 25/PN/12, 33/PN/12, 37/PN/13 | Several polymorphisms |
D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L, 372(VDSSSS-YAGL)383 |
Full length type |
| 10/PN/14 | Several polymorphisms | T103S, K115T, F170L |
Full length type | |
| 7/PN/15 | Several polymorphisms | S57E, S59R, V127L, E185Q, P186G, V189T, E202Q, I210A, E230K, S240T, N262T, T276A, A281G, K296Q, Q301E, R310E, A315G, L347M, 372(VDSSSS-YAGL)383, S403A, Q424E | Full length type | |
|
Resistant strains |
10/PN/12, 13/PN/12 | Several polymorphisms Deletion of 37 nt at position 1046 nt |
D43N, T103S, K115T, F170L |
Frameshift mutation, premature stop codon (shorter, nonfunctional protein) |
| 16/PN/12, 53/PN/13 | Insertion of 1239 nt at position 215 nt | Premature stop codon and porin loss | ||
| 482/PA/15 | Several polymorphisms Insertion of 1337 nt at position 428 nt |
S57E, S59R, V127L |
Premature stop codon and porin loss |
aIn bold, mutations which determine a charge change
+/-: K115T, V127L, V189T, A267S, V359L, Q424E
-/+: S59R, E185Q, E202Q, E230K, T276A, S403A
+/neutral: P186G, A281G, R310G, A315G
An insertion sequence (IS) element of 1239 bp, showing 99% identity with a transposase (CP008861.1, range from 3835714 to 3836949) was found in 16/PN/12 and 53/PN/13 strains at the nt 215 from the transcription start site. The IS presence caused a premature termination of translation after 84 aa and the loss of the OprD porin. Moreover, four amino acidic substitutions were detected in both protein sequences. An IS of 1,337 bp (99% identity with the transposase annotated in CP008857, ranging from 505,750 to 507,078) and 3 amino acidic substitutions were detected in 482/PA/15. Also in this case, the IS, at nt 428 from the transcription start, caused a premature stop codon at the 151 aa position and protein loss.
Discussion
The evaluation of the microbiological quality of water aims to protect the general population from illness caused by contact with or ingestion of water that may contain pathogens such as P. aeruginosa. This opportunistic pathogen can be present in municipal water supplies and in water circuits offering suitable conditions for growth, and it poses potential health risks to particular segments of the population such as immunocompromised individuals, pregnant women, young children /infants and the elderly.
The present study investigates the presence of P. aeruginosa in different types of water obtained from a range of different environments. The supplementary bacteriological parameter P. aeruginosa was quantified for each water sample in accordance with Italian regulations for potable water [14], application of EC directive 98/83.
A very low overall prevalence of 0.63% was found in more than 8,000 samples collected over a 4-year period in swimming pool water, healthcare and accommodation facilities, municipal waterworks and residential buildings in the Marches Region.
The highest isolation rate (4.83%) of P. aeruginosa was observed in swimming pool water. This finding could be explained by the fact that colonized or infected individuals may use swimming pools [19].
In this study 7 out of the 41 (17%) investigated swimming pools were contaminated with at least one P. aeruginosa strain, and 2 out of the 41 (4.9%) were contaminated with two or more strains, although isolated in different samplings.
RAPD fingerprinting was used in order to identify sequence diversity and epidemiological relationships among isolates. The dendrogram (Fig 1) allowed us to cluster all isolates in 12 groups without any clear correlation with their origins. However, strains with identical RAPD profiles were isolated from the same location in the same year or even in different years. This result shows that a water system can remain colonized by one strain for long periods and the contamination may be difficult to eradicate.
Moreover, it highlights the importance of continuous monitoring and good maintenance of swimming pools because sand filtration and inactivation system may be inadequate to prevent pathogen contamination.
To achieve adequate chemical and microbiological quality and prevent the occurrence of undesirable transformations during storage and distribution, drinking water treatment is frequently recommended. Chlorination is commonly used to disinfect water because it is easy to apply at a moderate cost [28, 29]. Some studies have reported that water chlorination selects for antibiotic-resistant bacteria (ARB) [30–32]; however, this finding remains controversial [33,34].
In the present investigation, of a total of 53 P. aeruginosa strains isolated from 5 different locations, 7 (13.2%) isolates exhibited resistance to one or more antibiotics. Most of the strains (5/7, 72%) were resistant to imipenem. These strains were identified as carbapenemase-negative P. aeruginosa isolates. Resistance to imipenem has been associated with inactivating mutations within the oprD gene [35,36]. In particular, mutations in oprD caused by nucleotide insertions or deletions in the OprD structural gene have been found to be the major mechanisms leading to inactivation of OprD with a concomitant loss of porins from the P. aeruginosa outer membranes and increases in carbapenem MICs [37].
A recent study examining molecular mechanisms associated with carbapenem resistance in carbapenemase-negative P. aeruginosa isolates [38] showed no mutations in the promoter region of the oprD gene, but several in the coding region. Hence, sequencing analysis of the oprD gene was conducted to determine the mechanisms leading to imipenem resistance in 5 carbapenemase-negative P. aeruginosa isolates. Indeed, porin OprD is the specific point of entrance for carbapenems in P. aeruginosa [39], and mutational inactivation of the related gene is the main mechanism of carbapenem resistance in the absence of acquired carbapenemases [40]. The oprD sequence of 6 other imipenem sensitive strains isolated from recreational water in the present investigation was also determined for comparison. The sequence analysis of the susceptible strains revealed several amino acid substitutions, which can be clustered into three groups. In particular, the group of 4 strains composed of isolates 4/PN/12, 25/PN/12, 33/PN/12 and 37/PN/13, and strain 10/PN/14, showed the same amino acidic substitution patterns as the T1-VI and T1-II OprD types previously described by Ocampo-Sosa et al. [35], respectively. The 7/PN/15 isolate had the same alterations as the T1-VII group, except for the presence of P186G and the absence of D437E substitution [35]. Although some of the amino acid substitutions led to charge changes, the function of OprD porins was not affected, confirming the results of a previous study [35]. Moreover, the amino acid substitutions of the OprD sequence, described for the susceptible strains, were found in neither external loop 2 or 3, which are fundamental for imipenem entrance, nor in the deletion sites previously analyzed and related to imipenem susceptibility [37]. While loop 2 includes the imipenem binding site, loop 3 is more likely to be involved in the imipenem passage channel within OprD [37].
The 16/PN/12, 53/PN/13, and 482/PA/15 resistant strains encoded incomplete OprD proteins due to the presence of a stop codon caused by IS in the oprD gene, leading to the loss of the OprD ability to function as a porin. The IS elements were transposases inserted in different locations within the oprD, compared to other investigations [41,42].
In both the 10/PN/12 and 13/PN/12 isolates the oprD sequences showed 100% homology, with a 37-nucleotide deletion detected at 1,046 nt from the beginning of the coding region. The frameshift mutation determined an aberrant sequence of 72 aa from amino acid 349 positioned in loop 8, which begins at amino acid 345 [37]. This alteration may result in an abnormal protein structure and the loss of OprD function. Moreover, it should be noted that strains 10/PN/12 and 13/PN/12, which were isolated from the same swimming pool (from pool water and inlet water, respectively), also showed identical RAPD banding patterns (Fig 1), strongly suggesting an epidemiological correlation.
Conclusion
The possible presence of P. aeruginosa in water distribution systems and swimming pools deserves attention given their potential as reservoirs or carriers of resistance or as opportunistic pathogens.
This study has revealed the presence of P. aeruginosa in potable and recreational water, including resistant strains, especially in swimming pools. Moreover, the present work identifies specific oprD mutations which are probably responsible for imipenem resistance and underlines the role of porins as a contributing factor in carbapenem resistance in Gram-negative bacteria.
Although imipenem is still considered a front-line antibiotic against P. aeruginosa, the results of this investigation confirm the continuous spread of resistance against the drug. This trend may pose a serious threat, especially if we consider that swimming pools are frequented by people with heterogeneous health statuses, including those who are potentially susceptible to opportunistic infections.
Supporting information
(DOCX)
Acknowledgments
We wish to thank Professor Timothy C. Bloom for his linguistic revision of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Balasubramanian D, Schneper L, Kumari H, Mathee K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013; 41: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefebvre A, Bertrand X, Quantine C, Vanhems P, Lucet JC, Nuemi G, et al. Association between Pseudomonas aeruginosa positive water samples and healthcare-associated cases: nine-year study at one university hospital. J Hosp Infect. 2017; 96: 238–243. doi: 10.1016/j.jhin.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 3.Felföldi T, Tarnóczai T, Homonnay ZG. Presence of potential bacterial pathogens in a municipal drinking water supply system. Acta Microbiol Immunol Hung. 2010; 57: 165–179. doi: 10.1556/AMicr.57.2010.3.2 [DOI] [PubMed] [Google Scholar]
- 4.Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med. 2002; 162: 1483–92. [DOI] [PubMed] [Google Scholar]
- 5.Huhulescu S, Simon M, Lubnow M, Kaase M, Wewalka G, Pietzka AT, et al. Fatal Pseudomonas aeruginosa pneumonia in a previously healthy woman was most likely associated with a contaminated hot tub. Infection. 2011; 39: 265–269. doi: 10.1007/s15010-011-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guida M, Di Onofrio V, Gallè F, Gesuele R, Valeriani F, Liguori R, et al. Pseudomonas aeruginosa in swimming pool water: evidences and perspectives for a new control strategy. Int J Environ Res Public Health. 2016; 15: 13(9). pii: E919. doi: 10.3390/ijerph13090919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amagliani G, Parlani ML, Brandi G, Sebastianelli G, Stocchi V, Schiavano GF. Molecular detection of Pseudomonas aeruginosa in recreational water. Int J Environ Health Res. 2012; 22: 60–70 doi: 10.1080/09603123.2011.588325 [DOI] [PubMed] [Google Scholar]
- 8.Berthelot P, Grattard F, Mallaval FO, Ros A, Lucht F, Pozzetto B. Epidemiology of nosocomial infections due to Pseudomonas aeruginosa, Burkholderia cepacia and Stenotrophomonas maltophilia. Pathol Biol. 2005; 53: 341–348. doi: 10.1016/j.patbio.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol. 2009; 201: 71–115. doi: 10.1007/978-1-4419-0032-6_3 [DOI] [PubMed] [Google Scholar]
- 10.Blanc DS, Nahimana I, Petignat C, Wenger A, Bille J, Francioli P. Faucets as a reservoir of endemic Pseudomonas aeruginosa colonization/infections in intensive care units. Intensive Care Med. 2004; 30: 1964–1968. doi: 10.1007/s00134-004-2389-z [DOI] [PubMed] [Google Scholar]
- 11.Kimata N, Nishino T, Suzuki S, Kogure K. Pseudomonas aeruginosa isolated from marine environments in Tokyo bay. Microb Ecol. 2004; 47: 41–47. doi: 10.1007/s00248-003-1032-9 [DOI] [PubMed] [Google Scholar]
- 12.Hunter PR. The microbiology of bottled natural mineral waters. J Appl Bacteriol. 1993; 74: 345–352. [DOI] [PubMed] [Google Scholar]
- 13.Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int. 2015; 2015:759348 doi: 10.1155/2015/759348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Italian Republic. Legislative Decree No. 31, 2 February, implementing Directive 98/83/EC on the quality of water intended for human consumption. Official Gazette No. 52 of 3rd March 2001, Rome.
- 15.Italian Republic. Legislative Decree No. 27, 2 February, amending and supplementing Legislative Decree No. 31 of 2 February 2001 implementing Directive 98/83/EC on the quality of water intended for human consumption. Official Gazette No. 58 of 9th of March 2002, Rome.
- 16.Italian Republic. Permanent Council State-Regions and Autonomous Provinces. Accordo Stato–Regioni e Province Autonome 16-1-2003. Official Gazette No. 51 of 3rd of March 2003, Rome.
- 17.De Francesco MA, Ravizzola G, Peroni L, Bonfanti C, Manca N. Prevalence of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa in an Italian hospital. J Infect Public Health. 2013; 6: 179–185. doi: 10.1016/j.jiph.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Riera E, Cabot G, Mulet X, Garcia-Castillo M, Del Campo R, Juan C, et al. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother. 2011; 66: 2022–2027. doi: 10.1093/jac/dkr232 [DOI] [PubMed] [Google Scholar]
- 19.Lutz JK, Lee J. Prevalence and Antimicrobial-Resistance of Pseudomonas aeruginosa in Swimming Pools and Hot Tubs. Int J Environ Res Public Health. 2011; 8: 554–564. doi: 10.3390/ijerph8020554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2015. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2017. [Google Scholar]
- 21.Livermore DM. Interplay of impermeability and chromosomal β- lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992; 36: 2046–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoneyama H, Nakae T. Mechanism of efficient elimination of protein D2 in outer membrane of imipenem resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993; 37: 2385–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirnay JP, De Vos D, Mossialos D, Vanderkelen A, Cornelis P, Zizi M. Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ Microbiol. 2002; 4: 872–882. [DOI] [PubMed] [Google Scholar]
- 24.UNI EN ISO 16266: 2008 Water quality. Detection and enumeration of Pseudomonas aeruginosa. Method by membrane filtration. International Organization for Standardization, Technical committee ISO/TC 147, Subcommittee SC 4.
- 25.Amagliani G, Schiavano GF, Stocchi V, Bucci G, Brandi G. Application of real-time PCR to Pseudomonas aeruginosa monitoring in a public swimming pool. Microchem J. 2013; 110: 656–659. [Google Scholar]
- 26.Lanotte P, Watt S, Mereghetti L, Dartiguelongue N, Rastegar-Lari A, Goudeau A, et al. Genetic features of Pseudomonas aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins. J Med Microbiol. 2004; 53: 73–81. doi: 10.1099/jmm.0.05324-0 [DOI] [PubMed] [Google Scholar]
- 27.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0, 2015. http://www.eucast.org.
- 28.Huang JJ, Hu HY, Tang F, Li Y, Lu SQ, Lu Y. Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Res. 2011; 45: 2775–2781. doi: 10.1016/j.watres.2011.02.026 [DOI] [PubMed] [Google Scholar]
- 29.Sharma VK, Johnson N, Cizmas L, McDonald TJ, Kim H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere. 2016; 150: 1–13. doi: 10.1016/j.chemosphere.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 30.Xi C, Zhang Y, Marrs CF, Ye W, Simon C, Foxman B, et al. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl Environ Microbiol. 2009; 75: 5714–5718. doi: 10.1128/AEM.00382-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueira V, Serra EA, Vaz-Moreira I, Brandão TRS, Manaia CM. Comparison of ubiquitous antibiotic-resistant Enterobacteriaceae populations isolated from wastewaters, surface waters and drinking waters. J Water Health. 2012; 10: 1–10. doi: 10.2166/wh.2011.002 [DOI] [PubMed] [Google Scholar]
- 32.Bai X, Ma X, Xu F, Li J, Zhang H, Xiao X. The drinking water treatment process as a potential source of affecting the bacterial antibiotic resistance. Sci Total Environ. 2015; 533: 24–31. doi: 10.1016/j.scitotenv.2015.06.082 [DOI] [PubMed] [Google Scholar]
- 33.Shi P, Jia S, Zhang XX, Zhang T, Cheng S, Li A. Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Res. 2013; 47: 111–120. doi: 10.1016/j.watres.2012.09.046 [DOI] [PubMed] [Google Scholar]
- 34.Armstrong JL, Calomiris JJ, Seidler RJ. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl Environ Microbiol. 1982; 44: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ocampo-Sosa AA, Cabot G, Rodríguez C, Roman E, Tubau F, Macia MD, et al. Alterations of OprD in carbapenem-intermediate and–susceptible strains of Pseudomonas aeruginosa isolated from patients with bacteremia in a Spanish multicenter study. Antimicrob Agents Chemother. 2012; 56: 1703–1713. doi: 10.1128/AAC.05451-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wołkowicz T, Patzer JA, Kamińska W, Gierczyński R, Dzierżanowska D. Distribution of carbapenem resistance mechanisms in Pseudomonas aeruginosa isolates among hospitalised children in Poland: Characterisation of two novel insertion sequences disrupting the oprD gene. J Glob Antimicrob Resist. 2016; 7: 119–125. doi: 10.1016/j.jgar.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 37.Li H, Luo YF, Williams BJ, Blackwell TS, Xie CM. Structure and function of OprD protein in Pseudomonas aeruginosa: from antibiotic resistance to novel therapies. Int J Med Microbiol. 2012; 302: 63–68. doi: 10.1016/j.ijmm.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalhoub H, Sáenzb Y, Rodriguez-Villalobos H, Denis O, Kahl BC, Tulkens PM., et al. High-level resistance to meropenem in clinical isolates of Pseudomonas aeruginosa in the absence of carbapenemases: role of active efflux and porin alterations. Int J Antimicrob Agents. 2016; 48: 740–743. doi: 10.1016/j.ijantimicag.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 39.Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011. 2, 65 doi: 10.3389/fmicb.2011.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavalcanti FL, Mirones CR, Paucar ER, Montes LÁ, Leal-Balbino TC, Morais MM, et al. Mutational and acquired carbapenem resistance mechanisms in multidrug resistant Pseudomonas aeruginosa clinical isolates from Recife, Brazil. Mem Inst Oswaldo Cruz. 2015; 110: 1003–1009. doi: 10.1590/0074-02760150233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanbongi Y, Shimizu A, Suzuki T, Nagaso H, Ida T, Maebashi K, et al. Classification of OprD sequence and correlation with antimicrobial activity of carbapenem agents in Pseudomonas aeruginosa clinical isolates collected in Japan. Microbiol Immunol. 2009; 53: 361–367. doi: 10.1111/j.1348-0421.2009.00137.x [DOI] [PubMed] [Google Scholar]
- 42.Wolter DJ, Hanson ND, Lister PD. Insertional inactivation of oprD in clinical isolates of Pseudomonas aeruginosa leading to carbapenem resistance. FEMS Microbiol Lett. 2004; 236: 137–143. doi: 10.1016/j.femsle.2004.05.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.