Abstract
Rationale: Understanding long-term outcomes of critically ill patients may inform shared decision-making in the intensive care unit (ICU).
Objectives: To quantify 6-month functional outcomes of general ICU patients, and develop a multivariable model comprising factors present during the first ICU day to predict which patients will return to their baseline function 6 months later.
Methods: We conducted a prospective cohort study in three medical ICUs and two surgical ICUs in three hospitals. We enrolled patients who spent at least 3 days in the ICU and received mechanical ventilation for more than 48 hours and/or vasoactive infusions for more than 24 hours.
Results: We measured 6-month outcomes including survival, return to original place of residence, and physical and cognitive function. Of 303 enrolled patients, 299 (98.7%) had complete follow-up at 6 months. Among the 169 patients (56.5%) who survived to 6 months, 82.8% returned home, 81.9% were able to toilet, 71.3% were able to ambulate 10 stairs, and 62.4% reported normal cognition. Overall, 31.1% of patients returned to their baseline status on these measures. Factors associated with not returning to baseline included higher APACHE III score, being a medical patient, older age, nonwhite race, recent hospitalization, prior transplantation, and a history of cancer or of neurologic or liver disease. A model including only these Day 1 factors had good discrimination (area under receiver operating characteristic curve, 0.778; 95% confidence interval, 0.724–0.832) and calibration (difference between observed and expected P value, 0.36).
Conclusions: Among patients spending at least 3 days in an ICU and requiring even brief periods of life-sustaining therapy, nearly one-half will be dead and less than one-third will have returned to their baseline status at 6 months. Of those who survive, the majority of patients will be back at home at 6 months. Future research is needed to validate this multivariable model, including readily available patient characteristics available on the first ICU day, that seems to identify patients who will return to baseline at 6 months.
Keywords: long-term outcomes, function, intensive care unit, post–intensive care syndrome
Patients hospitalized for an acute illness are at risk for worsening cognitive and physical function after hospital discharge (1–3), outcomes that are of utmost importance to patients (4, 5). Those who require life-sustaining therapy in an intensive care unit (ICU) appear to be at even greater risk of experiencing disability and death (6). Indeed, studies of ICU patients who require prolonged mechanical ventilation (7, 8) or develop acute respiratory distress syndrome (9) have found substantial impairments in health-related quality of life and increased health care use that commonly persist for months to years after ICU discharge (10).
Many of the sequelae of critical illness have been grouped under the term “post–intensive care syndrome” (11). Post–intensive care syndrome highlights three major domains of dysfunction after critical illness: physical, cognitive, and psychiatric. Discussing these potential outcomes with patients or surrogates in the ICU may help set expectations and inform shared decision-making, given that ICU-based conversations about prognosis often focus on functional outcomes rather than mortality (12). Indeed, guidelines recommend meeting with patients’ families for such purposes early in an ICU admission (13). However, despite investigation of patients with prolonged mechanical ventilation (7, 8) or acute respiratory distress syndrome (9), evidence to guide discussions of future disabilities and other outcomes is largely unavailable for general ICU patients early in their ICU admission. The lack of information for outcomes of a general ICU population serves as a recognized barrier to facilitating conversations about long-term prognosis (14).
We therefore performed a prospective cohort study of patients spending at least 3 days in an ICU and receiving at least a brief period of mechanical ventilation or vasopressors. We sought to describe such patients’ survival and functional outcomes 6 months after their ICU admission, and to evaluate the possibility that readily ascertained patient characteristics might predict which patients would return to their prior residence and baseline level of function 6 months after ICU admission.
Methods
Patients
We conducted a prospective cohort study in five ICUs (three medical, two surgical) in three hospitals within the University of Pennsylvania Health System. These hospitals vary in their levels of specialization and incorporation of trainees into patient care. ICU clinicians’ discriminative accuracy in predicting outcomes for patients in this prospective cohort study has been reported previously (15). Patients were enrolled from October 2013 to May 2014, and 6-month follow-up was completed in December 2014. The University of Pennsylvania Institutional Review Board approved this study.
Patients were screened daily (excluding weekends and holidays), using the electronic medical record to identify those who met the inclusion criteria. We included adult patients admitted to one of the participating ICUs who spent at least three calendar days in the ICU and used life-sustaining therapy. We chose to include patients requiring 3 days of ICU care so as to make the results generalizable to a substantial fraction of ICU patients, while still capturing patients with appreciable risks for short-term death (16). For purposes of this study, we defined life-sustaining therapy as mechanical ventilation for more than 48 consecutive hours, vasoactive infusions for more than 24 consecutive hours, or both, within the first 6 days of being in the ICU. In a secondary analysis, we restricted the cohort to patients who were eligible and enrolled on Days 3 or 4, given the possibility that patient characteristics collected on Day 1 would apply less well to patients who only began using life-sustaining therapy later in the ICU stay. We excluded patients for whom no family member was available for consent, for whom the attending physician reported that the patient’s goals of care were transitioning to palliation/withdrawal of life-sustaining therapy, or who met other, less common criteria (see Table E1 in the online supplement). We sought patients’ or surrogates’ consent for the patient to participate, and surrogates’ consent for researchers to contact them directly during follow-up.
Baseline Data Collection
We collected patients’ clinical and demographic data through interviews with patients or surrogates at the time of enrollment and via the electronic medical record (including admission and consult notes, discharge summaries, and ICU flow sheets). When patients were enrolled, we asked patients or surrogates to describe the patient’s physical and cognitive function 1 month before ICU admission. The measures used to describe such baseline function included two physical outcomes (i.e., ambulating up 10 stairs [17] and toileting independently [18]) and one cognitive outcome (a composite assessment of executive function, memory, and clarity of thought that is part of the Health Utility Index) (19). We also collected patients’ major medical comorbidities (Table 1), functional comorbidity index (20), employment status, and Acute Physiology and Chronic Health Evaluation (APACHE) III score (21).
Table 1.
Baseline characteristics and outcomes of patients
| Characteristic | Value |
|---|---|
| Age (yr),* median (IQR) | 62 (53–71) |
| Male sex,* n (%) | 173 (57.1%) |
| Race/ethnicity,† n (%) | |
| White | 191 (63.0%) |
| African American | 98 (32.3%) |
| Other | 14 (4.7%) |
| Level of education,† n (%) | |
| Elementary or middle school | 15 (5.1%) |
| High school | 133 (45.1%) |
| Some college | 61 (20.7%) |
| Bachelor’s degree | 56 (19.0%) |
| Masters or terminal degree | 30 (10.2%) |
| Marital status,† n (%) | |
| Married or living with partner | 150 (50.1%) |
| Unmarried | 101 (34.0%) |
| Separated or divorced | 19 (6.4%) |
| Widowed | 27 (9.1%) |
| Employment status,† n (%) | |
| Employed | 85 (28.4%) |
| Unemployed | 34 (11.4%) |
| Retired | 114 (38.1%) |
| Disabled | 64 (21.4%) |
| Student | 2 (0.7%) |
| Place of residence before hospital admission,† n (%) | |
| Home | 283 (94.1%) |
| Long-term acute care hospital | 9 (3.0%) |
| Nursing home | 9 (3.0%) |
| Retirement home | 1 (0.3%) |
| Insurance status,* n (%) | |
| Private | 106 (35.6%) |
| Medicaid | 25 (8.4%) |
| Medicare | 161 (54.0%) |
| Uninsured | 4 (1.3%) |
| Other | 2 (0.7%) |
| Normal baseline function,† n (%) | 204 (68.0%) |
| Able to ambulate up 10 stairs before hospitalization,† n (%) | 243 (80.7%) |
| Toilet before hospitalization,† n (%) | 263 (88.3%) |
| Normal cognition before hospitalization,† n (%) | 249 (83.0%) |
| Functional comorbidity index,†‡ n (%) | |
| 0 | 13 (4.3%) |
| 1 | 30 (9.9%) |
| 2 | 50 (16.5%) |
| 3 | 57 (18.8%) |
| 4 | 53 (17.5%) |
| 5 | 45 (14.9%) |
| 6 or more | 55 (18.2%) |
| Medical comorbidities before ICU admission,* n (%) | |
| Neurologic condition | 59 (19.5%) |
| Congestive heart failure | 114 (37.6%) |
| Coronary artery disease | 103 (34.0%) |
| Peripheral vascular disease | 61 (20.1%) |
| COPD | 66 (21.8%) |
| Renal failure requiring dialysis | 30 (9.9%) |
| Liver disease | 35 (11.6%) |
| Obesity | 106 (35.0%) |
| Rheumatologic condition | 50 (16.5%) |
| Psychiatric condition | 104 (34.3%) |
| Malignancy (treated for cure) | 65 (21.5%) |
| Malignancy (metastatic or palliative) | 40 (13.2%) |
| Transplantation history§ | 21 (6.9%) |
| ICU type,* n (%) | |
| MICU | 190 (62.7%) |
| SICU | 113 (37.3%) |
| ICU admitting diagnosis,* n (%) | |
| Respiratory failure | 83 (27.4%) |
| Sepsis | 66 (21.8%) |
| Nonemergency surgery | 54 (17.8%) |
| Emergency surgery | 34 (11.2%) |
| Cardiac (nonsurgical) | 18 (5.9%) |
| Hemorrhagic shock | 11 (3.6%) |
| Neurologic | 7 (2.3%) |
| Illicit drug/alcohol related | 6 (2.0%) |
| Liver failure | 5 (1.7%) |
| Other | 19 (6.3%) |
| APACHE III score,* median (IQR) | 96 (75–120) |
| Goals of care made palliative in the ICU,* n (%) | 73 (24.1%) |
| Hospital discharge disposition,* n (%) | |
| Dead | 72 (23.8%) |
| Home | 90 (29.7%) |
| Rehabilitation | 25 (8.3%) |
| Long-term acute care hospital | 23 (7.6%) |
| Inpatient hospice | 21 (6.9%) |
| Other acute care hospital | 11 (3.6%) |
| Skilled nursing facility | 6 (19.1%) |
| Other | 3 (1.0%) |
| 6-Month disposition,† n (%) | |
| Dead | 130 (42.9%) |
| Original residence | 138 (45.5%) |
| Different residence | 9 (3.0%) |
| Rehabilitation Facility | 6 (2.0%) |
| Nursing home | 5 (1.7%) |
| Long-term facility | 4 (1.3%) |
| Other | 7 (2.3%) |
| Unknown | 4 (1.3%) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; IQR = interquartile range; MICU = medical ICU; SICU = surgical ICU.
Adapted by permission from Reference 15.
Abstracted from chart.
Data acquired from patient or surrogate.
The functional comorbidity index assesses 18 possible diagnoses, and assigns one point for the presence of each (20).
Transplantation includes lung, liver, kidney, and bone marrow.
Outcome Assessment
Hospitalization outcomes included survival, discharge disposition, ICU and hospital length of stay, exposure to selected medications, and the use of mechanical ventilation, vasopressors, and renal replacement therapy. For patients who survived the hospitalization, we assessed additional outcomes 6 months after enrollment by administering a standardized interview to survivors or their surrogates via telephone or e-mail. We first sought to contact patients themselves. If initial attempts to contact the patient were unsuccessful, we then simultaneously attempted to contact the patient and surrogate twice per week for up to five attempts, and completed the interview with the first person we reached. If we failed to contact either the patient or surrogate after a total of 10 attempts, we considered the patient lost to follow-up.
At 6 months we assessed the same cognitive outcome (19), and two well-described physical outcomes (ability to ambulate up 10 stairs independently [17] and toileting independently [18]) assessed at baseline. Using these measures, as well as whether or not patients returned to their baseline place of residence at 6 months, we also prospectively defined a composite summary outcome of “return to baseline” at 6 months. We considered patients to have returned to baseline if they were alive, living in the pre-ICU place of residence, and exhibiting the same or better function for cognition, stair ambulation, and toileting independence compared with 1 month before their critical illness. We defined patients as having “normal baseline function” if they were living at home with no self-reported deficits in cognition or abilities to ambulate up 10 stairs and toilet independently before ICU admission.
Statistical Analysis
All outcomes were summarized using medians (interquartile ranges) and proportions. We used χ2 or Mann–Whitney U tests as appropriate to compare outcomes between groups defined a priori: medical versus surgical ICU patients, patients with and without normal baseline function, and patients who did versus did not return to baseline function at 6 months. These analyses were conducted with Stata version 13.0 (StataCorp, College Station, TX).
We also constructed a multivariable model to determine which patient demographic and clinical variables were most predictive of patients’ abilities to return to baseline at 6 months. We selected variables eligible for inclusion based on prior literature, clinical judgment, and reliability of measurement (Table E2). We limited consideration to those variables that are readily available early in an ICU patient’s course so as to optimally inform prognostic guidance and decision-making.
The model was derived using the stepAIC algorithm available in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria), which applies a stepwise procedure guided by the Akaike information criterion (AIC) of each potential combination of variables. The AIC value permits comparison of the relative fit between a range of regression models against each other (22). Lower AIC values indicate better model fit, accounting for a penalty that is applied for each additional variable required to achieve that fit. As such, the AIC value supports the selection of a parsimonious model by ranking different models with different numbers of variables and selecting the one that maximizes the balance between parsimony and goodness of fit. The model was run forward and backward to determine whether different variables were included based on the variable selection procedure. To assess the predictive performance of our parsimonious model in the absence of an external validation cohort we conducted leave-one-out cross-validation and fivefold cross-validation (23, 24). Discrimination of this model was evaluated using the area under the receiver operating characteristic (ROC) curve. Calibration of the model was evaluated using the Hosmer–Lemeshow test.
We performed two secondary analyses. First, we restricted the model to patients enrolled on Days 3 and 4. Second, we constructed a model using only variables that would be available before ICU admission, thereby excluding APACHE III and ICU type. The goal was to determine whether this latter model could predict return to baseline 6 months before ICU admission, such that it might be useful in guiding triage.
Results
Baseline Patient Data
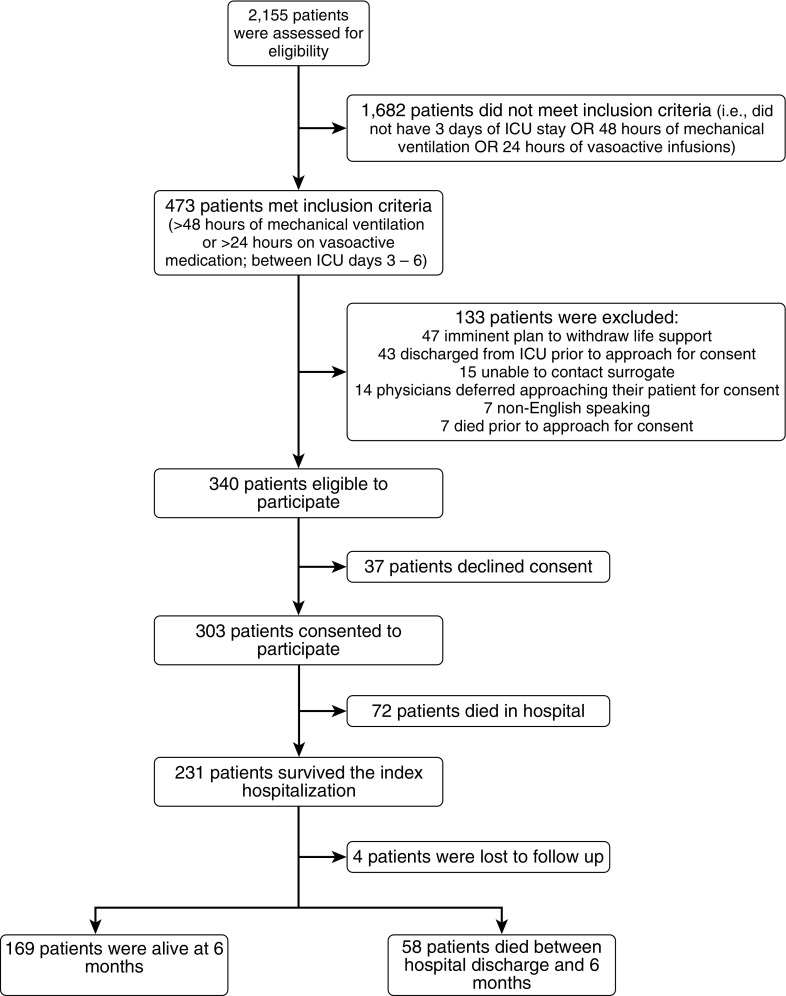
Of the 303 enrolled patients (Figure 1), the median age was 62 years (interquartile range, 53–71 yr), 57.1% were male, and 32.3% were African American (Table 1). The majority of patients (94.0%) lived at home before their critical illness, and 28.4% were employed. Most patients had normal baseline function before their ICU admission (68.0%), with 88.3% toileting independently, 80.7% ambulating up 10 stairs independently, and 83.0% reporting normal cognition (Table 1). Patients’ most common primary admitting diagnoses were respiratory failure (27.4%), sepsis (21.8%), and nonemergency surgery (17.8%). The median APACHE III score was 96 (75–120) with 91.1% patients receiving mechanical ventilation, 81.5% of patients receiving vasoactive infusions, and 72.6% of patients receiving both (Table 1; and see Tables E3 and E4).
Figure 1.
Flow diagram for study cohort. ICU = intensive care unit.
Survival and Functional Outcomes
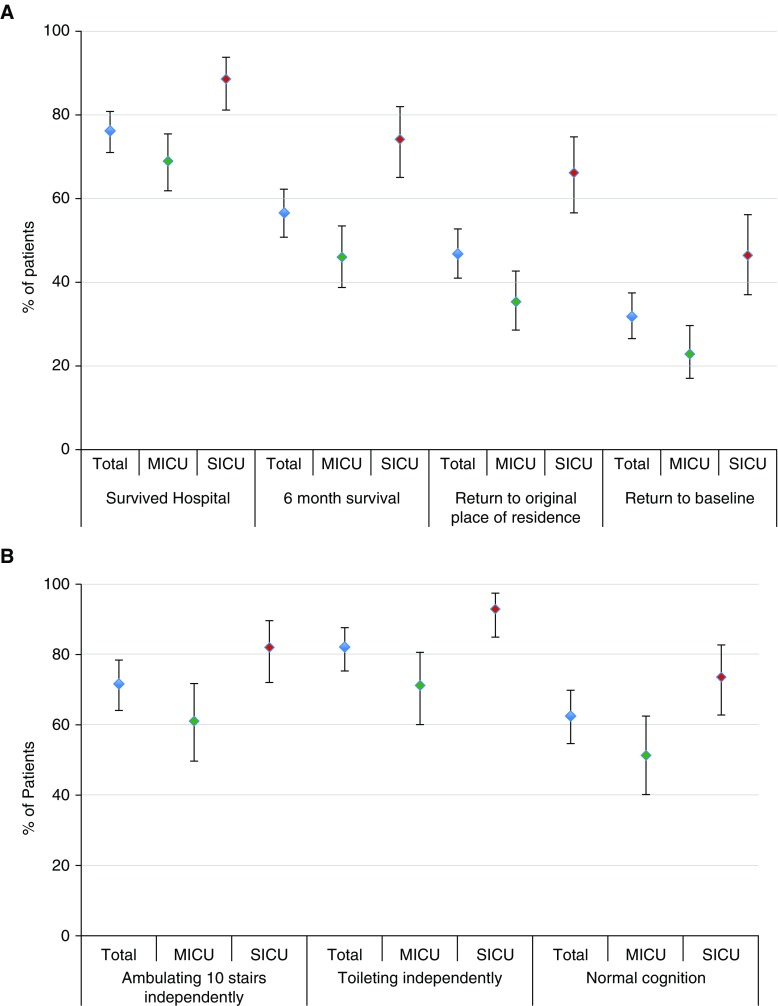
Of the 303 enrolled patients, 72 (23.8%) died in the hospital, and 21 (6.9%) were discharged to inpatient hospice (Table 1). Between hospital discharge and 6-month follow-up, 58 patients (19.1%) died, leaving 173 of the original 303 (57.1%) alive and eligible for 6-month assessment. Survival to 6 months was higher for SICU patients (74%) than for MICU patients (46%) (P < 0.001) (Figure 2).
Figure 2.
Outcomes of the total population, medical and surgical populations. (A) Mortality, return to original place of residence, and return to baseline. (B) Functional outcomes among survivors. Error bars represent approximate 95% confidence intervals of the proportions. P < 0.001 (difference between MICU and SICU) for hospital survival, 6-month survival, return to original place of residence, return to baseline, and toileting independently. P = 0.003 (difference between MICU and SICU) for ambulating 10 stairs and cognition. MICU = medical intensive care unit; SICU = surgical intensive care unit.
Of the 173 patients who were alive at 6 months, we completed follow-up assessments for 166 patients (96.0%) and at least partial follow-up for 169 patients (97.8%). Of these, 85 (50.3%) were completed by patients and 84 (49.7%) by surrogates. Of the surviving patients, 138 (82.6%) returned to their original place of residence by 6 months. The 29 patients who did not return to their original residence reported living in another person’s home (n = 9), an acute care hospital (n = 7), a rehabilitation facility (n = 6), a nursing home (n = 3), or a long-term care facility (n = 4). Reasons given for why ICU survivors were unable to return home included ongoing requirements for medical or nursing care (n = 11), being unable to look after oneself physically (n = 15) or cognitively (n = 1), and living in an inpatient substance abuse treatment facility (n = 2).
Among survivors, 71.3% were able to ambulate 10 stairs independently and 81.9% were able to toilet independently. Normal cognition was reported for 62.4% of survivors (Figure 2). Among the 85 patients who were employed before their critical illness, 57 (67.1%) were alive at 6 months. Of these, 21 (36.8%) had returned to work at the same capacity as before their critical illness, and 7 (12.3%) had returned to work in a limited capacity.
All measured morbidity and mortality outcomes were better for surgical patients than for medical patients (Figure 2). Patients who had normal baseline function tended to have better risk-adjusted outcomes (Table E5). Physical and cognitive outcomes reported by patients were significantly better than those reported by surrogates (Table E6).
Return to Baseline Function
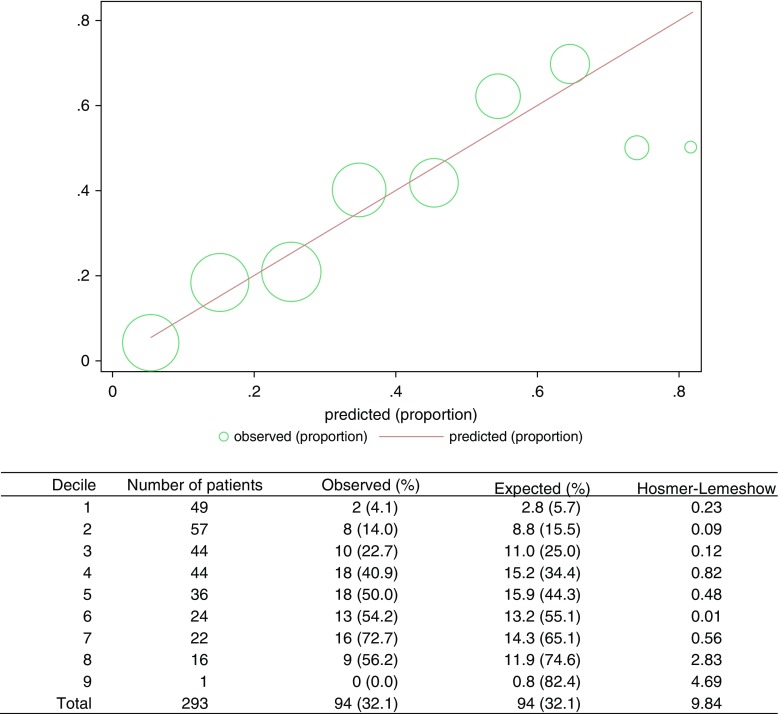
To be included in the multivariable logistic regression model, patients needed complete 6-month physical and cognitive outcome data as well as baseline characteristics. These variables were available for 293 of 303 enrolled patients (96.7%), who were ultimately included in the analysis designed to identify important predictors of return to baseline function. At 6 months, 91 patients (31.1%) had returned to baseline. More SICU patients (46%) than MICU patients (23%) returned to baseline (P < 0.0001) (Figure 2). Patients with normal function before ICU admission appeared to be slightly more likely to return to baseline at 6 months than patients with abnormal function at baseline (35 vs. 25%), but this comparison was not statistically significant (P = 0.079) (Figure E1). Unadjusted analyses highlight several differences between patients who returned to baseline and those who did not (Table E7). The multivariable model identified the following independent predictors of not returning to baseline function: older age, being a medical patient (compared with a surgical patient), nonwhite race, higher APACHE III score, hospitalization in the prior year, and a past history of cancer, liver disease, a neurologic condition, or any type of transplantation (Table 2). This model had an area under the ROC curve of 0.778 (95% CI, 0.724–0.832). The areas under the ROC curves for the leave-one-out cross-validation and fivefold cross-validation were 0.734 and 0.721, respectively. The forward and backward models were identical in their results. In secondary analyses, the “restricted” model including only the 212 patients enrolled through the fourth ICU day showed similar results to the primary analysis (area under the ROC curve, 0.800; 95% CI, 0.739–0.860) (Table E8). The analysis restricted to variables available only before ICU admission had comparable results (area under the ROC curve, 0.725; 95% CI, 0.664–0.787) (Table E9). The Hosmer–Lemeshow test illustrated good calibration of the primary model (P = 0.36) (Figure 3), secondary “restricted” model (P = 0.20) (Figure E2), and the model of variables available before ICU admission (P = 0.96) (Figure E3).
Table 2.
Predictors of 6-month return to baseline: n = 293 patients
| Variable* | Odds Ratio | 95% CI | AIC increase |
|---|---|---|---|
| APACHE III† | 0.85 | 0.77–0.93 | 11.1 |
| History of cancer | 0.47 | 0.24–0.90 | 3.2 |
| Race (nonwhite) | 0.49 | 0.26–0.91 | 3.2 |
| History of transplantation‡ | 0.15 | 0.01–0.81 | 3.1 |
| Age | 0.98 | 0.96–1.00 | 2.5 |
| Medical patient | 0.53 | 0.24–1.16 | 2.2 |
| Hospitalized in prior year | 0.58 | 0.32–1.09 | 1.2 |
| Neurological disease | 0.54 | 0.24–0.97 | 0.5 |
| Liver disease | 0.44 | 0.13–1.02 | 0.4 |
Definition of abbreviations: AIC = Akaike information criterion; APACHE = Acute Physiology and Chronic Health Evaluation; CI = confidence interval.
The stepAIC procedure in R selects the best model based on the combination of variables that achieves the lowest Akaike information criterion (AIC). The AIC for the best fitting model was 327.2. The AIC shown in the last column indicates the absolute increase in AIC (from 327.2 in the best model) if that single variable was removed from the full model shown in the table. They are ordered by the biggest change, indicating the relative predictive improvement related to that predictor.
For APACHE III score, this is represented as each 10-point increase.
Transplantation includes lung, liver, kidney, and bone marrow.
Figure 3.
Hosmer–Lemeshow plot and table comparing observed rates of returning to baseline with predicted rates of this outcome.
Discussion
Among patients spending at least 3 days in an ICU and requiring at least 48 hours of mechanical ventilation or 24 hours of vasoactive infusions we found that almost one-half had died within 6 months, and that less than one-third had returned to their baseline level of function 6 months later. These degrees of morbidity and mortality were greater among medical than surgical ICU patients. Overall, these data among general ICU patients complement and extend the results of prior studies that have been restricted to participants in randomized clinical trials (25, 26) or to patients with more prolonged courses of ICU stays or mechanical ventilation (7, 8). They suggest substantial degrees of near-term morbidity and mortality even among patients exposed to lower “doses” of critical care than evaluated in prior studies.
Indeed, our observed 6-month mortality rate of 43% actually underestimates overall mortality because we excluded patients who died or had their goals of care transitioned to full comfort within a few days of ICU admission, before enrollment. In a study of patients spending at least 3 days in a single medical ICU in Chicago, 69% of patients had died by 6 months when early deaths and transitions to palliation were included (27). Importantly, because 94% of patients in our sample were residing at home before ICU admission and 69% reported normal baseline function, the observed rates of adverse outcomes are unlikely to be attributable to our enrollment of a particularly high-risk sample.
A second important finding of this study is that among the 57% of patients who survived to 6 months, a great majority had resumed living in their original place of residence (83%). This rate is more favorable than observed among patients receiving prolonged mechanical ventilation (7), further suggesting that data from studies on such unique populations cannot be generalized to broader cohorts of ICU patients.
Third, we found that among patients who survived to 6 months, cognitive impairments were more common than physical impairments. The similarity of these rates of long-term cognitive and physical dysfunction to those observed in other cohorts of ICU patients (28, 29), despite differences in methods of assessment, supports the robustness of these findings. Further, such reaffirmation of long-term outcome rates is important for reducing uncertainty among ICU physicians when they guide patients and families in establishing expectations for future disabilities.
Fourth, this study identified a set of easily ascertainable clinical factors that together may help identify which patients will not return to their baseline function 6 months after ICU admission. The inclusion of only readily available factors during the first ICU day, and implementation of a variable selection process that prioritized parsimony, both augment the potential clinical usefulness of this model. Indeed, this easily replicated nine-variable model showed properties of good discrimination that were reproducible using two cross-validation techniques, and excellent calibration in determining who will and will not return to baseline 6 months later. However, further research is required to determine the model’s external validity in other cohorts.
Finally, the finding that blacks were less likely to return to baseline is concerning. Future research is needed to determine whether this may be attributable to racial differences in social supports and access to post-ICU care, differences in physical and occupation therapy use in the ICU or afterward, or other potential explanations.
Importantly, this model was designed to predict a novel outcome—whether or not patients will return to their baseline function within 6 months—that has several appealing features for assessing the outcomes of critical care. In contrast to commonly used outcome measures such as short-term survival or ICU length of stay (30), return to baseline provides a more complete picture of the survivorship experience. In addition, whereas measuring longitudinal outcomes such as quality of life poses numerous statistical and interpretive challenges due to informative censoring (31), this dichotomous measure incorporates survival and function without making any problematic assumptions. Finally, this outcome accords with patients’ and family members’ perspectives on what outcomes are important (32), and with the types of prognostic guidance that clinicians commonly provide to families (12). Thus, if the model is validated, it may improve the accuracy of the type of prognostic guidance that clinicians are giving anyway.
A potential limitation of this study is that because we conducted 6-month follow-up interviews by phone, we could not complete objective measurements of function, such as 6-minute walk distance. However, the functional outcomes we assessed were clearly defined and easily understood and completed by patients and surrogates, thereby minimizing missing or erroneous data. This pragmatic approach to participant follow-up knowingly sacrifices objectivity and measurement precision to augment real-world applicability, and has been used previously (27). Future studies are warranted to determine whether the quality of patient or surrogate decision-making can be optimized by providing prognostic information based on these less quantitative but more generalizable measures, versus more objective and comprehensive measures obtained from more selected samples.
A second limitation is that surrogates reported outcomes for one-half of the surviving patients. The outcomes reported by patients were generally more favorable than those reported by surrogates. This does not indicate that surrogates’ ratings were biased because such differences would be expected by virtue of the fact that patients who were in worse states of health were likely less able to complete their own assessments. However, because we lack data on how patients and surrogates rated outcomes for the same patients, we cannot rule out the possibility that surrogates’ ratings were systematically more pessimistic than patients’ ratings. This limitation also applies to assessment of baseline function, which was reported by surrogates on behalf of patients who were intubated or delirious, although premorbid assessments by patients and proxies appear to be highly correlated (33).
These limitations should also be considered in the context of this study’s strengths. In particular, the cohort included a much broader range of medical and surgical critical illness than has been represented in prior studies of long-term outcomes of ICU patients. And because we confirmed mortal and functional outcomes for 98.7% of enrolled patients, these results are not susceptible to the biases associated with loss to follow-up that may have been present in prior longitudinal cohorts. These strengths provide confidence in the core findings that patients undergoing even brief periods of life-sustaining therapy are at risk for high rates of mortality and functional decline out to 6 months later, and that several easily collected patient factors during the first ICU day may help predict which patients will return to their baseline function at this time point. If future work externally validates this predictive model and confirms that the outcome of return to baseline is important to all stakeholders, these insights may guide physicians in providing desirable prognostic guidance to patients and family members, and may provide a patient-centered, feasibly measured and analyzed survivorship outcome.
Supplementary Material
Acknowledgments
Acknowledgment
On behalf of all the authors, the corresponding author (M.E.D.) states that there is no conflict of interest. The authors acknowledge Steven P. Gale, Ph.D., for technical assistance with figure preparation. Dr. Gale received no financial compensation for his contribution.
Footnotes
Supported by an Institutional National Research Service Award grant from the National Heart, Lung, and Blood Institute, administered by the National Institutes of Health (T32-HL098054; M.E.D.); supported by the National Heart, Lung, and Blood Institute (F31-HL127947; M.O.H.).
Author Contributions: M.E.D. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: M.E.D., S.D.H., D.F.B.; acquisition, analysis or interpretation of the data: all authors; drafting of the manuscript: M.E.D., S.D.H.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: M.E.D., N.B.G., S.J.R., M.O.H.; study supervision: S.D.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med. 2016;176:1557–1559. doi: 10.1001/jamainternmed.2016.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, Friedrich JO, Mehta S, Lamontagne F, Levasseur M, et al. RECOVER Program Investigators (Phase 1: towards RECOVER); Canadian Critical Care Trials Group. The RECOVER Program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194:831–844. doi: 10.1164/rccm.201512-2343OC. [DOI] [PubMed] [Google Scholar]
- 9.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 10.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 11.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 12.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician–family discussions about limiting life support in intensive care units. Crit Care Med. 2007;35:442–448. doi: 10.1097/01.CCM.0000254723.28270.14. [DOI] [PubMed] [Google Scholar]
- 13.Davidson JE, Powers K, Hedayat KM, Tieszen M, Kon AA, Shepard E, Spuhler V, Todres ID, Levy M, Barr J, et al. American College of Critical Care Medicine Task Force 2004–2005; Society of Critical Care Medicine. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35:605–622. doi: 10.1097/01.CCM.0000254067.14607.EB. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull AE, Davis WE, Needham DM, White DB, Eakin MN. Intensivist-reported facilitators and barriers to discussing post-discharge outcomes with intensive care unit surrogates: a qualitative study. Ann Am Thorac Soc. 2016;13:1546–1552. doi: 10.1513/AnnalsATS.201603-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detsky ME, Harhay MO, Bayard DF, Delman AM, Buehler AE, Kent SA, Ciuffetelli IV, Cooney E, Gabler NB, Ratcliffe SJ, et al. Discriminative accuracy of physician and nurse predictions for survival and functional outcomes 6 months after an ICU admission. JAMA. 2017;317:2187–2195. doi: 10.1001/jama.2017.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meadow W, Pohlman A, Frain L, Ren Y, Kress JP, Teuteberg W, Hall J. Power and limitations of daily prognostications of death in the medical intensive care unit. Crit Care Med. 2011;39:474–479. doi: 10.1097/CCM.0b013e318205df9b. [DOI] [PubMed] [Google Scholar]
- 17.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310:1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 18.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 19.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 22.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 23.Witten IH, Frank E, Hall MA. Data mining: practical machine learning tools and techniques. 3rd ed. Boston: Elsevier; 2011. [Google Scholar]
- 24.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12:132–139. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 25.Needham DM, Wozniak AW, Hough CL, Morris PE, Dinglas VD, Jackson JC, Mendez-Tellez PA, Shanholtz C, Ely EW, Colantuoni E, et al. National Institutes of Health NHLBI ARDS Network. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The Adult Respiratory Distress Syndrome Cognitive Outcomes Study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meadow W, Pohlman A, Reynolds D, Rand L, Correia C, Christoph E, Hall J. Power and limitations of daily prognostications of death in the medical ICU for outcomes in the following 6 months. Crit Care Med. 2014;42:2387–2392. doi: 10.1097/CCM.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 28.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harhay MO, Wagner J, Ratcliffe SJ, Bronheim RS, Gopal A, Green S, Cooney E, Mikkelsen ME, Kerlin MP, Small DS, et al. Outcomes and statistical power in adult critical care randomized trials. Am J Respir Crit Care Med. 2014;189:1469–1478. doi: 10.1164/rccm.201401-0056CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum PL. Comment: the place of death in the quality of life. Stat Sci. 2006;21:313–316. [Google Scholar]
- 32.Lyon SM, Auriemma C, Hilbert C, Cooney E, Sterlec L, Kent S, Barg F, Halpern SD. Defining patient- and surrogate-centered outcomes for critical care research [abstract; accessed 4 July 2017] Am J Respir Crit Care Med. 2014;189:A2181. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A2181. [Google Scholar]
- 33.Hofhuis J, Hautvast JL, Schrijvers AJ, Bakker J. Quality of life on admission to the intensive care: can we query the relatives? Intensive Care Med. 2003;29:974–979. doi: 10.1007/s00134-003-1763-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.