Abstract
With the emerging interest in documenting and understanding muscle atrophy and function in critically ill patients and survivors, ultrasonography has transformational potential for measurement of muscle quantity and quality. We discuss the importance of quantifying skeletal muscle in the intensive care unit setting. We also identify the merits and limitations of various modalities that are capable of accurately and precisely measuring muscularity. Ultrasound is emerging as a potentially powerful tool for skeletal muscle quantification; however, there are key challenges that need to be addressed in future work to ensure useful interpretation and comparability of results across diverse observational and interventional studies. Ultrasound presents several methodological challenges, and ultimately muscle quantification combined with metabolic, nutritional, and functional markers will allow optimal patient assessment and prognosis. Moving forward, we recommend that publications include greater detail on landmarking, repeated measures, identification of muscle that was not assessable, and reproducible protocols to more effectively compare results across different studies.
Keywords: muscle atrophy, muscle thickness, muscle cross-sectional area, intensive care, critical illness
The field of acute muscle wasting in critical illness has expanded significantly with an understanding and characterization of functional impairment in survivors of critical illness. Muscle wasting is one of the greatest problems that survivors exhibit, which contributes to muscle weakness. Both quality-of-life questionnaires and functional walking tests have demonstrated muscle weakness as a primary contributor to functional disability (1–10). However, the underlying mechanisms of muscle weakness in the intensive care unit (ICU) survivors are poorly understood.
Muscle weakness is driven by loss of muscle mass, metabolic and physiological dysregulation of skeletal muscle, degradation in skeletal muscle architecture, and dysfunctional central and peripheral neural signals. Because these elements represent a composite of muscle health, it is important to evaluate them as a whole rather than independently. Many of these variables often require volitional assessment, which in many cases can be invasive and expensive. Obtaining objective and feasible measures of muscle health at admission and during the trajectory of critical illness is challenging, given the unstable and weakened condition of patients; therefore, nonvolitional methods and surrogate measures are needed to assess muscle health (11). Previous reports highlight that up to 60% of critically ill patients fail common screening processes for manual muscle testing at awakening (11, 12) and that approximately 12% are unable to complete the test, owing to death, pain, or refusal (12). There is also a time-critical period following ICU admission that occurs prior to awakening (on average, 7–12 d) when patient participation in assessments is not possible but during which accelerated rates of muscle wasting are known to occur (13–15).
Features of skeletal muscle (including muscle quantity measures such as mass and cross-sectional area and muscle quality measures such as architecture and evidence of myonecrosis) may provide a more feasible and objective approach to assessing muscle health in ICU patients. With the ultimate focus on physical activity and functional outcomes, it is not surprising that the quadriceps muscles, which are an important weight-bearing muscle group, have been the most extensively studied region of the body. Lower limb muscle mass and strength have repeatedly been shown to correlate in diverse populations, including in ICU survivors (13, 16–18). Although muscle mass may technically be a surrogate measure of muscle function, not all studies support this relationship (19, 20). Objective quantifications of muscle (which include but are not limited to muscle mass, thickness, and cross-sectional area) that are sufficiently sensitive to detect small changes over acute time frames may ultimately facilitate evaluation of interventions to counter muscle atrophy and weakness.
Over the last decade, common imaging technologies such as ultrasound (13, 21–23) and computed tomography (CT) (23–27) have been translated to the ICU for quantification of muscle. The transformational application of these techniques has advanced understanding of the muscle characteristics of ICU patients at admission and during the ICU trajectory. Quantification of skeletal muscle (13), an example of this transformational application, allows for identification of those with low muscle quantity (21). Low muscle quantity in turn has been related to poor clinical outcomes, including increased mortality as well as reduced ventilator-free days and ICU-free days (25–27).
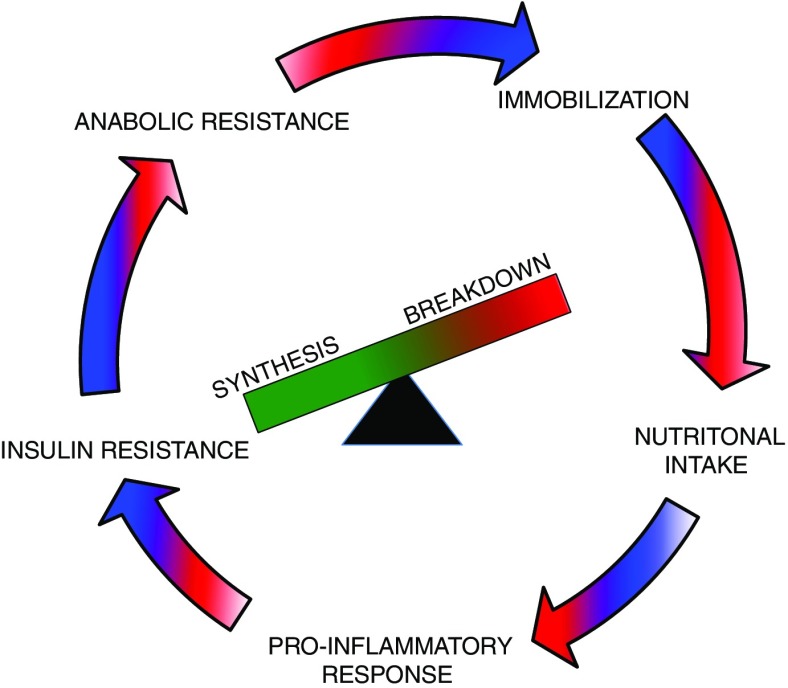
Approximately 63% of ICU patients have low muscle cross-sectional area as measured by CT at the time of ICU admission (25), and this prevalence increases to approximately 70% when ICU patients are aged 65 years or older (26). Ultrasonography has also demonstrated approximately 8 to 30% loss of muscle within the first 7–10 days of ICU admission (13, 14), and muscle atrophy has been associated with degree of organ failure as well as increased ICU length of stay (14, 28, 29). Muscle weakness may independently predict clinical outcomes, including mortality (30, 31), ventilator-dependent time (15, 30, 32), and hospital length of stay (30). Muscle atrophy and weakness are likely related to catabolic processes such as inflammation during and following critical illness (33) as well as immobility or lack of activity (Figure 1) (34, 35). The fact that muscle wasting and muscle weakness occur is not new to clinicians; Asher alluded to this phenomenon in his treatise on bed rest in 1947 and placed “beds and graves in the same category” (36, p. 967).
Figure 1.
Depiction of diverse factors that contribute to the progressive cycle of reduced protein synthesis and increased protein breakdown leading to muscle atrophy in critically ill patients.
Need for Precise and Accurate Modalities for Muscle Quantification
Precise and accurate muscle quantification at a single time point and for longitudinal analysis is valuable for (1) characterizing metabolic and functional changes in lean tissue, (2) understanding the underlying mechanisms of muscle wasting, and (3) assessing the success or failure of therapeutic interventions. Adverse metabolic adaptations secondary to muscle atrophy may also contribute to these poor functional and clinical outcomes, but the measurement of muscle metabolism is elusive in critically ill patients and survivors.
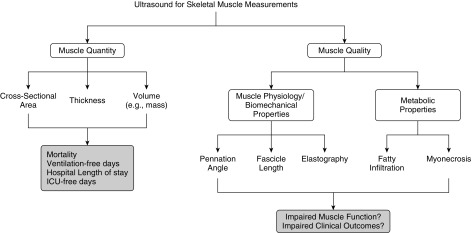
Skeletal muscle is fundamental to immune and cytokine function (37) and accounts for greater than 75% of glucose disposal (38). Thus, ICU-associated muscle atrophy and deleterious changes in muscle integrity are expected to complicate glucose regulation and various processes affected by inflammation. Maintaining and restoring skeletal muscle becomes challenging, given that patients exhibit a vicious cycle of attenuated protein synthesis and accelerated protein breakdown. This vicious cycle is driven by ICU-related bed rest (33), reduced caloric and protein intake (39, 40), a proinflammatory state (41, 42), and anabolic and insulin resistance (defined as the reduced ability to take up amino acids and glucose into muscle, respectively) (43, 44) (Figure 1). Body composition measures, specifically ultrasonography, present the potential opportunity to noninvasively characterize muscle health by measuring change in muscle quantity (e.g., muscle mass) and muscle quality (e.g., development of myonecrosis, fatty infiltration) (Figure 2).
Figure 2.
Several quantitative and qualitative features of skeletal muscle can be measured using ultrasound that are associated with clinical and functional outcomes. ICU = intensive care unit.
Measuring Up: Methods for Muscle Quantification
Body mass index (BMI), bioelectrical impedance analysis (BIA), dual-energy X-ray absorptiometry (DXA), CT, magnetic resonance imaging (MRI), and ultrasonography are currently available as tools for assessing muscle mass. Research focused on understanding muscle atrophy has been impeded by the lack of available tools for measuring muscle mass that (1) are easily deployable within the constraints of an ICU, (2) have low/no risk to patients, and (3) are able to accurately track both muscle atrophy and recovery. Body composition, often coupled with additional metabolic/biochemical biomarkers, is increasingly used to quantify changes in muscle mass and integrity. Next, we briefly discuss the merits and challenges of these modalities, which are more elaborately reviewed elsewhere (23, 45).
Historically, weight, BMI, or BIA has been used in various nutrition studies to describe changes in body composition and evaluate intervention efficacy. Pronounced edema and fluid shifts in patients, combined with the lack of specificity and variability in these measures, impede the usefulness of these tools in understanding specific muscle changes in ICU patients and recovery in survivors. Importantly, weight and BMI are general measures of body size and cannot distinguish between fat and lean tissue. Weight lost during hospitalization may be regained within 1 year of ICU discharge, but as fat mass rather than lean mass (46, 47). Such changes in weight can increase risk of morbidity and reduce quality of life, demonstrating the importance of accurate tracking of muscle and fat mass. Studies that examine muscle atrophy and weakness in ICU survivorship therefore need to include specific and accurate quantification of muscle.
BIA is commonly used to determine total body water, which is then used to estimate fat-free mass using body weight (48). Given its reliance on the conductive properties of water for estimating lean tissue mass, BIA is susceptible to inaccuracies secondary to large fluxes in fluid status that present in critically ill patients (49–51). This, coupled with the lack of reliable weight measures in critical care, lack of predictive equations for this cohort, and limitations in the positioning of the patient for accurate measures, reduces the usefulness of this modality. However, emerging work suggests that BIA may provide information on fluid shifts (by calculating impedance ratio) as well as cell membrane integrity and body cell mass (based on phase angle calculations). When these measures are combined with commonly collected covariates, they may predict muscularity in this population (52).
DXA is performed with a scanner that uses two X-ray beams of different energy levels to distinguish lean and fat tissue. It is a powerful modality for accurately and precisely measuring lean tissue with little radiation exposure. It permits the compartmentalization of specific regional lean tissue measures where there may be interest in understanding whether muscle atrophy is universal or regional in ICU patients as well as evaluating potential regional improvements following targeted rehabilitation. Its use has been limited in the ICU, given the sparse accessibility of the equipment and the logistics of patient transfer. In only a single study to date, researchers evaluated lean tissue mass in 49 ICU patients using DXA (50); however, the data were used to validate BIA methods and thus have little relevance to muscle atrophy.
CT and MRI offer precise, accurate, and reliable measures of muscle cross-sectional area and volume as well as muscle integrity (quality), but their use is limited in the critical care setting. Most studies are based on retrospective analysis of computed tomographic images of the third lumbar vertebral (L3) region. Although studies in healthy (53) and cancer populations (54) suggest that the L3 region is representative of whole-body skeletal muscle or lean tissue distribution, it is unknown whether muscle atrophy occurs globally or in specific regions of the body. Although De Jonghe and colleagues (32) showed that respiratory and limb weakness are associated, the magnitude and etiology of muscle atrophy in proximal versus distal muscle groups may be distinct. Future work is needed to investigate whether muscle atrophy occurs in multiple locations and to elucidate the underlying mechanisms that drive these losses.
In addition, the retrospective nature of most studies using CT or MRI prohibits the proper design of longitudinal studies. Selection bias is also introduced by restricting the study population to those patients who require abdominal CT or MRI for diagnostic purposes. Prospective computed tomographic or MRI analysis is associated with patient transport outside the ICU (and its accompanying risks), cost, and safety concerns (e.g., CT radiation dose), all of which preclude their use in research studies and, for now, clinical evaluation of muscle mass and quality. Researchers in one study used prospective computed tomographic imaging (24), but the study’s limited sample size (n = 8) precludes extrapolation. Despite these limitations, knowledge of muscle atrophy in critically ill patients has been significantly advanced by these modalities (21, 25, 26).
B-mode ultrasound has emerged as an attractive method, being easily accessed by researchers as a result of its central and expanding role in a variety of clinical bedside procedures. Skeletal muscle ultrasound is not a new technique; reports of its use in the ICU setting date back 2 decades, and it has been used in pediatric muscle disease for more than 30 years. More recent work employing this technique has been focused on understanding muscle loss in aging (55, 56) and chronic disease populations (17, 57). Two imaging aspects are of interest: muscle quantification (e.g., muscle layer thickness or cross-sectional area of an individual muscle or muscle group; Figure 3) and muscle quality (echogenicity). On one hand, in healthy volunteers and ambulant disease states (e.g., chronic obstructive pulmonary disease, chronic heart or renal failure), measures of muscle cross-sectional area correlate with strength (57), and this assessment may have a conceivable use in guiding rehabilitation. Echogenicity, on the other hand, offers the opportunity to potentially evaluate the integrity of muscle (i.e., muscle biomechanics, fatty infiltration, presence of myonecrosis; Figure 2) (58, 59).
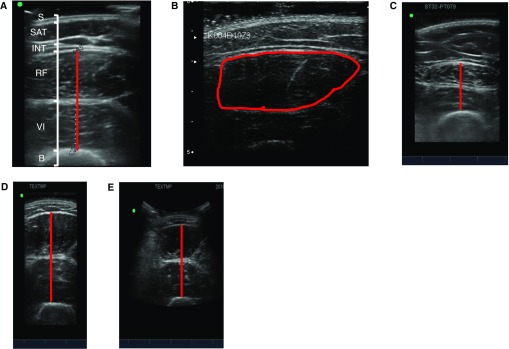
Figure 3.
Illustrations of diverse patient and scan characteristics obtained by ultrasonography. (A) Anatomical labels are identified in a scan depicting muscle layer thickness of a 23-year old non–intensive care unit female with a body mass index of 22.8 kg/m2. Muscle layer thickness would include rectus femoris and vastus intermedius depicted in red. (B) Example of cross-sectional area of a rectus femoris muscle highlighted in red. (C) Muscle layer thickness is shown for a non–intensive care unit individual who has a body mass index of 28.9 kg/m2 and body fat percentage of 45.6% (measured using dual-energy X-ray absorptiometry). The red line depicts muscle layer thickness. (D) Muscle layer thickness is imaged using a linear probe and depicted in red. (E) Muscle layer thickness is imaged using a curvilinear probe, depicted in red, in the same individual as in (D). B = bone; INT = interface between subcutaneous adipose tissue and rectus femoris; RF = rectus femoris; S = skin; SAT = subcutaneous adipose tissue; VI = vastus intermedius.
Current State of the Translational Use of Critical Care Muscle Ultrasound
In the past 5 years (2011–2016), 11 observational studies (13, 14, 19, 28, 59–65) and 3 interventional studies (66–68) using ultrasound measures of skeletal muscle as an outcome in critically ill patients have been published. Currently, an additional 13 trials using ultrasound measures as outcomes are registered with www.clinicaltrials.gov, suggesting that ultrasonography is being rapidly adopted by clinicians in multiple disciplines for measuring muscle features. However, the technique of critical care skeletal muscle ultrasound lags behind other critical illness research domains where standardization of definitions (60), gradation of severity (60, 61), and technical skills (62) have allowed assessment of external validity of data produced.
Challenges in Critical Care Muscle Ultrasound Studies
Although ultrasonography is an attractive tool for evaluating muscle health, several uncertainties and limitations need to be considered (summarized in Table 1). The discussion below offers an overview and is intended to highlight areas to be addressed in future work. This brief discussion is critically important, given the rapid expansion in the adoption of ultrasound imaging in clinical and research practice as well as the lack of standardization in the approaches used.
Table 1.
Summary of limitations and challenges in using ultrasound for assessing muscle health
| Challenge or Limitation | Description of Challenge or Limitation |
|---|---|
| Identification of landmarks | • Appropriate training is needed for consistent identification of landmarks. |
| • Standardized protocols on how to identify anatomical landmarks for measurement are lacking. | |
| • Reporting standards (in publications) are lacking for landmarking. | |
| • Reliability testing for landmarking is either lacking or included within the entire data acquisition process (i.e., probe placement, image analysis). | |
| Reliability testing | • Reporting on reliability testing is often lacking. |
| • Standardization on how a reliability test is performed and what is included in the testing procedure is lacking. | |
| • Intra- and interreliability tests are either both lacking or only one of the two tests is performed using ultrasound and published in research studies. | |
| Muscle site | • Different muscles are often measured across different studies (rectus femoris vs. vastus lateralis, upper limb vs. lower limb, or a combination). |
| • Different methods are used for muscle quantity assessment (i.e., cross-sectional area vs. muscle layer thickness). | |
| • Best site to capture muscle groups consistently are unclear (i.e. mid-thigh versus 2/3 femur length versus 3/5 femur length) | |
| Image acquisition and analysis | • Form of analysis varies across studies (i.e., muscle layer thickness, cross-sectional area, echogenicity). Some studies use a combination of these methods. |
| • Different body composition phenotypes present distinct challenges (e.g., obese individuals may have indistinguishable fascial boundaries, fatty infiltration may affect muscle thickness). | |
| • Fluid status may affect measurements: use of minimal versus maximal compression. | |
| Equipment use | • Appropriate use and reporting of curvilinear versus linear probes |
| • Identification of a minimal level of resolution for echogenicity | |
| • Software choice for image analysis varies | |
| Normative data and interpretation of results | • Limited studies that provide a healthy, homogeneous cohort for comparison |
| • Measurement reporting is essential for longitudinal evaluation but is currently missing in the literature. | |
| • Identification of the smallest detectable change for clinically meaningful longitudinal analysis is needed. |
Training
Although muscle ultrasound is relatively straightforward to perform, appropriate training is required (63). Currently, there is no formal training program or standardized protocol used to educate clinicians, health care providers, or students. Importantly, reliability, reproducibility, and accuracy should not be assumed; thus, data pertaining to these aspects should be made available in studies, and general reporting standards should be developed. Within this context, standardized protocols are needed in terms of the following (additional details can be found in Table 2):
-
•
Blinding for reliability tests
-
•
Number of repeated measures needed
-
•
Reliability tests for landmarking
-
•
Reliability tests for measuring thickness or cross-sectional area
-
•
Inclusion of intra- and interreliability measures
-
•
Consideration of patient positioning (i.e., supine vs. semireclined positions may generate different results for various muscle groups)
-
•
Criteria to be used to label a muscle as “unassessable” (where unassessable may refer to poor image quality or ability to distinguish fascial borders for precise and accurate quantification)
Table 2.
General ultrasound protocol and reporting characteristics important to include in publications
| Reporting Characteristics | Description of Information |
|---|---|
| Data acquisition | |
| Identification of landmarks | • Diverse papers use various landmarks. |
| • Choose the appropriate landmarks for your study and refer to the paper(s) that have used or validated these landmarks. | |
| Number of repeated measures | • Identify the number of times that the measurements (thickness or cross-sectional area) were performed. |
| • Identify whether the largest, smallest, or average of the repeated measures was reported. | |
| Identification of “unassessable muscle” | • Outline of the criteria used to determine whether the quality of the image would provide an unreliable measure: “unassessable muscle” |
| • Example criteria may include inability to distinguish fascial borders between muscle and fat. | |
| Reliability testing | |
| Reliability tests for landmarking | • Description of how the landmark was tested for reliability |
| Reliability tests for measuring thickness or cross-sectional area (CSA) | • Description of thickness or CSA reliability tests performed |
| Blinding for reliability tests | • Description of how the analyst was blinded to “relandmarking” the area of interest (e.g., if a mark had been made on a given limb, was that mark removed?) |
| • Describe how the analyst was blinded to the reliability test for using the measuring tools (e.g., screen/electronic calipers). | |
| Intra- versus interreliability | • Identify the number of repeated tests that were performed for reliability. |
| • Identify the coefficient of variation for the inter- and intrareliability tests. | |
The majority of reliability measures have been focused on image analysis as opposed to the fundamental data quality of image acquisition. Validation and reliability work is essential to confirming that measurements are consistent and specific to permit comparisons across different studies.
Muscle Site
Heterogeneity exists in parameters measured and reported, and this is probably the most significant barrier to external validity. Standardization of specific muscles and muscle groups to be analyzed is lacking. Muscle mass changes have been focused on both composite and individual muscles: rectus femoris (either as cross-sectional area or thickness; Figure 2) (17) or vastus lateralis (thickness) (13) or muscle limb thickness composed of rectus femoris and vastus intermedius (Figure 2) (21) or individual muscle groups (13). More recently, Fischer and colleagues reported data using multiple images and multiple sites (66). The best image acquisition site also remains unknown, with midthigh, 2/3, and 3/5 of femur length all being used for the quadriceps.
The number of images needed for adequate analysis of biomechanical measures and metabolic integrity also remains unstandardized. These diverse measures could lead to a more targeted approach not only to identifying patients at risk of poor outcomes but also to understanding underlying mechanisms in muscle atrophy in this patient population. Recent work has begun to address this, demonstrating that muscle layer thickness may underestimate muscle loss and cross-sectional area may relate to strength to a greater degree (69).
Image Acquisition and Analysis
There is variability in the data collected across studies, which may include some or all of the following: muscle layer thickness, cross-sectional area, echogenicity, or a combination of these and other measurements. Within each of these ultrasound measurements, patients in the ICU have diverse body composition phenotypes that present distinct challenges in quantifying muscle. Obese patients typically require greater scanning depth, with subsequent loss of resolution. Fatty infiltration within the muscle will confound muscle thickness measures unless echogenic factors are concurrently considered. Echogenic factors, which are variables that influence the “bounce back” or reflection of ultrasound waves, differ across diverse ultrasound devices. This heterogeneity in echogenic factors may be challenging and may limit the ability to compare results without a phantom standard (a standard tool that is used for calibration of multiple pieces of equipment). Fluid status may also affect echogenic factors, but there are conflicting reports in this area of work (59, 63). To overcome the issue of fluid status, some researchers have used full compression of the probe in an attempt to remove the confounding effect of edema (21, 28). However, full compression may alter the size and shape of muscle variably. Other researchers have used minimal or no compression whereby an excess of ultrasound gel is used to minimize the distortion of the image that typically results from skin indentations caused by pressure of the ultrasound probe (13, 59). Still others have not reported their approach. Consequently, these distinct techniques make it difficult to compare results across studies.
Image acquisition is also confounding where interfaces of the muscle boundaries can be challenging and add to error in reliability and accuracy in aged and/or morbid individuals. Cannulae from external devices may also limit the available scanning site (e.g., extracorporeal membrane oxygenation cannulae overlying the quadriceps) and prohibit image acquisition. Clarity is also needed in determining the use of bilateral versus unilateral limb measurements as well as the number of measures to be performed (and whether the average or peak measure is reported).
Equipment Use
Linear arrays offer a range of resolutions determined by transducer frequency. Though direct comparisons have been made between curvilinear and linear probes (Figures 2D and 2E) (65), an as yet unestablished minimum level of resolution is required for echogenicity analysis of muscle quality and detection of necrosis. Multiple software platforms capable of image analysis are used, but the impact of subtle analytical differences (e.g., in pixelation analysis) or equipment mode selections (e.g., gain, depth) may also preclude direct comparison of datasets.
Normative Data and Interpretation of Results
There are a limited number of studies that provide a healthy, homogeneous cohort for comparison. As a consequence, there is an essential need for normative data to identify patients with low muscle mass. Case–control studies are methodologically difficult, given the interindividual variability in muscle size. Measurement reporting needs to be considered, especially in the context of longitudinal evaluation. Percentage changes may be more meaningful, but these percentages may be misleading in the context of small muscle groups or atrophied muscles. For example, a loss of cross-sectional area of 5 cm2 in a muscle group that was 40 cm2 at baseline equates to a 13% loss, compared with a muscle that was 80 cm2 at baseline, which equates to a 7% loss. Clear distinctions need to be made between statistically significant differences and clinically relevant changes while respecting the intrinsic accuracy and variability of the technique (66). Determining the smallest detectable change that is permitted with skeletal muscle ultrasound and assessing the validity of this value is fundamental to distinguishing true change from artifact. Overall, investigators need to be clear with regard to the quality, accuracy, and reproducibility of data collected.
Perspectives: Optimizing Use and Interpretations of Muscle Ultrasound in the ICU
Muscle quantification at ICU admission and changes over time are potential surrogate outcome measures for muscle function as well as overall muscle health. Muscle function and strength require voluntary participation of the patient, and these measurements are often precluded by barriers such as sedation and delirium. Nonvolitional measurements are attractive in their ability to set a baseline measurement at the onset of critical illness. This relationship between muscle mass and function should be examined more thoroughly in the critically ill to guide method development using ultrasonography. The most optimal method of understanding muscle health in the ICU will likely require a combination of several assessments, including systemic, nutritional, functional, clinical, and metabolic biomarkers, as well as muscle quantity (e.g., thickness, cross-sectional area, mass) and quality (including echogenic factors). Algorithms for the most predictive approaches should be considered in future studies, but a first step is identifying a universal protocol for characterizing muscle quantity and quality.
Mitigating the degree of muscle wasting during acute critical illness and regaining muscle mass via rehabilitation are core targets for improving patient outcomes in critical care, and they are strongly associated (13). Accurate and precise quantification of muscle mass is one step toward identifying patients who have low muscle mass at ICU admission. In combination with other measures, future work in muscle quantification may help to identify those who would benefit most from targeted nutrition and rehabilitative therapy. Intervention evaluation aimed at preventing or reducing muscle loss requires an accurate and precise set of tools. Ultrasound has several merits in providing a noninvasive, nonvolitional, and accessible approach to measuring quantity and quality of muscle. Although ultrasound represents a potential option for measurement of muscle mass in real time in clinical facilities, first a universal protocol needs to be identified and validated that can be easily implemented in the critical care setting to enhance and advance studies whose aim is to improve muscle rehabilitation in survivors of critical illness.
Supplementary Material
Footnotes
Supported by a National Health and Medical Research Council Early Career Fellowship (1111640) and a short-term European Respiratory Society fellowship in 2016 (S.P.), as well as by a National Institute of Health Research (NIHR) postdoctoral research fellowship and the NIHR Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London (B.C.). The views expressed are those of the authors and are not necessarily those of the NHS, the NIHR, or the Department of Health.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbertson BH, Roughton S, Jenkinson D, MacLennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 4.Myhren H, Ekeberg Ø, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med. 2010;38:1554–1561. doi: 10.1097/CCM.0b013e3181e2c8b1. [DOI] [PubMed] [Google Scholar]
- 5.Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Mendez-Tellez PA, Shanholtz C, Ciesla ND, Pronovost PJ, Needham DM. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–1566. doi: 10.1007/s00134-016-4530-1. [DOI] [PubMed] [Google Scholar]
- 6.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 7.Poulsen JB, Møller K, Kehlet H, Perner A. Long-term physical outcome in patients with septic shock. Acta Anaesthesiol Scand. 2009;53:724–730. doi: 10.1111/j.1399-6576.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, Denehy L, Harrold M, Higgins A, Presneill J, Saxena M, et al. TEAM Study Investigators. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, Casaer MP, Meersseman P, Debaveye Y, Van Cromphaut S, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190:410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 10.Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, Nollet F, van Schaik IN, Schultz MJ, Horn J, van der Schaaf M. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. doi: 10.1186/s13054-015-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly BA, Jones GD, Curtis AA, Murphy PB, Douiri A, Hopkinson NS, Polkey MI, Moxham J, Hart N. Clinical predictive value of manual muscle strength testing during critical illness: an observational cohort study. Crit Care. 2013;17:R229. doi: 10.1186/cc13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15:R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, Annoni R, Puthucheary Z, Gordon IR, Morris PE, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30:1151.e9–1151.e14. doi: 10.1016/j.jcrc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 15.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al. Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 16.de Bruin PF, Ueki J, Watson A, Pride NB. Size and strength of the respiratory and quadriceps muscles in patients with chronic asthma. Eur Respir J. 1997;10:59–64. doi: 10.1183/09031936.97.10010059. [DOI] [PubMed] [Google Scholar]
- 17.Seymour JM, Ward K, Sidhu PS, Puthucheary Z, Steier J, Jolley CJ, Rafferty G, Polkey MI, Moxham J. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 18.Young A, Stokes M, Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984;14:282–287. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashida I, Tanimoto Y, Takahashi Y, Kusabiraki T, Tamaki J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLoS One. 2014;9:e111810. doi: 10.1371/journal.pone.0111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 21.Paris MT, Mourtzakis M, Day A, Leung R, Watharkar S, Kozar R, Earthman C, Kuchnia A, Dhaliwal R, Moisey L, et al. Validation of bedside ultrasound of muscle layer thickness of the quadriceps in the critically ill patient (VALIDUM Study): a prospective multicenter study. JPEN J Parenter Enteral Nutr. 2017;41:171–180. doi: 10.1177/0148607116637852. [DOI] [PubMed] [Google Scholar]
- 22.Bunnell A, Ney J, Gellhorn A, Hough CL. Quantitative neuromuscular ultrasound in intensive care unit-acquired weakness: a systematic review. Muscle Nerve. 2015;52:701–708. doi: 10.1002/mus.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paris M, Mourtzakis M. Assessment of skeletal muscle mass in critically ill patients: considerations for the utility of computed tomography imaging and ultrasonography. Curr Opin Clin Nutr Metab Care. 2016;19:125–130. doi: 10.1097/MCO.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 24.Poulsen JB, Møller K, Jensen CV, Weisdorf S, Kehlet H, Perner A. Effect of transcutaneous electrical muscle stimulation on muscle volume in patients with septic shock. Crit Care Med. 2011;39:456–461. doi: 10.1097/CCM.0b013e318205c7bc. [DOI] [PubMed] [Google Scholar]
- 25.Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, Beishuizen A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12–R18. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, Bulger E, Kozar RA Nutrition and Rehabilitation Investigators Consortium (NUTRIC) Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206–R213. doi: 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Troy KL, Ajanaku FC, Patel A, Sclamberg JS, Wang Z. Exploitation of diagnostic computed tomography scans to assess the impact of nutrition support on body composition changes in respiratory failure patients. JPEN J Parenter Enteral Nutr. 2014;38:880–885. doi: 10.1177/0148607113500505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruther W, Benesch T, Zorn C, Paternostro-Sluga T, Quittan M, Fialka-Moser V, Spiss C, Kainberger F, Crevenna R. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med. 2008;40:185–189. doi: 10.2340/16501977-0139. [DOI] [PubMed] [Google Scholar]
- 29.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali NA, O’Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, et al. Midwest Critical Care Consortium. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 31.Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, Cerf C, Outin H, De Jonghe B Groupe de Réflexion et d’Etude des Neuromyopathies En Réanimation. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37:3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 32.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 33.Griffith DM, Vale ME, Campbell C, Lewis S, Walsh TS. Persistent inflammation and recovery after intensive care: a systematic review. J Crit Care. 2016;33:192–199. doi: 10.1016/j.jcrc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(10 Suppl):S422–S428. doi: 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 35.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asher RA. The dangers of going to bed. BMJ. 1947;2:967. doi: 10.1136/bmj.2.4536.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 39.Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32:350–357. doi: 10.1097/01.CCM.0000089641.06306.68. [DOI] [PubMed] [Google Scholar]
- 40.Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C. Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. JPEN J Parenter Enteral Nutr. 2016;40:45–51. doi: 10.1177/0148607115583675. [DOI] [PubMed] [Google Scholar]
- 41.Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol. 1991;260:E727–E730. doi: 10.1152/ajpendo.1991.260.5.E727. [DOI] [PubMed] [Google Scholar]
- 42.Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol. 2011;589:3883–3895. doi: 10.1113/jphysiol.2011.206193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rennie MJ. Anabolic resistance in critically ill patients. Crit Care Med. 2009;37(10 Suppl):S398–S399. doi: 10.1097/CCM.0b013e3181b6ec1f. [DOI] [PubMed] [Google Scholar]
- 44.Saberi F, Heyland D, Lam M, Rapson D, Jeejeebhoy K. Prevalence, incidence, and clinical resolution of insulin resistance in critically ill patients: an observational study. JPEN J Parenter Enteral Nutr. 2008;32:227–235. doi: 10.1177/0148607108316195. [DOI] [PubMed] [Google Scholar]
- 45.Teigen LM, Kuchnia AJ, Mourtzakis M, Earthman CP. The use of technology for estimating body composition: strengths and weaknesses of common modalities in a clinical setting. Nutr Clin Pract. 2017;32:20–29. doi: 10.1177/0884533616676264. [DOI] [PubMed] [Google Scholar]
- 46.Reid CL, Murgatroyd PR, Wright A, Menon DK. Quantification of lean and fat tissue repletion following critical illness: a case report. Crit Care. 2008;12:R79. doi: 10.1186/cc6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dos Santos C, Hussain SN, Mathur S, Picard M, Herridge M, Correa J, Bain A, Guo Y, Advani A, Advani SL, et al. MEND ICU Group; RECOVER Program Investigators; Canadian Critical Care Translational Biology Group. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay: a pilot study. Am J Respir Crit Care Med. 2016;194:821–830. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 48.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, et al. Composition of the ESPEN Working Group. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23:1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Foley K, Keegan M, Campbell I, Murby B, Hancox D, Pollard B. Use of single-frequency bioimpedance at 50 kHz to estimate total body water in patients with multiple organ failure and fluid overload. Crit Care Med. 1999;27:1472–1477. doi: 10.1097/00003246-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Savalle M, Gillaizeau F, Maruani G, Puymirat E, Bellenfant F, Houillier P, Fagon JY, Faisy C. Assessment of body cell mass at bedside in critically ill patients. Am J Physiol Endocrinol Metab. 2012;303:E389–E396. doi: 10.1152/ajpendo.00502.2011. [DOI] [PubMed] [Google Scholar]
- 51.Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30:180–193. doi: 10.1177/0884533614568155. [DOI] [PubMed] [Google Scholar]
- 52.Kuchnia A, Earthman C, Teigen L, Cole A, Mourtzakis M, Paris M, Looijaard W, Weijs P, Oudemans-van Straaten H, Beilman G, et al. Evaluation of bioelectrical impedance analysis in critically ill patients: results of a multicenter prospective study. JPEN J Parenter Enteral Nutr. doi: 10.1177/0148607116651063. [online ahead of print] 24 May 2016; DOI: 10.1177/0148607116651063. [DOI] [PubMed] [Google Scholar]
- 53.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 54.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 55.Abe T, Sakamaki M, Yasuda T, Bemben MG, Kondo M, Kawakami Y, Fukunaga T. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J Sports Sci Med. 2011;10:145–150. [PMC free article] [PubMed] [Google Scholar]
- 56.Minetto MA, Caresio C, Menapace T, Hajdarevic A, Marchini A, Molinari F, Maffiuletti NA. Ultrasound-based detection of low muscle mass for diagnosis of sarcopenia in older adults. PM R. 2016;8:453–462. doi: 10.1016/j.pmrj.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, Shaheen AA, Qamar H, Mansoor N, Carbonneau M, et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480.e3. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 58.Connolly B, MacBean V, Crowley C, Lunt A, Moxham J, Rafferty GF, Hart N. Ultrasound for the assessment of peripheral skeletal muscle architecture in critical illness: a systematic review. Crit Care Med. 2015;43:897–905. doi: 10.1097/CCM.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 59.Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, Moxham J, Harridge S, Hart N, Montgomery HE. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43:1603–1611. doi: 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 60.Bloch SA, Donaldson AV, Lewis A, Banya WA, Polkey MI, Griffiths MJ, Kemp PR. MiR-181a: a potential biomarker of acute muscle wasting following elective high-risk cardiothoracic surgery. Crit Care. 2015;19:147. doi: 10.1186/s13054-015-0853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloch SA, Lee JY, Wort SJ, Polkey MI, Kemp PR, Griffiths MJ. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit Care Med. 2013;41:982–989. doi: 10.1097/CCM.0b013e318274671b. [DOI] [PubMed] [Google Scholar]
- 62.Campbell IT, Watt T, Withers D, England R, Sukumar S, Keegan MA, Faragher B, Martin DF. Muscle thickness, measured with ultrasound, may be an indicator of lean tissue wasting in multiple organ failure in the presence of edema. Am J Clin Nutr. 1995;62:533–539. doi: 10.1093/ajcn/62.3.533. [DOI] [PubMed] [Google Scholar]
- 63.Cartwright MS, Kwayisi G, Griffin LP, Sarwal A, Walker FO, Harris JM, Berry MJ, Chahal PS, Morris PE. Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve. 2013;47:255–259. doi: 10.1002/mus.23525. [DOI] [PubMed] [Google Scholar]
- 64.Grimm A, Teschner U, Porzelius C, Ludewig K, Zielske J, Witte OW, Brunkhorst FM, Axer H. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit Care. 2013;17:R227. doi: 10.1186/cc13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23:273–280. doi: 10.1016/S0261-5614(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 66.Fischer A, Spiegl M, Altmann K, Winkler A, Salamon A, Themessl-Huber M, Mouhieddine M, Strasser EM, Schiferer A, Paternostro-Sluga T, et al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care. 2016;20:30. doi: 10.1186/s13054-016-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13:R161. doi: 10.1186/cc8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gruther W, Kainberger F, Fialka-Moser V, Paternostro-Sluga T, Quittan M, Spiss C, Crevenna R. Effects of neuromuscular electrical stimulation on muscle layer thickness of knee extensor muscles in intensive care unit patients: a pilot study. J Rehabil Med. 2010;42:593–597. doi: 10.2340/16501977-0564. [DOI] [PubMed] [Google Scholar]
- 69.Puthucheary ZA, McNelly AS, Rawal J, Connolly B, Sidhu PS, Rowlerson A, Moxham J, Harridge SD, Hart N, Montgomery HE. Rectus femoris cross-sectional area and muscle layer thickness: comparative markers of muscle wasting and weakness. Am J Respir Crit Care Med. 2017;195:136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.