Abstract
Objective
To determine the safety and efficacy of the novel combination of docetaxel, oxaliplatin, and bevacizumab as first-line treatment of advanced cancer of the ovary, peritoneum or fallopian tube after initial debulking surgery.
Methods
Eligible patients (stage IB-IV) were treated with 6 cycles of oxaliplatin (85 mg/m2), docetaxel (75 mg/m2), and bevacizumab (15 mg/kg) every 3 weeks, followed by single-agent bevacizumab 15 mg/kg every 3 weeks to complete one year of therapy. The primary endpoint was 12-month progression-free survival (PFS).
Results
A total of 132 patients (80 with measurable disease at baseline; 52 with non-measurable, evaluable disease at baseline) enrolled and received study treatment. At diagnosis, 76.5% of patients had stage III disease and 20% had stage IV. 62.9% were optimally cytoreduced. The most common grade 3/4 adverse events were neutropenia (42.4%), leukopenia (13.6%), hypertension (8.3%), fatigue (6.1%), and nausea (6.1%). One patient (0.8%) had a fatal gastrointestinal perforation. The best overall confirmed response rate (complete response + partial response [measurable disease subgroup]) was 58.6% (95% CI 49%, 67%). CA-125 response rates for the measurable and non-measurable disease subgroups were 83.0% and 81.5%, respectively. The 12-month PFS rate for the measurable disease subgroup was 65.7% (95% CI 53.4%, 76.7%); median PFS was 16.3 (95% CI 12.6, 19.6) months. Median overall survival was 47.3 (95% CI 34.1, upper limit not applicable) months.
Conclusions
This novel treatment regimen may provide a promising therapeutic approach for women with ovarian, primary peritoneal, or fallopian tube carcinoma. No unanticipated safety concerns were identified.
Keywords: Oxaliplatin, Docetaxel, Bevacizumab, Ovarian cancer, Primary peritoneal cancer, Fallopian tube carcinoma
Introduction
Approximately 22,000 cases of epithelial ovarian cancer, most of which present in advanced stages, occur in the United States each year, resulting in over 15,000 deaths [1]. Standard first-line therapy typically consists of approximately 6 cycles of combination therapy including a taxane and platinum compound [2].
Docetaxel and oxaliplatin have independent activity in ovarian cancer and each demonstrates excellent activity in recurrent platinum-sensitive and resistant ovarian cancer [3]. Although current data are limited, this novel combination provides a rationale justifying a study of its administration in the front-line setting based upon the potential for improved efficacy in lieu of responses in drug resistant patients [4]. The efficacy of docetaxel versus paclitaxel as first-line therapy for ovarian cancer was evaluated in a randomized trial of 1077 patients in combination with carboplatin and demonstrated similar progression-free survival (PFS), with more hematologic but less neurologic toxicity [5]. In a smaller randomized trial, 182 patients received either oxaliplatin or cisplatin combined with cyclophosphamide as first-line therapy for ovarian cancer [6]. The median PFS was 13.0 and 13.3 months, respectively, and overall survival (OS) was 36.0 and 25.1 months, respectively (no statistical significance) [6]. However, there was significantly less grade 3/4 myelosuppression and nausea/vomiting, and less neurological toxicity with the oxaliplatin combination [6]. While the activity of the oxaliplatin and cisplatin combinations in advanced ovarian cancer patients was comparable, there was a better safety profile for the oxaliplatin-containing regimen [6]. In a phase II study of second-line docetaxel and oxaliplatin in patients with platinum-sensitive recurrent ovarian cancer, rates of neurotoxicity and alopecia were favorable [3]. From these studies, it was anticipated that there would be less neurotoxicity and alopecia with docetaxel and oxaliplatin compared with other platinum/taxane regimens. Additionally, agents that target the vascular endothelial growth factor (VEGF) pathway, e.g., the neutralizing anti-VEGF monoclonal antibody bevacizumab, have the potential to improve the outcomes of advanced epithelial ovarian cancer treatment. Bevacizumab has demonstrated activity in terms of PFS and clinical response either alone or in combination with a taxane- or platinum-based chemotherapy in several advanced tumor types, with an acceptable toxicity profile [7–11].
In this open-label, single-arm phase II study, we examined the safety and efficacy of the novel taxane/platinum doublet (docetaxel/oxaliplatin), combined with bevacizumab, as first-line therapy for patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB through IV ovarian, primary peritoneal, or fallopian tube carcinoma after primary surgical debulking. The primary objective was to evaluate the efficacy of docetaxel, oxaliplatin, and bevacizumab combination therapy as assessed by 12-month PFS. Secondary objectives were to assess safety as well as tumor response rate, PFS, recurrence-free survival (RFS), CA-125 response rate, and OS.
Methods
Study population
Eligible patients were 18 years of age or older; had undergone standard ovarian cancer surgery with a histological diagnosis of ovarian, primary peritoneal, or fallopian tube carcinoma, Stage IB-IV; and had either optimal (≤ 1 cm maximal diameter any remaining lesion thus encompassing those with complete resection and small volume residual disease) or suboptimal residual disease (> 1 cm maximal diameter any remaining lesion) following initial surgery. Adequate bone marrow reserve, as well as normal renal, hepatic, and neurological function by standard indices, was required. Patients with significant cardiovascular disease including uncontrolled hypertension, previous cancer history, prior radiotherapy to any portion of the abdominal cavity or pelvis, any prior anticancer chemotherapy or biologic therapy, known bleeding disorder or coagulopathy were excluded.
Study design and treatment
All patients received docetaxel (75 mg/m2), oxaliplatin (85 mg/m2), and bevacizumab (15 mg/kg). Cytotoxic treatment was initiated at a minimum of 28 days after initial surgery. All patients were treated in 3-week cycles until disease progression, unacceptable toxicity, or voluntary withdrawal of consent. After completing 6 cycles of doublet chemotherapy plus bevacizumab, patients continued on maintenance bevacizumab every 3 weeks to complete one year of therapy. Docetaxel and oxaliplatin were provided by Sanofi US LLC, and bevacizumab was provided by Genentech. Patient survival was followed for 3 years from the date of enrollment.
The protocol and its amendments were reviewed and approved by each institution’s Institutional Review Board (IRB), and all patients provided signed informed consent as per local IRB guidelines. The study was conducted under Good Clinical Practice guidelines as per Food and Drug Administration guidelines and was registered with clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00296816).
Assessments
Efficacy was evaluated through tumor burden assessment. Patients with measurable disease at baseline, defined as ≥ 1 target lesions, were assessed for response by Gynecologic Oncology Group Response Evaluation Criteria in Solid Tumors (GOG RECIST) [12] using computed tomography (CT) or magnetic resonance imaging (MRI), while patients with non-measurable disease at baseline, defined as 0 target lesions, were assessed for response by Gynecologic Cancer Intergroup CA-125 response criteria [13]. CT or MRI of the abdomen and pelvis was performed at baseline, after the 3rd and 6th cycles during the chemotherapy treatment period, and then every 3 months during the bevacizumab-only and follow-up periods, unless clinically indicated. Chest CT/MRI was performed at baseline and as clinically indicated. Patients with normal abdominopelvic CT/MRI scans at baseline did not have a CT/MRI scan after completing cycle 3 of chemotherapy unless CA-125 levels increased or remained elevated during chemotherapy. CA-125 was assessed at baseline and on day 1 of every cycle. Patients with a pretreatment sample ≥ 2× upper limit of normal within 2 weeks of first study treatment were considered evaluable for CA-125 response (defined as a ≥50% reduction in CA-125 level from a pretreatment level, confirmed and maintained for ≥28 days). Hematology, blood chemistry, coagulation, urinalysis and urine protein/creatinine ratio (UPCR) were assessed at baseline and on day 1 of every cycle during chemotherapy and bevacizumab-only treatment periods; hematology was also assessed on day 8 of every cycle during the chemotherapy treatment period. Adverse events (AEs) and toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), Version 3.0 [14].
Dose reductions and administration of hematopoietic growth factors
Dose reductions for oxaliplatin and docetaxel were made depending upon individual patient tolerance; dosing of bevacizumab remained fixed at 100% of the recommended dose. If docetaxel and/or oxaliplatin were withheld, bevacizumab was continued unless the patient’s medical condition precluded this. If bevacizumab was withheld, docetaxel and oxaliplatin were continued. Patients requiring more than one dose reduction of docetaxel were discontinued from the drug.
Routine primary prophylactic colony-stimulating factors could be used at the discretion of the investigator. Investigators could administer secondary prophylactic administration of hematopoietic growth factors in patients experiencing recurrent difficulties with neutropenia in subsequent cycles or therapeutic use of these agents in patients with serious neutropenic complications following the 2000 American Society of Clinical Oncology (ASCO) guidelines [15].
Statistical analysis and sample size determination
Data were summarized using descriptive statistics, with 95% confidence intervals (CIs) for the efficacy analyses calculated using the exact binomial method. The primary efficacy analysis assessed the rate of PFS at 12 months after first study treatment in the subgroup with measurable disease at baseline. Survival time intervals were assessed using the Kaplan-Meier method [16]. Unless otherwise noted, efficacy summaries are based on the data for the intent-to-treat (ITT) population (all patients who met inclusion/exclusion criteria at the time of registration), while safety results are based on all enrolled patients who received at least part of 1 dose of any of the study drugs. One study site (Site 015) closed prematurely due to noncompliance with good clinical practice guidelines. Patients from this site were included only in the safety analysis as efficacy data were insufficient.
Based on the data from Vasey et al. showing a 1-year PFS of approximately 60% for patients with ovarian carcinoma treated with docetaxel/carboplatin or paclitaxel/carboplatin as first-line chemotherapy [5], the null hypothesis of interest in this study was that the 1 -year PFS proportion (rate) was 0.60 versus the alternative hypothesis that it was higher than 0.60. The null hypothesis was tested at the 1-sided 0.05 significance level using an exact binomial test assuming that the true PFS proportion was 0.75. Approximately 70 patients were originally to be enrolled in the study to provide 62 evaluable patients for PFS and ensure a power of 80% if the true 1 -year PFS proportion was 0.75. According to a review of the baseline distribution of patients in this study, 28 of 59 enrolled patients (48%) had non-measurable disease at baseline. In order to assess the PFS probability for patients with measurable disease, the planned sample size was increased to approximately 145 patients overall. Thus, enrollment was planned to continue until 62 patients with measurable disease at baseline or 145 patients overall were accrued, whichever came first.
Results
Demographics and baseline characteristics
Of the 132 enrolled patients, 80 had measurable and 52 had non-measurable disease at baseline. Median age was 58 years (range, 35–83 years); the majority of patients were Caucasian (81.8%). The demographic characteristics of patients with measurable and non-measurable disease were generally similar at baseline (Table 1).
Table 1.
Demographics and baseline characteristics (ITT population).
| Oxali + Doce + Bev
|
|||
|---|---|---|---|
| Measurable disease n = 80 | Nonmeasurable disease n = 52 | Total N = 132 | |
| Age, years | |||
| Median (range) | 57.0 (35–79) | 59.0 (42–83) | 58.0 (35–83) |
| Race, n (%) | |||
| Caucasian | 62 (77.5) | 46 (88.5) | 108 (81.8) |
| Black | 8 (10.0) | 2 (3.8) | 10 (7.6) |
| Asian/Pacific Islander | 1 (1.3) | 4 (7.7) | 5 (3.8) |
| Other | 8 (10.0) | 0 | 8 (6.1) |
| Missing | 1 (1.3) | 0 | 1 (0.8) |
| Cancer diagnosis primary site, n (%) |
|||
| Ovary | 65 (81.3) | 45 (86.5) | 110 (83.3) |
| Fallopian tube | 6 (7.5) | 5 (9.6) | 11 (8.3) |
| Peritoneum | 9 (11.3) | 2 (3.8) | 11 (8.3) |
| FIGO stage at diagnosis, n (%) | |||
| Stage IC | 0 | 3 (5.8) | 3 (2.3) |
| Stage IIC | 3 (3.8) | 5 (9.6) | 8 (6.1) |
| Stage IIIA | 3 (3.8) | 2 (3.8) | 5 (3.8) |
| Stage IIIIB | 2 (2.5) | 5 (9.6) | 7 (5.3) |
| Stage IIIC | 57 (71.3) | 32 (61.5) | 89 (67.4) |
| Stage IV | 15 (18.8) | 5 (9.6) | 20 (15.2) |
| Initial pathology cell type, n (%) | |||
| Serous adenocarcinoma | 62 (77.5) | 38 (73.1) | 100 (75.8) |
| Endometrioid adenocarcinoma | 6 (7.5) | 5 (9.6) | 11 (8.3) |
| Clear cell adenocarcinoma | 2 (2.5) | 6 (11.5) | 8 (6.1) |
| Mucinous adenocarcinoma | 3 (3.8) | 0 | 3 (2.3) |
| Adenocarcinoma NOS | 2 (2.5) | 0 | 2 (1.5) |
| Mixed epithelial carcinoma | 1 (1.3) | 1 (1.9) | 2 (1.5) |
| Other | 4 (5.0) | 2 (3.8) | 6 (4.5) |
| Histology grade, n (%) | |||
| Poorly differentiated | 51 (63.8) | 36 (69.2) | 87 (65.9) |
| Moderately differentiated | 14 (17.5) | 10 (19.2) | 24 (18.2) |
| Well differentiated | 1 (1.3) | 1 (1.9) | 2 (1.5) |
| Unknown | 14 (17.5) | 5 (9.6) | 19 (14.4) |
| Post-surgery residual disease, n (%) |
|||
| Optimal | 41 (51.3) | 42 (80.8) | 83 (62.9) |
| Suboptimal | 39 (48.8) | 10 (19.2) | 49 (37.1) |
| Median time from surgery to date of first dose, days | 39.0 | 41.5 | 40.0 |
| GOG performance status, n (%) | |||
| 0 | 46 (57.5) | 31 (59.6) | 77 (58.3) |
| 1 | 30 (37.5) | 16 (30.8) | 46 (34.8) |
| 2 | 1 (1.3) | 3 (5.8) | 4 (3.0) |
| Missing | 3 (3.8) | 2 (3.8) | 5 (3.8) |
Bev, bevacizumab; Doce, docetaxel; FIGO, International Federation of Gynecology and Obstetrics; GOG, Gynecologic Oncology Group; ITT, intent-to-treat; NOS, not otherwise specified; Oxali, oxaliplatin; max, maximum; and min, minimum.
The majority (83.3%) of patients had primary ovarian cancer, most frequently stages IIIC/IV (96.5%). Compared with the non-measurable disease group, more patients in the measurable disease group had stage IIIC and IV disease. Overall, following cytoreductive surgery, 62.9% of patients had optimal residual disease, with a larger proportion having non-measurable disease. In both disease groups, the majority of patients had a GOG performance status of either 0 or 1. (Patient disposition and extent of exposure is shown in Supplemental Fig. S1).
Adverse events
A total of 130 (98.5%) patients reported at least one AE during the study and 102 (77.3%) patients experienced a grade 3/4 AE. 130 (98.5%) patients reported AEs considered possibly related to chemotherapy (oxaliplatin and/or docetaxel) and 124 (93.9%) reported AEs considered possibly related to bevacizumab (Table 2a). A total of 117 (88.6%) patients started received all 6 cycles of combination therapy, and 45 (34.1%) received all 12 cycles of single-agent bevacizumab maintenance. The mean (SD) relative (actual/planned) dose intensities for oxaliplatin, docetaxel, and bevacizumab were all >90%, or 96.0% (docetaxel), 93.9% (oxaliplatin), and 96.4% (bevacizumab), respectively.
Table 2a.
Most common and Bev-attributed AEs.
| Event | Oxali + Doce + Bev N = 132 |
|||
|---|---|---|---|---|
| Overall AEsc
|
Bev-attributed AEs*
|
|||
| All grades, n (%) | Grades 3/4, n (%) | All grades, n (%) | Grade 3/4, n (%) | |
| Most common AEs (≥20% of patients) | ||||
| Patients with at least 1 AE | 130 (98.5) | 102 (77.3) | ||
| Fatigue | 101 (76.5) | 8 (6.1) | ||
| Nausea | 88 (66.7) | 8 (6.1) | ||
| Alopecia | 86 (65.2) | 5 (3.8) | ||
| Diarrhea | 78 (59.1) | 4 (3.0) | ||
| Neuropathy peripheral | 65 (49.2) | 5 (3.8) | ||
| Constipation | 59 (44.7) | 0 | ||
| Neutropenia | 59 (44.7) | 56 (42.4) | ||
| Arthralgia | 47 (35.6) | 1 (0.8) | ||
| Decreased appetite | 45 (34.1) | 4 (3.0) | ||
| Vomiting | 45 (34.1) | 6 (4.5) | ||
| Headache | 40 (30.3) | 1 (0.8) | ||
| Epistaxis | 39 (29.5) | 1 (0.8) | ||
| Abdominal pain | 38 (28.8) | 3 (2.3) | ||
| Lacrimation increased | 35 (26.5) | 0 | ||
| Nail disorder | 33 (25.0) | 0 | ||
| Peripheral sensory neuropathy | 33 (25.0) | 2 (1.5) | ||
| Dysgeusia | 32 (24.2) | 0 | ||
| Hypertension | 31 (23.5) | 11 (8.3) | ||
| Leukopenia | 31 (23.5) | 18 (13.6) | ||
| Urinary tract infection | 30 (22.7) | 0 | ||
| Neutrophil count | 12 (9.1) | 10 (7.6) | ||
| Selected AEs reported for Bev | ||||
| Hemorrhage | ||||
| Epistaxis | 39 (29.5) | 1 (0.8) | 30 (22.7) | 1 (0.8) |
| Eye hemorrhage | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Gastrointestinal hemorrhage NECa | 5 (3.8) | 2 (1.5) | 1 (0.8) | 1 (0.8) |
| Hematoma | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Respiratory tract hemorrhage | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Subdural hematoma | 1 (0.8) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| Urogenital hemorrhage | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Vaginal hemorrhage | 7 (5.3) | 0 | 4 (3.0) | 0 |
| Hypertension | ||||
| Hypertension | 31 (23.5) | 11 (8.3) | 29 (22.0) | 10 (7.6) |
| Proteinuria | ||||
| Proteinuria | 11 (8.3) | 1 (0.8) | 10 (7.6) | 1 (0.8) |
| Infusion reactions (systemic) | ||||
| Drug hypersensitivity | 2 (1.5) | 0 | 1 (0.8) | 0 |
| Hypersensitivity | 8 (6.1) | 3 (2.3) | 1 (0.8) | 0 |
| Gastrointestinal perforations | ||||
| Intestinal perforation | 1 (0.8) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| Thromboembolic events | ||||
| Embolism and thrombosisb | 4 (3.0) | 1 (0.8) | 1 (0.8) | 0 |
| Portal vein thrombosis | 1 (0.8) | 1 (0.8) | 0 | 0 |
| Pulmonary embolism | 5 (3.8) | 5 (3.8) | 3 (2.3) | 3 (2.3) |
| Encephalopathy | ||||
| Encephalopathy | 1 (0.8) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| Fistulae | ||||
| Colonic fistula | 1 (0.8) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| Female genital tract fistula | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Healing complications | ||||
| Impaired healing | 1 (0.8) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| Wound dehiscence | 1 (0.8) | 0 | 1 (0.8) | 0 |
AE, adverse event; Bev, bevacizumab; Doce, docetaxel; Oxali, oxaliplatin; and NEC, not elsewhere classified.
AEs were defined as new or worsening AEs with an onset date occurring on or after first dose date of study medication, inclusive to 30 days post-last dose.
Most common AEs are sorted by the decreasing frequency of the all-grades column.
High level group term.
High level term.
Cannot exclude combined effects with chemotherapy.
A total of 52 (39.4%) patients died during the study (Table 2b); 45 (34.1%) died from progressive disease, 2 (1.5%) from AEs, and 5 (3.8%) from other causes >30 days after last study treatment. Two AEs that resulted in death were pulmonary embolism (deemed unrelated to treatment) and subdural hematoma (possibly from bevacizumab). Serious AEs occurred in 33 (25.0%) patients, 29 (22.0% overall) of whom had at least 1 serious grade 3/4 event. The most common serious AEs (all grades) are listed in Table 2b. AEs that led to permanent discontinuation of study treatment are summarized in Supplemental Table S1.
Table 2b.
Serious AEs (safety population) (≥2% of patients).
| Preferred term | Oxali + Doce + Bev N = 132 |
|
|---|---|---|
| All grades | Grades 3/4 | |
| n (%) | n (%) | |
| Patients with at least 1 serious AE | 33 (25.0) | 29 (22.0) |
| Nausea | 7 (5.3) | 5 (3.8) |
| Vomiting | 7 (5.3) | 5 (3.8) |
| Pulmonary embolism | 5 (3.8) | 5 (3.8) |
| Small intestinal obstruction | 5 (3.8) | 5 (3.8) |
| Febrile neutropenia | 4 (3.0) | 4 (3.0) |
| Abdominal pain | 3 (2.3) | 1 (0.8) |
| Dehydration | 3 (2.3) | 3 (2.3) |
AE, adverse event; Bev, bevacizumab; Doce, docetaxel; and Oxali, oxaliplatin. AEs were defined as new or worsening AEs with an onset date occurring on or after first dose date of study medication, inclusive to 30 days post-last dose.
Events are sorted by the decreasing frequency of the all-grades column.
Grade 3/4 abnormalities of laboratory values were reported in 92 (69.7%) patients, 6 of whom had grade 3/4 toxicity at baseline. Shifts from grade 2 or less to grade 3/4 were reported most frequently for neutrophil count (53.4%), white blood cell count (35.9%), lymphocyte count (10.7%), and magnesium level (7.1%). Four patients had febrile neutropenia. Although allowed by the protocol, no patient was treated with primary G-CSF prophylaxis. Two of these patients received secondary G-CSF prophylaxis and two did not. None of these patients had subsequent febrile neutropenia. UPCRs were rated as moderate (≥ 2 and <3) for 8 patients (6.3%) and as severe (≥3) for 9 patients (7.0%). No cases of oxaliplatin associated cold hyperalgesia were observed.
Efficacy
Efficacy data were analyzed separately for ITT patients with measurable and non-measurable disease at baseline, with the exception of OS results.
Response rates
Table 3 summarizes overall response rates for the ITT measurable disease subgroup according to the investigator assessment using GOG RECIST. The unconfirmed best overall response rate was 72.9% (95% CI 64%, 80%), with a complete response (CR) rate of 32.9% and partial response (PR) rate of 40.0%. The confirmed best overall response rate was 58.6% (95% CI 49%, 67%), with a CR rate of 30.0% and a PR rate of 28.6%. There was an 83% (95% CI 70%, 92%) CA-125 response rate among the evaluable 53 patients in measurable disease subgroup, and an 82% (95% CI 62%, 94%) CA-125 response rate among the 27 patients evaluable in the non-measurable disease subgroup.
Table 3.
Best overall study response (ITT measurable disease subgroup).
| Parameters | Oxali + Doce + Bev N = 70 n (%) |
|---|---|
| Unconfirmed best overall response | |
| Response rate (CR + PR) | 51 (72.9) |
| Complete response (CR) | 23 (32.9) |
| Partial response (PR) | 28 (40.0) |
| Stable disease | 9 (12.9) |
| Progressive Disease | 5 (7.1) |
| Inevaluable for response | 5 (7.1) |
| Confirmed best overall response | |
| Response rate (CR + PR) | 41 (58.6) |
| Complete response | 21 (30.0) |
| Partial response | 20 (28.6) |
| Inevaluable for response | 0 |
Bev, bevacizumab; Doce, docetaxel; ITT, intent-to-treat; and Oxali, oxaliplatin. Site 015 data were not included in this table.
Progression-free survival
PFS was calculated from the time of enrollment. The primary efficacy variable, PFS rate at 12 months for the ITT measurable disease subgroup, was 65.7% (95% CI 53.4%, 76.7%) whereas the 18- and 24-month PFS rates (95% CI) for the measurable disease subgroup were 42.9% (31.1%, 55.3%) and 34.3% (23.3%, 46.6%), respectively. Kaplan-Meier estimates of the PFS rate (95% CI) at 12, 18, and 24 months were 66.6% (53.1%, 77.1%), 43.2% (29.8%, 55.9%), and 34.9% (22.3%, 47.7%), respectively (Fig. 1). Median PFS (95% CI) was 16.3 (12.6, 19.6) months. Patients with optimal residual disease after primary debulking surgery generally had a similar probability of PFS as patients with suboptimal residual disease (Fig. 2).
Fig. 1.
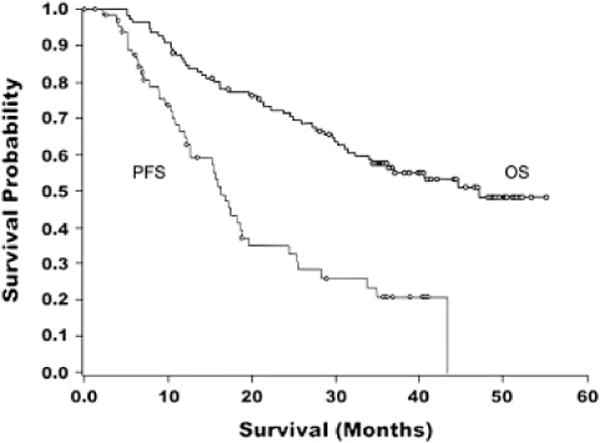
Kaplan–Meier curves for progression-free survival (ITT measurable disease subgroup) and overall survival (ITT population).
Fig. 2.
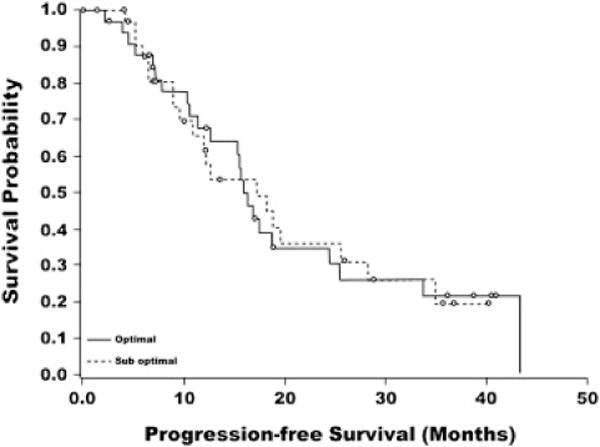
Kaplan–Meier curve for progression-free survival by pre-study residual disease status (ITT measurable disease subgroup).
Overall survival
Median OS (95% CI) for the ITT population (both measurable and non-measurable disease subgroups) was 47.2 months (34.1, upper limit not applicable) (Fig. 1). OS rates (95% CI) at 12, 18, and 24 months were 85.5% (77.5%, 91.5%), 77.3% (68.3%, 84.7%), and 71.8% (62.4%, 80.0%), respectively. There was no statistical OS difference between the measurable disease subgroup and the non-measurable subgroup (Supplemental Fig. S2).
Discussion
Despite high response rates observed in front-line adjuvant treatment of ovarian cancer, cure has remained elusive, especially for those who present at advanced stages of disease where recurrence rates and cancer-related mortality remain high. Novel combinations that improve efficacy, mitigate toxicities, or provide an alternative therapeutic regimen are indicated. To this end, this study evaluated the efficacy and safety of the novel combination of oxaliplatin, docetaxel, and bevacizumab as first-line systemic therapy for stages IB through IV epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. The median PFS was 16.3 months in the measurable disease group, and is comparable to that reported for standard regimens in patients with high-risk and advanced stage disease [17,18]. Median PFS was 20.7 months with carboplatin plus paclitaxel and 19.4 months with cisplatin plus paclitaxel in GOG 158 [22], 15.0 months with carboplatin plus docetaxel and 14.8 months with carboplatin plus paclitaxel in Scottish Randomised Trial in Ovarian Cancer (SCOTROC) where 80% of patients were stage III/IV versus 96.5% in this trial [5], and 11.2 months with carboplatin plus paclitaxel plus bevacizumab initiation and 14.1 months with carboplatin plus paclitaxel plus bevacizumab throughout in GOG 218 [10]. The Kaplan-Meier estimate of the12-month PFS was 60% with carboplatin plus paclitaxel and 59% with carboplatin plus docetaxel in SCOTROC [5]. The non-measurable disease group had a median RFS time of approximately 21 months, although this group had smaller proportions of patients with advanced stage and suboptimal residual disease (Table 1) than the measurable disease group.
As assessed by the primary endpoint of 1-year PFS (65.7% [95% CI 53.4%, 76.7%]) in the measurable disease group—although not reaching statistical significance—the combination of oxaliplatin, docetaxel, and bevacizumab showed promising efficacy as first-line systemic therapy for patients with stages IB through IV epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. The treatment combination also demonstrated efficacy with respect to tumor response rates, PFS at 18 and 24 months, and OS.
Obviously, each clinical trial has a unique study population that makes cross-trial comparisons problematic. For ovarian cancer, there have been 2 recently reported phase III trials that incorporated bevacizumab along with platinum and taxane agents [9,10]. Additionally, 2 prior phase II trials have incorporated bevacizumab with chemotherapy and as maintenance for the first-line treatment ovarian cancer [19,20]. Micha et al. also studied the addition of bevacizumab to first-line therapy in ovarian cancer, but that study did not include a maintenance-bevacizumab component and reported primarily the short-term toxicities of the combination and not PFS [9]. The two large trials, GOG 218 and International Collaboration on Ovarian Neoplasms (ICON) 7, had median a PFS of 14.1 and 19.0 months, respectively, in the bevacizumab arms (Table 4) [10,11,21,22]. Combination oxaliplatin, docetaxel, and bevacizumab in the current trial demonstrated a median PFS of 16.3 months, which is intermediate between the 2 large studies. The CIs do overlap, suggesting that this combination does not appear to be clinically inferior, although a randomized trial would be required to confirm this hypothesis.
Table 4.
Studies of a platinum compound plus a taxane with or without bevacizumab.
| GOG 218 [9] | ICON-7 [10] | GOG 182 [20] | GOG 158 [21] | TEACO | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment arm | Carb + Pac + Bev initiation (N = 625) | Carb + Pac + Bev throughout (N = 623) | Carb + Pac + placebo (N = 625) | Carb + Pac (N = 764) | Carb + Pac + Bev (N = 764) | Carb + Pac (N = 864) | Carb + Pac (N = 392) | Oxali + Doce + Bev (N =132) |
| Study population | ||||||||
| Cell type | 83.0% serous adenocarcinoma, 2.2% endometroid, 3.7% clear cell, 0.8% mucinous, 10.2% other or not specified | 84.1% serous adenocarcinoma, 3.9% endometroid, 3.2% clear cell, 1.3% mucinous, 7.5% other or not specified | 86.6% serous adenocarcinoma, 3.4% endometroid, 1.9% clear cell, 1.0% mucinous, 7.2% other or not specified | 69% serous, 2% mucinous, 7% endometroid, 8% clear cell, 6% mixed, 7% other | 69% serous, 2% mucinous, 8% endometroid, 9% clear cell, 5% mixed, 7% other | Not reported | 74% serous adenocarcinoma 9% endometroid adenocarcinoma 2% mucinous adenocarcinoma 5% clear-cell carcinoma 9% other | 75.8% serous adenocarcinoma, 8.3% endometroid, 6.1% clear cell, 2.3% mucinous, 1.5% mixed epithelial, 1.5% adenocarcinoma not otherwise specified, 4.5% other |
| Residual disease | 32.8% ≤1 cm, 41.0% > 1 cm | 34.7% ≤1 cm, 38.8% >1 cm | 34.9% ≤1 cm, 40.6% >1 cm | 74% ≤ 1 cm, 26% > 1cm | 74% ≤1 cm, 26% >1 cm | 21.6% measurable | 35% none or microscopic 65% gross | 62.9% ≤1 cm, 37.1% > 1 cm |
| Age | Median 60 year | Median 60 year | Median 60 year | Median 57 year | Median 57 year | Median 57.7 year | 1% 21–30 year 7% 31–40 year 21%41–50 year 33% 51–60 year 25% 61–70 year 12% 71–80 year 1% 81–90 year |
Median 58.0 year |
| PS | 50.4% GOG PS 0 43.2% GOG PS 1 6.4% GOG PS 2 |
49.0% GOG PS 0 42.9% GOG PS 1 8.2% GOG PS 2 |
49.8% GOG PS 0 43.5% GOG PS 1 6.7% GOG PS 2 |
47% EGOG PS 0 47% ECOG PS 1 6% ECOG PS 2 |
45% EGOG PS 0 49% ECOG PS 1 6% ECOG PS 2 |
Not reported | 43% GOG PS 0 49% GOG PS 1 8% GOG PS 2 |
58.3% GOG PS 0 34.8% GOG PS 1 3.0% GOG PS 2 3.8% missing |
| Major efficacy endpoints | ||||||||
| PFS | Median 11.2 mos | Median 14.1 mos | Median 10.3 mos | Median 17.3 mos | Median 19.0 mos | Median 16.0 mos | Median 20.7 mos | Median 16.3 mos |
| OS | Median 38.7 mos | Median 39.7 mos | Median 39.3 mos | Not reported | Not reported | Median 44.1 mos | Median 57.4 mos | Median 47.2 mos |
| Toxicity | ||||||||
| Neurologic | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 7% grade 3, 0% grade 4; 28% grade 2–4 | Neuropathy peripheral: 3.8% grade ≥3 Peripheral sensory neuropathy: 1.5% grade ≥3 |
| Marrow | Neutropenia: 63.3% grade ≥4 | Neutropenia: 63.3% grade ≥ 4 | Neutropenia: 57.7% grade ≥ 4 | Neutropenia: 15% grade ≥ 3 Thrombo-cytopenia: 2% grade ≥ 3 |
Neutropenia: 17% grade ≥3 Thrombo-cytopenia: 3% grade ≥3 |
Not reported | Leukopenia: 53% grade 3, 6% grade 4 Thrombo-cytopenia: 19% grade 3, 20% grade 4 Granulocytopenia: 17% grade 3, 72% grade 4 |
Neutropenia: 42.4% grade ≥ 3 Leukopenia: 13.6% grade ≥ 3 Neutrophil count: 7.6% grade ≥3 |
| GI (nausea and diarrhea) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 5% grade 3, 5% grade 4 | Nausea: 6.1% grade ≥ 3 Vomiting: 4.5% grade ≥ 3 Diarrhea: 3.0% grade ≥3 |
| Hypertension | 16.5% grade ≥2 | 22.9% grade ≥2 | 7.2% grade ≥2 | <1% grade ≥3 | 6% grade ≥3 | Not reported | Not reported | 8.3% grade ≥3 |
| GI perforations | 2.8% grade ≥2 GI-wall disruption (perforation, fistula, necrosis, or anastomotic leak) | 2.6% grade ≥2 GI wall disruption (perforation, fistula, necrosis, or anastomotic leak) | 1.2% grade ≥2 GI wall disruption (perforation, fistula, necrosis, or anastomotic leak) | 0.4% grade ≥3 | 1.3% grade ≥3 | Not reported | Not reported | 0.8% grade ≥ 3 |
| Dose reductions | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 14.4% |
| Dose delays | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 23.5% |
| Both dose reductions and dose delays | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 4.5% |
| Discontinuation | 15% discontinued due to AEs | 17% discontinued due to AEs | 12% discontinued due to AEs | 22 (2.9%) stopped chemotherapy early due to AEs or intercurrent illness | 15 (2.0%) stopped chemotherapy early due to AEs or intercurrent illness, 112 (14.7%) stopped Bev early due to AEs or intercurrent illness | 73 (8.4%) discontinued due to toxicity or refusal | Not reported | 15.9% discontinued due to AEs, 12.1% discontinued due to grade ≥3 AEs |
AE, adverse event; Bev, bevacizumab; Carb, carboplatin; Doce, docetaxel; ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; GOG, Gynecologic Oncology Group; OS, overall survival; Oxali, oxaliplatin; Pac, paclitaxel; PFS, progression-free survival; and PS, performance status.
Hypertension was reported in 23.5% of patients, but was grade 3/4 in only 8.3% of patients. Hypertension resulted in discontinuation of treatment in 2.3% of patients (Supplemental Table S1), which compares to 2.4% of patients in GOG 218 [10]. Proteinuria was seen in 8.3% of patients, in whom only 0.8% was grade 3/4 in (Table 2a), which compares to 1.6% seen in GOG 218 [9]. Rates of grade ≥ 3 thromboembolic events range across the trials from 2.4% to 6.8% [10,11,19,20]. However, results from the 2 randomized trials that had control arms without bevacizumab did not clearly suggest an increased risk of venous thrombosis. No arterial thrombosis was noted in the current trial. However, arterial thrombosis is a relatively rare event, seen in 3% of patients in the ICON 7 trial who received bevacizumab and 1% of controls, but less than 1% of patients in all arms of GOG 218 [10,11]. In the current trial, peripheral neuropathy was seen in 49.2% of patients but was grade ≥3 in 3.8% of patients. Based on SCOTROC, docetaxel and carboplatin is less neuropathic than paclitaxel and carboplatin [4]. However, no randomized trial directly compares oxaliplatin, docetaxel, and paclitaxel. GOG 182 reported incidence of ≥grade 2 peripheral neuropathy in approximately 25% of patients receiving carboplatin plus paclitaxel [21]. Peripheral neuropathy was not recorded in GOG 218 or ICON 7 [9,10]. Because of the lower neurotoxicity of docetaxel, docetaxel and oxaliplatin or the combination of docetaxel and carboplatin might be preferred in patients with preexisting neuropathy.
Risk of gastrointestinal perforation with combination oxaliplatin, docetaxel, and bevacizumab in this trial was less than 1% (Table 2a). This was lower than the rate observed in other trials and may simply be within the CIs of all trials for ovarian cancer treatment with bevacizumab (Table 4) [10,11,21,22]. Alternatively, drug interaction may modulate this adverse effect. The observation is particularly notable in that this trial allowed for administration of bevacizumab with the first cycle of chemotherapy. Median time from surgery to the first cycle was 40.0 days; 2 patients had wound complications (1 impaired healing; 1 wound dehiscence). Most trials withhold bevacizumab during cycle 1 of chemotherapy for concern of wound and bowel anastomotic healing [10,11].
In terms of study limitations, this was a single arm phase II study. Moreover, data were missing for 15 patients (11.4%) (Supplemental Fig. S1). While additional subanalyses of data based on stage or progression risk have been performed in larger phase III studies [10,11], the small sample sizes in the current study precluded effective subanalyses. In addition, the follow-up for OS was relatively short (24 months).
In summary, the combination of oxaliplatin, docetaxel, and bevacizumab was well tolerated. The AEs seen in the study are consistent with the known toxicities of the study drugs, with no unexpected safety concerns. This novel treatment regimen showed promising efficacy as first-line therapy for stages IB though IV ovarian, primary peritoneal, or fallopian tube carcinoma. However, further study in a randomized fashion would be required to confirm these phase II observations.
Supplementary Material
HIGHLIGHTS.
Combining oxaliplatin/docetaxel with bevacizumab appears to be promising; 12-month PFS data were similar to SCOTROC.
Median PFS (16.3months) was similar to that in the phase III GOG 218 and ICON 7 trials.
There were no unanticipated safety concerns, and the incidence of severe peripheral/sensory neuropathy appears to be low.
Acknowledgments
Study funding was provided by Sanofi U.S. LLC. Medical editorial writing assistance was provided by Maria Soushko, PhD, Phase Five Communications Inc., and supported by Sanofi U.S. LLC. The authors retained full editorial control over the content of the manuscript and received no compensation from any party for their work. Bevacizumab was provided by Roche-Genentech.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2014.01.035.
Conflict of interest statement
Thomas J. Herzog has consulted for Roche, Johnson & Johnson, and Morphotek, Inc. Bradley J. Monk received a research grant from Genentech, Inc., for whom he is a consultant and speakers bureau member. Robert M. Wenham provides investigator trial support for investigator-initiated research. Angeles Alvarez Secord received grant support for two clinical trials from Sanofi-aventis and is a Genentech, Inc., advisory board member. Barrett H. Childs was an employee of Sanofi U.S. LLC. throughout the study and during preparation of the manuscript. Peter G. Rose, Patricia Braly, Jeffrey F. Hines, Maria C. Bell, Lynda D. Roman, Mark H. Einstein, and Richard D. Drake declare that there are no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21(4):750–5. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 3.Ferrandina G, Ludovisi M, De Vincenzo R, Salutari V, Lorusso D, Colangelo M, et al. Docetaxel and oxaliplatin in the second-line treatment of platinum-sensitive recurrent ovarian cancer: a phase II study. Ann Oncol. 2007;18(8):1348–53. doi: 10.1093/annonc/mdm136. [DOI] [PubMed] [Google Scholar]
- 4.Seliger G, Mueller LP, Kegel T, Kantelhardt EJ, Grothey A, Groe R, et al. Phase 2 trial of docetaxel, gemcitabine, and oxaliplatin combination chemotherapy in platinum- and paclitaxel-pretreated epithelial ovarian cancer. Int J Gynecol Cancer Nov. 2009;19(8):1446–53. doi: 10.1111/IGC.0b013e3181b62f38. [DOI] [PubMed] [Google Scholar]
- 5.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96(22):1682–91. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 6.Misset JL, Vennin P, Chollet PH, Pouillart P, Laplaige PH, Frobert JL, et al. Multicenter phase II–III study of oxaliplatin plus cyclophosphamide vs. cisplatin plus cyclophosphamide in chemonaive advanced ovarian cancer patients. Ann Oncol. 2001;12(10):1411–5. doi: 10.1023/a:1012556627852. [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33):5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 8.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–6. doi: 10.1200/JCO.2007.12.0782. [Erratum in: J Clin Oncol. 2008;26(10):1773] [DOI] [PubMed] [Google Scholar]
- 9.Micha JP, Goldstein BH, Rettenmaier MA, Genesen M, Graham C, Bader K, et al. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer. 2007;17(4):771–6. doi: 10.1111/j.1525-1438.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 10.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 11.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96(6):487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Institute Common Terminology. Criteria for Adverse Events (NCI CTCAE), Version 3.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 15.Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol. 2000;18(20):3558–85. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 17.National Cancer Institute. Ovarian Epithelial Cancer Treatment (PDQ), Health Professional Version. Stage III and stage IV ovarian epithelial cancer treatment. 2012 Jun 22; Available from: http://www.cancer.gov/cancertopics/pdq/treatment/ovarianepithelial/HealthProfessional/page5.
- 18.Rubin SC, Sabbatini P, Viswanathan AN. Ovarian cancer. In: Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, editors. Cancer Management: A Multidisciplinary Approach. 13th. London, UK: UBM Medical LLC; Mar 25, 2011. [Available from: http://www.cancernetwork.com/display/article/10165/1802681] [Google Scholar]
- 19.Penson RT, Dizon DS, Cannistra SA, Roche MR, Krasner CN, Berlin ST, et al. Phase II study of carboplatin, paclitaxel, and bevacizumab with maintenance bevacizumab as first-line chemotherapy for advanced mullerian tumors. J Clin Oncol. 2010;28(1):154–9. doi: 10.1200/JCO.2009.22.7900. [DOI] [PubMed] [Google Scholar]
- 20.Konner JA, Grabon DM, Gerst SR, Iasonos A, Thaler H, Pezzulli SD, et al. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J Clin Oncol. 2011;29(35):4662–8. doi: 10.1200/JCO.2011.36.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27(9):1419–25. doi: 10.1200/JCO.2008.19.1684. [Erratum in: J Clin Oncol 2009;27(13): 2305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


