Abstract
Background
Data on second-line treatment options for pediatric patients with immune thrombocytopenia (ITP) are limited. Thrombopoietin receptor agonists (TPO-RA) provide a non-immunosuppressive option for children who require an increased platelet count.
Procedure
We performed a multi-center retrospective study of pediatric ITP patients followed at ITP Consortium of North America (ICON) sites to characterize TPO-RA use.
Results
Seventy-nine children had a total of 87 treatments (28 eltrombopag, 43 romiplostim, and 8 trialed on both). The majority had primary ITP (82%) and most (60.8%) had chronic ITP. However, 22% had persistent ITP and 18% had newly diagnosed ITP. During the first three months of treatment, 89% achieved a platelet count ≥ 50 × 109/l (86% romiplostim, 81% eltrombopag, p=0.26) at least once in the absence of rescue therapy. The average time to a response was 6.4 weeks for romiplostim and 7.0 weeks for eltrombopag (p=0.83). Only 40% of patients demonstrated a stable response with consistent dosing over time. An intermittent response with constant dose titration was seen in 15%, and an initial response that waned to no response was seen in 13%. Significant adverse events were minimal with the exception of two patients with thrombotic events and one who developed a neutralizing antibody.
Conclusions
Our results demonstrate that TPO-RA agents are being used in children with ITP of varying duration and severity. The response was similar to clinical trials, but the sustainability of response varied. Future studies need to focus on the ideal timing and rationale for these medications in pediatric patients.
Keywords: Immune Thrombocytopenia, Pediatrics, Treatment, Thrombopoietin Receptor Agonists, ICON
Introduction
Immune thrombocytopenia (ITP) is one of the most common conditions encountered by the pediatric hematologist. Current first-line therapy includes observation without drug therapy, corticosteroids, intravenous immune globulin, and anti- Rh (D) immunoglobulin[1, 2]. A minority of patients are refractory to first-line pharmacotherapy or develop chronic disease and require a second-line agent to improve health-related quality of life (HRQoL) and/or decrease bleeding symptoms. For pediatric patients who require second-line treatment, options include ongoing intermittent use of first-line agents, splenectomy, rituximab, or alternative immunosuppressive therapy.[1, 2] Each of these options carries a substantial risk and benefit profile that must be considered, and there remains a need for improved therapeutic approaches in these patients.
Recognition of impaired platelet production by megakaryocytes in patients with ITP led to the development of the thrombopoietin receptor agonists (TPO-RA).[3, 4] Two agents, romiplostim and eltrombopag, are currently FDA approved for adults with chronic ITP. Eltrombopag is also approved for children ≥ 1 year. Several clinical trials examining the efficacy of TPO-RAs in children with primary ITP have been published.[5-8] In a randomized phase I/II trial of pediatric patients with primary ITP for > 6 months, 88% of patients receiving romiplostim maintained a platelet count > 50 × 109/L for a median of 7 weeks compared to 0 patients in the placebo group.[5] Results from eltrombopag randomized clinical trials showed that approximately 40% of patients were able to achieve a platelet count ≥50 × 109/L for the majority of study visits compared to 0 - 3% of patients in the placebo group. In the open-label phase of these trials, 80% of patients were able to achieve a platelet count ≥50 × 109/L at least once.[6, 7] Additional data about the use of these agents in pediatric ITP is limited to small single center retrospective reviews and case series focused on children with persistent and chronic ITP.[9-11]
Despite a paucity of literature regarding TPO-RA use in children, physicians are prescribing TPO-RAs to pediatric patients off-label and outside of clinical trials. Furthermore, physicians may not be reserving these agents exclusively for chronic primary ITP, the only group in whom these agents have been investigated and are indicated. We present here multi-center retrospective data on TPO-RA administration to characterize the use of these agents in clinical practice.
Methods
Patient Sample
A retrospective chart review was performed at 12 sites in the Pediatric Immune Thrombocytopenia Consortium of North America (ICON). ICON is a research collaborative, wherein clinicians and researchers interested in pediatric ITP initiate and conduct collaborative studies. Boston Children’s Hospital serves as the coordinating study center.
The study was IRB approved at each site. Eligible patients were ages 0 to <18 years (y) treated with either eltrombopag or romiplostim between January 1, 2009 and November 31, 2014. Patients could have newly diagnosed, persistent, or chronic primary or secondary ITP. Patients were excluded if they were ever treated with a TPO-RA on a clinical trial or had non-immune thrombocytopenia.
Data Collection
Demographic and baseline ITP data were obtained. Information regarding TPO- RA initiation included the agent used, starting dose, platelet count at initiation, and reason for starting a TPO-RA. For each drug, information was collected on dosing, maximum dose, and platelet counts. Safety data focused on thrombosis, bone marrow reticulin, cataracts, and transaminitis (ALT > 3x normal). Discontinuation data included duration of TPO-RA use, reason for discontinuation, follow-up platelet counts, development of rebound thrombocytopenia (platelet count at or below baseline within 1 week of discontinuing TPO-RA), and status of ITP at both the time of discontinuation and data collection. Data were collected twice and patients are represented twice if they had been separately trialed on both agents.
Definitions
ITP status was classified using the international consensus guidelines as newly diagnosed (< 3 months), persistent (≥3 months and ≤ 12 months) and chronic (> 12 months).[12] The baseline platelet count was the pretreatment value closest to the first dose of drug; which could represent recent additional therapy.
Platelet count response was defined two different ways to allow for comparison to other clinical trials. Consecutive response was a platelet count ≥ 20 × 109 above baseline for 2 consecutive weeks without requiring any new or increased concomitant ITP treatment. Single response was any platelet count ≥ 50 × 109/L without rescue therapy in the previous 7 days. The quality of response was described subjectively as stable, waned, or intermittent response with high variability in the platelet count. To verify integrity of this variable the platelet count response was reviewed by two independent authors (CN, RG, JD, ML, KN, KS, KH) for determination of response pattern. Thrombocytosis was defined as a platelet count ≥ 400 × 109/l.
Statistical Methods
Data were entered into REDCap and analyzed with SAS 9.3. For comparisons of the two drug regimens, the eight subjects who received both drugs were treated as independent. For continuous variables, the two drug regimens were compared using the Wilcoxon two-sample test. For categorical variables, Fisher’s exact test was used. Spearman correlations were used to test for association between patient age, starting dose, and highest dose achieved. Fisher’s exact test was used to compare reasons for starting TPO-RA treatment between the three ITP status groups (newly diagnosed, persistent, and chronic). T-tests were used to compare the ages of patients with and without physician concerns about low platelet count and to compare the ages of responders and non-responders. Fisher’s exact test was used to compare responders and non-responders on splenectomy status and ITP status (newly diagnosed, persistent, and chronic).
Results
A total of 79 patients were eligible for inclusion. Twenty-eight patients received only eltrombopag, 43 patients received only romiplostim, and 8 patients were trialed on both agents separately for a total of 87 treatments. Table I shows the demographic and baseline clinical data. Figure 1 shows the age distribution of TPO-RA use. Patients receiving romiplostim were significantly younger (romiplostim 10.9 y, eltrombopag 13.8 y; p=0.002), and patients with newly diagnosed ITP were more likely to be treated with romiplostim (13 romiplostim, 1 eltrombopag; p< .001). Fifteen children (19%) had secondary ITP including Evans syndrome (n=10), medication associated (n=1), Crohns disease (n=1), immunodeficiency (n=1) and multiple sclerosis (n=1). Although most children (61%) had chronic ITP at the time of TPO-RA initiation, 18% (n=14) had newly diagnosed ITP. All newly diagnosed patients had received additional therapies prior to starting a TPO-RA (median 3, range 1-6), including steroids (n=13), IVIG (n=14), splenectomy (n=3), and rituximab (n=5). At the time of last follow up (median length of follow up: 1.4 mo), 8 of the newly diagnosed patients continued to have active ITP, and 6 had resolved ITP.
Table I.
Patient Demographics and Baseline ITP Data
| Total (n= 79)* | Romiplostim (n= 51) | Eltrombopag (n= 36) | p value+ | |
|---|---|---|---|---|
|
| ||||
| Age, years (mean, SD) | 11.9, 4.8 | 10.9, 5.2 | 13.8, 3.4 | 0.002 |
|
| ||||
| Gender (Female) | 50 (63.3%) | 33 (64.7%) | 22 (61.1%) | 0.82 |
|
| ||||
| Race | ||||
| White | 63 (79.7%) | 41 (80%) | 29 (81%) | |
| Black | 11 (13.9%) | 5 (10%) | 7 (19%) | 0.08 |
| Asian/Other | 5 (6.3%) | 5 (10%) | 0 | |
|
| ||||
| Ethnicity (Hispanic or Latino) | 15 (19%) | 10 (20%) | 5 (14%) | 0.29 |
|
| ||||
| Primary ITP | 65 (82.3%) | 42 (82%) | 30 (83%) | 1.0 |
|
| ||||
| Baseline platelet count (median, range) | 10 × 109/l, 0 - 258 × 109/l | 10 × 109/l, 2 - 226 × 109/l | 13 × 109/l, 0 - 258 × 109/l | 0.68 |
|
| ||||
| Disease status at start of TPO-RA | ||||
|
| ||||
| Newly Diagnosed | 14 (17.7%) | 13 (25.5%) | 1 (2.8%) | 0.013 |
| Persistent | 17 (21.5%) | 10 (19.6%) | 9 (25%) | |
| Chronic | 48 (60.8%) | 28 (54.9%) | 26 (72.2%) | |
|
| ||||
| Duration of ITP, months (mean, SD) | 25.0, 29.4 | 20.7, 23.0 | 33.0, 35.2 | 0.07 |
|
| ||||
| Prior Splenectomy | 15 (19%) | 12 (23.5%) | 3 (8.3%) | 0.09 |
|
| ||||
| Simultaneous ITP treatment at start of TPO-RA | ||||
|
| ||||
| Corticosteroids | 28 (35%) | 21 (42%) | 9 (25%) | 0.12 |
|
| ||||
| IVIg | 23 (29%) | 18 (35%) | 7 (19%) | 0.15 |
|
| ||||
| Rituximab | 6 (8%) | 6 (11%) | 1 (3%) | 0.23 |
|
| ||||
| Mycophenolate | 3 (4%) | 1 (2%) | 2 (6%) | 0.57 |
|
| ||||
| 6-Mercaptopurine | 3 (4%) | 3 (6%) | 1 (3%) | 0.64 |
|
| ||||
| Dual TPO-RA use while switching | 5 (0.6%) | |||
|
| ||||
| Other | 7 (9%) | 6 (12%) | 5 (14%) | 0.76 |
includes 8 patients who received both romiplostim and eltrombopag,
comparing romiplostim to eltrombopag
Figure 1.
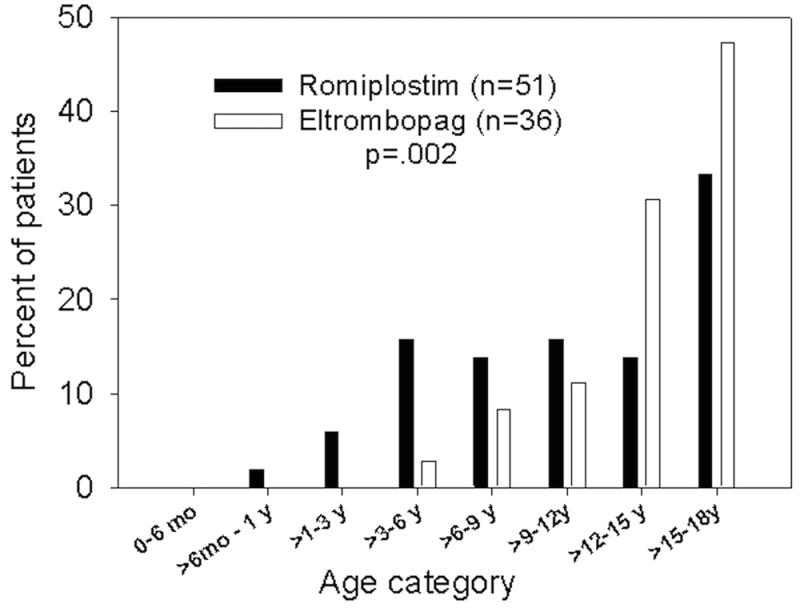
Age distribution of children receiving TPO-RAs
The most important factors for initiating a TPO-RA are shown in Table II. Bleeding was a more common reason earlier in the ITP course with 93% of newly diagnosed, 47% of persistent, and 65% of chronic patients having bleeding symptoms listed as the reason for starting (p=0.02). Physician concern as a reason for starting treatment was associated with younger age (concern: 9.7 y (SD 5.3); other: 12.9 y (SD 4.3), p=0.006). A goal platelet count was not stated in the chart for the majority of patients (n=49, 56%). For the 38 treatments in which a goal was stated, 7 had a goal of 100 × 109/l, 30 had a goal of 50 × 109/l, and 1 had a goal of 30 × 109/l.
Table II.
Initiation Data
| Total (n= 79)* | Romiplostim (n= 51) | Eltrombopag (n= 36) | p value+ | |
|---|---|---|---|---|
| Reason for starting a TPO-RA | ||||
| Bleeding symptoms | 29 (37%) | 23 (45%) | 9 (25%) | 0.07 |
| Failed other therapies | 23 (29%) | 14 (27%) | 11 (31%) | 0.81 |
| Resume activities | 8 (10%) | 2 (4%) | 7 (19%) | 0.02 |
| Improve health-related quality of life | 6 (8%) | 4 (8%) | 3 (8%) | 0.31 |
| Provider concern about low platelet count | 5 (6%) | 4 (8%) | 1 (3%) | 0.24 |
| Avoid splenectomy | 1 (1%) | 1 (2%) | 1 (3%) | 0.49 |
| Parental request | 2 (3%) | 0 | 2 (6%) | 0.17 |
| Prior to surgical procedure | 4 (5%) | 3 (6%) | 1 (3%) | 0.34 |
includes 8 patients who received both romiplostim and eltrombopag,
comparing romiplostim to eltrombopag
Dosing Data
Fifty-one patients received romiplostim for a median of 6.3 mo (range 0.2 – 67.7) and 15 (29%) were still receiving drug at the conclusion of data collection. The median starting dose was 2 mcg/kg/week (range 1-9) and median highest dose achieved was 8 mcg/kg/week (range: 3 - 11). 35% of patients were escalated to the maximum dose of 10 mcg/kg/week, including 2 patients who received a dose of 11 mcg/kg/week. There was no association between patient age and starting dose or highest dose achieved. During the first 3 months of therapy, the median number of romiplostim dose adjustments was 5 (range 0-11) and the majority of patients were increased by 1 mcg/kg/week; however, 4 patients were increased by larger dose increments of 2-4 mcg/kg/week. Eight patients (16%) had received at least one dose of romiplostim at home.
Thirty-six patients received eltrombopag for a median of 7.4 mo (range 0.6-46.9) and 20 (56%) were still receiving drug at the end of data collection. The median starting dose was 50 mg/day (range 12.5-50 mg/day). The median highest dose achieved was 75 mg/day (range 50-125 mg/day). Seventy-two percent of patients were escalated to the highest dose of 75 mg/day, including one patient that received a maximum dose of 125 mg/day.
Response Data
Response data are shown in Table III. During the first three months of treatment, 71% of patients had a consecutive platelet count response and 84% had a single response. There was no difference in age, splenectomy status, or duration of ITP between responders and non-responders. Forty percent of patients demonstrated a stable response with fairly consistent dosing over time. There was no difference in response pattern between the two medications (p=0.59). The median platelet counts during the first 3 months of romplostim and eltrombopag are shown in Figure 1. Nine patients experienced thrombocytosis (romiplostim= 2, eltrombopag=7; p=0.03). Thrombocytosis occurred 2-23 weeks after initiation and at doses ranging from 1-7 mcg/kg for romiplostim and 25-75 mg of eltrombopag. In the majority of cases, thrombocytosis was managed by holding or reducing doses or decreasing concomitant medications. In two cases, the thrombocytosis was associated with rescue medication administration.
Table III.
Response Data
| Overall (N=87) | Romiplostim (n=51) | Eltrombopag (n=36) | p-value+ | |
|---|---|---|---|---|
| Consecutive response during the first 3 months | 62 (71%) | 37 (73%) | 26 (72%) | 1.0 |
| Average time (weeks) to consecutive response (mean, SD) | 6.7 (10.4) | 6.4,(9.8) | 7.0 (11.4) | 0.83 |
| Average dose at time of consecutive response (mean) | n/a | 5 mcg/kg | 52 mg | n/a |
| Single response during the first 3 months | 73 (84%) | 44 (86%) | 29 (81%) | 0.54 |
| Subjective response | 0.60 | |||
| Responder with fairly consistent dosing over time | 35 (40%) | 24 (47%) | 11 (30%) | 0.17 |
| Intermittent response with frequent dosing titration | 12 (14%) | 9 (17%) | 3 (8%) | |
| No response | 11 (13%) | 6 (12%) | 5 (14%) | |
| Initial response that waned to no response over time | 11 (13%) | 6 (12%) | 5 (14%) | |
| Other | 15 (17%) | 5 (10%) | 10 (28%) | |
| Missing | 3 (3%) | 1 (2%) | 2 (6%) |
‘Other’ responses included: response only in the setting of additional therapy (n=4, 5%); intermittent response with concerns about adherence (n=3, 4%); unable to assess due to a brief course or loss to follow-up (n=2, 2%); response only following splenectomy (n=2, 2%); intermittent response with consistent dosing (n=2, 2%); decline in platelet count with initiation (n=1, 1%); loss of response due to antibody formation (n=1, 1%)
comparing romiplostim to eltrombopag
Most patients on corticosteroids (21/30, 70%), taking 6-mercaptopurine (3/4, 75%), and all patients taking mycophenolate (n=3) were able to discontinue therapy during TPO-RA use. A total of 26 patients (10 eltrombopag, 16 romiplostim) suffered 40 bleeding events requiring rescue therapy. The most common rescue medications were corticosteroids and IVIg. The median time to first bleeding event was 7.4 weeks (range 0.1 – 125). Bleeding events requiring rescue therapy occurred at a median dose of 3 mcg/kg of romiplostim (range 0 – 11) and 50 mg of eltrombopag (range 12.5 – 75).
Discontinuation
Of the 87 patients on a TPO-RA, 54% (33 romiplostim, 14 eltrombopag p=0.03) discontinued the medication. TPO-RA was weaned in 32% and abruptly discontinued in 66%. Reasons for discontinuation were; lack of efficacy (32%), bridging to another agent (23%), spontaneous resolution of thrombocytopenia (17%), concern over frequency of labs and visits (n= 6, 13%), loss of response (13%), side effects (13%), and desire to know the baseline platelet count (9%). There were no subjects with reported rebound thrombocytopenia. From an intention to treat perspective, 5% (n=4) of all 79 treated patients demonstrated a documented durable response or remission once TPO-RA was discontinued.
Adverse Events and Surveillance
Objective adverse events included thrombosis (eltrombopag=2), development of a romiplostim neutralizing antibody (n=1). There were two pulmonary emboli (PE), which were not associated with thrombocytosis. One occurred in a 17-year-old female with persistent ITP two weeks after starting eltrombopag. She had undergone splenectomy at time of PE and had prothrombotic risk factors including post-operative state, obesity, concomitant steroid use, and oral contraceptive pill usage. Her platelet count was 60 × 109/l at the time of the PE. The other PE occurred 3 months after switching from romiplostim to eltrombopag in a 17-year-old female with chronic ITP. Her platelet count was 61 × 109/l two weeks prior to the PE. The neutralizing antibody was identified in a patient who did not attain a platelet count response following increasing romiplostim dose up to 10 mcg/kg. Neutralizing antibody was confirmed by testing performed by Amgen and the drug was withdrawn.
There were 53 (67%) patients who had at least one bone marrow biopsy performed (median= 1 bone marrow procedure each, range 1-4) for monitoring. Forty-seven (89% of the 53) were performed prior to or within 1 month of starting a TPO-RA. Of the 53 patients with bone marrow biopsies, 9 (17%) had increased bone marrow reticulin. Of the 9 children, 3 had increased reticulin noted prior to or within several days of starting treatment. Once reticulin was seen, annual follow up bone marrow biopsies were performed in 3 patients, drug was discontinued <6 months from the last bone marrow in 4 and the other 2 patients were lost to follow up. Of the patients on eltrombopag, there were no reported episodes of transaminitis. Seven (20%) patients on eltrombopag had an ophthalmology evaluation with none demonstrating cataract formation.
Discussion
We present the clinical use of the TPO-RAs, romiplostim and eltrombopag, in a large cohort of pediatric patients followed at 12 sites. Romiplostim is currently not FDA approved for use in pediatric ITP, and eltrombopag has a narrow indication for use in children ≥ 1 year of age who have chronic ITP. Our results highlight the diverse off-label use of these medications among tertiary care pediatric ITP practice sites in North America.
Current data on the use of TPO-RAs is limited to patients with chronic ITP, with only a few patients with persistent ITP represented in the PETIT study.[7] Patients with acute ITP represented nearly 20% of patients in our cohort, highlighting a subgroup of extremely refractory newly diagnosed patients that failed first-line therapy with IVIg or corticosteroids, and in some case even splenectomy, early in their disease. Given high early spontaneous remission rates upwards of 75% in pediatric ITP, an important use for these agents might be as short-term therapy for early refractory disease where spontaneous remission is anticipated. Additionally, unlike adults, avoidance of immunosuppression and splenectomy is desirable as seen by the low number of children (n= 15) in our study cohort who had undergone splenectomy prior to TPO-RA use. Further information from clinical trials is needed to guide the ideal patient population for TPO-RA use in pediatrics, which may differ from the adult population due to these variables.
Overall, our data support that these agents are effective at increasing the platelet count similar to clinical trial results (supplemental Table I). Both agents had a comparable profile with regard to time to response and efficacy. We observed a consecutive response rate of 73% for romiplostim, which approximates the 88% from the phase I/II trial using a platelet count ≥ 50×109/L on two consecutive weeks.[5] Similarly, we demonstrated a single response rate of 75% with eltrombopag; comparable to the 62% in the double blind phase and 80% in the open label phase of the PETIT studies.[6, 7] While there are a high number of responders based on platelet count, of the 79 treated patients, there were 21 (27%) that discontinued therapy because of either no response or a loss of response over time. An additional 12 patients (15%) discontinued because of the inconvenience of administration or side effects. Frequent dose modifications and waning responses were also frequently seen. Only 40% of patients demonstrated a stable platelet count and minimal dose changes of medication, which is similar to the complete response seen in children after treatment with rituximab[13] but significantly less than seen with splenectomy.[14-17] A subset of patients (13%) were identified who had a consecutive response initially, which waned over time. This type of individual response is not described in clinical trials and indicates that not all patients continue to respond with long-term use. These findings illustrate important differences between clinical trial and real world use.
Time to response and dosing data were also similar to clinical trials. The median romiplostim dose of 5 μg/kg at the time of response was identical to the median dose in the romiplostim randomized trial [5] and the dose of 52 mg for eltrombopag, prior to publication of pediatric dosing guidelines, was only slightly lower than the average dose of 63 mg in the PETIT2 double-blind phase.[6] While current dosing guidelines target a platelet count between 50- 200 × 109/l, one benefit of these medications is the ability to titrate the dose to a desired platelet count. In our retrospective cohort, platelet count targets were often at the lower end of this range, which could reduce the therapeutic dose, provide cost saving strategies, and minimize side effects. Some patients in our study initiated drug at higher doses and had higher dose increases. It is possible that these strategies may result in a faster response time. In addition, frequent dose adjustment and monitoring may be avoided as we gain better understanding of the risks associated with temporary changes in the platelet count. Further investigation is needed to determine the ideal starting dose to maximize effectiveness, minimize side effects, and expand the use of these agents to the setting of acute bleeding events and for peri-procedural care.
We found there were few differences between patients receiving eltrombopag and romiplostim, except that patients receiving eltrombopag were significantly older, likely because our study period was prior to publication of the PETIT/PETIT2 studies [6, 7] that provided dosing data for pediatric patients and release of a liquid formulation. In selecting a TPO-RA, one consideration should be the route and location of administration. Romiplostim currently is not approved for home administration requiring weekly physician visits for the majority of children. However, this should be balanced against adherence with a daily medication and dietary considerations for eltrombopag administration. Providers should individually assess patients to determine which agent will align best with the patient and family preference and maximize adherence.
Across clinical trials these agents appear to be well tolerated and have an acceptable safety profile. We report here episodes of thrombotic events and the first report of neutralizing antibody formation in pediatric patients. The FDA currently recommends that patients on romiplostim experiencing failure to maintain a platelet response undergo neutralizing antibodies testing coordinated with the pharmaceutical company and drug discontinuation. There is no mention at this time regarding development of or testing for neutralizing antibodies against eltrombopag. In our cohort, there were an additional 6 patients who had an initial response to romiplostim that waned over time. It is not known if these patients had antibody formation testing. Bone marrow reticulin formation was an infrequent finding in our study. Obtaining reticulin stain on marrows prior to initiation of TPO-RA may prove helpful to effectively assess TPO-RA induced reticulin, as it can be present at baseline. No patients developed cataracts while receiving eltrombopag, but few patients underwent assessment for this adverse event or had baseline screening. Ophthalmology examinations might be difficult in centers with limited access to a pediatric ophthalmologist. In PETIT/PETIT2, two subjects developed cataracts, both had been treated with corticosteroids and one had pre-existing cataracts.[6, 7]
Our study is limited in its retrospective design. We are unable to comment on the impact of these agents on HRQoL or specifics regarding bleeding symptoms which may guide treatment decisions. Because retrospective assessment of bleeding severity is difficult we used need for rescue therapy as a surrogate. In clinical trials a consistent improvement in HRQoL has not been appreciated, possibly representing the burden of clinical trial participation. [7, 18] Since improved HRQoL was endorsed as a reason for initiating a TPO-RA, application of HRQoL measures both a priori and throughout treatment may provide useful information. Prospective trials collecting information on decision- making, HRQoL, and bleeding assessment are critically needed.
Our results represent patients on treatment for variable periods of time, making conclusions about long-term therapy difficult. For this reason, we focused much of our analysis on the first three months of therapy, which is similar to the initial time period of many clinical trials. However, this did not allow for monitoring of long-term side effects, an area still needing attention. All patients were treated at tertiary care referral sites, which may not be generalizable, however we feel it unlikely that refractory patients would be managed without referral to a pediatric hematologist. Our data did not allow for evaluation of adherence to medications or to dietary restrictions while taking eltrombopag which may impact response. It is possible that some data are misclassified due to retrospective interpretation of medical documentation, for example the primary reason for starting a TPO-RA. Lastly, inherent to the landscape of trials in TPO-RAs, we subjectively defined response criteria. Supplemental Table I outlines the lack of uniformity in applying a standardize response criteria across both pediatric and adult trials[5-11, 19-36]. We therefore applied definitions common to the literature to allow for the best comparison between our data and the existing literature.
Our findings support that the TPO-RAs are effective at increasing the platelet count, at least temporarily, in the majority of patients, even those with newly diagnosed and secondary ITP. Benefits of the TPO-RAs include the ability to rapidly start and stop treatment and a desirable safety profile when compared to immunosuppressive treatments, such as rituximab and splenectomy. These benefits must be weighed alongside the cost of long-term use and patient and parent preferences. Consideration should be made to using these agents in newly diagnosed refractory patients as upfront bridging agents to remission and in secondary ITP. Future trials need to identify the population of pediatric patients who will benefit the most from the agents, novel dosing strategies to expand their use, and comparison of patient-related outcomes with other therapeutic options.
Supplementary Material
Supplemental Table I. Variability in response reporting across thrombopoeitin-receptor agonist trials
Figure 2.
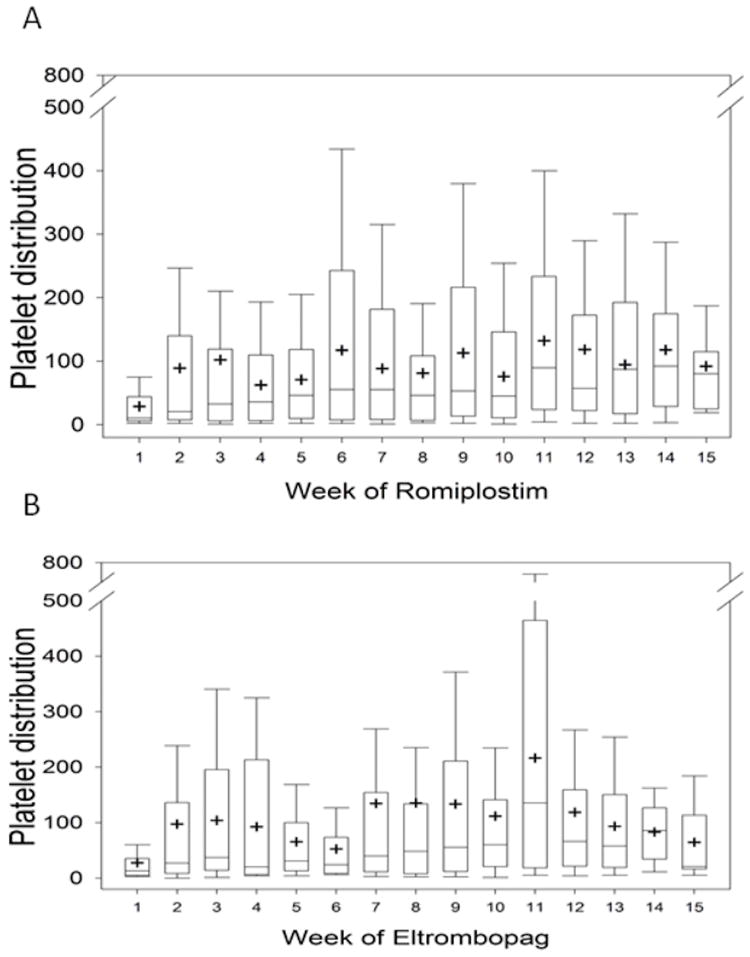
Median platelet counts during the first 3 months of romplostim (A) and eltrombopag (B)
Acknowledgments
This work was funded by the Terrana fund for ITP research (Boston Children’s Hospital); and NIH K12 HL087164 grant funding (RG). The authors would also like to thank George Buchanan and Ellis Neufeld for their scientific and editorial input as well as all members of ICON.
Abbreviation Key
- ITP
Immune thrombocytopenia
- TPO-RA
Thrombopoietin receptor agonists
- HRQoL
Health-related quality of life
- ICON
Pediatric Immune Thrombocytopenia Consortium of North America
- PE
Pulmonary emboli
Footnotes
Conflicts of Interest: CN, KS, YP, RG, KS, TB, JD, KH, JD, and PF have no conflicts of interest. RK- Amgen data monitoring committee for the Romiplostim 20080279 Study, chair of the bone marrow review panel for the Romiplostim study 20101221, and GlaxoSmithKline LLC data monitoring committee for Eltrombopag TRA108062 study. KN- employment with Janssen Research and Development. AT- research funding from Novartis, Amgen and Glaxo Smith Kline. CB- research funding from Amgen. ML- research funding from Glaxo Smith Kline and Astra Zeneca and consultant for Glaxo Smith Kline.
References
- 1.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA T.A.S.o. Hematology, The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 2.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, Grainger J, Greer I, Hunt BJ, Imbach PA, Lyons G, McMillan R, Rodeghiero F, Sanz MA, Tarantino M, Watson S, Young J, Kuter DJ. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 3.Chang M, Suen Y, Meng G, Buzby JS, Bussel J, Shen V, van de Ven C, Cairo MS. Differential mechanisms in the regulation of endogenous levels of thrombopoietin and interleukin-11 during thrombocytopenia: insight into the regulation of platelet production. Blood. 1996;88(9):3354–62. [PubMed] [Google Scholar]
- 4.Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, Nugent DJ. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102(3):887–95. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 5.Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, Wang YM, Nie K, Jun S. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118(1):28–36. doi: 10.1182/blood-2010-10-313908. [DOI] [PubMed] [Google Scholar]
- 6.Grainger JD, Locatelli F, Chotsampancharoen T, Donyush E, Pongtanakul B, Komvilaisak P, Sosothikul D, Drelichman G, Sirachainan N, Holzhauer S, Lebedev V, Lemons R, Pospisilova D, Ramenghi U, Bussel JB, Bakshi KK, Iyengar M, Chan GW, Chagin KD, Theodore D, Marcello LM, Bailey CK. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)61107-2. [DOI] [PubMed] [Google Scholar]
- 7.Bussel JB, Garcia de Miguel P, Despotovic J, Grainger JD. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (ITP): results from the randomised, multicentre, okacebo-controlled PETIT study. Lancet Haematology. 2015 doi: 10.1016/S2352-3026(15)00114-3. [DOI] [PubMed] [Google Scholar]
- 8.Bussel JB, Hsieh L, Buchanan GR, Stine K, Kalpatthi R, Gnarra DJ, Ho RH, Nie K, Eisen M. Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP) Pediatric blood & cancer. 2014 doi: 10.1002/pbc.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquet M, Aladjidi N, Guiton C, Courcoux MF, Munzer M, Auvrignon A, Lutz P, Ducassou S, Leroy G, Munzer C, Leverger G I.E. Centre de Reference National des Cytopenies Auto-immunes de. Romiplostim in children with chronic immune thrombocytopenia (ITP): the French experience. Br J Haematol. 2014;164(2):266–71. doi: 10.1111/bjh.12609. [DOI] [PubMed] [Google Scholar]
- 10.Mokhtar GM, Tantawy AA, El Sherif NH. Romiplostim therapy in children with unresponsive chronic immune thrombocytopenia. Platelets. 2012;23(4):264–73. doi: 10.3109/09537104.2011.619601. [DOI] [PubMed] [Google Scholar]
- 11.Marquinez-Alonso I, Escudero-Vilaplana V, Pernia S, Belendez Bieler C, Fernandez-Llamazares CM, Sanjurjo-Saez M. The treatment for primary immune thrombocytopenia with romiplostim in adult and paediatric patients: use experience at a Spanish university hospital. Journal of clinical pharmacy and therapeutics. 2014;39(4):376–82. doi: 10.1111/jcpt.12156. [DOI] [PubMed] [Google Scholar]
- 12.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kuhne T, Ruggeri M, George JN. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CM, Rogers ZR, Kinnamon DD, Bussel JB, Mahoney DH, Abshire TC, Sawaf H, Moore TB, Loh ML, Glader BE, McCarthy MC, Mueller BU, Olson TA, Lorenzana AN, Mentzer WC, Buchanan GR, Feldman HA, Neufeld EJ. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107(7):2639–42. doi: 10.1182/blood-2005-08-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantadakis E, Buchanan GR. Elective splenectomy in children with idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2000;22(2):148–53. doi: 10.1097/00043426-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Aronis S, Platokouki H, Avgeri M, Pergantou H, Keramidas D. Retrospective evaluation of long-term efficacy and safety of splenectomy in chronic idiopathic thrombocytopenic purpura in children. Acta Paediatr. 2004;93(5):638–42. [PubMed] [Google Scholar]
- 16.Donato H, Picon A, Rapetti MC, Rosso A, Schvartzman G, Drozdowski C, Di Santo JJ. Splenectomy and spontaneous remission in children with chronic idiopathic thrombocytopenic purpura. Pediatric blood & cancer. 2006;47(5 Suppl):737–9. doi: 10.1002/pbc.20982. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Xu M, Ji L, Yang R. Splenectomy for chronic idiopathic thrombocytopenic purpura in children: a single center study in China. Acta Haematol. 2006;115(1-2):39–45. doi: 10.1159/000089464. [DOI] [PubMed] [Google Scholar]
- 18.Klaassen RJ, Mathias SD, Buchanan G, Bussel J, Deuson R, Young NL, Collier A, Bomgaars L, Blanchette V. Pilot study of the effect of romiplostim on child health-related quality of life (HRQoL) and parental burden in immune thrombocytopenia (ITP) Pediatric blood & cancer. 2012;58(3):395–8. doi: 10.1002/pbc.23312. [DOI] [PubMed] [Google Scholar]
- 19.Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, Brainsky A E.S. Group. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121(3):537–45. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 20.Katsutani S, Tomiyama Y, Kimura A, Miyakawa Y, Okamoto S, Okoshi Y, Ninomiya H, Kosugi H, Ishii K, Ikeda Y, Hattori T, Katsura K, Kanakura Y. Oral eltrombopag for up to three years is safe and well-tolerated in Japanese patients with previously treated chronic immune thrombocytopenia: an open-label, extension study. Int J Hematol. 2013;98(3):323–30. doi: 10.1007/s12185-013-1401-1. [DOI] [PubMed] [Google Scholar]
- 21.Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short-term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP) Br J Haematol. 2013;160(4):538–46. doi: 10.1111/bjh.12169. [DOI] [PubMed] [Google Scholar]
- 22.Tomiyama Y, Miyakawa Y, Okamoto S, Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S, Ozaki K, Ikeda Y, Hattori T, Katsura K, Kanakura Y. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10(5):799–806. doi: 10.1111/j.1538-7836.2012.04695.x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 24.Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer B, Stone N, Arning M. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641–8. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 25.Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, Arning M, Provan D, Jenkins JM. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–47. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 26.Janssens A, Tarantino M, Bird RJ, Mazzucconi MG, Boccia RV, Lopez Fernandez MF, Kozak T, Steurer M, Te Boekhorst P, Dillingham K, Kreuzbauer G, Woodard P. Romiplostim Treatment in Adults with Immune Thrombocytopenia of Varying Duration and Severity. Acta Haematol. 2015;134(4):215–228. doi: 10.1159/000381657. [DOI] [PubMed] [Google Scholar]
- 27.Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, Viallard JF, Macik G, Rummel M, Nie K, Jun S. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411–23. doi: 10.1111/bjh.12260. [DOI] [PubMed] [Google Scholar]
- 28.Shirasugi Y, Ando K, Miyazaki K, Tomiyama Y, Iwato K, Okamoto S, Kurokawa M, Kirito K, Hashino S, Ninomiya H, Mori S, Yonemura Y, Usuki K, Wei H, Lizambri R. An open-label extension study evaluating the safety and efficacy of romiplostim for up to 3.5 years in thrombocytopenic Japanese patients with immune thrombocytopenic purpura (ITP) Int J Hematol. 2012;95(6):652–9. doi: 10.1007/s12185-012-1065-2. [DOI] [PubMed] [Google Scholar]
- 29.Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011;90(11):1341–4. doi: 10.1007/s00277-011-1172-9. [DOI] [PubMed] [Google Scholar]
- 30.Shirasugi Y, Ando K, Miyazaki K, Tomiyama Y, Okamoto S, Kurokawa M, Kirito K, Yonemura Y, Mori S, Usuki K, Iwato K, Hashino S, Wei H, Lizambri R. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized Phase III clinical trial. Int J Hematol. 2011;94(1):71–80. doi: 10.1007/s12185-011-0886-8. [DOI] [PubMed] [Google Scholar]
- 31.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–99. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 32.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–71. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 33.Shirasugi Y, Ando K, Hashino S, Nagasawa T, Kurata Y, Kishimoto Y, Iwato K, Ohtsu T, Berger DP. A phase II, open-label, sequential-cohort, dose-escalation study of romiplostim in Japanese patients with chronic immune thrombocytopenic purpura. Int J Hematol. 2009;90(2):157–65. doi: 10.1007/s12185-009-0361-y. [DOI] [PubMed] [Google Scholar]
- 34.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, Liebman HA, Slovick FT, de Wolf JT, Bourgeois E, Guthrie TH, Jr, Newland A, Wasser JS, Hamburg SI, Grande C, Lefrere F, Lichtin AE, Tarantino MD, Terebelo HR, Viallard JF, Cuevas FJ, Go RS, Henry DH, Redner RL, Rice L, Schipperus MR, Guo DM, Nichol JL. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 35.Newland A, Caulier MT, Kappers-Klunne M, Schipperus MR, Lefrere F, Zwaginga JJ, Christal J, Chen CF, Nichol JL. An open-label, unit dose-finding study of AMG 531, a novel thrombopoiesis-stimulating peptibody, in patients with immune thrombocytopenic purpura. Br J Haematol. 2006;135(4):547–53. doi: 10.1111/j.1365-2141.2006.06339.x. [DOI] [PubMed] [Google Scholar]
- 36.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, Wiznitzer I, Kelly R, Chen CF, Nichol JL. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672–81. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. Variability in response reporting across thrombopoeitin-receptor agonist trials


