Abstract
Control of dengue virus (DenV) transmission, primarily based on strategies to reduce populations of the principle vector Stegomya aegypti (= Aedes aegypti) (Diptera: Culicidae), is difficult to sustain over time. Other potential strategies aim to manipulate characteristics such as vector competence (VC), the innate capacity of the vector to transmit the virus. Previous studies have identified genetic factors, including differential expression of apoptosis-related genes, associated with the refractory and susceptible phenotypes in selected strains of S. aegypti from Cali, Colombia. The present study was designed to evaluate the variability of VC in selected strains against different DenV serotypes and to determine whether field-collected mosquitoes respond similarly to selected laboratory strains in terms of enhanced or reduced expression of apoptosis-related genes. Vector competence differed between strains, but did not differ in response to different DenV serotypes. Differences in VC were observed among mosquitoes collected from different localities in Cali. The overexpression of the pro-apoptosis genes, caspase 16 and Aedronc, was conserved in field-collected refractory mosquitoes and the selected laboratory refractory strain. The results suggest that the apoptosis response is conserved among all refractory mosquitoes to inhibit the development of all DenV serotypes.
Keywords: Apoptosis, caspases, dengue virus, innate immunity, vector competence
Introduction
Dengue virus (DenV) is a single positive stranded RNA virus transmitted by mosquitoes, especially Stegomyia aegypti (= Aedes aegypti) and Stegomyia albopicta (= Aedes albopictus), that manifests as dengue fever, a human disease for which there is no specific treatment. Although there have been major advances in vaccine development, no vaccines are 100% protective. Dengue is endemic in more than 100 countries in tropical and subtropical areas where 2.5 billion people are at risk and accounts for a global burden of 390 million infections per year, of which 96 million are symptomatic (Bhatt et al., 2013). In the Americas, dengue incidences have increased 30-fold over the last 50 years (World Health Organization, 2012).
Dengue control has traditionally focused on the reduction of vector densities through the application of insecticide and the elimination of mosquito larval habitats. However, the effectiveness of chemical control has been limited by the development of insecticide resistance, and the high costs of establishing and maintaining vector control programmes (Guzman & Harris, 2015). New strategies are therefore needed to decrease transmission. Advances in molecular biology and the availability of genome databases have opened new perspectives in the control of vectors, such as the genetic manipulation of mosquitoes (Fu et al., 2010), the development of transmission-blocking vaccines (Coutinho-Abreu & Ramalho-Ortigao, 2010) and the design of new insecticides. Some of these efforts are focused on modifying insect characteristics that determine their efficiency as vectors, such as vector competence (VC), the intrinsic ability of a vector to transmit a pathogen (Fu et al., 2010). However, the manipulation of an insect’s VC requires extensive knowledge of vector–pathogen interactions.
Vector competence depends on intrinsic genetic characteristics that influence the compatibility of the virus and vector (Fu et al., 2010; Schneider et al., 2011; Wise de Valdez et al., 2011). Incoming pathogens must avoid being eliminated by the innate immune response of the vector. Pattern recognition receptors (PRRs) in the insect recognize conserved pathogen-associated molecular patterns (PAMPs) on the surface of pathogens and activate multiple immune-related pathways that ultimately result in phagocytosis, melanization and the expression of reactive oxygen intermediates and antimicrobial peptides (AMPs) (Paradkar et al., 2012). The major pathways include the Toll (Fu et al., 2010), immune deficiency (Xi et al., 2008; Behura et al., 2011), RNA interference (RNAi) (Sánchez-Vargas et al., 2009), Janus kinase–signal transducer and activator of transcription (JAK-STAT) (Kleino et al., 2005; Souza-Neto et al., 2009) pathways, and autophagy and apoptosis (Behura et al., 2011). Although it has been traditionally considered that specific pathways respond to specific pathogens, there are shared molecules within and between multiple pathways that result in a generalized activation of multiple pathways by a single pathogen.
Dengue virus is an intracellular pathogen and, as such, is not exposed directly to many of the effector molecules of the innate immune system. Nonetheless, DenV infection in mosquitoes is recognized by the vector, resulting in the activation of the Toll, immune deficiency (Xi et al., 2008), JAK-STAT (Souza-Neto et al., 2009) and RNAi (Sánchez-Vargas et al., 2009) pathways, autophagy and apoptosis (Ocampo et al., 2013) that control, regulate or modulate DenV establishment and replication. There is significant molecular interplay between vector and virus that may increase or decrease immune responses and the subsequent success or failure of DenV to replicate within, and be transmitted by, an individual mosquito.
High variability in the VC of mosquitoes collected in Cali, Colombia has been previously demonstrated (Ocampo & Wesson, 2004), and strains with different levels of VC for DenV-2 have been selected (Caicedo et al., 2013) in order to understand potential molecular mechanisms associated with susceptible (Cali-S) or refractory (Cali-MIB) S. aegypti. Differences between Cali-S and Cali-MIB in the expression of specific genes, especially innate immune-related genes associated with apoptosis (Baron et al., 2010; Ocampo et al., 2013) have been demonstrated and the contributions of these genes to the phenotypes have been confirmed using RNAi silencing assays (Ocampo et al., 2013). These studies evaluated the innate immune response to DenV-2 and questioned whether this was serotype-specific or a general response to all DenV serotypes. The study described herein was performed to determine whether the observed responses associated with the VC of Cali-MIB were a product of the laboratory selection process itself or were mechanisms conserved and maintained in wild mosquitoes.
Materials and methods
Mosquito strains
The selected S. aegypti strains, Cali-S and Cali-MIB, were established in the laboratory facilities at the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) from larvae collected from several sites around the city of Cali, Colombia. The phenotypes were selected after exposure to DenV-2 New Guinea C (NGC) through isofamily selection as previously described (Caicedo et al., 2013). The selected strains were maintained in the laboratory and given exposure to DenV every two generations to maintain selection pressure.
Stegomyia aegypti larvae were collected from six localities around Cali, including Mariano Ramos, Valle Grande, Navarro la Y, Siloe, Antonio Nariño and Paso del Comercio, all of which are at least 5 km from one another. Larvae were collected from small containers in public places; all larvae collected within the same neighbourhood were combined and named by locality. Collected larvae were maintained at the CIDEIM insectary under standard rearing conditions at 27 ± 2 °C, 56% relative humidity and an LD photoperiod of 12 : 12 h. The larvae were kept in plastic containers at a density of ~ 300 larvae in 2 L of dechlorinated water and were fed with Tetramin® (Spectrum Brands, Inc., Middleton, WI, U.S.A.). Adults were provided with 10% sucrose solution ad libitum. Mosquitoes from each locality were allowed to mate and oviposit, and the subsequent experiments were carried out with mosquitoes from the F2 and F3 generations from each locality.
Viral propagation, experimental infections and VC determination
Cali-S and Cali-MIB females were exposed to four different DenV reference serotypes [DenV-1 Hawaii; DenV-2 New Guinea C (NGC), DenV-3H-87 (Philippines 56), DenV-4H-241 (Philippines 56)], all of which were supplied by Colorado State University in 2000. Levels of VC in the mosquitoes collected from different locations in Cali were determined using the reference strain DenV-2 NGC to compare the results of the present study with those reported previously in the selected strains (Ocampo et al., 2013).
Each DenV serotype was grown in S. albopicta C6/36 HT cells. Infected cells were incubated for 14 days at 32 °C in L-15 medium supplemented with 2% fetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine (Higgs et al., 1996). The virus and cells were harvested and collected in 15-mL conical centrifuge tubes. The virus suspension was mixed (1 : 1) with defibrinated rabbit blood, and adult female mosquitoes were exposed to this mixture through an artificial feeder using a pig intestine membrane. In order to determine viral concentration [median tissue culture infective dose per mL (TCID50/mL)], aliquots of the infected cell/blood suspension pre- and post-infection, as well as rabbit blood, were titrated to assess the amount of virus to which female mosquitoes were exposed (Bennett et al., 2005).
For each infection, approximately 50 females (aged 5–7 days) from each strain (Cali-MIB and Cali-S) were starved for 12 h and then exposed to the infectious bloodmeal. All feedings were performed in a Biosafety Level 2+ (BSL-2+) laboratory at CIDEIM. Mosquitoes were allowed to feed on the viral blood mixture for 2 h. Each individual serotype experiment was conducted four times. Fully engorged females were transferred to a separate cage and maintained for 15 days, the length of the extrinsic incubation period of the virus in the vector, under standard conditions as described above. Subsequently, the presence of disseminated virus in the head and abdomen of each mosquito was evaluated using indirect immunofluorescence (IIF) as described by Schoepp & Beaty (1984). Antibodies used in this study (anti-DenV-1 D2-1F1-3, anti-DenV-2 3H5-1-21, anti-DenV-3 D6-8A1-12, anti-DenV-4 1H10-6-7) were supplied by the Centers for Disease Control and Prevention (CDC), Puerto Rico.
Mosquitoes with positive head smears as determined by IIF were categorized as the susceptible phenotype. Mosquitoes with negative head smears were further dissected and their midguts assessed for the presence of DenV using IIF. Mosquitoes that were negative for DenV in both head and midgut smears were classified as refractory via a midgut infection barrier (MIB) phenotype, and those with negative head but positive midgut smears were classified as refractory via a midgut escape barrier (MEB) phenotype.
Vector competences were measured in the selected strains, Cali-S and Cali-MIB, and in the field-collected mosquitoes from six different localities in Cali.
Differences between the Cali-S and Cali-MIB strains in VC for each DenV serotype were measured using a binomial regression model to estimate the numbers of mosquitoes with susceptible and refractory phenotypes. The number of susceptible mosquitoes in each strain was independent of the number of refractory mosquitoes. The model used was: logit(p) = β0 + β1i x1i + β2j x2j, where β0 represents the intercept, β1i represents the log-odds ratios for the effects of strains (i = 1, 2; 1 = Cali-MIB; 2 = Cali-S), β2j represents the log-odds ratios for the effects of each serotype (j = 1, 2, 3, 4; 1 = DenV-2 (reference); 2 = DenV-1; 3 = DenV-3; 4 = DenV-4); x1i represents the strain, and x2j represents the serotype.
To determine whether there were differences in VC for DenV-2 among the field-collected S. aegypti from different neighbourhoods, chi-squared tests were performed to compare observed and expected frequencies of mosquitoes in relation to susceptible and refractory MIB phenotypes.
Gene expression studies
As previous studies had identified differential expression of apoptosis-related genes in Cali-S and Cali-MIB females (Ocampo et al., 2013), the expression levels of selected genes in pools of midguts of F2 or F3 generations from a sample of mosquitoes collected at each of the localities described above were measured.
Mosquitoes were exposed to either blood or blood + DenV-2, as previously described (Ocampo et al., 2013). Midguts of engorged females were dissected in diethylpyrocarbonate (DEPC)-treated water (Life Technologies, Inc., Carlsbad, CA, U.S.A.) at 0 h, 24 h, 36 h and 48 h post-feeding, and stored in pools of 10 midguts in 50 μL of RNAlater (Life Technologies, Inc.). Samples were stored for 24 h at 15 °C, 24 h at 0 °C and then at −70 °C to prevent the formation of crystals. Midguts were gradually thawed, RNAlater was removed from each vial and midgut RNA was extracted using the RNeasy® Mini Kit (Qiagen, Inc., Valencia, CA, U.S.A.). Total RNA was quantified using a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies LLC, Wilmington, DE, U.S.A.).
For cDNA synthesis, 100 ng of total RNA per time-point was reverse-transcribed in 20-μL reaction mixtures containing 5× first-strand buffer [50 mM Tris-HCl (pH 8.3)], 0.1 M DTT, 10 mM of each dNTP, 50 ng of Oligo(dT) primer (5′-CGGGCAGTGAGCGCAACGTTTTTTTTTTTTTT-3′) and 200 units of Superscript II Reverse Transcriptase (Life Technologies Corp., Grand Island, NY, U.S.A.). Reverse transcription was conducted at 42 °C for 50 min and then at 70 °C for 15 min. The resulting cDNAs were used in subsequent real-time quantitative polymerase chain reactions (qPCRs) using the primers in Table 1.
Table 1.
Primers used in quantitative polymerase chain reactions.
| Target gene | Transcript ID | Sequence | Temperature |
|---|---|---|---|
| Caspase 16 | AAEL005956-RA | 5′-TCCGCTATCTTCATATTGTATCCTTTG-3′ | 60 °C |
| Aedronc | AAEL011562–RA | 5′-CAACTTTCCAACTGCCTATAAATTGC-3′ 3′-CTCCACCGTATCGTTATTGTTCTTAG-5′ |
60 °C |
| Cathepsin-B | AAEL007585-RA | 5′-CAAAGCACTCCCTTCCATC-3′ 3′-CACGAGCGTCGAATGTATC-5′ |
57 °C |
| Niemann–Pick Type C-2 | AAEL015136-RA | 5′-GCACTCGTCCCAGCTGTAATG-3′ 3′-CACTGACCAGCGGATAGATGG-5′ |
57 °C |
| β-Actin | AAEL001928-RA | 5′-AAGGCTAACCGTGAGAAGATGAC-3′ 3′-GATTGGGACAGTGTGGGAGAC-5′ |
60 °C |
Gene expression was measured in 12.5-μL reactions prepared with 1 μL of cDNA product, 1 μL of each primer (10 μM) and 6.25 μL of SYBR Green ERTM Express (Life Technologies Corp.) in a Bio-Rad CFX96™ Real-Time System (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). The conditions were: 95 °C for 2 min, followed by 35 cycles of 95 °C for 10 s, 57 °C or 60 °C for 15 s (Table 1) and 72 °C for 20 s. Two biological replicates (three in Antonio Nariño samples) were analysed for collections from each locality and all qPCR reactions were performed in triplicate. The expression of each gene of interest was analysed in each of the F2/F3 generations collected from each of the different localities. Expression levels were normalized using an internal control [β-actin (AAEL001928)] to generate ΔCt values.
Gene expression was compared between strains (refractory vs. susceptible strain) after exposure to blood or blood + DenV-2 using 2−ΔCt refractory strain/2−ΔCt susceptible strain (Schmittgen & Livak, 2008). Results are presented as the mean ± standard deviation.
A multiple linear regression to identify differences in gene expression in the mosquitoes collected from the different localities was performed on the qPCR data, with the response variable being ΔCt = Ct (sample) − Ct [calibrator (β-actin)] and the independent factors being time, treatment (blood vs. blood + DenV-2) and strain. Robust standard errors were used to account for correlations between replicates. The model was: y = β0 + β1t x1t + β2i x2i + β3j x3j + εtij, where y represents ΔCt actin, β0 represents the intercept, β1t represents the coefficient for the effects between times (t = 1, 2, 3, 4; 1 = 0 h; 2 = 24 h; 3 = 36 h; 4 = 48 h), β2i represents the coefficient for the effects between treatments (i = 1, 2; 1 = blood; 2 = virus), β3j represents the coefficient for the effects between localities (j = 1, 2, 3; 1= Antonio Nariño; 2= Siloe; 3= Paso del Comercio), x1t represents time, x2i represents treatment, x3j represents locality and εtij represents random error. In the model, interactions between times and localities, and between treatments and localities were also evaluated. The overall differences in gene expression over time of each gene among strains and localities were observed with expression ratios (R) estimated by exponentiating 2−coefficient. Coefficients comparing ΔCt values estimate the corresponding ΔΔCt values.
In both analyses, a two-sided significance level of 0.05 was used. All statistical analyses were completed in R Version 3.1.1 (R Core Team, 2014) and STATA/SE Version 12.0 (StataCorp LP, College Station, TX, U.S.A.).
Ethical statement
All protocols for mosquito feeding were approved by CIDEIM’s Research Ethics Committee for Animal Experimentation.
Results
Vector competence for different DenV serotypes in selected strains
Viral load
Vector competence studies were carried out with the selected strains Cali-S (F31) and Cali-MIB (F30), which were 94 and 42% susceptible to DenV-2 infection, respectively. Ranges of virus concentration (TCID50/mL) measured pre- and post-feeding for each serotype were: 108.1–107.1 for DenV-1, 108.1–107.0 for DenV-2, 108.1–106.9 for DenV-3 and 106.9–105.9 for DenV-4.
The results of Cali-S and Cali-MIB mosquitoes infected with the different DenV serotypes are shown in Fig. 1. Figure 1(A) shows the percentage of mosquitoes with each phenotype [S (susceptible), MEB (midgut escape barrier), MIB (midgut infection barrier)] observed in the susceptible strain (Cali-S). Figure 1(B) shows the percentage of mosquitoes with each phenotype observed in the refractory strain (Cali-MIB). The results show that Cali-S maintains a high susceptibility to all DenV serotypes, whereas Cali-MIB maintains a low susceptibility to all DenV serotypes. There were highly significant differences between the Cali-MIB (reference) and Cali-S strains for all DenV serotypes [strains: odds ratio (OR) 0.11, Z = 7.6, P < 0.001]. There were no significant differences in susceptibility within the Cali-S or Cali-MIB strains to the different dengue serotypes, using DenV-2 as the reference category (DenV-1: OR 1.21, Z = 0.63, P = 0.53; DenV-4: OR 1.39, Z = 1.07, P = 0.29). Significant differences were observed with DenV-3, but with higher and lower susceptibility maintaining the phenotype of the strains (DenV-3: OR 2.08, Z = 2.45, P = 0.01).
Fig. 1.
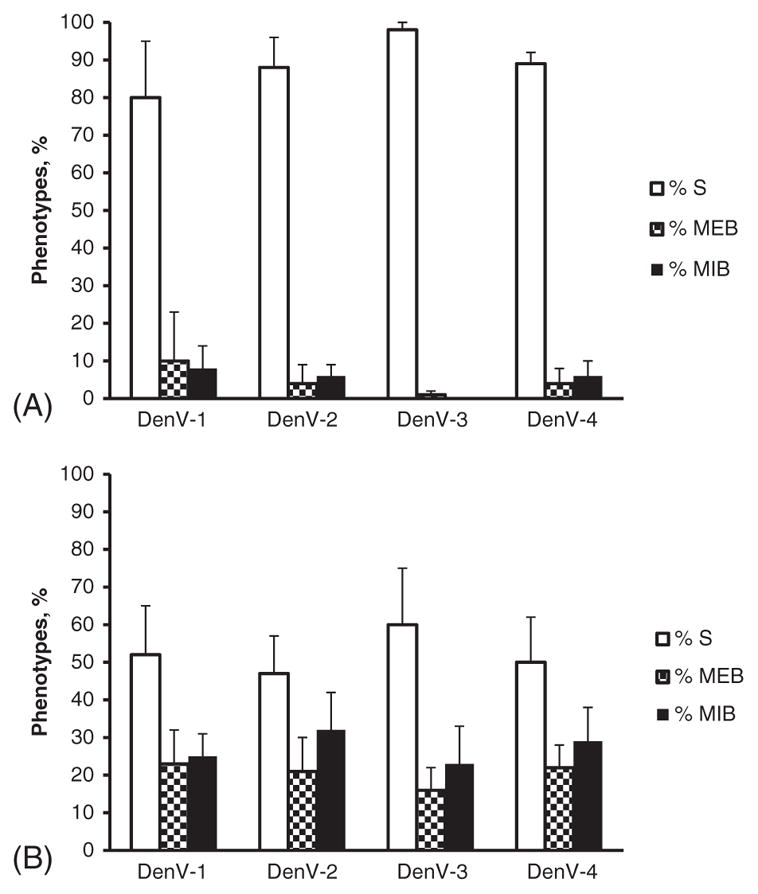
(A) Mean ± standard deviation percentages of mosquitoes with each phenotype after infection with DenV-1, DenV-2, DenV-3 and DenV-4 in (A) the susceptible strain (Cali-S) and (B) the refractory strain (Cali-MIB). DenV, dengue virus; S, susceptible; MEB, midgut escape barrier; MIB, midgut infection barrier.
Vector competence and immune response analysis in mosquitoes collected from different localities
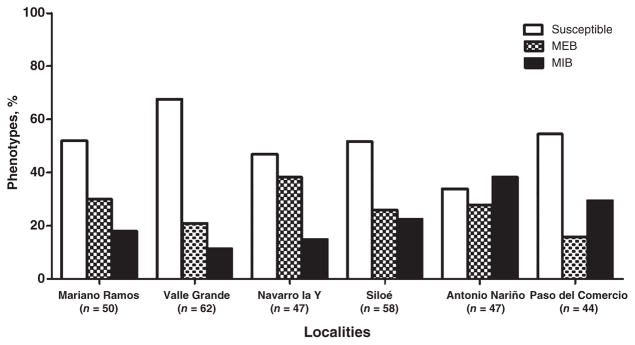
Vector competence for DenV-2 differed among the mosquitoes collected from the different localities around Cali (Fig. 2). Vector competence varied from 34% to 68% in the susceptible phenotype and from 11% to 38% in the refractory MIB phenotype. The largest percentage of susceptible mosquitoes was found in the neighbourhood of Valle Grande (68%, n = 62), followed by Paso del Comercio (55%, n = 44), Siloe (52%, n = 58), and Mariano Ramos (52%, n = 50). The neighbourhood of Antonio Nariño had the highest percentage of refractory mosquitoes (38%, n = 47) (Fig. 2). Statistical differences in VC were found among collection sites (χ2 = 15.65, P = 0.007). Pairwise comparisons highlighted significant differences in VC between the following localities: Paso del Comercio and Navarro la Y (χ2 = 6.63, P = 0.036), Antonio Nariño and Navarro la Y (χ2 = 6.59, P = 0.037), and Antonio Nariño and Valle Grande (χ2 = 14.71, P = 0.0006). In the temporal gene expression analysis, mosquitoes from Paso del Comercio and Siloe were selected as being more susceptible, and mosquitoes from Antonio Nariño as being more refractory.
Fig. 2.
Vector competence for dengue virus serotype 2 in colonized mosquitoes from each field collection site, showing percentages of mosquitoes observed with each phenotype. MEB, midgut escape barrier; MIB, midgut infection barrier.
Temporal gene expression analyses
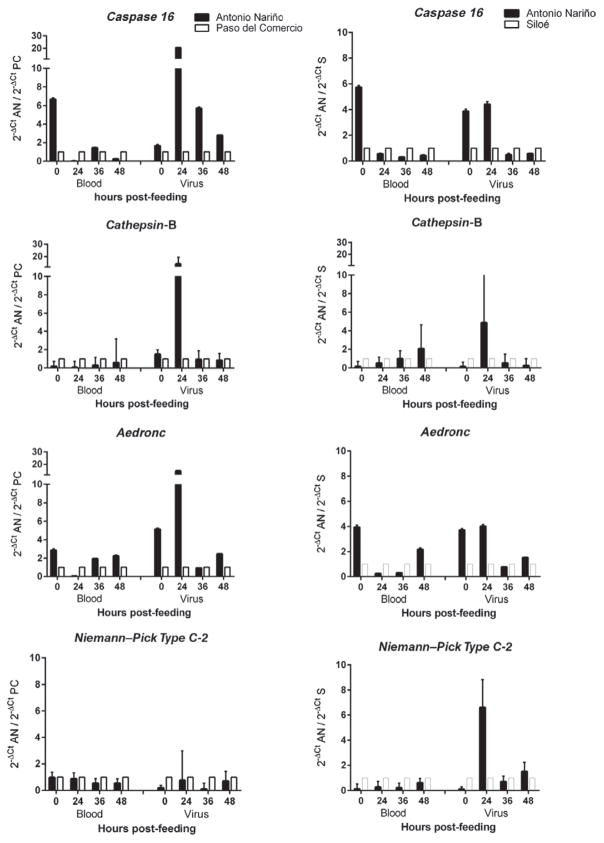
Differences in the expression of the selected genes between mosquitoes collected in Antonio Nariño (more refractory) and Paso del Comercio and Siloe (more susceptible) were measured, (Fig. 2). The expression levels of these genes in response to feeding on blood or blood + DenV-2 are shown in Fig. 3. With one exception, there were no statistically significant differences between gene expression levels in any of the mosquitoes at any time-point when all were fed on blood alone. When mosquitoes were exposed to DenV, higher levels of expression of caspase 16, cathepsin-B and Aedronc were observed, which were 20-fold higher at 24 h in mosquitoes collected from Antonio Nariño compared with mosquitoes from Paso del Comercio, and between four- and seven-fold higher than those in mosquitoes from Siloe (Fig. 3). With Niemann–Pick Type C-2, these differences in expression patterns were not observed between refractory Antonio Nariño and Paso del Comercio mosquitoes, but expression was six-fold higher in mosquitoes from Antonio Nariño than in those from Siloe.
Fig. 3.
Comparisons of midgut gene expression of caspase 16, cathepsin-B, Aedronc and Niemann–Pick Type C-2 between mosquitoes from Antonio Nariño (AN) and Paso del Comercio (PC) (left column) or Siloe (S) (right column) after feeding on blood or dengue virus-infected blood. In each pairwise comparison per time-point, expression levels in the Paso del Comercio or Siloe mosquitoes were arbitrarily set at 1, and expression levels in the Antonio Nariño mosquitoes represent relative fold-differences in expression.
The multiple linear regression model identified significant differences among collections from the various localities for caspase 16 across the evaluated times compared with time 0 (24 h: R = 0.45, t = 2.06, P = 0.042; 36 h: R = 0.33, t = 2.26, P = 0.007; 48 h: R = 0.33, t = 2.81, P = 0.006), treatments (blood vs. blood + DenV) (R = 1.95, t = −2.39, P = 0.019), and additionally evidenced significant differences between Antonio Nariño and Siloe mosquitoes (R = 0.31, t = 2.34, P = 0.02). Significant differences were also observed with Aedronc among the evaluated times compared with time 0 (24 h: R = 0.37, t = 2.15, P = 0.034; 36 h: R = 0.21, t = 3.41, P = 0.001; 48 h: R = 0.39, t = 2.04, P = 0.04), but not among treatments. Significant differences were also observed for Aedronc between Antonio Nariño and Paso del Comercio mosquitoes (R = 0.31, t = 2.02, P = 0.046). No statistical differences were observed for cathepsin-B and Niemann–Pick Type C-2 in either of the comparisons (data not shown).
Discussion
The selected S. aegypti strains, Cali-S and Cali-MIB, showed similar levels of VC for the four reference DenV serotypes, suggesting that the infection barrier in the refractory strain is serotype-independent. Previous studies in S. aegypti on VC for different serotypes showed contrasting results: some studies showed similar levels of VC among serotypes (Cox et al., 2011; Nguyen et al., 2013), whereas other studies suggested a dependency on the virus isolate (i.e. local viral serotype adaptations) (Lambrechts et al., 2009; Fansiri et al., 2013). In the present study, the similar VC among serotypes in the selected strains supports their use as a model for understanding vector–virus interactions observed in field mosquitoes from Cali. The low VC variation observed within the selected strains may be attributable to their laboratory maintenance for over 30 generations.
Field-collected mosquitoes showed significant variability in their VC for DenV-2; susceptibility ranged from 68% (Valle Grande mosquitoes) to 34% (Antonio Nariño mosquitoes) as expected based on previous studies (Ocampo & Wesson, 2004).
Variability in VC has been observed at national (Dickson et al., 2014), provincial (Carvalho-Leandro et al., 2012) and municipal (Ocampo & Wesson, 2004) levels. These changes demonstrate the high variability in VC exhibited by different geographic populations of S. aegypti. It is likely that field S. aegypti with different levels of VC will emerge as changes in the genetic structure of vector populations influence their ability to transmit dengue (Sim et al., 2013). Genetic studies on S. aegypti from Colombia suggest there are two overarching maternal lineages, the overall distribution of which has been influenced by microclimatic variables and reinvasion processes (Jaimes-Dueñez et al., 2015). The process of reinvasion and gene flow across countries can significantly increase population-level genetic variation (Lima & Scarpassa, 2009) and hence influence DenV VC.
The variability in VC in field-collected Cali mosquitoes allowed the selection of susceptible and refractory populations from different localities in which to analyse gene expression. Mosquitoes from Antonio Nariño overexpressed apoptosis-related genes earlier in the infection, similar to the comparisons between Cali-S and Cali-MIB (Ocampo et al., 2013). Expression of caspase 16 and Aedronc showed significant differences over time and treatment. This was not the case for cathepsin-B and Niemann–Pick Type C-2. The few statistically significant differences in the multiple regression models between localities may be associated with genetic variability in the field-collected mosquitoes.
Apoptosis-related genes such as caspase 16 and Aedronc may play important roles in the elimination of viruses. Apoptosis-mediated cell death has been reported previously in mosquito midguts and salivary glands in response to a wide range of arboviruses, and these mechanisms may be associated with decreased susceptibility (Vaidyanathan & Scott, 2006; Kelly et al., 2012; Ocampo et al., 2013).
Cathepsin-B is a cysteine protease produced by the fat body in response to blood feeding (Cho et al., 1999) and is secreted into the haemolymph during vitellogenesis (Price et al., 2011). Cathepsin-B has previously been associated with the regulation of cell death (Stoka et al., 2001). Although an overexpression of cathepsin-B at 24 h in mosquitoes from Antonio Nariño compared with both susceptible localities was observed (Fig. 3), the regression analysis does not show significant differences.
Niemann–Pick Type C-2 is a soluble protein with a lipid domain that appears to be important for pathogen recognition. Silencing this gene produced an increase in midgut resistance to DenV, suggesting that this gene plays a role as a DenV agonist (Jupatanakul et al., 2014). Niemann–Pick Type C-2 is a protein homologous to MD2 in mammals, and is a receptor required for activation of the Toll pathway (Dong et al., 2006; Sim et al., 2012).
In the present study, standard IIF was used as the reference standard method to determine the number of mosquitoes that have disseminated DenV (presence or absence of the virus) as a predictor of VC rather than of the intensity of infection. However, future studies could compare quantitative DenV viral loads with gene expression to elucidate correlations between levels of expression of apoptosis-related genes and VC in susceptible mosquitoes.
These results suggest that the overexpression of apoptosis-related genes observed in the Cali-MIB strain arose in field mosquitoes, is maintained in selected laboratory strains, and may contribute to the refractoriness of Cali-MIB to all four DenV serotypes. Evaluating the VC of Cali-MIB to other arboviruses, such as the Zika and Chikungunya viruses, will determine whether this is a general antiviral response, a response to flaviviruses, or a specific response to DenV. This study validates the use of the Cali-S and Cali-MIB strains as providing an appropriate model in which to study vector–DenV interactions, and corroborates the involvement of apoptosis as a viral elimination mechanism.
Acknowledgments
The authors thank Heather Coatsworth and Rachel Halipchuk (Department of Biological Sciences, Simon Fraser University) for language editing support, and Luis Ernesto Ramirez (CIDEIM) for technical support. This research was funded by COLCIENCIAS (contract no. 2229-569-33469 to CBO).
References
- Baron OL, Ursic-Bedoya RJ, Lowenberger CA, Ocampo CB. Differential gene expression from midguts of refractory and susceptible lines of the mosquito, Aedes aegypti, infected with Dengue-2 virus. Journal of Insect Science. 2010;10:1–23. doi: 10.1673/031.010.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Gomez-Machorro C, Harker BW, et al. Global cross-talk of genes of the mosquito Aedes aegypti in response to dengue virus infection. PLoS Neglected Tropical Diseases. 2011;5:e1385. doi: 10.1371/journal.pntd.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KE, Beaty BJ, Black WC., IV Selection of D2S3, an Aedes aegypti (Diptera: Culicidae) strain with high oral susceptibility to Dengue 2 virus and D2MEB, a strain with a midgut barrier to Dengue 2 escape. Journal of Medical Entomology. 2005;42:110–119. doi: 10.1603/0022-2585(2005)042[0110:SODAAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo PA, Baron OL, Pérez M, Alexander N, Lowenberger C, Ocampo CB. Selection of Aedes aegypti (Diptera: Culicidae) strains that are susceptible or refractory to Dengue-2 virus. The Canadian Entomologist. 2013;145:273–282. [Google Scholar]
- Carvalho-Leandro D, Ayres CFJ, Guedes DRD, et al. Immune transcript variations among Aedes aegypti populations with distinct susceptibility to dengue virus serotype 2. Acta Tropica. 2012;124:113–119. doi: 10.1016/j.actatropica.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Cho WL, Tsao SM, Hays AR, et al. Mosquito cathepsin B-like protease involved in embryonic degradation of vitellin is produced as a latent extraovarian precursor. Journal of Biological Chemistry. 1999;274:13311–13321. doi: 10.1074/jbc.274.19.13311. [DOI] [PubMed] [Google Scholar]
- Coutinho-Abreu IV, Ramalho-Ortigao M. Transmission blocking vaccines to control insect-borne diseases: a review. Memórias do Instituto Oswaldo Cruz. 2010;105:1–12. doi: 10.1590/s0074-02762010000100001. [DOI] [PubMed] [Google Scholar]
- Cox J, Brown HE, Rico-Hesse R. Variation in vector competence for dengue viruses does not depend on mosquito midgut binding affinity. PLoS Neglected Tropical Diseases. 2011;5:e1172. doi: 10.1371/journal.pntd.0001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson LB, Sanchez-Vargas I, Sylla M, Fleming K, Black WC., IV Vector competence in West African Aedes aegypti is flavivirus species and genotype dependent. PLoS Neglected Tropical Diseases. 2014;8:e3153. doi: 10.1371/journal.pntd.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathogens. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fansiri T, Fontaine A, Diancourt L, et al. Genetic mapping of specific interactions between Aedes aegypti mosquitoes and dengue viruses. PLoS Genetics. 2013;9:e1003621. doi: 10.1371/journal.pgen.1003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Lees RS, Nimmo D, et al. Female-specific flightless phenotype for mosquito control. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- Higgs S, Traul D, Davis B, Kamrud K, Wilcox C, Beaty B. Green fluorescent protein expressed in living mosquitoes without the requirement of transformation. BioTechniques. 1996;21:660–664. doi: 10.2144/96214st03. [DOI] [PubMed] [Google Scholar]
- Jaimes-Dueñez J, Arboleda S, Triana-Chávez O, Gómez-Palacio A. Spatio-temporal distribution of Aedes aegypti (Diptera: Culicidae) mitochondrial lineages in cities with distinct dengue incidence rates suggests complex population dynamics of the dengue vector in Colombia. PLoS Neglected Tropical Diseases. 2015;9:e0003553. doi: 10.1371/journal.pntd.0003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupatanakul N, Sim S, Dimopoulos G. The insect microbiome modulates vector competence for arboviruses. Viruses. 2014;6:4294–4313. doi: 10.3390/v6114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EM, Moon DC, Bowers DF. Apoptosis in mosquito salivary glands: sindbis virus-associated and tissue homeostasis. Journal of General Virology. 2012;93:2419–2424. doi: 10.1099/vir.0.042846-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleino A, Valanne S, Ulvila J, et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO Journal. 2005;24:3423–3434. doi: 10.1038/sj.emboj.7600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Chevillon C, Albright RG, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evolutionary Biology. 2009;9:160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima RS, Jr, Scarpassa VM. Evidence of two lineages of the dengue vector Aedes aegypti in the Brazilian Amazon, based on mitochondrial DNA ND4 gene sequences. Genetics and Molecular Biology. 2009;32:414–422. doi: 10.1590/S1415-47572009005000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NM, Kien DTH, Tuan TV, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo CB, Wesson DM. Population dynamics of Aedes aegypti from a dengue hyperendemic urban setting in Colombia. American Journal of Tropical Medicine and Hygiene. 2004;71:506–513. [PubMed] [Google Scholar]
- Ocampo CB, Caicedo PA, Jaramillo G, et al. Differential expression of apoptosis-related genes in selected strains of Aedes aegypti with different susceptibilities to dengue virus. PLoS ONE. 2013;8:e61187. doi: 10.1371/journal.pone.0061187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar PN, Trinidad L, Voysey R, Duchemin JB, Walker PJ. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18915–18920. doi: 10.1073/pnas.1205231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DP, Nagarajan V, Churbanov A, et al. The fat body transcriptomes of the yellow fever mosquito Aedes aegypti, pre-and post-blood meal. PLoS One. 2011;6:e22573. doi: 10.1371/journal.pone.0022573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2014. [Google Scholar]
- Sánchez-Vargas I, Scott JC, Poole-Smith BK, et al. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito’s RNA interference pathway. PLoS Pathogens. 2009;5:e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider JR, Chadee D, Mori A, Romero-Severson J. Heritability and adaptive phenotypic plasticity of adult body size in the mosquito Aedes aegypti with implications for dengue vector competence. Infection, Genetics and Evolution. 2011;11:11–16. doi: 10.1016/j.meegid.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp R, Beaty B. Titration of dengue viruses by immunofluorescence in microtiter plates. Journal of Clinical Microbiology. 1984;20:1017–1019. doi: 10.1128/jcm.20.5.1017-1019.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S, Ramirez JL, Dimopoulos G. Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathogens. 2012;8:e1002631. doi: 10.1371/journal.ppat.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S, Jupatanakul N, Ramirez JL, et al. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus–vector molecular interactions. PLoS Neglected Tropical Diseases. 2013;7:e2295. doi: 10.1371/journal.pntd.0002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Neto J, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoka V, Turk B, Schendel SL, et al. Lysosomal protease pathways to apoptosis cleavage of Bid, not procaspases, is the most likely route. Journal of Biological Chemistry. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Scott TW. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis. 2006;11:1643–1651. doi: 10.1007/s10495-006-8783-y. [DOI] [PubMed] [Google Scholar]
- Wise de Valdez MR, Nimmo D, Betz J, et al. Genetic elimination of dengue vector mosquitoes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4772–4775. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Strategy for Dengue Prevention and Control. 2012–2020. WHO; Geneva: 2012. [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathogens. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]