Abstract
Context:
There is little information regarding β cell mass in individuals at early stages of type 1 diabetes (T1D).
Objective:
To investigate both acute insulin response to arginine at hyperglycemia (AIRmax), as a correlate of β cell mass, and β cell function by the intravenous glucose tolerance test (IVGTT) in subjects at early stages of T1D.
Design/Setting/Participants:
Forty subjects were enrolled: (1) low-risk group: relatives of patients with T1D with 0 to 1 antibody (n = 21) and (2) high-risk group: relatives with ≥2 antibodies (n = 19).
Main Outcome Measure:
Acute insulin and C-peptide responses to IVGTT and to AIRmax. Participants underwent two IVGTT and AIRmax procedures on different days.
Results:
AIRmax was reproducible, well tolerated, and correlated to first-phase insulin response (FPIR) from IVGTT (r = 0.779). The high-risk group had greater impaired β cell function compared with the low-risk group, determined both by lower mean FPIR and a greater number of subjects below an established threshold for abnormal function [10 of 19 (52.6%) versus 4 of 21 (19%)]. There was a heterogeneous AIRmax response in these subjects with low FPIR, ranging from 38 to 250 μU/mL.
Conclusions:
There is significant variation in insulin secretory reserve as assessed by AIRmax in family members with low β cell function assessed by FPIR. As AIRmax is a functional measure of β cell mass, these data suggest heterogeneity in disease pathogenesis in which mass is preserved in relation to function in some individuals. The tolerability and reproducibility of AIRmax suggest it could be a useful stratification measure in clinical trials of disease-modifying therapy.
We report significant variation in insulin secretory reserve (β cell mass) by AIRmax in family members with low β cell function by FPIR. These data suggest heterogeneity in disease pathogenesis.
Based on the understanding that type 1 diabetes (T1D) results from an immune-mediated loss of pancreatic β cells, therapeutic trials to interrupt this process and preserve β cells have been designed. A major tool for predicting the onset of clinical disease and understanding the clinical course is measurement of β cell function. Current measures used to assess β cell function in individuals at early stages of disease (antibody-positive individuals prior to overt clinical diagnosis) include measurement of β cell response to oral glucose or intravenous (IV) glucose. Low insulin secretion is an important predictor of disease progression. Less information is known about β cell mass, or insulin secretory reserve, in antibody-positive individuals.
β cell mass cannot currently be directly measured in living individuals. The acute insulin response to arginine at hyperglycemia (AIRmax) is a dynamic test that measures the acute insulin response to arginine in the presence of marked hyperglycemia. It is considered to reflect β cell secretory reserve or β cell mass (1, 2). Studies in animal models and humans have demonstrated that AIRmax is well correlated with β cell mass in contrast to first-phase insulin response (FPIR) (1, 3–8).
The TrialNet Pathway to Prevention Study aims to gain information about the pathogenesis and natural history of T1D, as well as to facilitate the assessment and recruitment of individuals who might qualify for T1D prevention trials. The study screens relatives of individuals with T1D for diabetes-related autoantibodies to identify those at risk for disease (9).
The aim of this TrialNet ancillary study is to evaluate insulin secretory reserve as determined by AIRmax and IV glucose tolerance test (IVGTT)-derived FPIR in high-risk subjects for T1D. To our knowledge, this is the first report of AIRmax in antibody-positive relatives of individuals with T1D. We also assessed the reproducibility and tolerability of the AIRmax to determine whether it could serve as a suitable measure for risk stratification or endpoint in clinical trials of disease-modifying therapy.
Research Design and Methods
Subjects
After TrialNet Ancillary Studies Committee and Institutional Review Board approval, eligible subjects were identified through the TrialNet Pathway to Prevention Study, which tests first- and second-degree relatives for the presence of autoantibodies. Forty nondiabetic subjects were enrolled in the study according to islet cell antibody status: (1) relatives of patients with T1D having zero or one antibody defined as low risk for developing T1D (n = 21) and (2) relatives with two or more antibodies as high risk for T1D (n = 19).
Participants came for up to three visits. After informed consent, subjects underwent two tests of secretion on different days and an oral glucose tolerance test (OGTT) if not already done as part of the TrialNet Pathway to Prevention Study. All subjects were asked about their study experience following each test.
OGTT
Subjects consumed Glucola (at a dose of 1.75 g/kg body weight to maximum of 75 g) within 5 minutes. Samples were collected at −10, 0, 30, 60, 90, and 120 minutes.
Insulin secretion test
After overnight fasting, baseline samples were obtained, and 0.5 g/kg glucose was given (intravenously over 3 minutes). Samples were then collected at 1-, 3-, 5-, 7-, and 10-minute time points. FPIR was the sum of these 1- plus 3-minute insulin values. Low FPIR was defined as in Diabetes Prevention Trial-Type 1 (DPT-1) ≤100 μU/mL (10). IVGTT was then administered over 20 minutes to raise blood glucose to ≥300 mg/dL, at which time arginine (5 gm; IV over 1 min) was given. Samples were then collected at 2, 3, 4, 5, 7, and 10 minutes. AIRmax was calculated as mean insulin levels postbolus at 2 + 3 + 4 + 5 minutes minus baseline value. Additionally, the acute C-peptide responses to IVGTT and glucose-potentiated arginine were calculated, as noted for insulin.
Statistical analysis
FPIR and AIRmax between the groups were analyzed by Mann–Whitney test. Correlation between FPIR and AIRmax was assessed by Pearson correlation. Reproducibility of measurement of AIRmax was assessed with intraclass correlation. A significance of 0.05 was used in all tests.
Results
Demographic information of the 40 subjects who participated in this study is summarized in Table 1. There were no statistically significant differences between the groups with respect to age, body mass index, gender, or HLA. There were no statistical differences between groups with respect to fasting (P = 0.75) or 2-hour glucose (P = 0.18) during OGTT. Of the 40 subjects, 11 of them had impaired glucose tolerance (IGT), which included 3 subjects from low-risk group and 8 subjects from high-risk group. The rest of the 29 subjects had normal glucose tolerance (NGT). The fasting glucose at the time of IVGTT (P = 0.35) and the glucose prior to arginine stimulation (P = 0.15) were also comparable between the two groups.
Table 1.
Demographics of the Subjects in Each Group
| Low-Risk Group With ≤1 Antibodies (n = 21) | High-Risk Group With ≥2 Antibodies (n = 19) | |
|---|---|---|
| Age (mean ± SD) | 33.6 + 2.4 years | 35.9 + 2.5 years |
| BMI (mean ± SD) | 26.1 + 0.9 | 29.7 + 1.5 |
| Sex (male/female) | 12/9 | 9/10 |
| Race/ethnicity non-Hispanic white | 21 (100) | 19 (100) |
| IGT | 3/21 (14.3) | 8/19 (42.1) |
| Islet cell antibodies | ||
| GAD65 10/21 (47.6) | 18/19 (94.7) | |
| ICA 0 | 16/19 (84.2) | |
| ICA512 0 | 10/19 (52.3) | |
| mIAA 2/21 (9.5) | 2/19 (10.5) | |
| ZnT8 0 | 5/19 (26.3) | |
| HLA DR3 or DR4; not DQA1a0102 or DQB1a0602 | 9/14a (64.3) | 14/19 (73.7) |
| OGTT | ||
| Fasting glucose (mean ± SE) | 93.9 ± 2.025 mg/dL | 94.84 ± 2.459 mg/dL |
| 2-hour glucose (mean ± SE) | 112.6 ± 5.417 mg/dL | 128 ± 7.999 mg/dL |
| IVGTT | ||
| Fasting glucose at the time of IVGTT (mean ± SE) | 91.69 ± 1.885 mg/dL | 94.22 ± 1.981 mg/dL |
| AIRmax | ||
| Prestimulating glucose at the time of arginine (mean ± SE) | 391.6 ± 12.07 mg/dL | 410.1 ± 8.244 mg/dL |
Note: Values are mean ± SD/SE or n (%).
Abbreviations: BMI, body mass index; IGT, impaired glucose tolerance; SD, standard deviation; SE, standard error.
Only 14 of 21 subjects have HLA data in the group.
Acute insulin response to IVGTT and AIRmax as measures of β cell function and mass
Those with zero or one antibody and therefore low risk for disease progression had significantly higher FPIR than those with two or more antibodies (192.6 ± 32.7 vs 115.4 ±11.05 μU/mL, P = 0.02). In contrast, AIRmax was not significantly different between these groups (222.7 ± 24.19 vs 179.4 ± 23.97 μU/mL, P = 0.10).
Neither FPIR nor AIRmax was different between individuals with IGT (n = 11) and NGT (n = 29). FPIR was 158.4 ± 19.53 μU/mL in IGT and 149.4 ± 46.69 μU/mL in NGT group (P = 0.17), and AIRmax was 175.7 + 37.84 μU/mL in IGT and 212.1 ± 18.99 μU/mL in NGT (P = 0.37). Moreover, neither FPIR nor AIRmax was a significant predictor of 2-hour glucose values from the OGTT (data not shown).
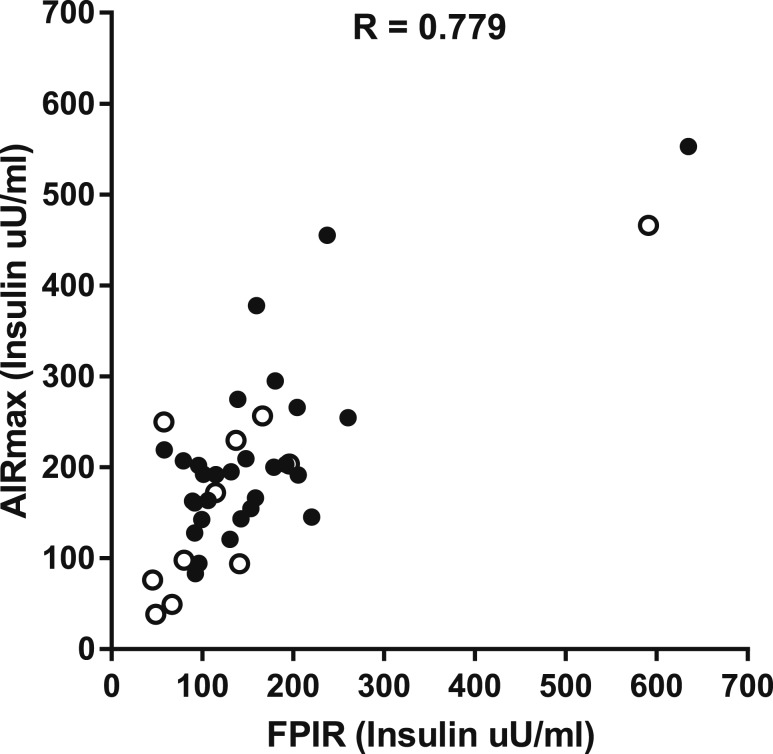
FPIR and AIRmax were correlated [r = 0.779, 95% confidence interval (0.617 to 0.877)] in all subjects (Fig. 1). Those individuals with low FPIR (n = 14) as defined during Diabetes Prevention Trial-Type 1 (DPT-1) had significantly lower AIRmax than the rest of the subjects (n = 26) (136.4 ± 17.74 vs 237.5 ± 21.86 μU/mL, P = 0.002).
Figure 1.
Correlation between AIRmax and FPIR in all subjects. The subjects with NGT are shown as solid dots, and subjects with IGT are shown as open circle.
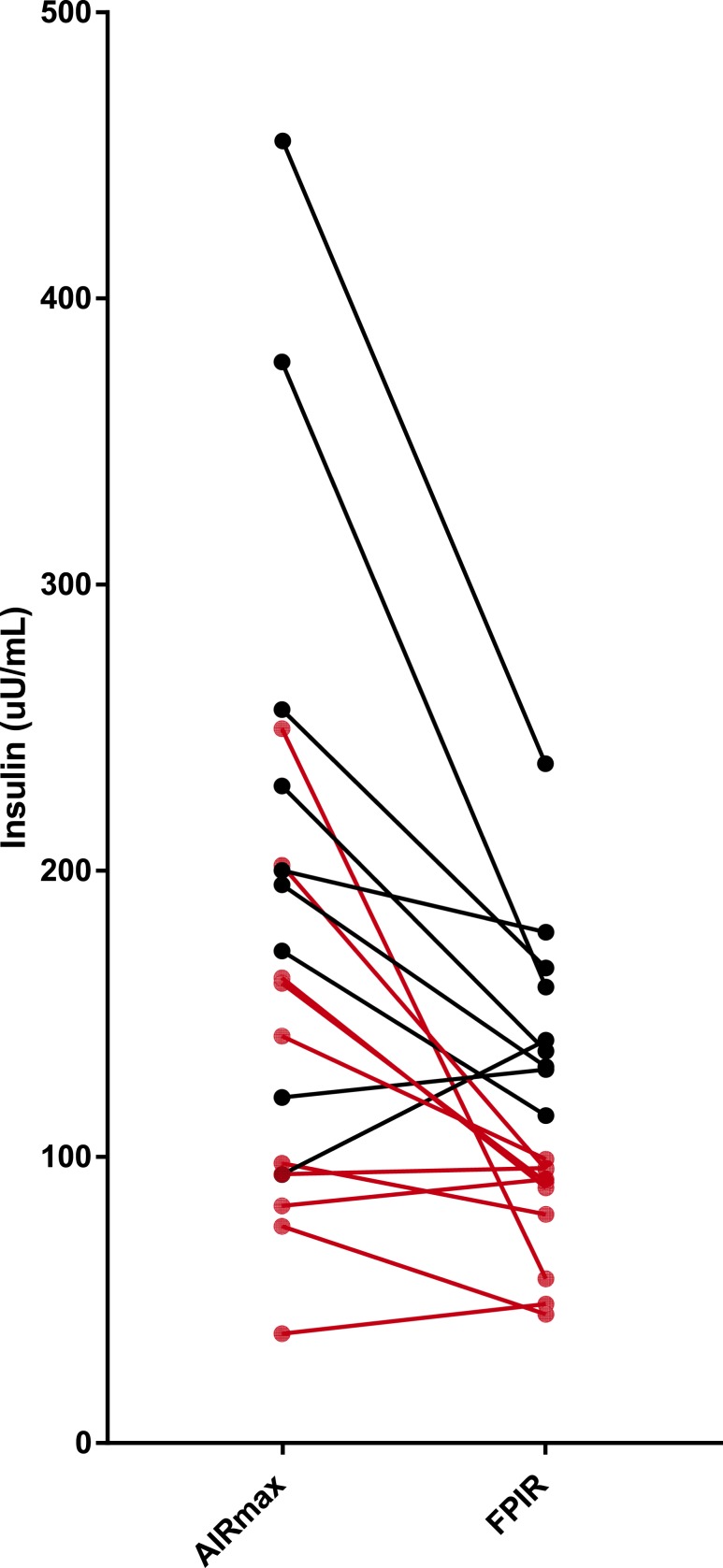
In those with two or more antibodies (high-risk group, n = 19), FPIR and AIRmax were correlated as well (r = 0.73). Using DPT-1 criteria for low FPIR as impaired β cell function, we identified 10 of 19 (52.6%) subjects in the high-risk group below this threshold (Fig. 2). As shown, these individuals had a wide range of AIRmax values. In those in the low-risk group (zero to one antibody, n = 21), only 4 of 21 (19%) individuals had low FPIR. The four subjects include one subject with no antibodies and three subjects with one antibody; one of these three subjects had IGT. These individuals also had a wide range of AIRmax values.
Figure 2.
AIRmax and FPIR by insulin value in each individual in the high-risk group. The red dot represents individuals with low FPIR (≤100 μU/mL) and their corresponding AIRmax values. The reference of normal AIRmax is ∼300 μU/mL.
Reproducibility and tolerability of IVGTT and AIRmax
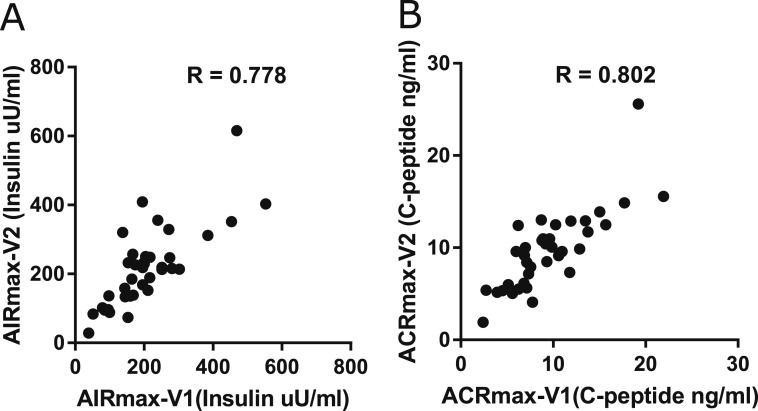
Thirty-eight of 40 participants underwent repeat IVGTT and AIRmax testing within 2 to 45 days. There was a high correlation for both insulin (r = 0.778) and C-peptide (r = 0.802) responses to glucose-potentiated arginine stimulation conducted on different days (Fig. 3). Similarly, the IVGTT tests conducted on different days showed good reproducibility with both insulin [FPIR (r = 0.93)] and C-peptide (r = 0.95) values. Intraclass correlation was close to 1.0 for each, although differences in replicate AIRmax values tended to increase with larger initial levels.
Figure 3.
Correlation of AIRmax and ACRmax between two AIRmax tests. (A) Comparison of AIRmax between the two tests (r = 0.778). (B) Comparison of ACRmax between the two tests (r = 0.802).
AIRmax was well tolerated. Most participants experienced no symptoms; some reported only mild symptoms, the most common of which were high glucose infusion-induced bladder fullness and flushed feeling, and the sensation of a transient metallic taste during IV arginine. In the study survey, 85% of participants responded that the test would be appropriate in young children (Table 2).
Table 2.
Tolerability of AIRmax by Participants’ Survey in Two AIRmax Visits
| Questions | Visit 1 (n = 40) N (%) | Visit 2 (n = 38) N (%) | |
|---|---|---|---|
| 1. How difficult was it for you to have two IVs placed to do the AIRmax test? | |||
| 1 | Not a problem | 34 (87.5) | 27 (71.1) |
| 2 | A little bit of a problem | 5 (12.5) | 10 (26.3) |
| 3 | Very difficult | 0 | 0 |
| 4 | Not sure | 1 (2.5) | 1 (2.6) |
| 2. In some people, the arginine can cause a “metallic” taste. Did you notice the metallic taste today? | |||
| 1 | No | 20 (50) | 19 (50.0) |
| 2 | Yes, didn't bother me | 12 (30) | 16 (42.1) |
| 3 | Yes, bothered a little | 8 (20) | 3 (7.9) |
| 4 | Yes, bothered a lot | 0 | 0 |
| 5 | Yes, not sure how much it bothered me | 0 | 0 |
| 3. High glucose can make your bladder full. Did you notice this? | |||
| 1 | No | 13 (32.5) | 16 (42.1) |
| 2 | Yes, didn't bother me | 9 (22.5) | 12 (31.6) |
| 3 | Yes, bothered a little | 16 (40) | 9 (23.7) |
| 4 | Yes, bothered a lot | 1 (2.5) | 1 (2.6) |
| 5 | Yes, not sure how much it bothered me | 0 | 0 |
| 4. IVGTT can make you feel warm or flushed. Did you notice this? | |||
| 1 | No | 13 (32.5) | 11 (28.9) |
| 2 | Yes, didn't bother me | 17 (42.5) | 21 (55.2) |
| 3 | Yes, bothered a little | 10 (25) | 6 (15.8) |
| 4 | Yes, bothered a lot | 0 | 0 |
| 5 | Yes, not sure how much it bothered me | 0 | 0 |
| 5. Would you be willing to come in for a second AIRmax test this month? | |||
| 1 | Yes | 40 (100) | |
| 2 | No | 0 | |
| 6. What is the youngest age group that you feel would be okay to do the AIRmax test? | |||
| 1 | Under age 5 | 6 (15.8) | |
| 2 | Age 5 to 8 | 9 (23.7) | |
| 3 | Age 8 to 12 | 18 (47.4) | |
| 4 | Age 12+ | 3 (7.9) | |
| 5 | Not sure | 2 (5.3) |
Conclusions
In this study, we report significant variation in insulin secretory reserve as assessed by AIRmax in family members with low β cell function assessed by FPIR. As AIRmax is a functional measure associated with β cell mass, these data suggest heterogeneity in disease pathogenesis in which mass is preserved in relation to function in some individuals.
β cell dysfunction is known to occur as antibody-positive individuals progress to clinical type 1 diabetes. β cell function has been measured by assessing the early insulin (<10-minute) response after an IVGTT bolus (FPIR). In DPT-1, 60% of antibody-positive relatives with low FPIR progressed to clinical disease within 5 years (10). Moreover, FPIR was lower in those with IGT as compared with NGT. Yet, in DPT-1, FPIR explained only 14% of the variance in OGTT glucose response, and many with low FPIR did not progress during this time period (11). Similarly, C-peptide or insulin during an OGTT is a poor predictor of disease progression, remaining relatively stable until just prior to diagnosis (12). Although these data suggest that an acute change in function occurs in the peri-diagnostic period, other longitudinal functional data support either linear decline or waxing and waning of function potentially consistent with disease flares (13).
In contrast to the breadth of studies describing β cell function during disease progression, little information is available about β cell mass. Studies in animal models and humans support the concept that FPIR is poorly correlated with β cell mass in contrast to AIRmax (3–5). In baboons treated with varying doses of streptozocin, AIRmax was highly correlated with β cell mass (4). Moreover, studies from human autologous islet transplantation demonstrate that AIRmax is highly correlated with the numbers of transplanted islets (1, 2, 6–8).
In the current study, relatives with multiple antibodies and impaired insulin secretion have a wide range of AIRmax responses (38 to 250 μU/mL) up to 6.5-fold. Although a limitation of the current study is that there was no healthy normal control group, several independent studies have found remarkably similar values of AIRmax in healthy adults of ∼300 μU/mL (1, 6, 7, 14, 15). When studied in otherwise healthy subjects who underwent hemipancreatectomy and thus have ∼50% of normal β cell mass, AIRmax was markedly impaired at 95 μUml (16). We previously reported that individuals with early, but asymptomatic type 1 diabetes have normal fasting and arginine-stimulated insulin response when normoglycemic, but markedly reduced AIRmax at ∼90 μU/mL (15). Thus, it is likely that the low AIRmax values in some of the subjects in the current study represent markedly impaired functional β cell mass.
It is now accepted that nearly all of those with two or more antibodies will eventually progress to clinical disease (17). This study demonstrates that AIRmax is well tolerated and reproducible in adults. Further work will require longitudinal studies of AIRmax that include children whose rate of progression at each stage of disease is more rapid than adults. Development of a marker that further predicts the rate of progression or that can detect early effects of therapy on disease progression is needed. Because AIRmax is a surrogate measure of β cell mass, whereas FPIR represents functional measure of β cells, using combination of the two measurements could test the hypothesis that the rate of progression to disease in subjects with both low FPIR and low AIRmax is more rapid than those with only low FPIR.
Two recent reports using the data from the network for pancreatic organ donors indicate that β cell mass was preserved in nondiabetic autoantibody-positive subjects (18, 19), but there is controversy as to the interpretation and relevance of these data positing relative intact, but dysfunctional β cells underlying disease progression in contrast to chronic β cell destruction. This controversy emphasizes that relationship of dysfunction to mass and the kinetics of disease progression is not well understood. Our data indicate that both perspectives are correct; whereas function and mass are correlated in our study, in some individuals there is relatively preserved mass and in others it is severely depressed. This heterogeneity is evident in those both with and without multiple antibodies. These studies once again emphasize the need to understand the multiple pathways to T1D to develop targeted therapies. For example, it is possible that those with a severe functional defect, but relatively intact mass, may be more likely to respond to disease-modifying therapy. The tolerability and reproducibility of AIRmax suggest it could be a useful stratification measure in clinical trials to address these questions.
Acknowledgments
The authors thank Kim Varner, Diabetes Clinical Research Program study coordinator, for her outstanding efforts on this project.
Financial Support: This work was supported by National Institutes of Health Grant DP3 DK096744-01. Subjects for this TrialNet Ancillary Study were recruited through the TrialNet TN-01 Pathway to Prevention Study, which is currently supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, and UC4 DK106993, and the Juvenile Diabetes Research Foundation International.
Author Contributions: W.H. researched the data and wrote the manuscript. A.W. and C.B. contributed statistical support and reviewed the manuscript. H.T.B. contributed statistical support. J.P.P. discussed the data and reviewed and edited the manuscript. C.J.G. designed the study and edited the manuscript. W.H. and C.J.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIRmax
- acute insulin response to arginine at hyperglycemia
- DPT-1
- Diabetes Prevention Trial-Type 1
- FPIR
- first-phase insulin response
- IGT
- impaired glucose tolerance
- IVGTT
- intravenous glucose tolerance test
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- T1D
- type 1 diabetes.
References
- 1.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient [published correction appears in Diabetes. 2007;56:2420–2424]? Diabetes. 2007;56(10):2420–2424. [DOI] [PubMed] [Google Scholar]
- 2.Robertson RP, Raymond RH, Lee DS, Calle RA, Ghosh A, Savage PJ, Shankar SS, Vassileva MT, Weir GC, Fryburg DA; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium . Arginine is preferred to glucagon for stimulation testing of β-cell function. Am J Physiol Endocrinol Metab. 2014;307(8):E720–E727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward WK, Wallum BJ, Beard JC, Taborsky GJ Jr, Porte D Jr. Reduction of glycemic potentiation: sensitive indicator of beta-cell loss in partially pancreatectomized dogs. Diabetes. 1988;37(6):723–729. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch DK, Koerker DJ, Kahn SE, Bonner-Weir S, Palmer JP. Correlations of in vivo beta-cell function tests with beta-cell mass and pancreatic insulin content in streptozocin-administered baboons. Diabetes. 1991;40(6):673–679. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch DK, Raghu PK, Johnston C, Klaff LJ, Kahn SE, Beard JC, Ward WK, Benson EA, Koerker DJ, Bergman RN, Palmer JP. Defects in beta-cell function and insulin sensitivity in normoglycemic streptozocin-treated baboons: a model of preclinical insulin-dependent diabetes. J Clin Endocrinol Metab. 1988;67(4):785–792. [DOI] [PubMed] [Google Scholar]
- 6.Robertson RP, Bogachus LD, Oseid E, Parazzoli S, Patti ME, Rickels MR, Schuetz C, Dunn T, Pruett T, Balamurugan AN, Sutherland DE, Beilman G, Bellin MD. Assessment of β-cell mass and α- and β-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes. 2015;64(2):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson RP. Islet transplantation for type 1 diabetes, 2015: what have we learned from alloislet and autoislet successes? Diabetes Care. 2015;38(6):1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47(3):324–330. [DOI] [PubMed] [Google Scholar]
- 9.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, Bingley PJ, Bonifacio E, Palmer JP, Eisenbarth GS, Wolfsdorf J, Skyler JS; TrialNet Natural History Committee Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10(2):97–104. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Trial—Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–1691. [DOI] [PubMed] [Google Scholar]
- 11.Barker JM, McFann K, Harrison LC, Fourlanos S, Krischer J, Cuthbertson D, Chase HP, Eisenbarth GS; DPT-1 Study Group. Pre-type 1 diabetes dysmetabolism: maximal sensitivity achieved with both oral and intravenous glucose tolerance testing. J Pediatr. 2007;150(1):31–36.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2006;29(3):643–649. [DOI] [PubMed] [Google Scholar]
- 13.Koskinen MK, Helminen O, Matomäki J, Aspholm S, Mykkänen J, Mäkinen M, Simell V, Vähä-Mäkilä M, Simell T, Ilonen J, Knip M, Veijola R, Toppari J, Simell O. Reduced β-cell function in early preclinical type 1 diabetes. Eur J Endocrinol. 2016;174(3):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh S, Gudipaty L, De Leon DD, Hadjiliadis D, Kubrak C, Rosenfeld NK, Nyirjesy SC, Peleckis AJ, Malik S, Stefanovski D, Cuchel M, Rubenstein RC, Kelly A, Rickels MR. Reduced β-cell secretory capacity in pancreatic-insufficient, but not pancreatic-sufficient, cystic fibrosis despite normal glucose tolerance. Diabetes. 2017;66(1):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51(4):951–957. [DOI] [PubMed] [Google Scholar]
- 16.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest. 1992;89(6):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diedisheim M, Mallone R, Boitard C, Larger E. β-Cell mass in nondiabetic autoantibody-positive subjects: an analysis based on the Network for Pancreatic Organ Donors Database. J Clin Endocrinol Metab. 2016;101(4):1390–1397. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, Castillo E, Lajevardi Y, Krogvold L, Dahl-Jørgensen K, von Herrath MG. Increase in pancreatic proinsulin and preservation of β-cell mass in autoantibody-positive donors prior to type 1 diabetes onset. Diabetes. 2017;66(5):1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]