Abstract
Context:
Maternal obesity in pregnancy has profound impacts on maternal metabolism and promotes placental nutrient transport, which may contribute to fetal overgrowth in these pregnancies. The fatty acid docosahexaenoic acid (DHA) has bioactive properties that may improve outcomes in obese pregnant women by modulating placental function.
Objective:
To determine the effects of DHA supplementation in obese pregnant women on maternal metabolism and placental function.
Design:
Pregnant women were supplemented with DHA or placebo. Maternal fasting blood was collected at 26 and 36 weeks’ gestation, and placentas were collected at term.
Setting:
Academic health care institution.
Subjects:
Thirty-eight pregnant women with pregravid body mass index ≥30 kg/m2.
Intervention:
DHA (800 mg, algal oil) or placebo (corn/soy oil) daily from 26 weeks to term.
Main Outcomes:
DHA content of maternal erythrocyte and placental membranes, maternal fasting blood glucose, cytokines, metabolic hormones, and circulating lipids were determined. Insulin, mTOR, and inflammatory signaling were assessed in placental homogenates, and nutrient transport capacity was determined in isolated syncytiotrophoblast plasma membranes.
Results:
DHA supplementation increased erythrocyte (P < 0.0001) and placental membrane DHA levels (P < 0.0001) but did not influence maternal inflammatory status, insulin sensitivity, or lipids. DHA supplementation decreased placental inflammation, amino acid transporter expression, and activity (P < 0.01) and increased placental protein expression of fatty acid transporting protein 4 (P < 0.05).
Conclusions:
Maternal DHA supplementation in pregnancy decreases placental inflammation and differentially modulates placental nutrient transport capacity and may mitigate adverse effects of maternal obesity on placental function.
Obese women supplemented with 800 mg/d DHA compared with placebo show changes in placental inflammation and nutrient transport related to DHA status of the placenta.
As populations in developed countries have become progressively heavier, an increasing number of women are obese when they enter pregnancy (1–3). Pregravid obesity is associated with an increased risk of adverse pregnancy complications, including gestational diabetes mellitus (GDM), preeclampsia, delivery by caesarean section, and fetal overgrowth (4, 5). The consequences of pregravid obesity are not limited to pregnancy alone because children of high body mass index (BMI) mothers are at increased risk for metabolic and cardiovascular disease, mental health disorders, and some types of cancer (6–9). Ample evidence suggests that changes in maternal nutrition/metabolism alter fetal development and growth mediated by modulation of placental function (10, 11).
Fetal overgrowth in obese women is associated with increased placental macronutrient transport capacity. Specifically, amino acid, fatty acid, and glucose transporter activity/expression have been reported to be increased in isolated syncytiotrophoblast plasma membranes in obese women (12–14). Obese, pregnant women are characterized by endocrine and metabolic perturbations, including insulin resistance, hyperlipidemia, and systemic inflammation (15–18). Such metabolic perturbations may affect placental function because the placenta expresses multiple cytokine and hormone receptors on the maternally facing microvillous membrane (MVM), which interacts with maternal signals. Recent studies have shown that maternal obesity increases placental inflammation, oxidative stress, and both mechanistic target of rapamycin (mTOR) and signal transducer and activator of transcription (STAT) signaling (12, 16–19). Some of these factors are established regulators of placental macronutrient transport capacity in vitro (20–26).
Given the dramatic increase in the number of pregnancies complicated by obesity, there is an urgent need to develop intervention strategies to break the vicious cycle of intrauterine transmission of metabolic and cardiovascular disease from mother to the next generation. Docosahexaenoic acid (DHA) supplementation during pregnancy reduces circulating and placental inflammatory markers in overweight/obese women (27). In addition, in vitro experiments suggest that DHA has a potential for reducing adverse effects of maternal obesity on the placental function (28–31). For instance, DHA attenuates mTOR signaling and reduces amino acid transport in cultured primary human trophoblast cells (32), which may help mitigate the activation of placental mTOR and amino acid transport in obese women delivering large babies (12).
Multiple studies have investigated the effects of maternal supplementation with DHA/fish oil during pregnancy on maternal and child outcomes (33). However, few studies have explored the effects of DHA supplementation on the placenta in human pregnancy (27, 34–37). Apart from a recent report suggesting alterations in placental fatty acid metabolism in DHA-supplemented mothers (37), the effects of maternal DHA supplementation on placental nutrient transport capacity and cellular signaling in obese women are largely unknown. Therefore, we tested the hypothesis that DHA supplementation in the second half of pregnancy in obese women modulates placental cellular signaling (insulin, inflammation, and mTOR) and nutrient transport capacity.
We determined circulatory, inflammatory, and metabolic markers at enrollment (26 weeks’ gestation) and after 10 weeks of DHA supplementation in a cohort of obese pregnant women. At term delivery, placental membrane DHA levels were assessed and analysis was performed on the relationship between placental DHA levels and cellular signaling and expression and activity of amino acid, glucose, and fatty acid transport proteins.
Methods
Ethics
Informed, written consent was obtained from all participating women after careful explanation of the study. The study protocol was approved by the Institutional Review Board at the University of Texas Health Science Center, San Antonio (IRB HCS20090506H).
Study participants
Obese women (pregravid BMI ≥30 kg/m2; n = 51) were enrolled at 25 to 29 weeks’ gestation (mean, 26.6 weeks) and randomized to either placebo (corn/soy oil) or DHA (800 mg/d) supplementation (DSM Nutritional Products, Heerlen, The Netherlands) for the remainder of their pregnancy. Inclusion and exclusion criteria are presented in Supplemental Table 1 (35.9KB, docx) . Fasting maternal blood samples were collected at enrollment and at 36 weeks’ gestation from 38 women who completed the study. All women were also seen at 30 weeks’ gestation for a compliance check. Placentas were collected from 35 subjects who delivered at term (≥37 weeks’ gestation). Thirteen participants were not included in the maternal circulatory factor analysis (nine women developed GDM, and four women chose to not complete the study), and three participants were not included in the placenta analysis study (one woman delivered preterm, one placenta was not collected at delivery, and one woman declined placenta collection). Hence, the final number of participants was 38 for the maternal blood analysis and 35 for the placental analysis [n = 31 for MVM and basal plasma membrane (BM) parameters, due to membranes not successfully isolated from four placentas]. Data were obtained from the participants’ medical records, including medications taken during pregnancy, medical diagnoses, gestational age at delivery, and weight, length, and head circumference of the neonate.
Measurement of fatty acids
Fatty acid content (expressed as a percentage of total phospholipid fatty acids) was measured in maternal erythrocytes and total placental membrane preparations by gas chromatography/mass spectrometry (GC/MS), using well-established protocols (38). Briefly, samples were saponified with methanolic sodium hydroxide and the fatty acids converted to methyl esters using boron trifluoride in methanol. After addition of saturated sodium chloride solution and hexane, the hexane fraction was transferred into a vial that contained sodium sulfate for drying, then transferred to a second vial for analysis by GC/MS on a Thermo Fisher DSQ using electron impact ionization. Fatty acid identification was made by comparison of the electron ionization mass spectra and GC retention times with those of known standards. Peak areas were determined by manual integration using the DSQ Xcalibur software.
Maternal blood collection and analysis
At enrollment and at 36 weeks’ gestation, fasting maternal venous blood samples were obtained and processed immediately for glucose and lipid analysis by standard clinical assays. In addition, erythrocytes and plasma were collected. Fatty acid composition was measured in erythrocyte membranes. Maternal cytokine and hormone levels [insulin, leptin, total adiponectin, interleukin-6 (IL-6), and tumor necrosis factor α] were determined by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Homeostasis model assessment of insulin resistance was calculated according to the following equation: [insulin (mIU/L) × glucose (mg/dL)] / 405 (39).
Placenta collection
Placentas were obtained immediately after delivery, and dissections were carried out on ice. After removing the decidua basalis and chorionic plate, ∼100 g of villous tissue was rinsed in ice-cold physiological saline. The villous tissue was transferred to ice-cold buffer D (250 mM sucrose, 10 mM Hepes, pH 7.4) containing protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO), then homogenized on ice. The placental homogenates were frozen in liquid nitrogen and stored at −80°C until processed further. Separate aliquots were used for isolation of syncytiotrophoblast plasma membranes, protein expression analysis, and isolation of nuclei.
Isolation of syncytiotrophoblast plasma membranes
The isolation of syncytiotrophoblast BM and MVM was performed according to a previously described protocol (40). All procedures were performed on ice. After an initial isolation of all cellular membranes (used for determining placental membrane fatty acid levels), the MVM was purified by magnesium chloride precipitation, and BM was purified by sucrose gradient centrifugation. Alkaline phosphatase activity was, on average, almost 18-fold greater in isolated MVM vesicles, and ferroportin expression was >30-fold higher in BM vesicles compared with activity/expression in placental homogenates. There were no differences in enrichment in DHA- vs placebo-treated groups.
Western blotting
Western blot was performed as previously described (41). Commercially obtained primary antibodies used are listed in Supplemental Table 2 (35.9KB, docx) . The l-type amino acid transporter (LAT) 1 and LAT2 antibodies were the kind gifts of Dr. Y. Kanai (Osaka University, Japan), and the sodium-dependent neutral amino acid transporter (SNAT) 2 antibody was from Dr. P. D. Prasad (University of Georgia). The SNAT4 antibody was generated by Eurogentec (peptide sequence: YGEVEDELLHAYSKV; Seraing, Belgium).
Nuclear factor–κB p65 and peroxisome proliferator-activated receptor γ activity
Nuclei were isolated from homogenates of placental villous tissue using a nuclear isolation kit (Active Motif, Carlsbad, CA). DNA binding activity of nuclear factor–κB (NF-κB) p65 (20 µg) and peroxisome proliferator-activated receptor γ (PPARγ) (10 µg) was measured in nuclear extracts with transcription factor assay kits (Active Motif, Carlsbad, CA). Each sample was measured in duplicate.
Amino acid and glucose transporter activities
System A and system L amino acid transporter activities in MVM vesicles, as well as glucose transporter activity in BM and MVM vesicles, were assessed using radioisotope-labeled substrates and protocols described in detail previously (12, 13). Each condition was studied in triplicate (amino acid uptakes) or quadruplicate (glucose uptakes) for all the uptake experiments.
Lipase activity
Placental lipase activity in MVM vesicles was measured with a commercial kit (LPL activity assay; Roar Biomedicals, Inc., New York, NY), and 20 μg of MVM vesicles was incubated with the fluorescent substrate solution for 60 minutes according to the manufacturer’s instructions.
Oxidative stress
Placental free radical and hydrogen peroxide levels and total antioxidant capacity were measured by a commercially available kit (Oxiselect; Cell Laboratories Inc., San Diego, CA).
Data presentation and statistics
Differences between DHA-supplemented and placebo control groups were evaluated by t test. Spearman correlation was used to identify associations between all continuous variables. To evaluate relationships between key variables, including DHA status (maternal erythrocyte DHA content, placental membrane DHA content, and treatment assignment), variables were grouped into nine nonredundant major categories (presented in Table 1). For each category the first principal component of the scaled variables was computed to capture the major axis of variation. These first principal components were correlated to each of the key variables and outcomes using the t test for discrete factors (treatment assignment), with Spearman correlation for the continuous outcomes (placental membrane DHA content). We applied the Benjamini-Hochberg false discovery rate adjustment to account for multiple testing. All tests were two-sided, and P values <0.05 and false discovery rate values <0.2 were considered significant. Statistical calculations were performed in R v2.18 (Vienna, Austria) or GraphPad Prism 5 (version 5.04; GraphPad Software, La Jolla, CA).
Table 1.
Correlations Between Placental Membrane DHA Levels and Groups of Placental Factors (Principal Component Analysis)
| Characteristic | Variables Included | Spearman R Value | P Value | FDR |
|---|---|---|---|---|
| Amino acid transport | System A activity, system L activity, SNAT1, SNAT2, SNAT4, LAT1, LAT2 | −0.511 | 0.003 | 0.02 |
| Fatty acid transport | Lipase activity, FAT/CD36, FATP2, FATP4 | 0.432 | 0.02 | 0.05 |
| Fetal factors | Birth weight, head circumference, length, placental weight, ponderal index | −0.077 | 0.66 | 0.74 |
| Glucose transport | Glucose uptake, GLUT1, GLUT9 | 0.257 | 0.16 | 0.24 |
| Inflammation | COX-2, GPR120 (MVM), IκBα, JNK, NF-κB p65 activation, p38α | −0.585 | 0.0002 | 0.002 |
| Insulin signaling | AKT, ERK, FOXO 1/3, GRB2, GSK3α, insulin receptor β, IRS1, PI3K p85 | 0.256 | 0.14 | 0.24 |
| mTOR signaling | 4EBP1, AMPK, mTOR, REDD1, rpS6 | 0.201 | 0.25 | 0.32 |
| Oxidative stress | Free radicals, H2O2, total antioxidant capacity | −0.040 | 0.82 | 0.82 |
| STAT signaling | SOCS3, STAT3, STAT5 | 0.289 | 0.09 | 0.21 |
Abbreviations: 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; AMPK, AMP-activated protein kinase; COX-2, cyclooxygenase 2; ERK, extracellular signal-regulated kinase; FAT, fatty acid translocase; FATP, fatty acid transport protein; FDR, false discovery rate; FOXO 1/3, forkhead box protein 1/3; GLUT, glucose transporter; GRB2, growth factor receptor-binding protein 2; GSK3, glycogen synthase kinase 3; IRS, insulin receptor substrate; PI3K, phosphatidylinositide 3-kinase; REDD1, regulated in development and DNA damage responses 1; rpS6, ribosomal protein S6; SOCS3, suppressor of cytokine signaling 3.
Results
Clinical characteristics
Maternal age, prepregnancy BMI, and erythrocyte DHA levels at enrollment did not differ between the placebo (n = 19) and DHA-supplemented (n = 19) groups (Table 2). After 10 weeks of supplementation, maternal erythrocyte DHA levels were significantly higher in the DHA-supplemented group (P < 0.0001; Table 2). Fetal growth (measured by birth weight, length, ponderal index, and head circumference) did not differ between the placebo and DHA-supplemented groups (Table 2). In both groups, there were more male than female fetuses. Placental weight tended to be heavier in the DHA-supplemented group (P = 0.07; Table 2).
Table 2.
Clinical Characteristics of Study Subjects
| Characteristic | Placebo | DHA Supplemented | P Value |
|---|---|---|---|
| Mother | |||
| n | 19 | 19 | |
| Age (y) | 28.5 ± 1.2 | 27.8 ± 1.2 | 0.72 |
| BMI (kg/m2) | 32.9 ± 1.0 | 31.5 ± 1.1 | 0.35 |
| Ethnicity (Hispanic/not Hispanic), n | 16/3 | 18/1 | 0.60 |
| RBC DHA (%), baseline | 5.8 ± 0.5 | 6.0 ± 0.5 | 0.82 |
| RBC DHA (%), 36 weeks | 6.1 ± 0.5 | 10.0 ± 0.6 | <0.0001 |
| Gestational length (d) | 275.4 ± 2.2 | 277.9 ± 1.7 | 0.37 |
| Delivery mode (caesarean section/vaginal), n | 6/13 | 11/8 | 0.19 |
| Newborn | |||
| n | 19 | 19 | |
| Sex (female/male), n | 6/13 | 6/13 | >0.99 |
| Birth weight (g) | 3503 ± 94 | 3452 ± 94 | 0.70 |
| Length (cm) | 51.1 ± 0.5 | 51.2 ± 0.4 | 0.87 |
| Ponderal index (g × 100/cm3) | 2.62 ± 0.04 | 2.57 ± 0.05 | 0.46 |
| Head circumference (cm) | 34.7 ± 0.2 | 34.6 ± 0.3 | 0.69 |
| Placenta | |||
| n | 17 | 18 | |
| Weight (g) | 708 ± 26 | 791 ± 35 | 0.07 |
| Membrane DHA (%) | 6.8 ± 0.5 | 9.8 ± 0.9 | 0.006 |
| Membrane AA (%) | 38.4 ± 1.0 | 34.9 ± 1.0 | 0.02 |
| Fetal/placental weight ratio | 5.1 ± 0.2 | 4.5 ± 0.1 | 0.01 |
Data are presented as mean ± standard error of the mean unless otherwise noted. Differences between groups were evaluated by t test or Fisher’s exact test.
Abbreviations: AA, arachidonic acid; RBC, red blood cell.
Maternal circulatory factors
Maternal circulating levels of adiponectin, glucose, IL-6, insulin, leptin, and tumor necrosis factor α did not differ between the two groups either at the time of enrollment or after 10 weeks of supplementation (Supplemental Table 3 (35.9KB, docx) ). Similarly, the homeostasis model assessment of insulin resistance index was not affected by DHA supplementation (Supplemental Table 3 (35.9KB, docx) ). At enrollment, maternal levels of cholesterol, high-density lipoprotein, and low-density lipoprotein were similar between the two groups. However, triglyceride and very low-density lipoprotein levels at the start of the study were significantly higher in the DHA-supplemented group (P < 0.05; Supplemental Table 3 (35.9KB, docx) ). This was not anticipated and is likely due to small sample sizes. Importantly, after 10 weeks of supplementation, there was no major difference in any of the maternal lipid levels between the two groups (Supplemental Table 3 (35.9KB, docx) ).
Placental membrane DHA
Placental membrane DHA levels were significantly higher in the DHA-supplemented group compared with the placebo group (P < 0.01; Table 2); placental membrane DHA levels correlated with maternal erythrocyte DHA levels (P < 0.05; Fig. 1). Analysis of the outcomes by treatment group (t test, DHA- vs placebo-supplemented women) indicated no statistically significant differences in placental signaling or transport between the two groups (data not shown).
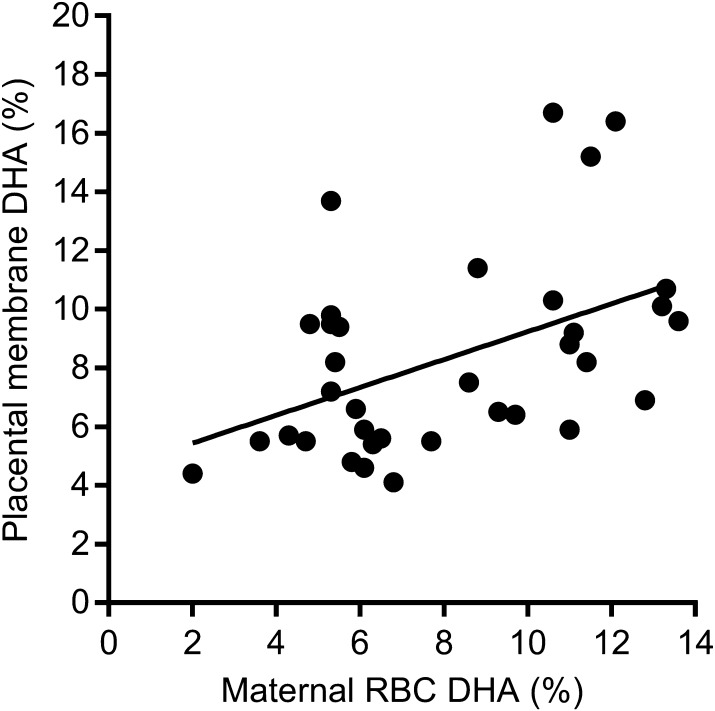
Figure 1.
Placental membrane DHA level correlates with maternal red blood cell (RBC) DHA levels. Maternal RBC DHA levels were measured at 36 weeks’ gestation and placental membrane DHA levels at term. Spearman r = 0.372, P < 0.05, best-fit line, n = 35.
DHA status is dependent on several factors, including initial levels, compliance with study protocol in taking supplements, diet, genetics, and other variations in lipid metabolism. Therefore, we found large variation in the DHA levels in both the placebo and supplemented groups at 36 weeks. It has been suggested that it is more appropriate to report outcomes of nutritional supplementation studies based on acquired nutritional status (i.e., DHA levels at study completion) rather than by group assignment (42). Therefore, in our secondary analysis, fetal growth, placental cellular signaling, and transport capacity were analyzed by correlation with the continuous variable placental DHA levels at delivery, irrespective of supplementation group assignment.
Fetal growth
Birth weight, length, ponderal index, and head circumference did not correlate with placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ). Principal component analysis (PCA) confirmed the lack of association between fetal growth parameters and DHA levels in the placental membranes (Table 1).
Inflammatory signals
The PCA indicated an inverse association between placental inflammation and DHA levels in the placental membranes (P < 0.001, Table 1). The inflammatory signaling pathways in placental homogenates included expression of IκBα, which was inversely correlated (P < 0.01; Table 3), and NF-κB p65 DNA binding activity, which tended to be positively correlated (P = 0.055; Table 3) with placental membrane DHA levels. Furthermore, there was a trend toward an inverse correlation between placental membrane DHA levels and p38α mitogen-activated protein kinase (MAPK) phosphorylation (P = 0.059; Table 3) and a significant inverse correlation with total p38 MAPK expression (P < 0.05; Table 3). The expression of cyclooxygenase 2 correlated positively with placental membrane DHA levels (P < 0.01; Table 3). There was no significant association between placental membrane DHA levels and JNK expression or phosphorylation, or MVM expression of GPR120 (Supplemental Table 4 (35.9KB, docx) ).
Table 3.
Correlations Between Placental Membrane DHA Levels and Placental Cellular Signaling and Nutrient Transport Capacity
| Characteristic | Spearman R Value | P Value | FDR |
|---|---|---|---|
| Amino acid transporter expression | |||
| LAT1 (MVM) | −0.636 | 0.0001 | 0.01 |
| Inflammation | |||
| IκBα | −0.488 | 0.003 | 0.04 |
| COX-2 | 0.463 | 0.01 | 0.05 |
| p38α (total) | −0.389 | 0.02 | 0.10 |
| NF-κB p65 activation | 0.328 | 0.05 | 0.17 |
| p38α (phospho; T180/Y182) | −0.322 | 0.06 | 0.17 |
| Insulin signaling | |||
| IRS1 (total) | −0.404 | 0.02 | 0.10 |
| GRB2 | −0.385 | 0.02 | 0.10 |
| AMPK (total) | −0.359 | 0.03 | 0.13 |
| ERK (total) | 0.342 | 0.04 | 0.16 |
| AKT (total) | 0.310 | 0.07 | 0.19 |
| STAT signaling | |||
| STAT5 (total) | −0.525 | 0.001 | 0.02 |
| mTOR signaling | |||
| mTOR (total) | −0.403 | 0.02 | 0.10 |
Abbreviations: AMPK, AMP-activated protein kinase; COX-2, cyclooxygenase 2; ERK, extracellular signal-regulated kinase; FDR, false discovery rate; IRS1, insulin receptor substrate 1.
Insulin signaling
Insulin signaling has been implicated as a key regulator of amino acid transport activity in trophoblast cells. To assess insulin signaling, we studied the protein expression of several insulin pathway intermediates. Expression of insulin receptor substrate 1 (P < 0.05; Table 3) and growth factor receptor-binding protein 2 (GRB2) (P < 0.05; Table 3) was inversely correlated with placental membrane DHA levels. There was a positive correlation between extracellular signal-regulated kinase 1/2 expression and placental membrane DHA levels (P < 0.05; Table 3), and AKT expression tended toward a positive association with placental membrane DHA levels (P = 0.07; Table 3). AMP-activated protein kinase expression was negatively associated with placental membrane DHA levels (P < 0.05; Table 3). Phosphorylation of AMP-activated protein kinase, AKT, extracellular signal-regulated kinase 1/2, forkhead box protein 1/3, glycogen synthase kinase 3, and insulin receptor substrate 1 and expression of glycogen synthase kinase and phosphatidylinositide 3-kinase p85 were not related to placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ). Expression of insulin receptor β in isolated MVM and BM vesicles did not correlate with placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ). PCA indicated a lack of association between insulin signaling and placental membrane DHA levels (Table 1).
mTOR signaling
There was an inverse correlation between mTOR expression and membrane DHA levels in the placentas (P < 0.05; Table 3). Components of the mTOR signaling pathway (eukaryotic translation initiation factor 4E-binding protein 1, regulated in development and DNA damage responses 1, and ribosomal protein S6) showed no association between expression/phosphorylation and placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ). No association between mTOR signaling and placental membrane DHA levels was found with PCA (Table 1).
Oxidative stress
Indicators of oxidative stress (free radicals and hydrogen peroxide levels) and total antioxidant capacity were not associated with placental membrane DHA levels (Table 1 and Supplemental Table 4 (35.9KB, docx) ).
PPARγ activity
PPARγ DNA binding activity did not correlate with placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ).
STAT signaling
STAT5 expression in placental homogenates correlated inversely with placental membrane DHA levels (P < 0.01; Table 3). There was no association between DHA levels in the placental membranes and expression of suppressor of cytokine signaling 3, phosphorylation of STAT3 or STAT5, or total STAT3 (Supplemental Table 4 (35.9KB, docx) ). The PCA analysis revealed no association between STAT signaling and placental membrane DHA levels (Table 1).
Amino acid transport
The PCA suggested an inverse association between placental amino acid transport capacity and placental membrane DHA levels (P = 0.003, Table 1). System A amino acid transporter activity in the isolated MVM inversely correlated with placental membrane DHA levels [P < 0.05; Fig. 2(a)]. Similarly, MVM system L activity [P < 0.001; Fig. 2(b)] and the MVM expression of the system L transporter LAT1 (P < 0.0001; Table 3) were also inversely correlated with placental membrane DHA levels. MVM expression of the system A transporters SNAT1, SNAT2, and SNAT4, as well as BM expression of the system L transporter LAT2, did not correlate with placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ).
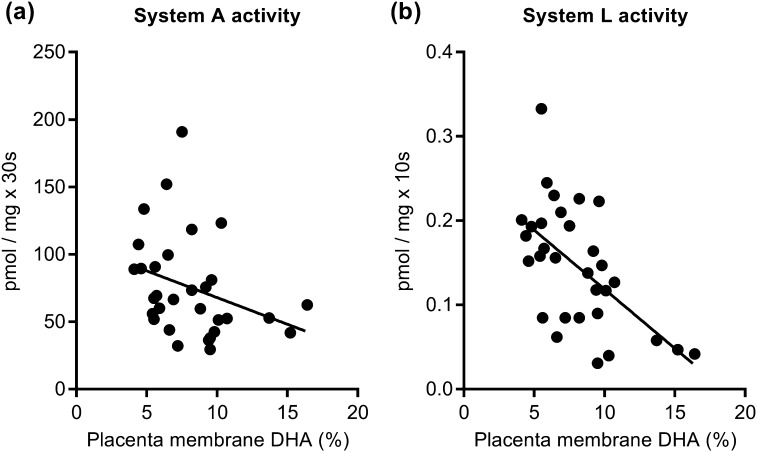
Figure 2.
Higher placental DHA levels are associated with reduced placental amino acid transport. In isolated MVM vesicles, (a) system A (R = −0.413, P < 0.05, false discovery rate < 0.099) and (b) system L (R = −0.576, P < 0.001, false discovery rate < 0.02) amino acid transport capacities were inversely correlated with placental membrane DHA levels. Spearman, best-fit line, n = 31.
Fatty acid transport
Placental capacity to transport fatty acids correlated with placental membrane DHA levels (PCA, P = 0.02, Table 1). MVM expression of fatty acid transport protein (FATP) 4 [P < 0.01; Fig. 3(a)] and BM FATP4 expression [P = 0.059; Fig. 3(b)] correlated positively with placental membrane DHA levels. Expression of CD36/fatty acid translocase, FATP2, and lipase activity were, however, not related to placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ).
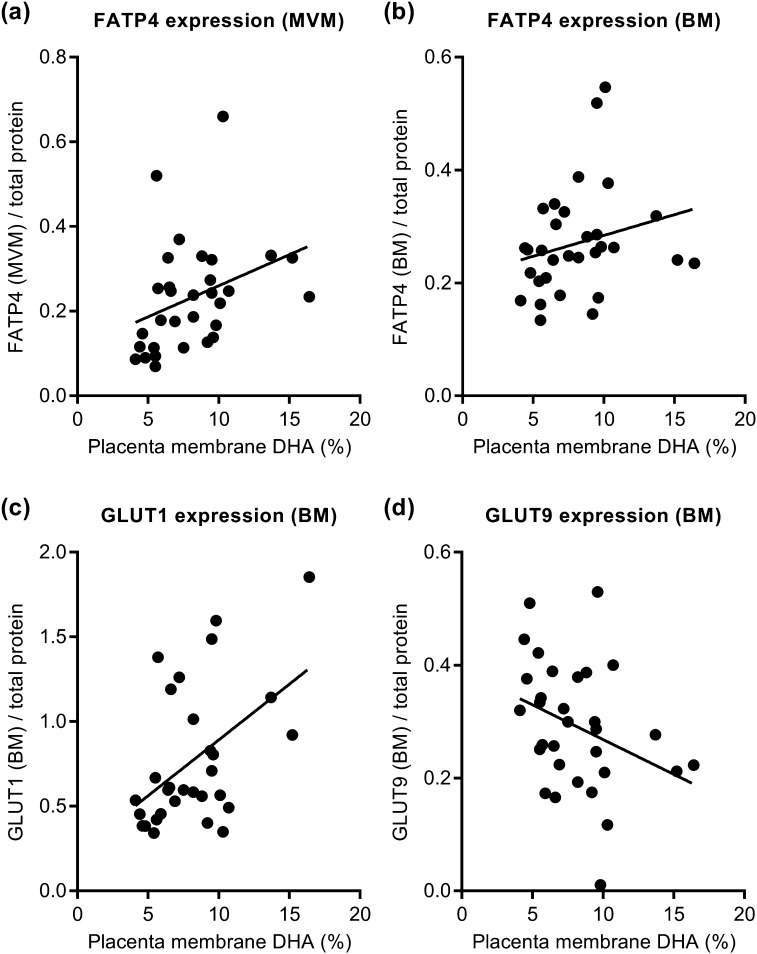
Figure 3.
Placental membrane DHA levels and fatty acid and glucose transporter expression. Expression of FATP4 in isolated (a) MVM vesicles [R = 0.488, P < 0.01, false discovery rate (FDR) < 0.05] and (b) BM vesicles (R = 0.343, P = 0.059, FDR < 0.18) correlated positively with placental membrane DHA levels. Expression of glucose transporter (c) GLUT1 (R = 0.434, P < 0.05, FDR < 0.096) and (d) GLUT9 (R = −0.396, P < 0.05, FDR < 0.11) in isolated BM vesicles correlated with placental membrane DHA levels. Spearman, best-fit line, n = 31.
Glucose transport
In the BM, expression of glucose transporter (GLUT) 1 correlated positively with placental membrane DHA levels [P < 0.05; Fig. 3(c)], whereas GLUT9 BM expression was inversely correlated [P < 0.05; Fig. 3(d)]. MVM expression of GLUT1 and GLUT9, as well as glucose uptake in either plasma membrane, was not related to placental membrane DHA levels (Supplemental Table 4 (35.9KB, docx) ). The PCA analysis suggests no association between placental glucose transport capacity and placental membrane DHA levels (Table 1).
Discussion
To our knowledge, this is the first detailed report on the effects of DHA supplementation in obese pregnant women on placental functions such as amino acid, glucose, and fatty acid transport capacity. We demonstrate that increasing maternal DHA results in higher placental membrane DHA content. Moreover, the increase in placental membrane DHA was associated with a reduction in placental amino acid transport and inflammatory markers, along with an increase in fatty acid transport capacity. We propose that DHA supplementation in obese women with low DHA intake may mitigate some of the adverse effects of obesity on placental function by decreasing placental inflammation and modulating the composition of nutrients transported to the fetus. Furthermore, some of these effects may represent direct cause-and-effect relationships because we have previously reported similar responses to physiological levels of DHA in cultured human trophoblast cells (32).
As expected, DHA supplementation resulted in higher maternal erythrocyte DHA levels. Obesity in pregnancy without diagnosis of GDM has been suggested to result in a metabolic profile of chronic inflammation, insulin resistance, and hyperlipidemia (43). The ω-3 long-chain polyunsaturated fatty acids have been reported as beneficial for metabolic syndrome disorders such as dyslipidemia and inflammation (44). However, in general agreement with a previous study (27), DHA supplementation in the second half of pregnancy did not affect maternal circulating inflammatory or metabolic markers or insulin sensitivity. At enrollment, triglyceride and very-low-density lipoprotein levels were significantly higher in the group assigned to DHA supplementation. This difference was inadvertent and likely due to the relatively low number of women enrolled in this study. Importantly, however, these differences had disappeared following 10 weeks of DHA supplementation, suggesting that DHA, if anything, may improve maternal lipid profiles.
With the lack of significant change in maternal metabolic parameters, it was not surprising that a groupwise comparison of placental variables resulted in no significant changes. Maternal DHA supplementation significantly increased placental membrane DHA levels, confirming previously reported findings (34, 35). Together, these observations strongly suggest that maternal intake of DHA is reflected in the placental membrane DHA content. This is further supported by the positive correlation between maternal and placental DHA levels. Analyzing cellular signaling and functional characteristics based on the placental membrane DHA content is important because DHA status depends on not only DHA intake but also other factors, such as diet and genetic variation related to lipid metabolism (45, 46). Supplementation studies, such as ours, are further complicated by variable compliance and potentially modified dietary intake in both the placebo and supplemented groups. Indeed, the overall outcome of our study at 36 weeks was a continuum of maternal erythrocyte DHA levels rather than a clear distinction between the two groups (as shown in Fig. 1). Therefore, as in a previous study correlating Na+K+ATPase activity to cell membrane DHA levels (47), we conducted correlation analyses to determine the impact of variation in placental DHA levels on functional transport capacity and cellular signaling. This study provides a comprehensive evaluation of placental cellular signaling and nutrient transport capacity in a cohort of obese women with a variation in placental DHA content.
In this study, placental membrane DHA content was measured in samples containing a pool of all membranes, rather than in the isolated syncytiotrophoblast cellular membranes. This approach was selected due to the low yield of purified BM, which restricted what studies could be performed. Another limitation in the current study is that the effect of DHA supplementation on fetal endothelial cells has not been specifically addressed. Further studies are required to delineate the effects of maternal DHA supplementation on endothelial cell function, fetal adiposity, and net transport of DHA and other nutrients to the fetus.
Maternal obesity during pregnancy is associated with increased placental inflammation (16–18) such as increased p38 MAPK phosphorylation (18). With increased DHA content, we found reduced p38 MAPK phosphorylation, indicating that DHA mitigates obesity-related placental inflammation. This observation may represent a cause-and-effect relationship because we have previously demonstrated that DHA reduces p38 MAPK phosphorylation in vitro using cultured primary trophoblast (32). We also found a nonsignificant trend toward increased NF-κB nuclear translocation, reduced IκBα expression, and increased cyclooxygenase 2 expression with higher DHA levels, suggesting that not all inflammatory pathways are improved with increasing placental DHA content.
Maternal obesity is linked with impairment in placental insulin signaling (48). In the current study, activation of the insulin signaling pathway (measured by phosphorylation of pathway intermediaries) was not significantly correlated with placental DHA levels. However, total expressions of critical signaling nodes were modified in association to changes in placental DHA. What the consequences are of these altered expression levels for overall cellular insulin signaling remain to be established. In myotubes, decreased expression of GRB2 increases insulin sensitivity (49). This suggests that the lower GRB2 expression associated with higher placental DHA levels may improve placental insulin sensitivity.
DHA levels were not associated with alterations in phosphorylation of placental STAT3 or downstream functional readouts of mTOR (eukaryotic translation initiation factor 4E-binding protein 1, ribosomal protein S6). Total mTOR expression was negatively correlated with placental DHA content. In a recent study, we demonstrated that DHA reduces signaling through mTOR and STAT3 pathways in cultured primary human trophoblast cells (32). mTOR and STAT3 are known positive regulators of placental amino acid transport (20, 24, 25). However, the lack significant change in mTOR and STAT3 in response to increased placental DHA content in this study suggests other cellular signaling systems may link DHA and placental amino acid transport in vivo. It should also be noted that placental mTOR (but not STAT3) signaling is reduced in response to labor (41). Therefore, we cannot exclude that any effects of DHA may have been obscured in this cohort where a majority of deliveries were vaginal.
A concern of DHA supplementation in pregnancy is that it may cause increased oxidative stress (31). In vitro placental models have suggested exposure to high levels of DHA increases cellular oxidative stress (31, 50). Because maternal obesity has been associated with elevated oxidative stress in the placenta (19), further exaggerating this condition could have detrimental effects on placental function. However, we did not find any evidence of increased oxidative stress in response to DHA supplementation, suggesting that increasing placental DHA levels do not aggravate placental oxidative stress in pregnancies of obese women.
Placental amino acid transporter activity is closely associated with alterations in fetal growth (11); for instance, in the syncytiotrophoblast MVM system A, amino acid transport activity correlates positively with birth weight, and SNAT2 expression is positively associated with maternal BMI (12). We have previously demonstrated that a diverse array of maternal metabolic parameters associated with obesity (insulin, leptin, IL-6, hyperlipidemia) can activate amino acid transporter system A in cultured primary human trophoblast cells (20, 22, 51). In the MVM isolated from placentas in our study cohort, amino acid transport was reduced in association with increasing placental DHA levels. We have previously shown that DHA treatment reduces amino acid transport in cultured primary human trophoblast cells (32). If enhanced transport of amino acids contributes to fetal overgrowth in pregnancies complicated by maternal obesity, increasing placental DHA levels represents a potential mechanism to prevent overgrowth. Fetal overgrowth is linked to adverse medical complications at birth, including operative delivery, stillbirth (52), and long-term metabolic disease in the offspring (6, 7). It is important to note that birth weights were not affected in the study subjects. However, due to the small sample size, we could not address the frequency of large for gestational age infants. Although the sample is small, the extensive characterization of the placentas accomplished in this study would not have been possible in a larger cohort. These data suggest that the impact of DHA on amino acid transport capacity in women of varying BMIs should be carefully evaluated in a larger cohort of both normal-weight and obese mothers.
Contrary to amino acid transport, placental capacity to transfer fatty acids was increased with elevated placental DHA levels. In support of our finding, DHA has been reported to stimulate fatty acid uptake in BeWo choriocarcinoma cells (53). Transfer of fatty acids across the placental barrier is a complex process, involving multiple steps. It has previously been shown that FATP4 messenger RNA expression correlates positively with placental phospholipid DHA levels and that the FATP4 may facilitate ω-3 fatty acid uptake (34). In general agreement with this report, we demonstrate a positive correlation between placental DHA concentrations and FATP4 protein expression in the plasma membranes of the syncytiotrophoblast.
Glucose transfer across the BM of the syncytiotrophoblast is believed to be the rate-limiting step for transplacental glucose transport, and GLUT1 is likely the major glucose transporter at term in human placenta (54). BM expression of GLUT1 was positively associated with placental DHA content. The higher GLUT1 expression with increasing placental DHA levels implies that glucose delivery to the fetus may be enhanced in response to increased DHA levels, but we could not demonstrate an increased activity in the isolated BM vesicles. Currently, preferred substrates for GLUT9 are in debate. Transport capacity for glucose, fructose, and urate has been described (55). Recently, the ability of GLUT9 to transport glucose has been challenged (56). Further studies are needed to elucidate the importance and function of GLUT9 in human placenta.
In conclusion, maternal DHA supplementation in pregnancies complicated by obesity did not modulate maternal circulatory markers of inflammation or insulin sensitivity. Maternal DHA supplementation increased placental membrane DHA levels, which was associated with modulation of placental nutrient transport capacity and decreased placental inflammatory markers. Increasing placental DHA may be beneficial in obese pregnancies by attenuating excessive placental amino acid transport, which otherwise contributes to fetal overgrowth. In contrast, increased placental DHA was associated with upregulation of fatty acid transporters that are believed to be responsible for delivering critically needed ω-3 fatty acids to the fetus. We speculate that maternal DHA supplementation in obese pregnant women with low DHA intake may mitigate some of the adverse effect of obesity on placental function.
Acknowledgments
The authors are grateful to patients for participation in this study; the staff at Labor & Delivery, University Hospital, San Antonio, Texas; and E. Dudley for outstanding technical assistance. The GC/MS analyses were conducted in the University of Texas Health Science Center, San Antonio, Institutional Mass Spectrometry Laboratory.
Financial Support: This study was supported by grants from National Institutes of Health R21HL093532 (to D.A.K. and T.L.P), 8UL1TR000149, UL1 TR001120, and the Mike Hogg Fund (to T.L.P.), the Swedish Research Council (to S.L.), and the Swedish Society of Endocrinology (to S.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov no. NCT00865683 (registered 19 March 2009).
Author Contributions: S.L., V.I.R., D.A.K., and T.L.P. designed the research; S.L., V.I.R., O.A., C.M., E.M., F.G., F.J.R., and K.H. conducted the research; S.L., V.I.R., and S.T.W. analyzed the data; J.A.L.G. and S.L. performed the statistical analysis; S.L., D.A.K., and T.L.P. wrote the paper; and T.L.P. had primary responsibility for final content. All authors read and approved the final manuscript.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BM
- basal plasma membrane
- BMI
- body mass index
- DHA
- docosahexaenoic acid
- FATP
- fatty acid transport protein
- GC/MS
- gas chromatography/mass spectrometry
- GDM
- gestational diabetes mellitus
- GLUT
- glucose transporter
- GRB2
- growth factor receptor-binding protein 2
- IL-6
- interleukin-6
- LAT
- l-type amino acid transporter
- MAPK
- mitogen-activated protein kinase
- mTOR
- mechanistic target of rapamycin
- MVM
- microvillous membrane
- NF-κB
- nuclear factor–κB
- PCA
- principal component analysis
- PPARγ
- peroxisome proliferator-activated receptor γ
- SNAT
- sodium-dependent neutral amino acid transporter
- STAT
- signal transducer and activator of transcription.
References
- 1.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes. 2009;34(3):420–428. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre HD, Gibbons KS, Flenady VJ, Callaway LK. Overweight and obesity in Australian mothers: epidemic or endemic? Med J Aust. 2012;196(3):184–188. [DOI] [PubMed] [Google Scholar]
- 3.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56(6):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133. [DOI] [PubMed] [Google Scholar]
- 5.Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004–2011. BJOG. 2013;120(8):932–939. [DOI] [PubMed] [Google Scholar]
- 6.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–e36. [DOI] [PubMed] [Google Scholar]
- 8.Newman L, Judd F, Olsson CA, Castle D, Bousman C, Sheehan P, Pantelis C, Craig JM, Komiti A, Everall I. Early origins of mental disorder—risk factors in the perinatal and infant period. BMC Psychiatry. 2016;16(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nat Rev Cancer. 2012;12(7):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis. 2012;4(2):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol. 2015;212(2):227.e1–e7. [DOI] [PubMed] [Google Scholar]
- 14.Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta. 2016;40:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231–4237. [DOI] [PubMed] [Google Scholar]
- 16.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–254. [DOI] [PubMed] [Google Scholar]
- 17.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aye ILMH, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, Powell TL. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva K, Barker G, Riley C, Bailey MJ, Permezel M, Rice GE, Lappas M. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol. 2012;48(2):139–149. [DOI] [PubMed] [Google Scholar]
- 20.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297(5):C1228–C1235. [DOI] [PubMed] [Google Scholar]
- 21.Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells. Diabetes. 2010;59(5):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lager S, Gaccioli F, Ramirez VI, Jones HN, Jansson T, Powell TL. Oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by toll-like receptor 4. J Lipid Res. 2012;54(3):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lager S, Jansson N, Olsson AL, Wennergren M, Jansson T, Powell TL. Effect of IL-6 and TNF-α on fatty acid uptake in cultured human primary trophoblast cells. Placenta. 2011;32(2):121–127. [DOI] [PubMed] [Google Scholar]
- 24.von Versen-Höynck F, Rajakumar A, Parrott MS, Powers RW. Leptin affects system A amino acid transport activity in the human placenta: evidence for STAT3 dependent mechanisms. Placenta. 2009;30(4):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol. 2008;296(1):C142–C150. [DOI] [PubMed] [Google Scholar]
- 26.Baumann MU, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2007;293(1):C477–C485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haghiac M, Yang XH, Presley L, Smith S, Dettelback S, Minium J, Belury MA, Catalano PM, Hauguel-de Mouzon S. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: a randomized double-blind controlled clinical trial. PLoS One. 2015;10(9):e0137309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species–dependent manner. J Biol Chem. 2009;284(40):27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TM, Hsieh SC, Chen JW, Chiang AN. Docosahexaenoic acid and eicosapentaenoic acid reduce C-reactive protein expression and STAT3 activation in IL-6-treated HepG2 cells. Mol Cell Biochem. 2013;377(1–2):97–106. [DOI] [PubMed] [Google Scholar]
- 31.Stark MJ, Clifton VL, Hodyl NA. Differential effects of docosahexaenoic acid on preterm and term placental pro-oxidant/antioxidant balance. Reproduction. 2013;146(3):243–251. [DOI] [PubMed] [Google Scholar]
- 32.Lager S, Jansson T, Powell TL. Differential regulation of placental amino acid transport by saturated and unsaturated fatty acids. Am J Physiol Cell Physiol. 2014;307(8):C738–C744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larqué E, Gil-Sánchez A, Prieto-Sánchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr. 2012;107(Suppl 2):S77–S84. [DOI] [PubMed] [Google Scholar]
- 34.Larqué E, Krauss-Etschmann S, Campoy C, Hartl D, Linde J, Klingler M, Demmelmair H, Caño A, Gil A, Bondy B, Koletzko B. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am J Clin Nutr. 2006;84(4):853–861. [DOI] [PubMed] [Google Scholar]
- 35.Klingler M, Blaschitz A, Campoy C, Caño A, Molloy AM, Scott JM, Dohr G, Demmelmair H, Koletzko B, Desoye G. The effect of docosahexaenoic acid and folic acid supplementation on placental apoptosis and proliferation. Br J Nutr. 2007;96(1):182–190. [DOI] [PubMed] [Google Scholar]
- 36.Keelan JA, Mas E, D’Vaz N, Dunstan JA, Li S, Barden AE, Mark PJ, Waddell BJ, Prescott SL, Mori TA. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction. 2014;149(2):171–178. [DOI] [PubMed] [Google Scholar]
- 37.Calabuig-Navarro V, Puchowicz M, Glazebrook P, Haghiac M, Minium J, Catalano P, Hauguel deMouzon S, O’Tierney-Ginn P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr. 2016;103(4):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38(3):514–515. [Google Scholar]
- 39.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24(9):1602–1607. [DOI] [PubMed] [Google Scholar]
- 40.Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990;1029(2):218–226. [DOI] [PubMed] [Google Scholar]
- 41.Lager S, Aye ILMH, Gaccioli F, Ramirez VI, Jansson T, Powell TL. Labor inhibits placental mechanistic target of rapamycin complex 1 signaling. Placenta. 2014;35(12):1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galli C, Brenna JT. Omega-3 fatty acid supplementation and cardiovascular disease events. JAMA. 2013;309(1):27–29. [DOI] [PubMed] [Google Scholar]
- 43.Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthén L, Powell TL, Jansson T. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87(6):1743–1749. [DOI] [PubMed] [Google Scholar]
- 44.Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res. 2011;50(4):372–387. [DOI] [PubMed] [Google Scholar]
- 45.Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138(11):2222–2228. [DOI] [PubMed] [Google Scholar]
- 46.Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2010;93(1):211–219. [DOI] [PubMed] [Google Scholar]
- 47.Turner N, Else PL, Hulbert AJ. Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: implications for disease states and metabolism. Naturwissenschaften. 2003;90(11):521–523. [DOI] [PubMed] [Google Scholar]
- 48.Colomiere M, Permezel M, Riley C, Desoye G, Lappas M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol. 2009;160(4):567–578. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Liu M, Zhang J, Bai X, Ramos F, Van Remmen H, Richardson A, Liu FY, Dong LQ, Liu F. Downregulation of Grb2 contributes to the insulin-sensitizing effect of calorie restriction. Am J Physiol Endocrinol Metab. 2009;296(5):E1067–E1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoji H, Franke C, Demmelmair H, Koletzko B. Effect of docosahexaenoic acid on oxidative stress in placental trophoblast cells. Early Hum Dev. 2009;85(7):433–437. [DOI] [PubMed] [Google Scholar]
- 51.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88(3):1205–1211. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Decker A, Platt RW, Kramer MS. How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol. 2008;198(5):517.e1–e6. [DOI] [PubMed] [Google Scholar]
- 53.Johnsen GM, Weedon-Fekjaer MS, Tobin KA, Staff AC, Duttaroy AK. Long-chain polyunsaturated fatty acids stimulate cellular fatty acid uptake in human placental choriocarcinoma (BeWo) cells. Placenta. 2009;30(12):1037–1044. [DOI] [PubMed] [Google Scholar]
- 54.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77(6):1554–1562. [DOI] [PubMed] [Google Scholar]
- 55.Doblado M, Moley KH. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metab. 2009;297(4):E831–E835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebert K, Ludwig M, Geillinger KE, Schoberth GC, Essenwanger J, Stolz J, Daniel H, Witt H. Reassessment of GLUT7 and GLUT9 as putative fructose and glucose transporters. J Membr Biol. 2017;250(2):171–182. [DOI] [PubMed] [Google Scholar]