Abstract
Both type 1 and type 2 diabetes adversely affect the microvasculature in multiple organs. Our understanding of the genesis of this injury and of potential interventions to prevent, limit, or reverse injury/dysfunction is continuously evolving. This statement reviews biochemical/cellular pathways involved in facilitating and abrogating microvascular injury. The statement summarizes the types of injury/dysfunction that occur in the three classical diabetes microvascular target tissues, the eye, the kidney, and the peripheral nervous system; the statement also reviews information on the effects of diabetes and insulin resistance on the microvasculature of skin, brain, adipose tissue, and cardiac and skeletal muscle. Despite extensive and intensive research, it is disappointing that microvascular complications of diabetes continue to compromise the quantity and quality of life for patients with diabetes. Hopefully, by understanding and building on current research findings, we will discover new approaches for prevention and treatment that will be effective for future generations.
This scientific statement reviews and discusses the microvascular complications of diabetes on an organ-by-organ basis.
The cellular elements of the microvasculature appear to be particularly sensitive to injury from sustained hyperglycemia. This injury (and responses by the body directed toward its repair) cause tissue/organ dysfunction that affects the quality and duration of life for persons with either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). Despite the disparate pathogenesis of these two common forms of diabetes, they (along with secondary forms of diabetes resulting from genetic mutations or pharmaceutical or surgical interventions) all share microvascular injury/dysfunction as a chronic outcome. This scientific statement provides an up-to-date overview of the general pathogenesis of microvascular disease in diabetes, as well as its impact on specific tissues. As such, this statement provides readers with a clear understanding of how microvascular injury adversely affects the normal physiologic function of multiple tissues within the body. This statement does not attempt to provide a compendium of all of the organ-specific treatments for limiting microvascular damage that are in use or in development. Nor do we attempt to review/critique the more general systemic approaches to treatment designed to control glycemia, blood pressure (BP), lipids, or oxidative stress.
At the outset, we are reminded that the very diagnosis of diabetes rests on identifying the level of blood glucose that associates with microvascular injury to the eye. In addition, much of the impetus for developing effective glycemic therapy arises from clinical trials that demonstrate that improved glycemic control decreases the incidence and progression of microvascular injury.
The body’s microvasculature is a diffuse target whose properties differ considerably between different tissues and organs. The response of the microvasculature to injury/repair likewise differs across tissues and organs. For this reason we chose to use an organ-based organizational structure for this scientific statement. However, although we discuss the microvascular complications of diabetes on an organ-by-organ basis, we recognize that in the individual patient all organs are affected simultaneously to a greater or lesser degree (i.e., evident microvascular dysfunction found in one organ is a sentinel of systemic injury, which may be preclinical).
Biochemical Pathways of Microvascular Injury
Introduction
Vascular complications are the major cause of morbidity and mortality in diabetic patients. These result from interactions between systemic metabolic abnormalities, such as hyperglycemia, dyslipidemia, genetic and epigenetic modulators, and local tissue responses to toxic metabolites. Macrovascular complications involve atherosclerotic/thrombotic obstructions, such as those that occur in coronary, cerebral, and peripheral artery diseases. Classic microvascular pathologies include retinopathy, nephropathy, and neuropathy, but brain, myocardium, skin, and other tissues are also affected. In this work, we focus on cellular/molecular mechanisms causing diabetic microvascular pathologies.
Hyperglycemia is the major systemic risk factor for diabetic microvascular complications. The Diabetes Control and Complications Trial (DCCT) in T1DM and the United Kingdom Prospective Diabetes Study (UKPDS) in T2DM clearly demonstrated that intensive blood glucose control delays the onset and retards the progression of diabetic microvascular complications (1, 2).
Hyperglycemia alone, however, is not sufficient to trigger generalized diabetic microvascular pathologies (e.g., only 20% to 40% of diabetic patients will ultimately develop chronic renal failure), suggesting that as yet unidentified genetic or other endogenous protective factors play important roles (3, 4). The Joslin Diabetes Center 50-Year Medalist Study of patients surviving >50 years with T1DM has shown that 30% to 35% are without significant microvascular complications, regardless of their hemoglobin A1c (HbA1c) levels and other classical risk factors thought to predict diabetic vascular complications (3). These patients may possess endogenous tissue factors that diminish the adverse microvascular effects of hyperglycemia.
Research has suggested that multiple biochemical pathways link the adverse effects of hyperglycemia with vascular complications. Cellular mechanisms include the following: nonenzymatic glycation and the formation of advanced glycation end products (AGEs); enhanced reactive oxygen production and actions; endoplasmic reticulum (ER) stress; and the activation of the polyol pathway, the diacylglycerol (DAG)–protein kinase C (PKC) pathway (5), Src homology-2 domain-containing phosphatase-1 (SHP-1), and the renin-angiotensin system (RAS) and kallikrein-bradykinin (BK) systems. It is likely that hyperglycemia-induced intracellular and extracellular changes alter signal transduction pathways, thus affecting gene expression and protein function and causing cellular dysfunction and damage.
Molecular mechanisms of injury
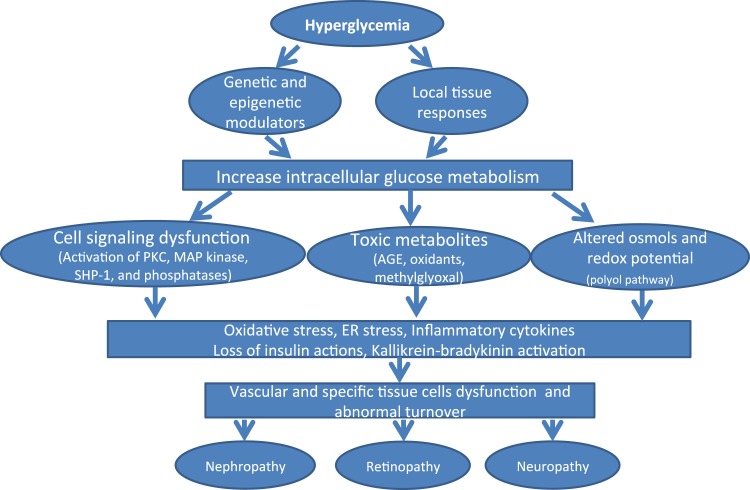
Research has described multiple abnormalities in cell signaling, gene expression, and the regulation of cell biology and physiology in diabetes, and it is likely that many of these abnormalities operate concurrently to cause various diabetic microvascular complications. These mechanisms may be active preferentially in some (although probably not all) vascular tissues or organs, but generally they are associated with the development of complications in several organs (Fig. 1). We will discuss seven mechanistic pathways that appear to be involved in diabetic microvascular injury, as well as several potentially protective factors. The emphasis in this study is on cellular mechanisms; we will cite mechanism-based efforts at clinical interventions but not analyze them in detail.
Figure 1.
Schema of hyperglycemia’s induced pathways to microvascular complications. MAP, mitogen-activated protein.
The activation of PKC in vascular tissues
PKC is a family of serine/threonine-related protein kinases that includes multiple isoforms and affects many cellular functions and signal transduction pathways (6). Phosphatidyl serine, calcium, DAG, and phorbol esters (such as phorbol-12-myristate-13-acetate) activate the conventional PKC isoforms PKCα, β1, β2, and γ. Phosphatidyl serine and DAG (but not calcium) also activate the novel PKC isoforms PKCδ, ε, ϕ, and η. Neither calcium nor DAG activates the atypical PKC isoforms PKCζ and ι/λ. Hyperglycemia, per se, modulates PKC activation. In addition, oxidants (e.g., H2O2 and superoxide) can also activate PKC in a manner unrelated to lipid second messengers (7, 8). Many abnormal vascular and cellular processes, including endothelial dysfunction, vascular permeability, angiogenesis, cell growth and apoptosis, vessel dilation, basement membrane (BM) thickening, extracellular matrix (ECM) expansion, and altered enzymatic activity of mitogen-activated protein kinase (MAPK), cytosolic phospholipase A2, Na+–K+–adenosine trisphosphatase (ATPase), and several transcription factors, are attributed to the activation of several PKC isoforms. Diabetes increases PKC activity in skeletal muscle and the renal glomeruli, retina, myocardium, and liver. Among the isoforms of PKC, the α, β, and δ isoforms are most consistently implicated in diabetic vascular complications.
The activation of DAG–PKC pathway and diabetes
DAG levels are elevated chronically in the hyperglycemic diabetic environment due to increased levels of glycolytic intermediate dihydroxyacetone phosphate. This intermediate is reduced to glycerol-3-phosphate, which subsequently increases de novo synthesis of DAG (9). In diabetes, studies reported that total DAG levels were elevated in the retina (10) and renal glomeruli (11). However, there is no consistent change in DAG levels in the central nervous system (CNS) or peripheral nerves (12). Cell culture studies have shown that as glucose levels rise from 5.5 to 22 mmol/L, DAG levels increase in a time-dependent manner in aortic endothelial cells (13), retinal pericytes (14), smooth muscle cells (9), kidney proximal tubular cells (15), and renal mesangial cells (16). Increased DAG synthesis can also occur from dihydroxyacetone phosphate that accumulates when poly adenosine 5′-diphosphate (ADP) ribosylation inhibits glyceraldehyde-3-phosphate dehydrogenase in the presence of high glucose concentrations (17). Elevated cytosolic glucose levels promote the accumulation of glyceraldehyde-3-phosphate, which can increase DAG and activate PKC (18). In an experimental model of diabetes, large doses of thiamine and thiamine monophosphate derivative (benfotiamine) appear to decrease the formation of DAG and mitigate PKC activation (19).
PKC activation and the development of diabetic nephropathy
Experiments in diabetic rodents support a role for PKC in the pathogenesis of diabetic nephropathy (DN). PKCα, β, and δ isoforms are activated in renal glomeruli isolated from streptozotocin (STZ)-induced diabetic rats (20) and mice, and 50% of the increase in PKC activity in renal glomeruli is prevented in PKCβ knockout mice (21). PKCα activation can upregulate vascular endothelial growth factor (VEGF) expression through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (22). PKCα knockout mice are protected against BM proteoglycan losses induced by VEGF (23). In wild-type mice, diabetes increases NADPH oxidase activity and induces the expression of endothelin 1 (ET-1), VEGF, transforming growth factor β (TGF-β), connective tissue growth factor, and collagen types IV and VI. These changes are partly prevented in PKCβ knockout mice (21). Mesangial expansion and albuminuria in mice with STZ-induced diabetes are reduced in both PKCβ (21) and PKCδ (24) knockout vs wild-type mice.
General PKC isoform inhibitors can interact with other kinases and can have significant toxic side effects. The PKCβ inhibitor ruboxistaurin (RBX) is a bisindolylmaleimide class agent that selectively inhibits PKCβ1 and PKCβ2 (25). Rottlerin (mallotoxin) has higher affinity for PKCδ but also inhibits other isoforms of PKC (26) and other non-PKC kinases, such as MAPKs, protein kinase A, and glycogen synthase kinase-3 (27). Orally administered RBX reversed glomerular hyperfiltration and reduced urinary albumin excretion in diabetic rodents without a change in DAG content (28). In addition, glomerular TGF-β1 expression, mesangial expansion, glomerulosclerosis, tubule-interstitial fibrosis, and renal function all improved (28). In Ren-2 diabetic rats, RBX attenuated macrophage recruitment, tubulointerstitial injury associated with TGF-β activation, and increases in PKC-induced osteopontin expression in tubular epithelial cells of the renal cortex (29).
Remarkably, PKCε may have effects on DN that are opposite to the effects of PKCα, β, and δ. One study showed that knockout of PKCε upregulated renal TGF-β1 and its downstream signaling and increased the expression of fibronectin and collagen type IV, which caused glomerular and tubulointerstitial fibrosis and the development of albuminuria (30). These changes were further aggravated by diabetes (30). Therefore, PKCε may act as a protective factor by reducing kidney damage.
Supporting the relevance of these findings to human DN, one study associated polymorphisms of the PKCβ gene with accelerated kidney disease (KD) in Japanese T2DM subjects (31), and another study associated polymorphisms in the PKCβ-1 gene with end-stage KD (ESKD) in Chinese patients with T2DM (32). However, efforts to treat DN by inhibiting PKC activation with RBX have generally been disappointing, as illustrated by a secondary analysis of three DN trials (33), which showed no differences in kidney outcomes with RBX treatment.
PKC activation and the development of diabetic retinopathy
The early stages of diabetic retinopathy (DR) are characterized by the loss of pericytes in capillaries of the retina, followed by weakness in the capillary wall, microaneurysm formation and fluid leakage, and increased adhesion of leukocytes and monocytes to the endothelium (34). Hyperglycemia activates PKCα, β, δ, and ε (18) in retinal tissues and alters ET-1 and VEGF activity and nitric oxide (NO) levels in endothelial cells, as well as levels of platelet-derived growth factor (PDGF), reactive oxygen species (ROS), and nuclear factor κB in pericytes (35). Administering RBX to diabetic rats can reduce retinal PKC activation and normalize retinal blood flow (RBF) (36, 37). In vessels isolated from diabetic animals, NO-dependent acetylcholine-induced vessel relaxation is delayed (38), and the PKC agonist phorbol-12-myristate-13-acetate impaired vascular relaxation in otherwise normal arteries (39).
The mechanism for reduced RBF mediated by PKCβ involves the upregulation of ET-1 synthesis in the retina of diabetic rats (40). RBX treatment can block this induction of retinal ET-1 (40). VEGF (through signaling involving PKCβ) (41) helps mediate diabetic macular edema to increase the phosphorylation of occludin (a component of tight junctions), leading to increased vascular permeability (42) and kallikrein activation (43). Hyperglycemia may also increase endothelial cell permeability via PKCα activation (44).
Recently, researchers have clarified the actions of PKC on vascular cell proliferation and death. Both PKCβ and PKCδ isoforms are translocated to the membrane fraction in total retinal lysates of diabetic mice, but the consequences of PKCβ, δ, and ε isoform activation are very different. PKCδ induces cell apoptosis (14), whereas PKCβ enhances cell growth (45). Accordingly, the elevation of membranous PKCδ levels in diabetes correlated with the appearance of retinal pericyte apoptosis in vitro and acellular capillaries in vivo. In vivo studies reported that the induction of PKCδ in the retinal capillaries of diabetic mice led to PDGF resistance; this was not true with PKCδ knockout mice. Hyperglycemia (through PKCδ action) promotes two distinct important pathways, as follows: (1) increasing ROS production and nuclear factor κ light chain enhancer of activated B cell (NF-κB) activity, and (2) decreasing the survival-signaling pathway of PDGF by upregulating SHP-1 expression. These findings suggest a pivotal role for PKCδ in regulating pericyte apoptosis and the formation of cellular capillaries (14).
In animal studies, inhibiting PKCβ ameliorated the decline of RBF typically associated with DR and decreased diabetes-induced vascular leakage (36). Similarly, the stimulus for neovascularization is suppressed in animals with reduced PKCβ levels (45, 46). More recently, Nakamura showed that subcutaneous RBX treatment reduced retinal neovascularization (induced in neonatal mice) by returning the retina to normoxia (21% O2) after exposure to hyperoxia (75% O2). In addition, Nakamura et al. (47) reported that the RBX antiangiogenic effects were due, in part, to suppressed phosphorylation of extracellular signal-regulated protein kinases 1 and 2 and Akt.
In phase II clinical trials (PKC-Diabetic Retinopathy and PKC-Diabetic Macular Edema Studies) (48), RBX failed to alter the primary outcome (loss of visual acuity). However, there was a significant reduction in the secondary endpoint—the progression of diabetic macular edema. A much larger clinical trial (PKC-Diabetic Retinopathy Study 2) that administered a single daily dose (again using the loss of visual acuity, as the primary endpoint) (49) reported that RBX treatment significantly prevented the loss of visual acuity for diabetic patients with moderate vision loss and decreased the onset of diabetic macular edema (50). These results suggest that PKC activation, especially of the β isoform, could participate in the development of nonproliferative DR. However, RBX did not delay the progression of vascular DR. This suggests that inhibiting the PKCβ isoform alone is not adequate to stop the metabolic changes that drive the progression of proliferative DR.
PKC and the development of diabetic peripheral neuropathy
Neuropathy is one of the most distressing complications of diabetes and involves the entire peripheral nervous system (51). Healthy nerves receive a rich supply of blood from the vasa nervorum (52). Hyperglycemia can damage neuronal cells by impairing vasodilation and increasing capillary BM thickening and endothelial hyperplasia, which diminish oxygen tension (52, 53). Additionally, hyperglycemia reduces Na+K+ ATPase activity, which is essential for maintaining normal nerve membrane resting potential, as well as providing neurotrophic support (54).
The contribution of PKC activation to diabetic peripheral neuropathy (DPN) is still unclear. Hyperglycemia does not increase DAG content in nerve cells, nor is there any consensus as to whether it increases, decreases, or has any effect on PKC activity (55). One study reported that high glucose concentrations in neurons can decrease phosphatidylinositol, thereby decreasing DAG levels and actually decreasing PKC activity. This diminished activity reduces the phosphorylation of Na+K+ ATPase, leading to a decrease in nerve conduction and regeneration. Immunochemical analysis demonstrated the presence of PKCα, β1, β2, γ, δ, and ε isoforms in nerves (56). A previous study that directly measured sciatic nerve tissues in STZ diabetic rats also reported a reduction of PKC activity (57). However, these results contrast with recent studies showing that treating diabetic animals with nonselective PKC isoform inhibitors, as well as selective PKCβ inhibitors, improved neural function (58). Some animal studies have reported that PKCβ inhibitor treatments improved nerve conduction as well as neuronal blood flow (59). Indeed, Cameron et al. showed that low-dose RBX treatments improve motor nerve conduction velocity, normalize nerve blood flow, and restore Na+K+ATPase activity in diabetic rats (60). In humans, 1 year of RBX treatment did not significantly affect vibration detection threshold and Neuropathy Total Symptoms Score-6, but may have benefitted a subgroup of patients with less severe symptomatic DPN (61, 62). More recently, Boyd et al. (63) reported that RBX produced significant improvements in large fiber measures, quality of life (QOL), and Neuropathy Total Symptoms Score-6 in diabetic patents.
In summary, there is substantial evidence that PKCβ mediates some of the micropathologies in the early stages of microvascular complications. However, it is also clear that the effective prevention or treatment of these microvascular complications may involve inhibiting multiple PKC isoforms, including α, β, and δ.
The polyol pathway and the pathogenesis of diabetic microvascular complications
Increased cellular glucose uptake elevates glucose flux through multiple pathways, including the polyol pathway (also known as the sorbitol pathway). Aldose reductase (AR), the first enzyme of this pathway, has a Km between 5 and 10 mM glucose, which allows it to be active only when intracellular glucose is elevated. This pathway consumes NADPH in the AR reaction and reduces nicotinamide adenine dinucleotide (NAD)+ in the sorbitol reductase reaction (64). A hyperactive polyol pathway may deplete cytosolic NADPH, which is necessary to maintain the primary intracellular antioxidant [glutathione (GSH)] in its reduced state. In mice, deleting AR−/− reduced retinal neovascularization and capillary permeability. Furthermore, the expression of VEGF, p-Erk, p-Akt, and p-IκB was significantly reduced in AR−/− retina (65). In diabetic mice induced to have retinal ischemia by transient middle cerebral artery occlusion, AR−/− leptin receptor-deficient diabetic (db/db) mice had significantly less retinal swelling than the db/db control mice; this correlated with a reduced expression of the water channel aquaporin 4 (66). Similarly, AR deficiency in the renal glomeruli protects the mice from the diabetes-induced ECM accumulation and collagen IV overproduction. Furthermore, AR deficiency completely or partially prevented diabetes-induced glomerular hypertrophy and activation of renal cortical PKC and TGF-β1. In diabetic AR−/− mice, loss of AR resulted in reduced urinary albumin excretion (67) and protection from the decreased motor and sensory nerve conduction velocities (NCVs) seen in diabetic AR+/+ mice. Sorbitol levels in the sciatic nerves of diabetic AR+/+ mice were increased significantly, whereas sorbitol levels in the diabetic AR−/− mice were significantly lower. In addition, the study reported signs of oxidative stress [such as increased activation of c-Jun NH(2)-terminal kinase, depletion of reduced GSH, increased superoxide formation, and DNA damage] in the sciatic nerves of diabetic AR+/+ mice, but not diabetic AR−/− mice. This indicates that the diabetic AR−/− mice were protected from oxidative stress in the sciatic nerve (68). Polymorphisms in the promoter gene region of AR are associated with susceptibility to neuropathy, retinopathy, or nephropathy, and these associations have been replicated in patients with either T1DM or T2DM, as well as across several ethnic groups (69).
Animal studies using AR inhibitors showed promise with regard to an effect on DN or DR, but clinical trials since the 1980s have generally not confirmed such effects in patients with diabetes, except in Japan, where AR inhibitor treatments are approved for DN.
Oxidative stress and the pathogenesis of diabetic microvascular complications
The production of superoxide and other ROS in vascular cells may play an important role in the pathogenesis of vascular diseases in general and particularly in the diabetic state. A major source of superoxide in vascular cells is the NOX family of NADPH oxidases that favors reduced NAD as a substrate (70). The elevation of oxidants and signaling enzymes, like PKC, can induce NOX 1, 2, 4, and 5 in endothelial and contractile vascular cells (70). The expression and activity of NOX are increased in the vascular tissue of rodents with T1DM (71) and T2DM (72). An increase in the reduced NAD/NAD+ ratio may activate NOX. In diabetes this may be caused by an increased flux through the polyol pathway (see previous description) or through the activation of poly (ADP-ribose) polymerase (73) or PKC (74). In animal models, Baicalein, a NOX inhibitor, reduced vascular hyperpermeability and improved retinal endothelial cell barrier dysfunction (75). However, the role of NOX isoforms in the pathogenesis of KD in diabetes is unclear. For example, NOX2 deficiency did not protect NOX2 knockout mice against DN, despite a reduction in macrophage infiltration (76). Administering apocynin, a NOX inhibitor, corrected the vascular conductance deficits and reversed the reduction of sciatic nerve motor conduction velocity and sensory saphenous nerve blood flow induced by diabetes (77).
Mitochondria are another important source of ROS. The elevated intracellular glucose concentration in diabetes can yield excessive mitochondrial-reducing equivalents, thus increasing the proton gradient. This inhibits the transfer of electrons from reduced coenzyme Q-10 (ubiquinone) to complex III of the electron transport chain (64). As a result, these electrons are transferred to molecular oxygen, which results in superoxide production.
By promoting DNA strand breaks, oxidative stress can activate poly(ADP-ribose) polymerase, which can activate NF-κB and cause endothelial dysfunction (73). Oxidative stress can also inhibit the proteasomal degradation of homeo-domain–interacting protein kinase 2, which promotes kidney fibrosis through the activation of p53, TGF-β, and Wnt (78).
When cultured rat mesangial cells are incubated with high glucose, adding an inhibitor of the tyrosine kinase c-Src (which is activated by oxidative stress) reduces type IV collagen accumulation (79). Similarly, in STZ-induced diabetic mice, inhibiting c-Src in vivo reduced albuminuria, glomerular collagen accumulation, and podocyte loss (79). Podocyte injury, a major contributor to the genesis of diabetic glomerulopathy, may (in part) result from excess ROS generation. Khazim et al. (80) reported that the antioxidant plant extract silymarin reduced the high glucose-induced apoptosis of cultured mouse podocytes. In type I diabetic mice, it reduces glomerular podocyte apoptosis and albuminuria. Another study reported that silymarin treatment reduced the urinary excretion of albumin in T2DM patients with macroalbuminuria and suggested silymarin as a treatment of preventing the progression of DN (81).
ROS overproduction can also cause major retinal metabolic abnormalities associated with the development of DR. NF-E2–related factor 2 (Nrf2) (a redox-sensitive factor) provides cellular defenses against the cytotoxic ROS. In stress conditions, Nrf2 dissociates from its cytosolic inhibitor [Kelch erythroid cell-derived protein with CNC homolog-associated protein (Keap) 1] and moves to the nucleus to regulate the transcription of multiple (>30) antioxidant genes, including the catalytic subunit of glutamyl cysteine ligase, a rate-limiting enzyme for reduced GSH biosynthesis (see section on antioxidant enzymes). Diabetes increased retinal Nrf2 and its binding to Keap1 but decreased the DNA-binding activity of Nrf2l, as well as its binding to the promoter region of glutamyl cysteine ligase. A study reported similar impairments in Nrf2-Keap-glutamyl cysteine ligase in endothelial cells exposed to high glucose and in the retina from donors with DR (82).
To date, large clinical trials using antioxidants have not shown that the vitamins E, C, or α-lipoic acid (ALA) have a significant effect on definitive clinical endpoints for preventing or treating DR and other vascular complications (83–85).
Motor and sensory neuron myelination and nerve conduction decline with DPN; however, the mechanisms responsible are poorly understood. Chronic oxidative stress is one potential determinant of demyelination, as lipids and proteins are important structural constituents of myelin and are highly susceptible to oxidation. Using the db/db mouse model of DPN and the superoxide dismutase 1 knockout mouse model of in vivo oxidative stress, Hamilton et al. (86) reported recently that oxidation-mediated protein misfolding and the aggregation of key myelin proteins may be linked to demyelination and reduced nerve conduction in peripheral neuropathies.
Some studies have reported high oxidative status and oxidative stress index together with low serum total antioxidant status in serum from DPN patients (87). In a double-blind placebo-controlled trial of DPN subjects, vitamin E improved electrophysiological parameters of nerve function, including motor NCV and tibial motor nerve distal latency (88). Furthermore, a meta-analysis of 15 randomized controlled trials (RCTs) reported that the antioxidant ALA significantly improved both NCV and positive neuropathic symptoms (89). Despite this, it is clear that we will need better antioxidants if they are to significantly delay the progression of diabetic microvascular pathologies.
Protein glycation and diabetic microvascular complications
Sugars, such as pentosidine, carboxymethyllysine, methylglyoxal, and pyraline, can cause AGE formation (90). AGE formation can occur via a nonenzymatic reaction between glucose and protein through the Amadori product 1-amino-1-deoxyfructose adducts to lysine. However, faster reactions take place between proteins and intracellularly formed dicarbonyls, including 3-deoxyglucosone, glyoxal, and methylglyoxal, which result in the cross-linking of proteins. Due to their long turnover rate, structural extracellular proteins (such as collagen) accumulate more AGE modification. AGEs are probably present in all tissues of diabetic and/or ageing patients. AGE modification of ECM proteins and signaling molecules may alter their function. In addition, AGE-modified extracellular proteins may bind to receptors, the most well-characterized being the receptor for AGE (RAGE) (91). Most cells express RAGE—including the following: endothelial cells, mononuclear phagocytes, smooth muscle cells, pericytes, mesangial cells, podocytes, and neurons—and RAGE may play a role in the regulation of these cells in homeostasis and/or their dysfunction in the development of diabetic complications (92). Binding to RAGE on the endothelial cell surface can stimulate NOX and increase ROS, p21 RAS, and MAPK. The AGE–RAGE interaction may also stimulate signaling via p38 MAPK and Rac/Cdc; however, its exact mechanism is unclear because RAGE is not an enzyme. A key target of RAGE signaling in the endothelium is NF-κB, which is translocated to the nucleus, where it increases the transcription of a number of different proteins, including ET-1, intercellular adhesion molecule-1, E-selectin, and tissue factor (93). The ability of RAGE signaling to cause diabetic complications has been reported in transgenic mice overexpressing both inducible NO synthase (iNOS) targeted to β cells (providing a model for T1DM) and RAGE in all cells. These double-transgenic mice develop accelerated glomerular lesions (94), which an AGE inhibitor prevents (94). Conversely, a soluble RAGE prevents the development of increased vascular permeability and atherosclerosis in experimental diabetes (95). Furthermore, RAGE fusion protein inhibitor administered to STZ-diabetic rats had beneficial effects on early DR or DN (96). Clinical trials are ongoing for small molecule antagonists of RAGE (97). Researchers have used other approaches to inhibit tissue accumulation of AGE in diabetes, including AGE formation inhibitors, such as aminoguanidine, ALT 946, and pyridoxamine, or putative cross-link breakers, such as ALT 711 (98).
Interestingly, not all AGEs or their actions affect vascular cells adversely. Several recent studies have reported inverse correlations of carboxymethyl-lysine and fructose-lysine with vascular complications (4).
The renin-angiotensin system and the pathogenesis of diabetic microvascular complications
A large number of clinical trials have clearly shown that angiotensin-converting enzyme (ACE) inhibitors, angiotensin type-1 receptor blockers, or the combination may delay the onset of renal disease or progression to renal failure (99). However, an analysis of renal biopsies from T1DM patients treated with these drugs did not report improved glomerular pathology, indicating that RAS inhibition may only delay the progression of functional impairment in DN (100). The kidney produces angiotensin I and angiotensin II (Ang II) locally, and part of the renoprotective effect of ACE inhibition (in addition to lowering systemic BP) is a decrease of glomerular capillary pressure. Ang II actions may also lead to kidney damage through the induction of local factors, including ECM protein synthesis via TGF-β and inflammatory cytokines (101). Ang II receptors mediate angiotensin action, including the activation of RAF kinase/MAPK and multiple inflammatory cytokines, such as tumor necrosis factor-α, interleukin 6, and others (102). Furthermore, RAS blockade may improve or delay the development of DR and macular edema in diabetic patients (103) and DR in normotensive, normal albuminuric T1DM patients (104). This suggests their beneficial effects may be more than just the reduction of BP. In animal models of diabetes, the renin inhibitor aliskiren provided similar or greater protection than ACE inhibition alone to decrease nonproliferative DR and proliferative neoangiogenesis in oxygen-induced retinopathy. In transgenic TGR(mRen-2)27 rats, which overexpress mouse renin in extrarenal tissues, aliskiren treatment reduced retinal acellular capillaries and leucostasis and normalized retinal VEGF expression (105).
ER stress and diabetic microvascular complications
The ER plays an important role in Ca+2 and redox homeostasis, lipid biosynthesis, and protein folding. Increases in protein synthesis, protein misfolding, or perturbations in Ca+2 and redox balance can disturb ER function, causing ER stress. This triggers a coordinated program (the unfolded protein response) that reduces translation and increases protein-folding capacity to restore ER homeostasis. With chronic, unresolved ER stress, the unfolded protein response can initiate signaling that promotes apoptosis. Unfolded protein response genes are upregulated in kidney tissue from patients with diabetes, and ER stress may be a mediator of DN. Mice with STZ-induced diabetes and knockout of C/EBP homologous protein are protected from DN (106). In the retina of rats with STZ-induced diabetes, ER stress is also involved in increased vascular permeability and the upregulation of inflammatory genes and VEGF (107). These and other findings have prompted the development of therapeutics to reduce ER stress. These include synthetic chaperones to promote protein folding, as well as inhibitors of CCAAT/enhancer-binding homologous protein and other molecules that interfere with protein folding (107).
Several studies have also implicated ER dysfunction in the pathogenesis of DPN. In cultured Schwann cells, knockout of antiapoptotic protein ORP150 promoted high glucose-induced Schwann cell apoptosis, whereas knockout of C/EBP homologous protein protected Schwann cells from apoptosis (108). In rat models of high-fat STZ diabetes, knockout of ORP150 induced DPN in early diabetes and exacerbated DPN after prolonged diabetes, whereas knockout of the proapoptotic protein C/EBP homologous protein ameliorated DPN in rats with prolonged diabetes.
The kallikrein-bradykinin system and the development of diabetic microvascular complications
Plasma kallikrein is a serine protease with well-characterized effects in innate inflammation and the intrinsic coagulation cascade (109). The majority of plasma kallikrein’s physiological actions are attributed to the cleavage of factor XII and high-molecular-weight kininogen. The conversion of factor XII to factor XIIa leads to the activation of factor XI and the intrinsic coagulation cascade, which results in fibrin production and thrombus stabilization. Kininogen cleavage releases the nonapeptide BK, which is the ligand for the G protein–coupled BK2 receptor (BK2R). Subsequent BK cleavage by carboxypeptidases generates des-Arg9-BK, which binds and activates the BK1 receptor (BK1R). The activation of BK2R by BK and the activation of BK1R by des-Arg9-BK are associated with nearly all the effects the plasma kallikrein-kinin system (KKS) has on inflammation, vascular function, BP regulation, and nociceptive responses (110). Plasma KKS is also associated with a variety of coagulation, vascular, and metabolic abnormalities in diabetes. However, most studies have examined the physiological effects of the KKS using BK receptor-targeted approaches.
The kallikrein-kinin system and diabetic retinopathy
Experimental studies have demonstrated that KKS activation can result in biological effects that also occur in DR (e.g., increased vascular permeability and edema); promote changes in vascular diameter and hemodynamics; and affect inflammation, angiogenesis, and neuronal functions.
Retinal vascular permeability and blood flow
Activating the KKS by injecting C1 esterase inhibitor into the vitreous increases retinal vascular permeability (RVP). The coinjection of C1-inhibitor (C1-INH) (a neutralizing antibody against plasma kallikrein) and a small-molecule plasma kallikrein inhibitor (1-benzyl-1H-pyrazole-4-carboxylic acid 4-carbamimidoyl-benzylamide) inhibited this response (111). Intravitreal plasma kallikrein’s effect is greater in diabetic rats compared with nondiabetic rats, suggesting that diabetes enhances the retinal responses to intraocular KKS activation (111). Systemic administration of ASP-440 decreased RVP both in diabetic rats and in rats subjected to Ang II–induced hypertension (111, 112). Intravitreal BK injection increased RVP in both diabetic and nondiabetic rats, whereas only diabetic rats demonstrated a RVP response to des-Arg9-BK (112, 113). A BK1R antagonist reduced RVP in STZ-induced diabetic rats (113, 114). These data suggest that activating KKS in the circulation, and/or locally in the retina and vitreous, can increase RVP via both BK1R and BK2R mediation, and that diabetes increases actions mediated via BK1R.
KKS can also regulate retinal vessel diameters and hemodynamics. Intravitreal or intravenous BK injections acutely increased retinal vessel diameters and blood flow in adult cats (115) and rats (116), respectively. Des-Arg9-BK increased vessel diameters in the retinal vessels of diabetic rats, but not in the retinal vessels of nondiabetic controls (117). These effects of BK1R and BK2R on retinal vessel dilation are dependent on NO and prostaglandin in vascular endothelial cells. BK1R blockade reduces the retinal expression of potential inflammatory mediators (including iNOS and cyclooxygenase-2), NG-nitro-l-arginine methyl ester, and indomethacin. In addition, the BK2R antagonist Hoe140 inhibited in vitro BK-induced vasodilation responses (117, 118). BK and Des-Arg9-BK increase intracellular free calcium by coupling Gα q/11 or Gα i/o through the BK2R or BK1R, respectively (119, 120). The increased Ca+2 can stimulate phospholipase A2 to liberate arachidonic acid from membrane phospholipids, which can increase prostacyclin (121) and increase NO synthase (NOS) phosphorylation via Ca+2/calmodulin-dependent activation. However, under inflammatory conditions, BK1R stimulation results in a much higher and prolonged NO production via Gα(i) activation of the MAPK pathway, leading to iNOS activation (120, 122). Endothelial NOS (eNOS) and iNOS activation can independently and additively increase NO production (120, 123). BK also activates the Src kinases and the subsequent vascular endothelial cadherin phosphorylation, leading to the quick and reversible opening of endothelial cell junctions and plasma leakage (124).
Kallikrein-kinin system inhibitors: novel therapeutic applications to diabetic retinopathy
Targeting the KKS could occur at multiple levels, including the inhibition of the contact system, selective inhibition of plasma kallikrein activity, and blockade of BK receptors. Plasma kallikrein inhibitors include endogenous inhibitors, engineered proteins, and small molecules. C1-INH is a primary physiological inhibitor of plasma kallikrein, factor XIa, factor XIIa, C1r, and C1s proteases. Both plasma-derived and recombinant forms of C1-INH are effective treatments for hereditary angioedema (125). Intravitreal injection of exogenous C1-INH reduced retinal vascular hyperpermeability induced by diabetes and by intravitreal carbonic anhydrase-1 in rats (111). Although C1-INH is detected in the vitreous, it is unknown whether intravitreal concentrations of this endogenous serpin protease inhibitor are sufficient to inhibit plasma kallikrein. Exogenously administered C1-INH into the vitreous may provide an opportunity to inhibit the KKS, as well as other proteases in the complement and intrinsic coagulation cascades. Selective plasma kallikrein inhibition could provide increased efficacy in targeting the inflammatory effects of the plasma KKS while preserving the potential beneficial effects of the tissue KKS.
The generation of peptides that can activate BK1Rs and BK2Rs (which are expressed in a variety of ocular cell types and tissues) in large part mediates the effects of the KKS. Because both plasma kallikrein– and tissue kallikrein–mediated pathways activate BK receptors, the antagonism of these receptors blocks the effects of both KKSs. Although both BK1Rs and BK2Rs can induce RVP, BK1R appears to increase plasma extravasation in DR. The selective peptide BK1R antagonist R-954 reduced vascular permeability in a variety of tissues from STZ-induced diabetic rats, including the retina (114). Treating STZ-induced diabetic rats with R-954 for 5 days at the end of the 4- and 12-week periods of diabetes reduced NO, kallikrein activity, and capillary permeability, whereas retinal Na+,K+-ATPase activity increased (126). Treating diabetic rats with FOV-2304, a nonpeptide BK1R antagonist administered via eye drops, reduced RVP and normalized the retinal messenger RNA (mRNA) expression of inflammatory mediators (127). Pouliot et al. have reported that one eye drop of the nonpeptide BK1R antagonist LF22-0542 reversed retinal plasma extravasation and RVP in the diabetic retina. These reports indicated that both local and systemic administrations of BK1R antagonists are effective in ameliorating retinal vascular abnormalities in diabetic rodents, which are similar to findings from studies using plasma kallikrein inhibitors (128).
Protection factors
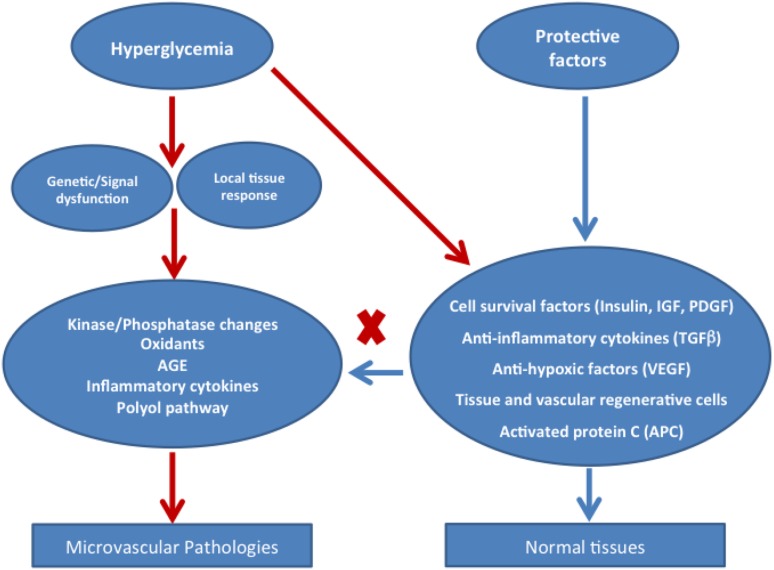
Clinical observational studies in patients with long-duration diabetes (e.g., the Joslin’s Medalist Study) tell us that, in addition to metabolic toxic factors, there may be equally important protective factors that spare the function and survival of cells involved in microvascular disease beyond the effect of glycemic control (3, 4). The finding that over half of diabetic patients with microalbuminuria have regression of this marker over 6 years of follow-up (129) also supports the possibility that endogenous protective factors are common in the general population. Researchers have only recently suggested that some factors with well-established functions were protective (Fig. 2).
Figure 2.
Interplay of hyperglycemia’s toxic mechanisms and tissues’ endogenous protective properties. IGF, insulinlike growth factor.
Insulin: selective insulin resistance on the vessel wall in diabetes
Insulin receptors are present on vascular cells and cells recruited to the vascular wall; these include endothelial cells, vascular smooth muscle cells, pericytes, macrophages, and all the glomerular cells. Insulin signal transduction in these cells occurs primarily by the activation of the insulin receptor substrate 1/2 (IRS1/2) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways, which have been shown to phosphorylate eNOS, induce the expression of VEGF and heme oxygenase-1, and decrease expression of VCAM-1. Insulin also activates the Src/MAPK pathway to induce the expression of ET-1 and the migration (and perhaps proliferation) of vascular contractile cells (130). In diabetes or insulin resistance, hyperglycemia or free fatty acids (FAs) activate PKCα, β, or δ to phosphorylate IRS2 and p85/PI3K and selectively inhibit the p-Akt pathway in the vessel wall with the loss of insulin’s anti-inflammatory and antioxidative effects (130), whereas insulin activation of the MAPK pathway persists. In the kidney, podocytes are critically important for maintaining the integrity of the glomerular filtration barrier and preventing albuminuria. Insulin receptor signaling has a surprisingly profound effect on podocyte survival. Mice with targeted knockout of the podocyte insulin receptor (131) after 5 weeks of age developed albuminuria, effacement of podocyte foot processes, and increased apoptosis, together with increased deposition of BM components. Some of these glomerular pathologies were similar to those observed in DN. Some animals also developed shrunken kidneys with scar tissue (similar to the macroscopic appearance of kidneys in late-stage DN), accompanied by mild worsening of kidney function. This is notable because kidney function is not affected by STZ-induced diabetes (the most commonly studied rodent model of diabetes), despite albuminuria and histopathological changes. One explanation for the importance of insulin action on podocytes is that insulin increases the expression of VEGF in several cell types, including podocytes (132). Insulin upregulates VEGF expression mostly via the IRS/Akt pathway, which may act as a survival factor for podocytes, endothelial cells, and mesangial cells. Recently, Hale et al. (132) reported that insulin directly increased VEGF-A mRNA levels and protein production in conditionally immortalized wild-type human and murine podocytes. Furthermore, when podocytes were rendered insulin resistant in vitro (using stable short hairpin RNA knockdown of the insulin receptor) or in vivo (using transgenic podocyte-specific insulin receptor knockout mice), podocyte VEGF-A production was impaired. Insulin could also prevent apoptosis by other mechanisms, including inhibiting proapoptotic molecule caspase-9 (133), inhibiting transcription factor FoxO (134), or upregulating the antioxidant activity of heme oxygenase-1 (18).
Researchers have also described the selective (IRS1/PI3K/Akt pathway) impairment of insulin action in the glomeruli of diabetic animals and patients, which may contribute to DN development. The IRS/PI3K/Akt pathway mediates many of insulin’s protective effects, including the upregulation of eNOS (135, 136) and heme oxygenase-1 (18). In contrast, the Ras/MAPK pathway mediates the induction of ET-1 (137). With diabetes or insulin resistance, elevated concentrations of glucose and free FAs can activate PKC and selectively inhibit insulin signaling through the PI3K pathway (138). Certain threonine/serine residues on IRS2 and on the p85 regulatory subunit of PI3K are substrates for PKC, and phosphorylation of these sites inhibits insulin-stimulated PI3K pathway signaling (139, 140). Hyperinsulinemia in T2DM may promote vascular disease through the induction of ET-1 (141) or other factors induced by MAPK signaling.
Antioxidant enzymes
Although extensive evidence from cell- and animal-based studies supports the role of oxidative stress in the development of vascular complications, nearly all clinical trials using antioxidants have failed to show efficacy with clinically significant vascular endpoints.
Nevertheless, tissue-specific endogenous antioxidant enzymes are most likely important to neutralize the increased levels of oxidants seen with hyperglycemia. This idea has stimulated clinical trials using bardoxolone methyl (142), a synthetic triterpenoid that activates Nrf2. This nuclear factor upregulates a gene program of molecules with antioxidant activity called phase 2 genes, which includes heme oxygenase 1 and enzymes in the GSH biosynthesis pathway. Keap1, a repressor that binds Nrf2 in the cytoplasm and promotes Nrf2 proteasomal degradation, inhibits Nrf2 translocation to the nucleus. Bardoxolone methyl interacts with cysteine residues on Keap1, preventing Nrf2 repression and allowing phase-2 gene transcription. Results from a trial of bardoxolone methyl in patients with advanced chronic KD (CKD) showed an improvement in glomerular filtration rate (GFR) up to 1 year after start of treatment (143). However, proteinuria was increased and researchers stopped phase-III trials due to safety issues. Researchers also reported that Nrf2 has a protective role in the retina against neuronal and capillary degeneration in retinal ischemia–reperfusion injury. In Nrf2+/+ mice, ischemia–reperfusion injury resulted in leukocyte infiltration of the retina and vitreous and increases in retinal levels of superoxide and proinflammatory mediators. These changes were greatly accentuated in Nrf2−/− mice (144).
PDGF and VEGF
PDGF expressed by retinal endothelial cells plays a role both in vascular cell survival and proliferative retinopathy (145). During sprouting angiogenesis, endothelial tip cells produce PDGF, which acts through PDGF receptor-β expressed by pericytes. This signal recruits pericytes to develop blood vessels. Pericytes, in turn, can support endothelial cell survival and inhibit its proliferation. Reports of pericyte loss and endothelial cell proliferation in PDGF knockout mouse embryos demonstrate this process (146). Mice with heterozygous deletion of the PDGF gene not only have an increased frequency of acellular capillaries (particularly after diabetes induction), but also an increased tendency for retinal neovascularization during ischemic retinopathy (147). As described previously, we have reported that hyperglycemia can inhibit the survival effects of PDGF by upregulating SHP-1, which causes dephosphorylation of the PDGF receptor in pericytes and possibly also in podocytes (14).
High glucose concentrations and diabetes can activate SHP-1 (a tyrosine phosphatase) in microvessels, including the retina and renal glomeruli. This leads to the dephosphorylation and deactivation of specific growth factor receptors critical for the survival of pericytes in the retina and podocytes in the kidney (14). One study reported that SHP-1 regulates AGE-related endothelial cell injury in vitro (148). In the retina of diabetic rodents, SHP-1 activation can desensitize pericytes to PDGF and cause pericyte apoptosis, an initiating step in the development of DR (14). In the renal glomeruli, the upregulation of SHP-1 expression can impair VEGF survival signaling and increase podocyte apoptosis and endothelial dysfunction (24). The upregulation of SHP-1 expression in diabetes depends on the activation of PKCδ and p38MAPKα transcription (14, 24), which is prevented in PKCδ knockout mice. These mice are protected from the apoptosis of retinal pericytes, mesangial expansion, and albuminuria (14, 24). Therefore, inhibiting SHP-1 is a potential novel approach to preserving survival signaling in vascular cells.
TGF-β1
TGF-β1 is a major inducer of profibrotic responses in diabetic kidneys. Diabetes increases the expression of TGF-β in blood vessels in many vascular beds, and one study suggests that it is a causative factor for the development of fibrosis in the kidney and other tissues (149). An earlier study has shown that treating C57BLKS/J db/db mice with neutralizing monoclonal TGF-β1 antibody decreases plasma TGF-β1, mesangial matrix expansion, and kidney mRNA levels of collagen IV and fibronectin (150). In addition, this therapy prevented a loss of renal function but had no effect on the elevated albuminuria in db/db mice. More recently, investigators have used an inhibitor of TGF-β receptor kinase activity, GW788388, to treat C57BLKS/J db/db mice (151). This therapy reduced glomerular collagen staining and kidney mRNA levels of plasminogen activator inhibitor 1 and types I and III collagen, but did not alter albuminuria (151).
However, numerous studies have reported that TGF-β has potent anti-inflammatory effects on macrophages and is a negative regulator of T cell and B cell activation. Therefore, TGF-β may have protective actions due to an anti-inflammatory effect, and its elevation may be a reaction to the inflammatory stress of diabetes. Thus, it is likely that diabetes-induced overexpression of TGF-β in many tissues could be an endogenous response to the inflammatory actions of hyperglycemia in vascular cells. These paradoxical roles of TGF-β make it a challenging drug target.
VEGF
VEGF expression changes paradoxically with diabetes, it increases in the retina and renal glomeruli, but it decreases in the myocardium, peripheral limbs, and nerves correlating with the extent of angiogenesis. VEGF neutralization is already a treatment of proliferative DR and macular edema, and one study suggests it as a therapy for DN (152). However, the increased levels of VEGF in both tissues are most likely an appropriate response to hypoxia, which results from loss of capillary function. It has been a longstanding concern that neutralizing VEGF could counteract survival signaling in retinal neurons. Interestingly, injecting low doses of VEGF accelerated the restoration of the physiological capillary bed and prevented preretinal neovascularization in a mouse model of proliferative retinopathy (153).
In the kidney, podocytes contain the highest level of VEGF expression, and some of the most insightful work describing a role for VEGF as a survival factor comes from studies of renal podocytes. The conditional deletion of VEGF in podocytes resulted in a complete lack of endothelial and mesangial cells in mature glomeruli and death within the first day of life (154). This finding strongly supports a role for VEGF in the maintenance of glomerular endothelial cells. The heterozygous knockout of VEGF in podocytes of mice resulted in proteinuria and ESKD in young adults (154) and was preceded by the disappearance of endothelial cell fenestrations, increases in necrosis, the effacement of podocyte foot processes, and a dramatic loss of mesangial cells (154). Inducing STZ diabetes in these mice exacerbated glomerular cell apoptosis, glomerulosclerosis, and proteinuria compared with nondiabetic controls (155). However, other studies reported that increased podocyte VEGF (156) expression worsens DN, characterized by glomerulosclerosis, microaneurysms, mesangiolysis, glomerular BM thickening, podocyte effacement, and massive proteinuria associated with hyperfiltration (156).
VEGF also has neuroprotective effects. Primary dorsal root ganglion cultures lacking VEGF-B or fms-like tyrosine kinase 1 (FLT-1) exhibited increased neuronal stress and are more susceptible to paclitaxel-induced cell death, and mice lacking VEGF-B or a functional FLT-1 develop more retrograde degeneration of sensory neurons. Conversely, adding VEGF-B (157) to dorsal root ganglia cultures antagonized neuronal stress, maintained the mitochondrial membrane potential, and stimulated neuronal survival. Mice overexpressing VEGF-B (157) or FLT-1 selectively in neurons were protected against distal neuropathy, whereas exogenous VEGF-B (157), delivered by either gene transfer or as a recombinant factor, was protective by directly affecting sensory neurons and not the surrounding vasculature (158). Identifying the prosurvival mechanisms in stressed neuronal cells revealed that protein kinase A functioned concurrently with the VEGF receptor-2 pathway to signal the activation of extracellular signal-regulated protein kinases 1/2 protection against caspase-3/7 activation and subsequent cell death (159).
Activated protein C
Activated protein C (APC) is an anticoagulant factor that acts as a survival factor for renal glomerular cells (160). Thrombomodulin, a procoagulant factor that activates protein C, is highly expressed in glomeruli of mice, but downregulated in diabetes (160). Diabetic mice with a loss-of-function thrombomodulin gene mutation had more albuminuria and more severe glomerular pathology than diabetic wild-type mice, whereas diabetic mice with a gain-of-function mutation of the protein C gene had less (160). The anticoagulant effects of APC did not account for its protective actions. Rather, APC was shown to counteract the apoptosis of endothelial cells and podocytes through the activation of two of its receptors (160). Therefore, endothelial-derived APC appears to act as a protective factor with local survival effects for both podocytes and endothelial cells in the glomerulus. The underlying mechanism for APC protection from renal dysfunction is still unknown. However, Li Calzi et al. (161) reported that APC-mediated protease suppressed lipopolysaccharide-induced increases in the vasoactive peptide adrenomedullin, suppressed infiltration of iNOS-positive leukocytes into renal tissue, and activated receptor-1 agonism. The anticoagulant function of APC was responsible for suppressing lipopolysaccharide-induced stimulation of the proinflammatory mediators ACE-1, interleukin-6, and interleukin-18, perhaps accounting for its ability to modulate renal hemodynamics (162).
Vascular progenitor cells
Bone marrow–derived cells, including endothelial progenitor cells (EPCs) and myeloid progenitors, may contribute to postnatal angiogenesis (163). EPCs may contribute by incorporating into newly formed blood vessels. However, it is likely the major action of EPCs is to release proangiogenic factors and temporarily associate with neovascular structures. In diabetic patients, both the number and function of EPCs are reduced (164), impairing the ability of EPCs to repair the vascular endothelium (165). The angiogenic potential of EPCs was also reduced in diabetic animals (166).
The differentiation of bone marrow–derived EPCs may also play a role (166). eNOS is necessary to mobilize EPCs from bone marrow (167). Uncoupling eNOS that favors superoxide rather than NO production may impair EPC function. Indeed, EPC function improves after eNOS is inhibited ex vivo in EPCs isolated from patients with diabetes (168). Interestingly, neuropathy in the bone marrow may reduce EPC mobilization. STZ-induced diabetes in rats reduced nerve terminals in bone marrow, and this correlated with increased EPCs in bone marrow and decreased EPCs released into the circulatory system. These abnormalities were associated with an increase in retinal acellular capillaries (169). Transplanting nondiabetic EPCs into diabetic animals can improve angiogenesis after peripheral ischemic injury (170).
These studies suggest that it may be possible to promote the repair of ischemic tissue in diabetes by improving the mobilization, differentiation, and function of EPCs or other progenitors. Recently, researchers have suggested that autologous EPC transplantation could be a potential therapy for DN. However, safety concerns regarding possible unwanted proliferation or differentiation of the transplanted stem cells might limit such treatment. An alternative approach is to stimulate endogenous bone marrow–derived EPC recruitment into ischemic lesions by administering stem cell mobilization agents or chemokines (171). Administering the EPC mobilization agent AMD3100 increased the local expression levels of vasculogenesis-associated factors and the number of newly formed endothelial cells in the sciatic nerve, which restored the sciatic vasa nervorum (171).
Circulating EPCs are markedly reduced in CKD patients (172), and EPCs have been shown to improve renal function, attenuate the proinflammatory response associated with renal injury, and improve damaged tubules and renal vascular segments during kidney injury while providing enhanced neoangiogenesis (173). An intact and healthy EPC niche, residing in the bone marrow but also found locally in renal vascular beds (i.e., in the adventitia layer of vessels), may be able to support normal vascular function, including maintenance and possible replacement of the endothelium (174).
Emerging studies in animal models suggest that EPCs help revascularize ischemic and injured retinas. Thus, EPCs could be a potential therapy for ischemic retinopathies in humans, which are a leading cause of blindness (161). In nonproliferative DR, EPCs may be less effective, as they do not recruit other EPCs and repair the acellular capillaries. In proliferative DR, the EPCs take on a proinflammatory phenotype and recruit too many EPCs, leading to pathological neovascularization.
For the last 10 years, many groups have focused on understanding the basic mechanism responsible for the diabetes-associated defect in EPC function. Correcting this defect may allow diabetic patients to use their own EPCs to repair injured retinal and systemic vasculature. Specifically in the retina, correction of this dysfunction may prevent early and intermediate stages of vasodegeneration (thus enhancing vessel repair), reverse ischemia, and prevent the progression to the late stages of DR. However, these findings on the changes of EPCs and their correlation to various complications in diabetes have been inconsistent. Clearly, we need more studies to clarify changes in diabetes and the role EPCs play before patients can use them therapeutically (161).
Summary
Hyperglycemia initiates its adverse effects by increasing its metabolites in vascular cells; this can cause specific changes in vascular functions, such as those mediated by PKC or ROS activation. However, increases in glucose metabolism can also generate nondiabetic specific toxic products (such as oxidants, AGE, and methylglyoxal), which accelerate the specific toxic actions of hyperglycemia and cause microvascular pathologies. The specific needs of various tissues (such as the retina, glomeruli, and the peripheral neuron), the importance of the various functions that are changed by hyperglycemia, and the protective responses generated by each tissue all modulate specific pathologic manifestations. Thus, treatments that prevent and delay the progression of diabetic microvascular complications must do the following: (1) eliminate hyperglycemia; (2) inhibit the major mechanisms that hyperglycemia activates to induce vascular dysfunction; (3) neutralize accelerants, such as inflammation and oxidative stress; and (4) activate tissue-specific protective factors.
Retinal Microvascular Disease
Introduction
DR and DN are considered the quintessential microvascular complications of diabetes. These complications are frequent and may result in severe visual impairment and renal failure and are associated with poor QOL. Plasma glucose and HgA1c concentration thresholds for the diagnosis of diabetes have been established based upon the correlation of these chemical indices to microvascular changes in the retina, as observed on fundus photography. We review in this study the natural history, pathogenesis, and epidemiology of DR development and progression. We also review the impact of risk factors and comorbidities on DR development and progression and briefly discuss clinical management.
Natural history of DR
We know that a number of subclinical changes in the physiology of the retinal vessels (retinal microaneurysms and blot hemorrhages that can be detected by ophthalmoscopy) occur in persons with diabetes prior to the appearance of the first clinical signs (175). These changes include disruption of the blood-retinal barrier and increased RBF, most likely due to disturbances in autoregulation. Clinicians do not routinely measure this. Another early change is widening of the retinal venules. One study (in the absence of any other clinical signs of DR) associated a widening of the retinal venules by 10 μm over a 4-year period, with a 26% increase in the risk of incident DR over the next 6 years (175). These data suggest that measuring venular diameter may provide an even earlier clinically measurable stage of DR than retinal microaneurysms and blot hemorrhages.
Retinal microaneurysms are small outpouchings of the retinal capillaries. Retinal blot hemorrhages often follow but may appear prior to microaneurysms. Both lesions are not pathognomonic of diabetes, as they may appear in 2% to 11% of persons aged 40 years or older without diabetes and are often associated with hypertension (176).
After the appearance of retinal microaneurysms and/or blot hemorrhages, retinopathy may progress with the appearance of other nonproliferative retinal abnormalities, such as retinal hard exudates (lipid deposits in the retina resulting from lipoprotein leakage from the retinal microvasculature), cotton wool spots [small localized infarctions of the nerve fiber layer of the retina (also called soft exudates)], intraretinal microvascular abnormalities (collateral dilated capillary channels in areas of retinal ischemia), and venous beading (irregular dilation of retinal veins associated with significant retinal ischemia). Retinopathy may further progress to the proliferative stage, characterized by the development of new retinal blood vessels and fibrous tissue at the optic disc or near venules elsewhere in the retina. These new retinal blood vessels may bleed, resulting in preretinal and vitreous hemorrhage, and the fibrovascular tissue can cause traction on the macula, resulting in loss of vision. Although the progression of proliferative disease in untreated eyes is the usual course, spontaneous regression of the new retinal vessels may occur at any stage. Macular edema (thickening of the retina in the macular area) may also develop and regress without treatment. Although clinicians can identify the source and extent of the leakage in the macula by fluorescein angiography, they now usually confirm the retinal thickness and response to treatment in eyes with macular edema by spectral domain optical coherence tomography (177). Visual loss may result from macular edema or proliferative retinopathy.
Although retinopathy is believed to result from the effects of hyperglycemia, hypertension, and high lipid levels on the retinal microvasculature (see the section on epidemiology), there is also growing evidence of concurrent early neurodegenerative changes of the retinal neuronal cells (e.g., retinal ganglion and Mueller cells, cones), which (in whole) we generally refer to as the neurovascular unit (178). The neurodegenerative changes are associated with impaired control of the metabolism of neurotransmitter glutamate, apoptosis in the ganglion cells and inner nuclear layer cells, and the activation of microglial cells, resulting in localized inflammation (178–180). These neuronal changes result in a loss of synaptic activity and loss of dendrites. Levels of brain-derived neurotrophic factor are also reduced (181, 182). Researchers have postulated that these neuronal changes contribute to the development of retinopathy by impairing autoregulation and vascular integrity in persons with T2DM (183, 184). Retinal flicker responses (a neurologic function) are impaired before the onset of retinopathy in people with T1DM (183, 185). Neuropathy may involve nerves in the cornea and pupil in addition to the retinal neuron. Retinal neurodegenerative changes may manifest clinically as a decreased ability to discriminate blue from yellow color, decreases in dark adaptation with decreases in the electroretinograph a-wave and b-wave amplitudes, changes in the oscillatory potentials generated by inner retinal neurons, and changes in contrast sensitivity (186). We have a poor understanding of the temporal and causative relationships between the neuropathic and retinopathic changes.
Pathogenesis
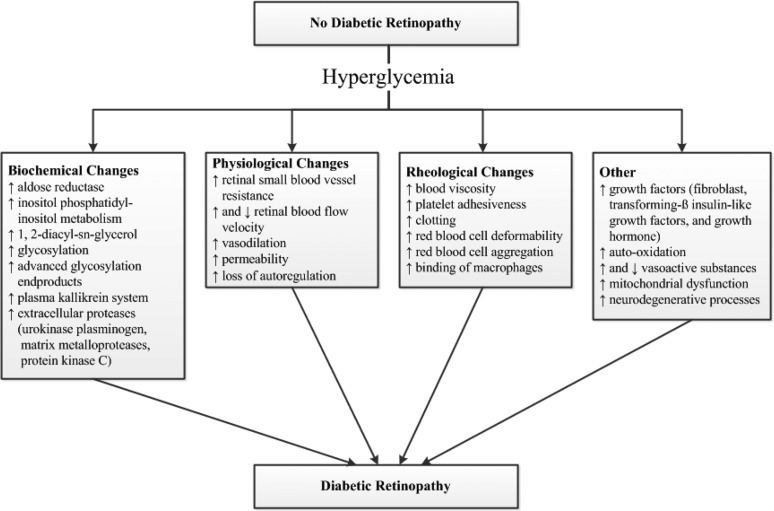
The pathogenesis of DR is complex (see Biochemical Pathways of Microvascular Injury). A number of possible mechanisms appear to contribute (157, 178, 187, 188) (Fig. 3). Hyperglycemia is an important initiator of the disease process. Studies have shown that hyperglycemia induces biochemical, physiological, rheological, hormonal, and other changes that are involved in the pathogenesis of DR (Fig. 3). These abnormalities are associated with the development of a number of anatomic changes in the diabetic retina, which include pericyte loss, endothelial cell abnormalities, acellular capillaries, increased BM thickness, and retinal pigment epithelial abnormalities.
Figure 3.
Conceptual diagram showing the effect of hyperglycemia on different mechanisms hypothesized to be involved in the pathogenesis of diabetic retinopathy.
It is likely that the initiation and progression of DR are due to a complex relationship among a number of these factors and pathways, which vary at different stages in the natural history of DR and also vary from individual to individual.
Epidemiology
Prevalence
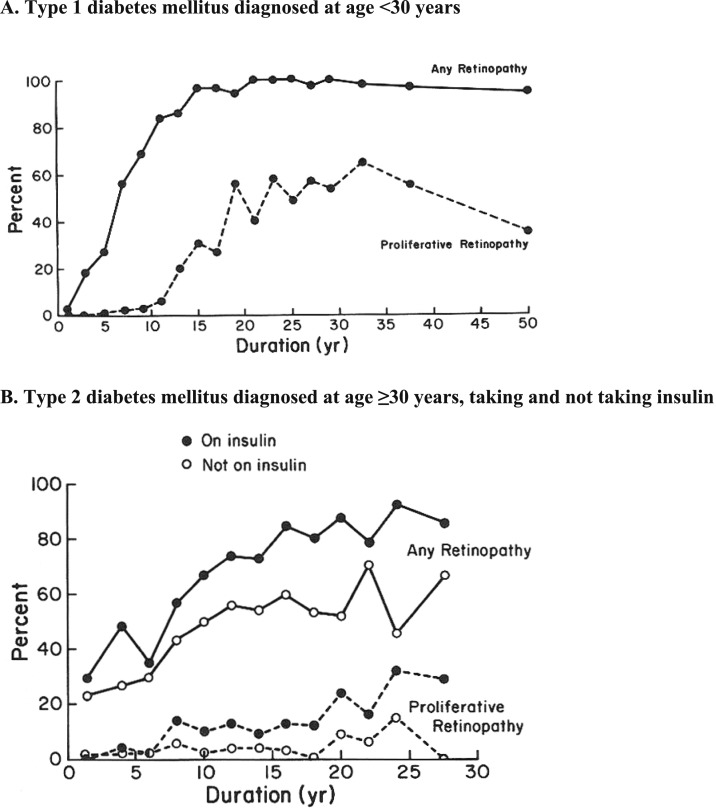
Epidemiologic population-based studies have provided important descriptive information on the prevalence, incidence, and progression of DR, as well as information on modifiable and potentially intervenable risk factors, such as glycosylated hemoglobin, BP, and lipid levels. The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) provided data on the prevalence and severity of DR by duration of diabetes (Fig. 4) and the 4-year incidence and progression of DR by age, sex, and duration of diabetes in younger-onset persons with T1DM and older-onset persons with T2DM (189–192). In the WESDR, the prevalence of DR in patients with T1DM was 17% in those with <5 years of diabetes vs 98% in those with 15 or more years of diabetes; proliferative retinopathy was absent in those with a shorter duration of diabetes, but present in 48% in those with 15 or more years of diabetes. For persons with older-onset T2DM for <5 years vs 15 or more years, the prevalence of any retinopathy was 28% vs 78% and the prevalence of any proliferative retinopathy was 2% vs 16%, respectively. The WESDR cohort is 99% white. Data indicate a higher prevalence of retinopathy in Mexican Americans and blacks with T2DM compared with whites (Table 1), although the data reflect prevalence estimates from different time periods (192, 193).
Figure 4.
Prevalence of any retinopathy and proliferative retinopathy in persons with diabetes by type/onset and duration in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. (A) T1DM diagnosed at age <30 years. (B) T2DM diagnosed at age ≥30 years, taking and not taking insulin.
Table 1.
Prevalence of Diabetic Retinopathy and Vision-Threatening Diabetic Retinopathy in US Individuals Age 40 and Older
| Crude Prevalence of DR |
|||||||
|---|---|---|---|---|---|---|---|
| Diabetes Population |
US Population |
||||||
| Characteristic | Na | Nb | Weighted Size (in Thousands)c | 95% CI | P Value | 95% CI | P Value |
| Total | 1006 | 324 | 4202 | 28.5 (24.9–32.5) | 3.8 (3.2–4.5) | ||
| Age, years | 0.64 | <0.001 | |||||
| 40–64 | 575 | 189 | 2588 | 28.0 (23.0–33.6) | 3.1 (2.4–3.9) | ||
| ≥65 | 431 | 135 | 1613 | 29.5 (25.4–33.9) | 6.1 (5.1–7.3) | ||
| Sex | 0.04 | 0.046 | |||||
| Male | 504 | 173 | 2257 | 31.6 (26.8–36.8) | 4.3 (3.5–5.3) | ||
| Female | 502 | 151 | 1944 | 25.7 (21.7–30.1) | 3.3 (2.7–4.1) | ||
| Race/ethnicity | 0.008 | <0.001 | |||||
| Non-Hispanic white | 396 | 107 | 2507 | 26.4 (21.4–32.2) | 2.9 (2.2–3.9) | ||
| Non-Hispanic black | 306 | 119 | 1006 | 38.8 (31.9–46.1) | 9.6 (7.7–11.9) | ||
| Mexican American | 197 | 70 | 401 | 34.0 (26.7–42.1) | 6.7 (5.4–8.4) | ||
| Other | 107 | 28 | 286 | 19.7 (12.5–29.7) | 3.3 (2.3–4.7) | ||
| Crude Prevalence of Vision-Threatening DR | |||||||
|---|---|---|---|---|---|---|---|
| Total | 1006 | 62 | 655 | 4.4 (3.5–5.7) | 0.6 (0.5–0.8) | ||
| Age, years | 0.41 | 0.009 | |||||
| 40–64 | 575 | 36 | 376 | 4.1 (2.8–5.8) | 0.4 (0.3–0.7) | ||
| ≥65 | 431 | 26 | 278 | 5.1 (3.5–7.3) | 1.0 (0.7–1.5) | ||
| Sex | 0.67 | 0.81 | |||||
| Male | 504 | 24 | 298 | 4.2 (2.8–6.1) | 0.6 (0.4–0.9) | ||
| Female | 502 | 38 | 356 | 4.7 (3.2–6.9) | 0.6 (0.4–0.9) | ||
| Race/ethnicity | 0.006 | <0.001 | |||||
| Non-Hispanic white | 396 | 13 | 304 | 3.2 (2.0–5.1) | 0.4 (0.2–0.6) | ||
| Non-Hispanic black | 306 | 28 | 241 | 9.3 (5.9–14.4) | 2.3 (1.5–3.6) | ||
| Mexican American | 197 | 16 | 85 | 7.3 (3.9–13.3) | 1.4 (0.8–2.7) | ||
| Other | 107 | 5 | 22 | 1.6 (0.6–3.8)d | 0.3 (0.1–0.6) | ||
Data were obtained from the National Health and Nutrition Examination Surveys, 2005 to 2008 (193).
Abbreviation: NHANES, National Health and Nutrition Examination Surveys.
Number of participants with diabetes in NHANES, 2005 to 2008.
Number of participants with diabetes who had DR or vision-threatening DR in NHANES, 2005 to 2008.
Weighted total number of US adult population who had DR or vision-threatening DR.
Estimate is considered unreliable because relative standard error is >30%.
Incidence
The duration of diabetes is associated with the incidence and progression of retinopathy in those with younger-onset T1DM. In the WESDR, half of the people with <5 years of diabetes at baseline and no retinopathy (n = 317) went on to develop retinopathy 4 years later (191). For those with >5 but <15 of years of diabetes at baseline, there were too few persons with no retinopathy at baseline to reliably calculate incidence by duration of diabetes; however, the longer the duration of diabetes, the greater the incidence of progression over the following 4 years (191). Within duration-specific groups, the incidence of retinopathy, proliferative retinopathy, and macular edema was higher in Mexican Americans with T2DM than in whites (194).
Risk factors
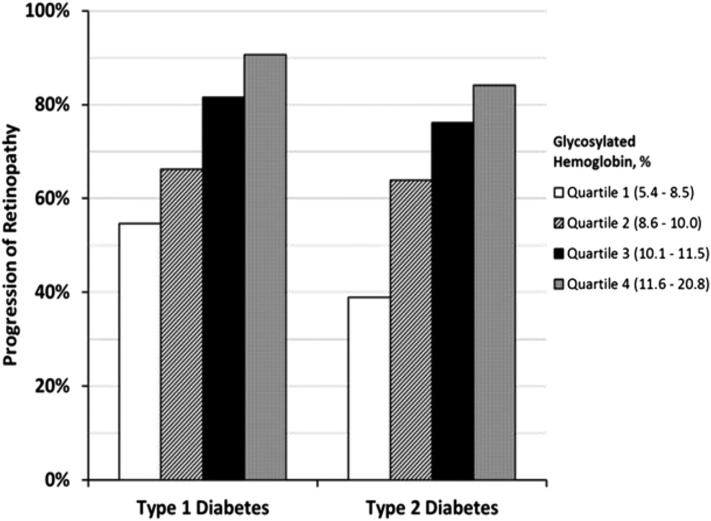
Glycemia
The WESDR (Fig. 5), DCCT, and the Epidemiology of Diabetes Interventions and Complications (EDIC) studies confirmed the role of glycemic control as a critical risk factor preceding the development and progression of DR in persons with T1DM.
Figure 5.
Test of trend P < 0.001 for both groups.
The UKPDS (195) and the Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Eye studies made the same conclusion regarding persons with T2DM (1, 196–198). One can see a decline in the levels of A1c when examining the trends over >30 years of follow-up of the group with T1DM in the WESDR (Table 2) (196).
Table 2.
Mean Glycosylated Hemoglobin A1c Levels (%) at Each Examination in the Wisconsin Epidemiologic Study of Diabetic Retinopathy, 1980–2013
| Baseline 1980–1982 | 4-Year 1984–1986 | 10-Year 1990–1992 | 14-Year 1994–1996 | 25-Year 2005–2007 | 33-Year 2012–2013 | |
|---|---|---|---|---|---|---|
| Glycosylated hemoglobin A1c, % | 10.1 | 9.4 | 9.3 | 8.9 | 7.6 | 7.6 |
ACCORD-Eye was a substudy of the ACCORD trial, a RCT comparing the effects of intensive glycemic control (A1c <6.0%) with standard glycemic control (A1c between 7.0% and 7.9%) that further randomized BP and lipid medication for high levels of each. The aim of this substudy was to examine the effects of the primary and secondary randomizations on the progression of DR in persons with T2DM. In a relatively short period (4 years), the study found a lower risk of DR progression (7.3%) in those in the intensive-glycemic-control group vs those in the standard-therapy group (10.4%) [adjusted OR 0.67; 95% confidence interval (CI): 0.51 to 0.87; P = 0.003] (190).
Researchers terminated the intensive glycemic-control phase of the ACCORD-Eye trial early because of a statistically significant 22% increase in overall mortality in the intensive glycemic-control group (196) of the larger study. This early closure of the intensive glycemic-control phase diminished the power to observe a protective effect for the severe microvascular endpoints, such as proliferative DR and clinically significant macular edema, which usually evolve over a longer period. In the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, intensive blood glucose control did not have any effect on any of the retinopathy and vascular outcomes in patients with T2DM (196).
The results of the UKPDS, ACCORD, ADVANCE, and the Veterans Affairs Diabetes trial (199) (a RCT of intensive glycemic control in people with T2DM) have advanced the way we think about managing hyperglycemia in people with T2DM. For intensive therapy, the American Diabetes Association Guidelines suggest a target A1c level of 7.0% to reduce the risk of visual loss from DR in persons with diabetes. Clinicians most likely used this guideline to help people with T2DM manage glycemia, as the National Health and Nutrition Examination Survey reported that the number of people with T2DM taking three or more hypoglycemic drugs increased from 1999 to 2006 (200). This has been accompanied by a decrease in mean A1c from 7.8% to 7.2% from 1996 to 1997 and an increase in the percentage (from 40% in 1996 to 1997 to 54% in 2004 to 2006) of persons aged 40 years or older with T2DM that had A1c levels <7% (200). Data from the ACCORD and ADVANCE trials and Veterans Affairs Diabetes trial suggest the need to tailor intensive treatment to the individual, especially in patients with long-standing T2DM who have or who are at risk for developing cardiovascular disease (CVD). The findings from these studies may lead to a reduction in the number of persons with T2DM meeting the American Diabetes Association Guidelines of having an A1c of <7%.
Hypertension
Uncontrolled hypertension in persons with both T1DM and T2DM is associated with both DR (201) and DN (202). Data suggest that its effect on blood flow damages the retinal capillary endothelial cells, resulting in the development and progression of DR (203).
The UKPDS was designed to test whether lowering BP is beneficial in reducing macrovascular and microvascular complications associated with T2DM (204). The study assigned hypertensive participants (defined at the time of the start of the trial in the 1980s as having a mean BP reading of 160/94 mm Hg) to tight BP control (aiming for <150/<85 mm Hg) and initial captopril or atenolol treatment (adding other agents as needed) or to less-tight BP control (aiming for <180/<105 mm Hg). Tight BP control resulted in a 35% reduction in retinal photocoagulation compared with the less-tight control group. After 7.5 years of follow-up, there was a 34% reduction in the rate of retinopathy progression and a 47% reduction in the deterioration of visual acuity. Atenolol and captopril were equally effective in reducing the risk of developing these microvascular complications, suggesting that BP reduction was more important than the type of medication used to reduce it. The effects of BP control were independent of the effects of glycemic control. These findings support the recommendations for BP control in patients with T2DM as a means of preventing visual loss from DR. Two years after completing the trial, follow-up of the UKPDS cohort showed that the reduction in BP was not sustained in the group that received tight BP control (205). This was associated with loss of reductions in relative risk present during the trial for diabetes-related end points, such as death, microvascular disease, and stroke in the group receiving tight BP control, as compared with the group receiving less-tight BP control.
In the ACCORD study, hypertensive persons with T2DM were randomized to intensive BP treatment targeted to lowering the systolic BP to <120 mm Hg or to standard treatment targeted to maintaining systolic BP <140 mm Hg (206). The average systolic BP was 119 mm Hg in the intensive group and 133 mm Hg in the standard group. Despite this 14 mm Hg difference, intensive BP control was not associated with decreased progression of DR, nor was it associated with a reduction in the hazard of developing moderate loss of vision (206). There were no statistically significant interactions with glycemic or lipid control.
Other RCTs have targeted specific types of antihypertensive agents, such as renin angiotensin system blockade. The EURODIAB Controlled Trial of Lisinopril in Insulin Dependent Diabetes Mellitus reported a borderline beneficial effect of renin angiotensin system blockade on the progression of DR in patients with T1DM, independent of BP (207). The Renin-Angiotensin System Study showed that the angiotensin-converting enzyme inhibitor enalapril and the Ang II receptor blocker (ARB) losartan were both associated with a reduced progression of retinopathy compared with those not randomized to BP medications, but these agents were not associated with the progression of nephropathy in subjects with T1DM (100). However, the Diabetic Retinopathy Candesartan Prevent and Protect trials reported that candesartan cilexetil did not result in a statistically significant reduction in the progression of DR in persons with T2DM (P = 0.0508) or in the incidence or progression in those with T1DM (208, 209). Neither the ACCORD nor the ADVANCE studies found that lowering BP in those with mild hypertension or in those already normotensive was of benefit in preventing the incidence and progression of DR.
Together, these data suggest that lowering BP in those who have poorly controlled hypertension provides the greatest benefit in preventing the progression of retinopathy, as shown in the UKPDS. The type of antihypertensive medication used was less important; however, the renin angiotensin system blockade had the greatest efficacy in those with T1DM at moderate risk of DR progression. Aggressive BP control <120 mm Hg was not indicated in persons with T2DM with mild or no hypertension. The American Diabetes Association recommends that people with hypertension should be treated to a systolic BP goal of <140 mm Hg and that patients with BP >120/80 mm Hg should be advised on lifestyle changes to reduce BP (210).
Serum lipids
Epidemiological studies have associated serum total and low-density lipoprotein (LDL) cholesterol and triglycerides with the severity of DR and diabetic macular edema (211–214). In the Early Treatment Diabetic Retinopathy Study, persons with higher levels of serum triglycerides, LDL cholesterol, and very LDL cholesterol at baseline had a 50% increased risk of developing hard exudates in the macula and decreased visual acuity and a 23% increased risk of developing proliferative DR (206). In the DCCT/EDIC Study, those with higher serum total cholesterol, LDL cholesterol, and triglycerides (fourth vs first quartile range) had a two- to threefold increase in the odds of developing macular edema (215).
Pilot studies to examine the efficacy of statin therapy in preventing or reducing the severity of macular edema have suggested a possible short-term benefit (216–218). In the Fenofibrate Intervention and Event Lowering in Diabetes Study, fenofibrate was shown to reduce the need for laser treatment of DR in persons with T2DM (hazard ratio 0.69 95% CI: 0.56, 0.84, P = 0.0002), although the effect was not clearly due to lowering of plasma lipid concentrations (219). Fenofibrate (in the context of simvastatin use in the ACCORD-Eye study) was associated with a significant decrease in the three-step or greater group [6.5% in the fenofibrate group vs 10.2% in the placebo group (hazard ratio 0.60 95% CI: 0.42 to 0.87, P < 0.006)]. There was no effect on moderate vision loss. A recent study, which used the HealthCore Integrated Research DatabaseSM containing administrative claims data for >35 million Americans, examined microvascular complications of diabetes (e.g., DR and DN). The incidence of microvascular complications of diabetes was lower in patients who attained their goal of lower serum LDL cholesterol, higher serum high-density lipoprotein cholesterol, and lower serum triglycerides compared with those who did not (220). In summary, accumulating evidence suggests that lipid lowering may have a role in limiting the development and progression of DR and macular edema, but the pathways leading to this protective effect are still unclear.
Other risk factors
There is evidence, mostly from clinical studies, that AGEs and oxidative stress are associated with complications of diabetes. AGEs result from the long-term exposure of proteins and lipids to hyperglycemia (via nonenzymatic glycation of these molecules). AGEs have been identified in renal lesions of persons with nephropathy as well as in atherosclerotic streaks in large blood vessels in persons with diabetes (221–224). The accumulation of AGEs in people with diabetes is thought to lead to retinopathy, nephropathy, neuropathy, CVD, and cognitive dysfunction by directly damaging the tissue. AGEs may also lead to increased oxidative stress, endothelial dysfunction, inflammation, thrombosis, and fibrinolysis, and they adversely affect the RAS. All of these processes are hypothesized to be pathogenetic mechanisms for these complications (93, 222, 225–228). Some, but not all, clinical studies have associated serum AGEs with diabetic complications, independent of A1c levels.
The body normally generates oxidizing compounds as an important component of the inflammation and tissue repair processes (229, 230). It represents part of the normal defense mechanism against invading microorganisms and malignant cells and occurs during tissue healing and remodeling. The retina exists in a highly oxidizing environment and is thought to be especially vulnerable to oxidative stress. Animal studies have shown a beneficial effect of antioxidants (e.g., nicanartine, vitamin E, and ALA) on retinopathy lesions in diabetic animals, suggesting that oxidative stress may be involved in the pathogenesis of DR (231–233). Data from some studies have led researchers to hypothesize that oxidative stress in persons with diabetes is involved in the pathogenesis of not only DR but also DN, myocardial infarction, and cognitive dysfunction (234–240). Oxidative stress in those with diabetes has been attributed to hyperglycemia with an increase in ROS through glucose auto-oxidation, nonenzymatic protein glycation, decreased antioxidant status, and reduced ROS removal (241).
Genetic factors
Studies have reported familial clustering of DR, and this is compatible with the notion that genetic factors may contribute to developing DR (242, 243). It is possible that similarities in retinopathy severity within families are related to how genes affect glycemia and BP (244, 245). Control of these factors may influence the apparent effect of genes on retinopathy. Also, because retinal microaneurysms and blot hemorrhages are not specific to diabetes, their presence (in the absence of signs of more severe retinopathy) may lead to misclassification, resulting in inconsistent associations of candidate genes with early stages of DR compared with more severe stages of DR (246).
DR has been associated with mitochondrial genes (69, 247), an AR gene (69, 248), endothelial NOS (249), paraoxonase (an enzyme that prevents oxidation of LDL cholesterol) (250), tumor necrosis factor-β NcoI gene (251), ε4 allele of the apolipoprotein E gene (252), intercellular adhesion molecule-1 (253), α2β1 integrin gene (involved with platelet function) (254), and cytokine VEGF genes, but subsequent studies have not consistently replicated these associations (255, 256). Two recent studies, one a meta-analysis (257) and one from separate studies in France and Denmark (258), failed to find definitive evidence of the effects of genes associated with serum levels of VEGF on DR.
Comorbidity and mortality
In the WESDR, the risk of developing systemic complications (e.g., myocardial infarction, stroke, lower extremity amputation, DN) was higher in those with proliferative DR compared with those with no or minimal retinopathy at baseline (Table 3) (259).
Table 3.
The Relative Risk for the Prevalence and 4-Year Incidence of Myocardial Infarction, Stroke, and Amputation of Lower Extremities Associated With Presence of Proliferative Diabetic Retinopathy, Adjusted for Age in the Wisconsin Epidemiologic Study of Diabetic Retinopathy
| Myocardial Infarction RR (95% CI) | Stroke RR (95% CI) | Amputation of Lower Extremity RR (95% CI) | |
|---|---|---|---|
| T1DM | |||
| Prevalence | 3.5 (1.5–7.9) | 2.6 (0.7–9.7) | 7.1 (2.6–19.7) |
| Incidence | 4.5 (1.3–15.4) | 1.6 (0.4–5.7) | 6.0 (2.1–16.9) |
| T2DM, taking insulin | |||
| Prevalence | 0.8 (0.4–1.4) | 1.2 (0.6–2.4) | 4.2 (2.3–7.9) |
| Incidence | 1.2 (0.5–3.4) | 2.9 (1.2–6.8) | 3.4 (0.9–13.2) |
| T2DM, not taking insulin | |||
| Prevalence | 0.3 (0.0–2.4) | 2.9 (0.9–9.4) | 5.2 (0.6–45.0) |
| Incidence | 1.5 (0.2–12.5) | 6.0 (1.1–32.6) | 7.0 (0.8–64.4) |
Source: Table 9 from Klein et al. (259).
Abbreviation: RR, relative risk.
In those with T1DM, while adjusting for age and sex, DR severity was associated with all-cause and ischemic heart disease mortality. In persons with T2DM, DR severity was associated with all-cause and ischemic heart disease mortality, as well as with stroke (260). After adjusting for systemic factors, these associations remained only for all-cause and stroke mortality in persons with T2DM. These findings suggest that severe DR is an indicator for increased risk of death, and may identify individuals who should be under care for CVD. Other studies have reported this finding (261–263). The higher risk of CVD in persons with more severe DR may be partially due to the association of severe retinopathy with CVD risk factors, such as hyperglycemia, hypertension, platelet aggregation, and chronic renal disease.
Prevention of incidence or progression of DR
The primary method currently used to prevent or retard the progression of DR is the judicious use of hypoglycemic agents. However, there is evidence that other treatments may also be protective. As noted previously, studies have reported that angiotensin-converting enzyme inhibitors targeting the RAS (100, 204, 207), as well as fenofibrate (206, 219), reduce the risk of progression of DR in those who are normotensive, independent of changes in BP and the lowering of uncontrolled BP (regardless of the antihypertensive medication used). However, RCTs of inhibitors of AR, PKC, and metalloproteinases have not shown efficacy in preventing the incidence and progression of DR in persons with diabetes (264).
Current treatment of severe DR
Standard treatment of proliferative DR is still panretinal photocoagulation (265); for diabetic macular edema it is focal laser treatment (266). RCTs have shown the efficacy of intravitreally administered VEGF inhibitors (267) and steroids in treating proliferative DR and diabetic macular edema (268). However, steroid injections are associated with increased risk of high intraocular pressure (269), glaucoma (270), and cataract surgery (271). There are times when retinopathy is so severe that vitrectomy is needed to attempt to maintain or restore vision after the nonresolution of a vitreous hemorrhage and to decrease the risk of tractional retinal detachment (272). Such treatment, although not without its own risks, has been found (on average) to be successful in maintaining visual function (273) and is still the best alternative for late-stage proliferative disease.
Summary
Although there is strong evidence of the efficacy of intensive glycemic and BP control in persons with diabetes, and therapeutic guidelines for these treatments exist, recent findings from clinical trials suggest the each person be treated individually, balancing microvascular and macrovascular risk against the risk of hypoglycemia and CVD mortality. The ACCORD trial clearly taught us that there is no single recipe for the glycemic management of CVD risk in T2DM. The old principles still hold: treat each patient as an individual and first do no harm (274).
Microvascular Disease and the Brain
Introduction
Cognitive impairment is a common complication in T2DM (275). Compared with the general population, the risk of dementia is 1.5 to 2.5 times greater for adults with T2DM (276–278). Recent data suggest that microvascular pathologies play an important role in the associations between T2DM, Alzheimer’s disease (AD), and vascular subtypes of dementia, but little is known about the microvascular mechanisms underlying these associations. There is increased interest in illuminating these mechanisms to tailor and implement intervention strategies aimed at preventing dementia, AD, and cognitive decline in T2DM, as well as in aging populations with T2DM risk factors.
Much work has focused on T2DM-related microvascular complications in peripheral organ systems, including the retina, kidney, and peripheral nerves. Although the brain is seldom discussed as a site of microvascular complications in T2DM, diabetes-associated vascular risk factors predispose individuals to both macrovascular and microvascular complications in the CNS (see Table 4). In particular, T2DM is an established risk factor for cerebral small vessel disease, as well as thrombo-embolic stroke (279). Cerebrovascular damage in the form of small vessel disease is likely to be a major factor in the association between T2DM and dementia and could explain the increased risk of vascular dementia. Furthermore, data from experimental studies also suggest that metabolic disturbances associated with T2DM may accelerate the development of AD-type pathologies (280, 281).
Table 4.
Select Studies Linking Diabetes Complications With Microvascular Complications in the Brain Through Shared Microvascular Mechanisms
| Peripheral Microvascular Complication | Shared Microvascular Mechanisms | Brain Outcomes |
||||
|---|---|---|---|---|---|---|
| Brain Atrophy | WMH | Infracts | CMBs | Cognitive | ||
| Retinopathy | Arteriovenous nicking | Cooper et al. (282) | Qiu et al. (283) | Qiu et al. (284) | ||
| Microhemorrhages | Cooper et al. (282) | Qiu et al. (283) | Qiu et al. (284) | |||
| Venular dilation | Ikram et al. (285) | Cooper et al.; Ikram et al. (282, 285) | Qiu et al. (284) | |||
| Nephropathy | Hypertension | Reviewed Beauchet et al. (286) | Verhaaren et al.; King et al. (287, 288) | Reviewed Loitfelder et al. (289) | Reviewed Beauchet et al. (286) | |
| Creatinine | Rajagopalan et al. (290) | Reviewed Vogels et al. (291) | ||||
| Cystatin C | Rajagopalan et al. (290) | (292) | Umemura et al. (293) | |||
| Glomerular filtration rate | Reviewed Vogels et al. (291) | Kurella Tamura et al. (294) | ||||
| Islet amyloid polypeptide | Jackson et al. (295) | |||||
| Neuropathy | Hyperglycemia | Manschot et al.; Launer et al.; Ursache et al. (296–298) | Manschot et al. (298) | Crane et al. (299) | ||
| AGEs, RAGE | Hudson et al. (300) | Hudson et al. (300) | ||||
| Vitamin B12 deficiency | Tangney et al. (301) | Feng et al.; de Lau et al.; Tangney et al. (301–303) | Kealey et al. (304) | Tangney et al. (301) | Smith et al. (303) | |
Unexplored common features in humans include the following: pericyte loss, microvascular reactivity, AGEs, ROS, etc.
Mechanisms for microvascular complications
Hyperinsulinemia and impaired insulin signaling
Insulin is known to have multiple functions in the CNS (305). Although there is some controversy regarding whether insulin is synthesized in the adult brain, circulating insulin in the bloodstream is readily transported across the blood-brain barrier (BBB) by a saturable receptor-mediated process (306–308). Hyperinsulinemia and insulin resistance may downregulate insulin transport across the BBB, leading to reduced insulin levels in the CNS (309). In select brain circuits (such as the hippocampus), insulin-containing neurons, insulin receptors, and glucose transporter isoforms 4 and 8 are colocalized (310, providing an infrastructure for insulin-stimulated glucose uptake into neurons to support cognitive function. In addition, other insulin-related mechanisms have also been implicated in normal hippocampal functioning (311). Insulin receptors are located in the synapses of both astrocytes and neurons, where insulin signaling contributes to synaptogenesis and synaptic remodeling (311). Insulin also modulates levels of neurotransmitters in the CNS (such as acetylcholine and norepinephrine) that influence cognitive function (312, 313). Given the multifactorial role of insulin in the brain, maintaining proper insulin homeostasis and insulin receptor activity may be essential for proper brain function and memory (310).
Chronic hyperinsulinemia is a key early factor in the process leading to insulin resistance and T2DM that may potentially mediate the relationships between T2DM and proinflammatory states, microvascular disease, and AD pathology. Whereas anti-inflammatory effects are observed with low doses of insulin, long-term hyperinsulinemia may exacerbate the inflammatory response and increase markers of oxidative stress (314).
Intravenous infusions of insulin to levels associated with insulin resistance increased the levels of F2-isopostanes and cytokines in cerebrospinal fluid (315). Hyperinsulinemia may also potentiate AD pathology [e.g., amyloid β (Aβ) plaques] by causing increased production but reduced extracellular degradation of Aβ, impaired insulin signaling, oxidative stress, inflammatory mechanisms, and coupling of neuronal components by AGEs (316). The amyloid precursor protein produces Aβ (a peptide of 36 to 43 amino acids). Although best known as a main component of amyloid plaques in association with AD (e.g., Aβ42), there is evidence that Aβ is a highly multifunctional peptide with significant nonpathological activity, including protecting against metal-induced ROS, modifying cholesterol transport, and potentially acting as a transcription factor (317). Hyperinsulinemia, at levels associated with insulin resistance, can elevate inflammatory markers and Aβ42 in the periphery and the CNS, which may increase the risk of AD (305). Interestingly, AD pathology may have direct effects on insulin receptors and their signaling. Soluble Aβ can disrupt brain insulin signaling by binding to the insulin receptor (318), suggesting interactions between T2DM, glucose metabolism in the brain, and AD pathology.
Aβ aggregation also occurs outside the CNS and often is associated with increased cell death (319). Aβ deposits also occur from the aggregation of the polypeptide hormone islet amyloid polypeptide (IAPP). IAPP aggregates into Aβ deposits and may induce the depletion of islet β-cells in T2DM (319). Aβ deposits are the most typical morphological islet lesion in T2DM. A recent study reported mixed IAPP and Aβ deposits in the brains of patients with T2DM and vascular dementia or AD (295). The study found IAPP oligomers and plaques in the temporal lobe gray matter, blood vessels, and perivascular spaces in T2DM patients, but not controls. The study also detected IAPP deposition in blood vessels and brain parenchyma of patients with late-onset AD without clinically apparent T2DM.
Hyperglycemia
In many prediabetic adults, the degree of insulin resistance increases as insulin secretion by pancreatic cells declines, resulting in hyperglycemia of sufficient magnitude to warrant a T2DM diagnosis. Extracellular and intracellular hyperglycemia are two general pathophysiologic mechanisms by which hyperglycemia leads to irreversible tissue damage, even in prediabetic states (320). Chronic hyperglycemia (both cellular and extracellular) leads to glycation end product formation (discussed later). This may have particular effects on the endothelial cell where intercellular glucose appears to be a significant driver of microvascular dysfunction (see Biochemical Pathways of Microvascular Injury). It also contributes to heart disease, microvascular complications, and intracellular hyperglycemia, and may increase the risk of developing dementia. Among participants without T2DM (random glucose <120 mg/dL), higher normal plasma glucose levels are associated with an increased risk of incident dementia (299). Individuals with T2DM also had a similar relationship at glucose levels >170 mg/dL (299). High glucose levels may contribute to an increased risk of dementia through several potential mechanisms, including acute and chronic hyperglycemia and insulin resistance (321) and increased microvascular disease of the CNS (322–325).
Extracellular hyperglycemia can lead to intracellular hyperglycemia through the increased flux of glucose freely across the cell membrane of many cell types. Excess intracellular glucose not used for energy will enter the polyol pathway, leading to decreased levels of NADPH (320). NADPH plays a central role in the production of NO and GSH. NO is an important vasodilator; therefore, reductions in NADPH may limit NO production with direct pathologic effects on vasodilation throughout the body, particularly in the kidneys and brain. NADPH also prevents ROS from accumulating and damaging cells, and thus reductions in NADPH can also increase oxidative stress (326).
Oxidative stress
Oxidative stress in the cerebral parenchyma and blood vessels plays a critical role in the processes associated with cerebrovascular dysfunction, with NADPH oxidase being a major source of ROS (327–329). ROS can alter vascular regulation through processes involving the formation of peroxynitrite from the reaction between NO and superoxide radical. Consequently, oxidative stress and ROS resulting from mitochondrial dysfunction have been strongly implicated in brain aging, AD, and vascular dementia (326, 330). Overall, several factors related to hyperglycemia may contribute to a chronic hypoperfusive state leading to microscopic tissue damage and regional specific syndromes (331).
Advanced glycation end products
Another direct result of high circulating levels of unbound glucose is the formation of AGEs. AGEs accumulate with age in the human brain, and may be one possible mechanism linking T2DM to cognitive impairment (332). One study found AGEs in hallmark neuropathologic features (e.g., neurofibrillary tangles and Aβ plaques) in patients with AD (333). Older adults with cerebrovascular disease have higher AGEs in cortical neurons and cerebral vessels, which are related to the severity of cognitive impairment (334). RAGE most likely plays an important role in the brain with respect to inflammation (335) and AD pathology. RAGE is expressed in astrocytes, microglia, and neurons, and is also highly expressed in the endothelial cells within the brain (336). RAGE expression in the endothelium has important consequences for vascular inflammation and BBB integrity (337). BBB integrity is an essential factor in Aβ equilibrium in the brain, which is regulated through LDL receptor-related protein 1 and RAGE. The RAGE protein mediates the influx, and the LDL receptor-related protein 1 mediates the efflux of amyloid protein through the BBB (336). Patients with T2DM not only produce endogenous AGEs at a higher rate, but they have an upregulation of RAGE expression in the brain (338). Increased RAGE expression in the T2DM brain might create an imbalance between the rates of influx and efflux of Aβ through the BBB, promoting uptake of Aβ into the brain and subsequent deposition of Aβ plaques (339).
Summary of mechanisms
The characteristic metabolic deregulation of T2DM promotes changes in insulin signaling, glucose uptake, ROS formation, and inflammation in the microvasculature that affect BBB integrity. In the brain, hyperinsulinemia promotes insulin resistance and reduces insulin signaling, which is essential to glucose uptake, amyloid regulation, and vascular function. T2DM-associated hyperglycemia leads to the formation of proinflammatory AGEs, which increase RAGE expression in the endothelium and brain. RAGE expression is thought to play a crucial role in BBB integrity through regulating inflammation and the flux of Aβ across the BBB. These factors most likely play important roles in the development of microvascular brain complications and AD pathology seen in T2DM. Considerable progress has been made in imaging microvascular complications and Aβ pathology in living humans, which has enabled a better characterization of microvascular disease in the brain.
Assessment of brain complications in T2DM
Neuroimaging is currently the best way to examine the effects of microvascular disease and other brain abnormalities on the human brain in vivo. Neuroimaging techniques aimed at studying microstructural cerebral small vessel disease are the most common. More recent advances enable the imaging and quantification of microstructural and functional abnormalities of the brain, including regional cerebral blood flow (CBF) and functional activation. Recent concerted efforts to standardize the study of small vessel disease have resulted in a position paper from the Standards for Reporting Vascular Changes on nEuroimaging (340). This paper sets the groundwork for the systematic evaluation of brain structure and function necessary for research using in vivo brain imaging.
Brain atrophy
Longitudinal observational cohorts of brain aging in the general population have shown that brain volume declines as people get older (341). After adolescence, the total brain volume tends to slowly decrease with age until the fifth to sixth decade of life when volume loss accelerates (342, 343). The average rate of decline is estimated to between 0% and 4%/year (343, 344). It is generally accepted that atrophy is not consistent across brain regions and tissue compartments (343, 345, 346). Brain atrophy is thought to be a result of both macrovascular and microvascular abnormalities in the brain.
T2DM is associated with greater total brain atrophy (347, 348), with possible preferential gray matter volume loss in cerebrum (348), putamen (348), medial temporal (349–351), and frontal (350, 352) regions. The longitudinal decline in total cerebral brain volume is associated with increasing age, T2DM, hypertension, current smoking, and evidence of cerebral small vessel disease (353). Cerebellar atrophy shares similar risk factors with longitudinal cerebral atrophy, including T2DM, higher serum glucose, and evidence of cerebral small vessel disease (353), but appears to be unrelated to hypertension and smoking or heavy drinking (344, 353). Although cerebral and cerebellar volumes do not entirely overlap, T2DM is the strongest common factor related to smaller volumes in both parts of the brain (353). T2DM is associated with enlargement of the ventricles and higher white matter hyperintensities (WMHs) (354). Individuals with T2DM also had greater longitudinal changes in these measures over 4 years of follow-up, resulting in smaller brain volumes and increases in WMHs and ventricle enlargement (354).
Recently, studies have directly assessed the relationship between insulin sensitivity and regional brain volume differences. Higher basal insulin resistance, measured by the homeostatic assessment of insulin resistance, was associated with less gray matter in hippocampus and prefrontal regions of the brain among adolescents and young adults without T2DM (297). In cohorts of late middle-aged adults, greater insulin resistance was associated with both increased atrophy in regions affected by early AD (355) and worse cognitive performance (356). In longitudinal studies, higher fasting insulin showed small but significant correlations with gray matter atrophy in orbitofrontal cortex and hippocampus (357). Some studies, however, have not found a relationship between insulin resistance and hippocampus volume in late middle-aged (358) or elderly (359) adults.
The pathological basis for this T2DM-associated global and regional brain atrophy still needs to be resolved. It is likely that T2DM-associated factors, including glucose homeostasis and insulin resistance, play central roles. Yet, the results from the ACCORD-Memory in Diabetes clinical trial provide an important caveat when considering the effect of glucose control on brain structure (296). They showed that intensive glycemic control targeting HbA1c to <6.0%, compared with standard strategy targeting HbA1c to 7.0% to 7.9%, resulted in slightly greater total brain volume, but did not enhance cognition. Intensive therapy was also associated with abnormal white matter and increased mortality in the intensive therapy arm. The findings from the ACCORD-Memory in Diabetes trial thus do not support intensive therapy to reduce the adverse effects of T2DM on the brain (296). However, these relationships are likely to be mediated by more detailed evidence of cerebrovascular disease and brain integrity. Atrophy is a relatively crude tool when used to assess microvascular complications in the brain.
Alterations in cerebral blood flow and glucose utilization
Clinicians can measure changes in innate brain function relating to cerebrovascular function and glucose utilization by positron emission tomography (PET) using the fludeoxyglucose F18 ligand-PET (FDG-PET) and by magnetic resonance imaging (MRI) through measures of CBF and functional connectivity known as functional MRI (Fig. 6). FDG-PET provides insight into regional glucose metabolism in the brain and changes in regional CBF.
Figure 6.
Neuroimaging measures related to cerebral small vessel disease and concomitant pathologies.
Changes in CBF and glucose utilization on FDG-PET are thought to reflect synaptic dysfunction among regional brain networks. AD is characterized by a pattern of reduced CBF and cerebral glucose hypometabolism. Reductions in FDG-PET are associated with increased AD risk and can be observed years before dementia onset (360, 361). Reductions in FDG-PET and CBF are also present in T2DM. Among individuals with prediabetes and T2DM, greater insulin resistance was associated with an AD-like pattern of reduced cerebral glucose metabolic rate in frontal, parietotemporal, and cingulate regions of the brain (362). Among cognitively normal individuals with a family history of AD, higher fasting glucose levels were significantly associated with lower cerebral glucose metabolic rate in areas differentially affected by AD (363). FDG-PET imaging studies suggest that hypometabolism of glucose in the brains of individuals with T2DM and those who go on to develop AD may be a product of a generalized metabolic dysregulation of glucose. A recent study showed that impaired glucose tolerance measured in midlife was associated with longitudinal changes in regional CBF (measured using [15]O-water PET scans) (364).
Although MRI-defined total CBF is associated with cognitive functioning, there appears to be no relative differences in total CBF between T2DM patients and controls (365). T2DM-related alterations in CBF may be regionally specific (366). In T2DM, total CBF is associated with impaired cognition and total brain volume in cross-sectional analyses, but does not appear to predict changes in cognition or brain volumes over time (367). Similar patterns have been reported with resting state functional MRIs for insulin-resistant adults (368). Individuals with T2DM have reduced functional connectivity in brain networks related to AD compared with control subjects, which was associated with insulin resistance in selected brain regions, even when there were no observed between-group differences in brain structure or cognition. Taken together, PET and MRI studies of CBF suggest early alterations occur throughout the brain in areas affected by AD, and these reductions in CBF colocalize with areas of reduced glucose utilization in the brain.
Microvascular ischemia
Ischemia due to microvascular disease manifests itself in several ways in T2DM, including WMHs, subtle alterations in white matter integrity, and lacunar or microinfarction (Fig. 6). White matter abnormalities are frequently detected as hyperintense regions on T2-weighted MRIs of the brain in an age-dependent fashion, especially in adults older than 60 years (369), and these abnormalities may be associated with increased relative risk of stroke and the presence of retinal microvascular abnormalities (369). Studies of WMH in T2DM show no consistent association. Discrepancies from early studies were attributed to methodological issues, including the use of crude visual rating scales (370). Studies using semiquantitative rating scales find more consistent relationships between WMH and T2DM, HbA1c, and diabetes duration (371). However, although some more recent studies using quantitative techniques to measure WMH volume show greater WMH volumes in T2DM compared with controls (372), others do not (348). Diabetes duration, HbA1c and insulin levels, BP, and the presence of infarcts have all been linked to WMH severity (371, 373).
WMH can be considered a downstream event of microscopic white matter abnormalities that exist before they can be visualized on T2-weighted MRI (374, 375). MRI can quantify white matter integrity in several ways. White matter swelling related to fluid influx can be visualized by magnetization transfer imaging (MTI). MTI is a quantitative MRI technique that detects subtle tissue differences that occur with brain aging, beyond the accumulation of WMH and brain atrophy. MTI correlates with macromolecular attenuation, and therefore is believed to largely reflect myelin content. Hypertension and T2DM are associated with abnormalities in MTI within the brain (376). Diffusion tensor imaging (DTI) is another form of MRI used to assess the microstructural integrity of the brain. DTI measures the diffusion (movement) of water molecules within each voxel. For example, water molecules restricted by dense membranes move less than unrestricted molecules. DTI is used to assess neuronal density in the gray matter. DTI enables the tractography of white matter tracts, which cannot be resolved using traditional MRI techniques. Furthermore, DTI enables the quantification of white matter integrity within the tracts in axonal and radial directions. Fractional anisotropy is a composite of the axonal and radial diffusivity of water molecules perpendicular to (radial) and along (axonal) the individual white matter tract. In simple terms, proper integrity of white matter tracts should result in high axonal diffusivity and high FAs along the tract. Axonal breaks and rarefication of the surrounding myelin result in lower diffusion of water molecules in the axonal plane and more diffusion in the radial plane, resulting in lower FAs. Several studies using DTI show white matter integrity may be compromised in children with T1DM (377, 378) and adults with T2DM (348, 379, 380). T2DM is associated with lower FAs in the total white matter, greater bilateral mean diffusivity for the hippocampus and dostolateral prefrontal cortex, and greater lateralized mean diffusivity for the posterior cingulate and right putamen (348). Studies reporting differences in white matter microstructure between controls and T2DM patients do not report significant differences in WMH derived by conventional MRI scans. The associations between T2DM and lower FAs along select white matter tracts extend to other T2DM-related conditions, including metabolic syndrome (381) and depression (382).
Lacunar infarction is another form of microvascular brain disease that produces a round or ovoid, subcortical, fluid-filled cavity. This cavity is visible by brain computed tomography or MRI (signal similar to cerebrospinal fluid) and is between 3 and 15 mm in diameter, consistent with a previous acute small subcortical infarct or hemorrhage in the territory of one perforating arteriole (340). It is estimated that lacunar infarcts account for 25% of all ischemic strokes, with an annual incidence of ∼15 per 100,000 people (383). A meta-analysis of studies with brain MRIs in patients with T2DM showed that there was a significant association between T2DM and lacunar infarcts. Compared with the general population, the odds of having lacunar infarction are 1.3 times higher among individuals with T2DM and 2.2 times higher for those with concomitant vascular disease (347). T2DM modifies the risk of short-term mortality and stroke recurrence among individuals with lacunar infarction. In patients with recent lacunar stroke, T2DM independently predicted ischemic stroke recurrence (384) and short-term and 5-year mortality (383).
Recent data suggest that T2DM may be more likely to contribute to the formation of small lacunes (385). Researchers have hypothesized that small lacunes (≤7 mm) probably have a lipohyalinotic etiology, and that larger lacunes (8 to 20 mm) result from microatheroma. The presence of lacunes ≤7 mm was significantly associated with age, black ethnicity, hypertension, ever-smoking, T2DM, and HbA1c. The same risk factors predicted infarcts with lacunes <3 mm. Interestingly, lacunes 8 to 20 mm in size had a risk factor profile more indicative of atherosclerosis that was not associated with T2DM. Taken together, factors related to T2DM (such as HbA1c) may be more likely to contribute to the formation of smaller lacunes (even those <3 mm) than the formation of larger lacunes. Further research focused on cardiometabolic risk factors contributing to lacunar infarction size will elucidate the mechanisms that atherosclerosis and T2DM share.
Even smaller, cerebral microinfarcts (CMIs) are attracting increasing attention in microvascular brain research. They are considered to be the single most widespread form of brain infarction and thus a major component of the causal pathway between microvascular disease and cognitive dysfunction (386, 387). Autopsies reveal CMIs in ∼43% of patients with AD, 62% of patients with vascular dementia, and 24% of nondemented elderly subjects (367). CMIs are typically defined as sharply delineated microscopic ischemic lesions accompanied by cellular death or tissue necrosis, often associated with gliosis and cavitation (388). CMIs can occur in both the white matter and subcortical regions of the brain, presumably more so in watershed areas (367). Because of their small sizes (ranging from 50 μm to a few mm), CMIs escape detection by regular clinical MRI protocols. The introduction of high-field-strength 7.0 Tesla MRI, with its high-resolution imaging and isotropic voxel sizes in the submillimeter range, permits clinicians to see CMIs in vivo (389).
In autopsy studies, CMIs were associated with increased measures of neuroinflammation, such as an elevated interleukin-6 concentration in the cortex. Of specific interest, in subjects with both T2DM and dementia, researchers observed different patterns between individuals who have or have not received medical diabetic therapy. Treated diabetic patients with dementia had the highest number of CMIs in the striatum, thalamus, and deep white matter (390). The association between cerebral injury and diabetes treatment in T2DM patients with dementia could have etiologic or therapeutic implications.
Nonischemic microvascular complications
Microvascular injury can also manifest as nonischemic pathology, such as cerebral microbleeds (CMBs), enlarged perivascular spaces (EPVS), evidence of BBB breakdown, and cerebral amyloid deposition. Human studies on aging and neurodegeneration currently use ever-developing technologies for measuring these lesions.
CMBs are visible in MRIs (391) (Fig. 6). CMBs commonly occur in patients with stroke, as well as in the general elderly population. The prevalence of CMBs in community-dwelling older adults is as high as 11.1% to 23.5% (392, 393). The presence of CMBs predicts the development of new CMBs (394). Some controversy remains as to whether T2DM predisposes individuals to CMBs (395). However, results from a meta-analysis showed that both T2DM and hypertension were associated with having more than a twofold increase in the odds of having CMBs (396). CMBs appear to colocalize with Aβ deposits in brain tissue samples from nondemented older adults, suggesting a shared etiology (397). PET imaging that uses amyloid-specific ligands (e.g., Pittsburgh compound B-PET) has openednew avenues to study amyloid deposits in the brain in vivo. One small study measured amyloid PET in AD patients with and without T2DM and found that the amyloid accumulation in AD patients was greater than in controls, but did not differ by T2DM status (398). A recent study using Pittsburgh compound B-PET in a convenience sample of older adults found no association between in vivo brain Aβ burden and serial measures of glucose intolerance or insulin resistance (399).
EPVS (also called Virchow-Robin spaces) are visible on T2-weighted MRIs and thought to represent the ex-vacuo dilatation that is secondary to cerebral tissue shrinkage after demyelination and axonal loss (400, 401) (Fig. 6). Once thought to be a normal phenomenon of aging, more recent studies show EPVS are associated with atherosclerosis (402), dementia, and other markers of microvascular disease (403, 404). EPVS are found in young patients with T1DM (405) and have yet to be examined in T2DM. EPVS may be indicative of perivascular cells (pericytes and vascular smooth muscle cells), which are important regulators of vascular formation, stabilization, remodeling, and function (406). Pericytes are integral components of the BBB and have a dramatic impact on microvascular integrity. They surround capillaries, contain contractile proteins, and are thought to regulate blood flow (407) and permeability (408). Notably, a loss of brain pericytes and the resulting BBB breakdown have been shown to impair CNS function through leakage and by depositing several potentially vasculotoxic and neurotoxic blood-derived macromolecules, including fibrin, thrombin, plasmin, and hemoglobin-derived hemosiderin, which causes accumulation of iron and ROS (409). Research suggests that the loss of pericytes in the brain parallels pericyte loss in DR, leading to the breakdown of the BBB (410). There are currently no neuroimaging techniques that enable us to see pericytes directly. Developing novelneuroimaging techniques, such as PET ligands specific for pericytes, may elucidate the in vivo role of pericyte loss in neurodegeneration and diabetes.
The cerebral microvascular endothelial cells, capillary BM, astrocyte endfeet, and pericytes are the structural components that comprise the BBB. The BBB regulates the normal neuronal and glial cell environment (411) by regulating the passage of circulating elements from blood into the brain. We are becoming increasingly more aware of changes in BBB integrity associated with aging and microvascular disease. Permeability of the BBB is an important aspect of microvascular complications in the brain and can be imaged in vivo using a MRI with intravenous gadolinium contrast enhancement (412). Postcontrast enhancement of brain parenchyma and increased signal intensity in the cerebrospinal fluid are presumed indicators of increased BBB permeability, and we see these changes in patients with T2DM (413). Postcontrast signal intensity increased more in the diabetic group than controls after administering gadolinium-diethylene triamine penta-acetic acid, particularly in the basal ganglia, an area known to be particularly vulnerable to cerebrovascular disease. The effects of T2DM on the BBB may contribute to increased risk of AD. Constituents of the endothelium, including RAGE, are important risk factors to consider when investigating T2DM-related BBB breakdown. Several novel neuroimaging techniques, including super paramagnetic nanoparticles and PET imaging ligands designed to image the BBB and its disruption, will provide useful tools for investigating BBB breakdown in the future.
Summary
Adults with T2DM have an increased risk of dementia as they age. T2DM and its associated factors predispose individuals to both microvascular and macrovascular complications throughout the body and brain. Recent neuroimaging studies show that patients with T2DM go on to develop structural and functional brain abnormalities similar to older adults with dementia. Many of the strongest neuroimaging markers of brain abnormalities seen in T2DM are related to microvascular disease. Furthermore, individuals who have evidence of metabolic deregulation (hyperglycemia and insulin resistance), but do not have T2DM, show similar structural and functional brain abnormalities to those with frank T2DM. There is evidence that individuals with T2DM-associated microvascular complications in the periphery have an elevated risk of having microvascular complications in the brain. Future research will determine whether the putative causal factors resulting in microvascular complications in the body (e.g., insulin resistance, hypertension, oxidative stress, and AGEs) mediate the observed associations between T2DM and brain microvascular abnormalities. Understanding the causes of microvascular disease in the brain associated with T2DM will provide targets for preventing cognitive decline and dementia in patients with T2DM.
The Microvasculture in Skeletal and Cardiac Muscle, Adipose, and Skin
Introduction
Diabetic microvascular disease is usually associated with eye, nerve, and renal injury. However, diabetes is a pervasive microvascular disease with functional consequences in tissues outside of those commonly associated with the disease. In this section, we review microvascular changes in skeletal and cardiac muscle, adipose, and skin, highlighting structural and functional changes that result from chronic hyperglycemia and from other factors that accompany diabetes. The retinal, renal, and neural pathology that accompanies diabetes arises in each case from disease/dysfunction of a very small mass of vessels in critical areas. However, for skeletal and cardiac muscle, adipose, and skin, we are dealing with a larger set of microvascular target vessels that when dysfunctional can impact general metabolic function.
Skeletal muscle microvasculature: structural and functional changes that accompany diabetes and insulin resistance
A study using electron-microscopic methods to examine pathologic changes in tissues from patients with diabetes reported that the BM of the microvasculature within skeletal muscle was thicker in diabetic than in healthy control subjects (414). The study also suggested that this change might occur early after the onset of diabetes or even precede frank hyperglycemia (414). However, subsequent work suggested that BM thickening correlated well with diabetes duration and glycemic control (415). Some initial confusion may have arisen as a result of different fixation methodologies for preparing tissues for electron microscopy (416), as well as differences related to which muscle group was biopsied (vastus lateralis, gastrocnemius, or neck muscles) (417, 418). Most experts now agree that the degree and duration of hyperglycemia appear to be important predictors of BM thickness (415). Research also indicates that, despite increased thickness of the BM, the peripheral vasculature in individuals with established diabetes appeared to be more leaky compared with nondiabetic controls. Studies most commonly defined leaky as the escape of radiolabeled albumin from the systemic circulation (419, 420). Although these studies used albumin as the tracer, other plasma proteins and lipoproteins also exit plasma at an accelerated rate. In the wall of larger arteries, this leakage from the vasa vasorum may contribute to the increased atherosclerosis that accompanies diabetes.
Over several decades, more has been learned about the composition of the BM (421) and how diabetes affects it. The principal proteins include type 4 collagen, laminin, perlecan, and nidogen/entactin. These proteins include several different isoforms, and the relative abundance and combinations of specific isoforms differ in various vascular beds. There are also a large number of other proteins present in smaller quantities. The biochemical composition of the BM in diabetes suggested that accumulation of glycosylated and cross-linked proteins contributed to the expanded membrane structure and disordered function (422). The nonenzymatic glycosylation of these proteins (like that of intracellular proteins), as well as cross-linking of the BM structural proteins by reactive carbonyl compounds like methylglyoxal (see Biochemical Pathways of Microvascular Injury), can affect endothelial cell/ECM interactions (422).
The pericyte is another important support component of the muscle microvasculature. As described elsewhere, pericyte loss is an early finding in the genesis of DR. In the kidney, changes in mesangial cell function (the glomerular cognate of the pericyte) contribute to the glomerular pathology of diabetes. Within skeletal muscle and many other tissues, pericytes and smooth muscle cells line the vessels down to the level of the capillary. Although originally thought to play principally a support and contractile function, it appears more likely that these cells may play a more dynamic role in regulating multiple functional aspects of the microvasculature (423). For example, although pericytes appear to play an important role in angiogenesis within the microvasculature, they also participate in the formation of the BM that envelops the endothelium. Within skeletal muscle, diabetes decreases the density of pericytes (417, 424). It is not certain whether this is one of the factors leading to muscle capillary rarefaction that is seen in diabetes (425), as well as in persons with hypertension or with simple obesity (426). It is of interest that insulin resistance is a common trait among these latter disorders, and impaired insulin action on muscle vascular elements may underlie this loss of capillary numbers. This has also been nicely demonstrated in animal models of obesity and insulin resistance in which decreased NO availability was implicated as potentially causative (427). In addition to changes in skeletal muscle capillary numbers, diabetes is associated with changes in the capillary architecture, which affect the perfusion pattern within the muscle (428).
One study reported that muscle-specific VEGF-deficient mice have capillary rarefaction in both skeletal and cardiac muscle, and this is accompanied by decreased insulin action on skeletal muscle glucose uptake during a euglycemic clamp (429). Glucose uptake in response to insulin was normal when these muscles were excised and studied in vitro, suggesting that the capillary rarefaction was responsible for at least a significant fraction of the metabolic insulin resistance observed. Likewise, a second study reported that mice deficient in insulin receptor substrate 2, specifically in the endothelium, have in vivo metabolic insulin resistance during the euglycemic clamp (430). This appears to be secondary to impaired insulin signaling to activate NOS and increase NO production. Interestingly, the study also reported that endothelial cells from control mice placed on a high-fat diet demonstrated decreased IRS-2 protein content, as well as impaired insulin-mediated glucose disposal. The study did not examine microvascular rarefaction. However, it did report that in control mice, insulin increased capillary recruitment (a process by which insulin, exercise, and other factors increase the fraction of capillaries within muscle that are perfused at any point in time); this did not occur in high fat–fed or endothelial cell–specific IRS-2 knockout mice.
These last two studies begin to probe the relationship between microvascular perfusion, capillary density, and metabolic function in muscle. Preceding that work, there are several decades of publications indicating a clear relationship between insulin’s metabolic actions in muscle and insulin’s action on vasculatures (both large conduit vessels, resistance vessels, and the microvasculature) that supply skeletal muscle. By the mid-1990s, it was reasonably established that changes in insulin concentrations (using the insulin clamp technique) could modify total blood flow to muscle, and that states of insulin resistance, including obesity (431), T1DM (432), and T2DM (433), blunted this effect. Presumably, this effect was principally mediated by actions on resistance arterioles, which (along with the microvasculature) regulate total blood flow to muscle. With the development of several methods to specifically measure muscle microvascular blood flow (434, 435) and the distribution of blood flow within the muscle, it became apparent that insulin also significantly enhanced the recruitment of capillaries that were relatively less perfused or unperfused within resting skeletal muscle. Both insulin’s effect on resistance arterioles and insulin’s effect on the smaller fourth- or fifth-order arterioles that regulate muscle flow distribution require intact signaling to NOS (436, 437). Insulin’s microvascular effect (like its effect on resistance arterioles) was impaired in states of insulin resistance (including diabetes) and correlated strongly with insulin’s metabolic effects within skeletal muscle in both experimental animals (438–440) and humans (441–445).
Even very modest exercise recruits capillaries within human skeletal muscle (446), and the effect appears stronger than that of insulin. Furthermore, the effect of exercise persists unabated in insulin-resistant states. The fact that expanding capillary surface enhances insulin (and nutrient) delivery to muscle (67) may, in part, explain the insulin-sensitizing effect of exercise.
Two aspects related to impaired skeletal muscle microvascular insulin action and its metabolic consequences are of particular interest. First, although this impairment is quite evident in individuals with diabetes, it is also apparent in other states of insulin resistance, like metabolic syndrome or simple obesity. As such, this microvascular dysfunction affects even a larger segment of the general population. This is of significant concern because both obesity alone and metabolic syndrome increase CVD risk (447). Second, clearly different mechanisms are most likely involved. Diabetes microvascular injury appears to be provoked (in many tissues) by excess glucose metabolism by the endothelial cell, which results in enhanced glycolytic activity, greater AGE formation, mitochondrial ROS production, and increased PKC activity (64). Because endothelial cell glucose metabolism occurs in an insulin-independent fashion, but is proportional to the degree of glycemia, it clearly involves a separate mechanism from that seen in normo-glycemic insulin-resistant obese or metabolic syndrome subjects. The latter may involve nutrient overload by FAs or other nutrients (448), although the relationship between obesity (or even T2DM) and increases in circulating concentrations of nonesterified FAs is not without controversy (449).
The myocardial microcirculation in diabetes
The coronary microvasculature plays a dynamic role in the regulation of coronary blood flow to meet the oxygen and nutrient demands of the myocardium. Because the heart’s microvascular bed provides endothelial surface area to facilitate the delivery of oxygen, nutrients, and hormones and the removal of metabolic end products from the myocardium, changes in the cardiac microvascular blood volume and flow could profoundly affect myocardial metabolism, function, and health.
The coronary circulation is composed of the arterial (epicardial coronary arteries down to 200 µm arterioles), microcirculatory (arterioles <200 μm, capillaries, and small venules <200 μm), and the venous (200 μm venules to coronary sinus) compartments with a total blood volume of ∼12 mL/100 g cardiac muscle, which is distributed near evenly among these three compartments (450, 451). Although most of the arterial and venous blood volumes are located on the epicardial surface of the heart, the microvascular compartment is exclusively located within the myocardium and constitutes ∼90% of the myocardial blood volume (450). Compared with other insulin-sensitive tissues (e.g., skeletal muscle and adipose tissue), myocardium has a much larger endothelial surface area (per gram of tissue). Within the myocardium, endothelial cells outnumber cardiomyocytes by three to one, and each mm2 myocardium contains 3000 to 4000 capillaries, which run parallel to cardiomyocytes (451, 452). At rest, only ∼50% of myocardial capillaries are perfused (453). When myocardial oxygen demand increases, myocardial blood flow velocity and/or volume increase to meet demand.
In addition to providing surface area for endothelial exchange, the myocardial microvasculature also actively regulates capillary hydrostatic pressure, which is critical for maintaining cellular homeostasis and health (454), resulting in a constant coronary blood flow over a wide range of coronary driving pressures (∼45 to 120 mm Hg) (451). The largest drop in pressure from the mean aortic pressure of ∼90 mm Hg to the capillary hydrostatic pressure of ∼30 mm Hg occurs in the arterioles smaller than 100 µm in diameter. Tone in these vessels responds to autonomic control and to local metabolites (455). Together, the arterioles confer ∼60% of total myocardial vascular resistance, whereas capillaries account for ∼25% and venules ∼15% (451, 453, 456). Unlike the arterioles that regulate myocardial blood flow, resistance, and volume by vasodilation or vasoconstriction, capillaries (which lack a smooth muscle component) do so via their recruitment or decruitment driven by arteriolar tone (453).
Many physiological factors regulate coronary blood flow, including catecholamines, adenosine, exercise, insulin, and glucagon-like peptide 1 (GLP-1). Adenosine is a potent vasodilator, which has been widely used clinically to assess coronary blood flow reserve. Exercise is probably the most important and potent physiological stimulus to increase myocardial blood flow. The increase in oxygen demand of the left ventricle during exercise is mainly met by augmenting coronary perfusion via capillary recruitment and the dilatation of coronary microvessels, as oxygen extraction is nearly maximal at rest (70% to 80%) (457). Insulin also increases coronary blood flow in humans (458–464), suggesting a vasodilatory action on coronary vasculature. Studies using myocardial contrast echocardiography (a noninvasive technology that employs perfluorocarbon gas–containing microbubbles to assess in vivo perfusion of the cardiac microvasculature) (465–467) have shown that insulin potently increases cardiac microvascular perfusion in healthy humans (443, 468, 469). This finding extends a prior report that mixed meal feeding significantly increased cardiac microvascular perfusion in healthy but not in T2DM humans (470). The postprandial increase in cardiac microvascular perfusion is most likely multifactorial. In addition to stimulating insulin secretion, the mixed meal induces the secretion of incretins, and at least one of these incretins (GLP-1) increases coronary blood flow independent of insulin (471, 472). It is very likely that GLP-1 also regulates coronary microvascular perfusion, as studies recently reported that GLP-1 recruits microvasculature and enhances insulin delivery and glucose use in skeletal muscle (473, 474). Researchers have yet to define the mechanisms underlying myocardial capillary recruitment, and these mechanisms most likely vary based on the particular stimuli. In skeletal muscle, insulin and GLP-1 recruit muscle microvasculature via a NO-dependent mechanism (67, 473, 475, 476), whereas exercise-induced muscle microvascular recruitment is largely NO independent (477). It is possible that in myocardium, both insulin and GLP-1 act via the NO-dependent mechanism, and exercise recruits myocardial capillaries perhaps by increased metabolic responses of the small arterioles.
Patients with diabetes have accelerated coronary artery disease and are prone to develop diabetic cardiomyopathy. Among many possible contributors are microvascular abnormalities. The morphological changes of small vessels seen in diabetic myocardium are extensive, including periarterial fibrosis, arteriolar thickening, focal constrictions, microvascular tortuosity, capillary BM thickening, capillary microaneurysms, and decreased capillary density (478, 479). In addition to structural abnormalities, coronary microvascular dysfunction also occurs in diabetes. Indeed, the maximal coronary flow reserve is reduced, and endothelium-dependent coronary vasodilation is clearly impaired in diabetes, even in the presence of angiographically normal coronary arteries and normal left ventricular systolic function (480, 481). The reduction in myocardial blood flow reserve correlates significantly with average fasting glucose concentrations and HbA1c (482), confirming the importance of glycemic control in the maintenance of cardiac health. In patients with T1DM and normal exercise echocardiography and autonomic nervous function, myocardial blood flow (measured with PET and [15O]H2O) is ∼30% lower, and total coronary resistance is 70% higher than normal healthy controls during hyperemia (483).
Endothelial dysfunction and insulin resistance, two core defects associated with diabetes, are both present in the coronary circulation and are most likely the major early cause of coronary microvascular dysfunction. Quantitative angiographic analysis of epicardial coronary artery responses to stepwise intracoronary acetylcholine infusion clearly demonstrates impairment in endothelium-dependent dilatation in diabetic patients with no significant coronary atherosclerosis (481). Vasodilation of the coronary microcirculation in response to sympathetic stimulation evoked by the cold pressor test is also impaired in T2DM patients in the absence of significant epicardial coronary artery lesions (484). Although insulin-mediated increases in coronary blood flow are maintained in young patients with T1DM without microvascular complications or autonomic neuropathy (459, 461), it is blunted in patients with obesity (485) or T2DM (460). Raising plasma insulin concentrations by ∼eightfold by ingesting a mixed meal not only fails to increase cardiac microvascular perfusion, as is seen in healthy humans, but actually induces a paradoxical decrease in many patients with diabetes (470, 486). In the acute insulin-resistant state induced by systemic lipid infusion, the myocardial microvascular response to insulin is clearly blunted (443). However, pretreatment with salsalate, an anti-inflammatory agent that inhibits the NF-κB pathway, preserves the microvascular response to insulin (468). These findings are consistent with a prior report that free FAs cause endothelial insulin resistance via NF-κB activation (487). Another potential contributor to coronary microvascular dysfunction is the increased blood viscosity commonly seen in patients with diabetes. Hypertriglyceridemia, a common feature of insulin resistance and insulin deficiency, increases blood viscosity and decreases coronary blood flow (488).
In patients with fixed stenoses in major coronary arteries due to coronary atherosclerosis, blood supply to the myocardium is limited. Although resting epicardial coronary blood flow remains normal until >85% of the lumen is obstructed, during hyperemia, total flow is reduced when the stenosis exceeds 50% (489–491), and both microvascular blood volume and flow velocity are depressed (490, 492). Under this circumstance, expansion of the coronary microvascular blood volume could markedly increase the endothelial exchange surface area. The presence of insulin resistance in the coronary microvasculature could further limit the microvascular blood volume and the capability of cardiac muscle to extract oxygen and nutrients and receive signals from circulating anabolic factors. This may explain partly why patients with diabetes tend to develop cardiac complications, including cardiomyopathy and heart failure. This also suggests that the coronary microvascular endothelial dysfunction and insulin resistance could be important therapeutic targets in reducing cardiac morbidity associated with diabetes.
Microvascular disease/dysfunction in the skin
The skin microvasculature plays an important physiologic role in the body’s defenses against thermal and mechanical injury and pathogen entry by maintaining the health of the keratinized epithelium of the epidermis, as well as the supporting dermis and subcutaneous tissues. Diabetes can injure the skin’s microvasculature, which could compromise the skin barrier and allow trans-cutaneous microbe migration. Pathologically, thickening of the BM and loss of pericyte coverage for microvessels characterize injury to the skin’s microvasculature, similar to responses in other tissues. The vessels leak plasma proteins, and, perhaps as a consequence of this leaking or as a consequence of hyperglycemia, the supporting connective tissue becomes more cross-linked and stiff. Microvascular injury may play a role in the development of the limited joint mobility syndrome (493) that is seen in both T1DM and T2DM and correlates with the progression of microvascular disease in the eye (494).
Beyond structural changes, there are abundant data regarding vascular dysfunction in the skin in diabetes (495). The skin (like the retina) is one of the few areas where clinicians can directly observe and functionally test the microvasculature. A significant body of data exists that details the changes in microvascular perfusion that occur as a result of diabetes or insulin resistance or components of metabolic syndrome. Researchers have used various techniques, including laser Doppler fluxmetry (496–498) and nail-bed capillaroscopy (499, 500), to characterize skin microvasculature in diabetes (500–502), obesity (503), insulin resistance, and metabolic syndrome (504). With well-established diabetes, the vascular hyperemic response to skin heating is impaired, as is the response to hypoxemia. There appears to be a clear relationship between impaired microvascular function and tissue metabolism in the feet of individuals with diabetes. Tissue oxygen saturation and high-energy phosphate stores are decreased in lower extremity skin in individuals with diabetes compared with controls, and this appears to be further aggravated when neuropathy is present (495).
Impaired function of the skin microvasculature is a key component contributing to delayed/deficient wound healing in diabetes. This applies to both postsurgical healing as well as spontaneous wound healing, as is seen with diabetic foot ulcers (505, 506). For the former, glycemic control is an important treatment intervention to improve wound healing. For the latter, which are more chronic wounds, there is a complex interplay between local tissue factors, infection, blood flow, and friction/pressure-related hyperkeratosis, each of which must be addressed to optimize the likelihood of successful treatment. Interestingly, recent work in experimental models has suggested that a defect in endothelial progenitor cell proliferation and subsequent recruitment to sites of injury may be playing a significant role. The generation of EPCs appears to depend on NO generation by eNOS within the bone marrow compartment, and diabetes impairs this (507). Beyond that, the recruitment of generated cells to the area of inflammation is dependent upon local tissue factors, which diabetes also decreases (507).
Microvascular dysfunction/disease in adipose tissue
Adipose tissue possesses an abundant microvasculature, and most adipocytes are within one cell diameter of a capillary. The resting blood flow to adipose tissue is similar to that of resting skeletal muscle (2 to 4 mL/min/100 g), and blood flow to each of these tissues increases following a meal in healthy subjects (508, 509). These changes can be blocked by β-blockade but not by α-blockade or NOS inhibition, suggesting an important role for adrenergic regulation. Inasmuch as adipose tissue is a principal repository for dietary fats that circulate in very LDL particles or chylemicrons, postprandial increases in flow could enhance nutrient delivery and storage. In muscle, meal ingestion increases both total blood flow and capillary recruitment. Similar blood flow and capillary recruitment may likewise occur postprandially in adipose tissue (510).
Interestingly, basal adipose blood flow is reduced, and postprandial adipose blood flow increases are blunted or absent in obese or T2DM subjects (510, 511). Subcutaneous adipose tissue capillary density (capillaries/mm2) is less in obese subjects (512), consistent with capillary rarefaction similar to that seen in skeletal muscle in diabetes, obesity, and hypertension. The larger fat cell size in subcutaneous tissue from obese diabetic subjects may, in part, explain this apparent rarefaction, which correlates well with the degree of insulin resistance measured using the euglycemic clamp method.
Whether diminished adipose vascularity might have metabolic consequences has been a topic of very recent interest. Several groups had reported decreased tissue oxygen tension within adipose tissue from obese rodents (512–514). These groups hypothesized that this decrease results in an oxidative stress within the tissue, which might contribute to the development of inflamed adipose tissues and the resultant release of inflammatory cytokines. This hypothesis has become more controversial with recent data suggesting that in obese humans, subcutaneous adipose tissue oxygen concentration was either minimally lower (512) or higher (508) than is seen in lean, age- and gender-matched control subjects. In the latter study, which also demonstrated decreased vascularity and reduced blood flow, the seemingly paradoxical adipose tissue hyperoxia appeared secondary to decreased mitochondrial activity and tissue energy expenditure. Whether these differences between rodents and humans represent species differences or differences between the methods used in the human compared with the rodent studies is uncertain. It is clear that the tissue pO2 in obese rodents is substantially lower (∼20 mm Hg) compared with obese humans (40 to 70 mm Hg). The levels of oxygen seen in humans would not be expected to trigger the same transcriptional program as seen in mice (e.g., enhanced expression of mRNA for HIF-1a, Glut-1, etc.).
The recognition of the close relationship between adipose tissue microvasculature and the adipocyte has recently provoked a number of very interesting investigations into the relationships between expanding fat mass and microvascular angiogenesis or involution and how both relate to body metabolic function. Over a decade ago, Rupnick et al. (515) observed that angiogenesis inhibitors could lead to significant weight loss in ob/ob mice. This was particularly intriguing, as the effect occurred using several different types of angiogenesis inhibitors, and the animals tolerated the treatment quite well. Withdrawal of the inhibitor allowed rapid regain of the lost weight. The animals treated with the angiogenesis inhibitors demonstrated increased apoptosis and decreased angiogenic activity in adipose tissue. The treated animals consumed less food than control animals; however, this did not entirely explain the weight loss, which was greater in the animals treated with the angiogenesis inhibitors compared with pair-fed controls. More recently, several intriguing studies have shown that adipose tissue endothelium specifically targeted with a proapoptotic peptide likewise caused weight loss in mice (516, 517). This treatment affected a decrease in food intake without other apparent toxicity. Importantly, it did not affect appetite or body weight in lean control animals (518). The mechanism responsible for this obesity-dependent appetite decrease is unclear. It is intriguing that treating mice with proapoptotic peptide, while causing vessel rarefaction in adipose tissue, improved glucose tolerance and insulin resistance (516). Studies targeting adipose angiogenesis did not assess tissue oxygenation, so it is unclear how this work relates to the hypothesis that adipose hypoxia causes a proinflammatory state and consequent insulin resistance.
Several other groups have reported finding a significant relationship between the microvasculature and the metabolic function of the adipocyte. When the VEGF gene is overexpressed in the adipocyte using an AP2-directed promoter, there is increased microvasculature development, specifically in adipose tissue (both white and brown) (518). These mice did not become obese when placed on a high-fat diet. They also showed decreased M1 macrophage infiltration of fat, increased thermogenesis, and improved glucose tolerance, and maintained greater insulin sensitivity when compared with high-fat–fed control mice. The complexity of this relationship is underscored by the observation that inhibiting VEGF-A expression (whole animal) using an inducible repression system also diminished weight gain on a high-fat diet and led to a browning of white adipose tissue. In addition, VEGF-B expression was increased in these mice, as were downstream FA transport proteins regulated by VEGF-B (519). This is particularly interesting in light of the report that knockout of VEGF-B (whole body) prevented the development of insulin resistance in db/db mice and improved glucose tolerance (520). In part, these salutary metabolic effects appeared due to decreased FA transport proteins in the endothelium of muscle and heart, which slowed ectopic lipid deposition at these sites.
Studies probing the relationship between VEGF expression and microvascular development have recently been extended to muscle. Bonner et al. (521) created a muscle-specific VEGF-A knockout mouse using the Cre/lox method. VEGF-A was absent in both cardiac and skeletal muscle, whereas plasma concentrations were decreased ∼25%. Accompanying this was nearly a 50% decline in capillary volume in both skeletal and cardiac muscle. Insulin sensitivity (euglycemic clamp) was diminished in the knockout animals due to a decline in insulin-stimulated glucose disposal. However, when muscles were excised and incubated in vitro with insulin and glucose, metabolism appeared normal. This suggests that the impairment in insulin-stimulated muscle glucose uptake was due to poor muscle perfusion.
It could be that there is an entirely different relationship between the microvasculature and adipose within skeletal muscle. This relates to the fact that small arterioles within both skeletal and cardiac muscle have a surrounding envelope of adipocytes, and the volume of this envelope increases with obesity. Nearly a decade ago it was proposed that a local signaling process occurs between perivascular adipose tissue and the microvasculature within skeletal muscle (522), and that adipokines released by adipose might influence muscle nutritive perfusion in a paracrine fashion. Such a process could provide an important linkage in our understanding of the relationship between adiposity, inflammation, and vascular dysfunction that is prevalent in diabetes. Increases in perivascular adipose tissue are not restricted to skeletal muscle. Indeed, several studies have noted an association between perivascular adipose tissue in the thoracic aorta and both extramural coronary circulation calcification and CVD prevalence (523, 524).
Summary
In summary, there is now abundant evidence that microvascular dysfunction/disease is by no means restricted to the traditional target tissues (i.e., retina, kidney, and peripheral nerve). Rather, it is a generalized phenomenon affecting multiple tissues throughout the body. This allows one to appreciate the pleiotropic effects of diabetes on health. In addition, dysfunction of the microvasculature in tissues like skeletal and cardiac muscle, skin, and adipose also occurs in settings related to insulin resistance and contributes to both metabolic and other functional defects in these tissues.
Microvascular Disease in the Kidney
Introduction
Microvascular renal disease is part of the classical triopathy of diabetes complications. It is a major contributor to the development of ESKD in the developed world. In addition to the morbidity/mortality provoked by DN per se, it associates strongly with CVD progression and mortality. In this study, we review the epidemiology of DN, its pathogenesis, and evolving information on genetic factors that either enhance or diminish the risk for development or progression of DN in diabetic patients. We also briefly highlight aspects of current treatment.
Epidemiology
Determining precise incidence and prevalence rates for KD in subjects with diabetes depends on the definition applied. Excessive albuminuria and/or reduced estimated GFRs (eGFRs) in subjects with diabetes are associated with diabetic KD (DKD), but also to nondiabetic KD, particularly in those with T2DM who have atypical clinical courses (short disease duration, severe hypertension, rapidly changing kidney function, or absence of DR). Between 2005 and 2010, National Health and Nutrition Examination Survey data revealed high rates of nephropathy among subjects with diabetes—19.3% had an eGFR <60 mL/min/1.73 m2 (using the Chronic Kidney Disease Epidemiology Collaboration formula), 29.9% an elevated urine albumin:creatinine ratio ≥30 mg/g, and 8.6% had both albuminuria and low eGFR (525). Based on these definitions, similar high rates of KD are present in patients with undiagnosed diabetes (526), and 17.7% of patients with prediabetes have KD (527). The incidence rate of CKD was significantly higher among those with metabolic syndrome (but lacking diabetes) in the Atherosclerosis Risk In Communities study, relative to those without metabolic syndrome (528). These studies demonstrate that hyperglycemia can lead to reduced kidney function and albuminuria prior to the onset of frank diabetes.
The incidence rate of diabetes-attributed nephropathy in the US diabetes population has been stable for the past 2 decades (526, 529). This is concerning because it occurred despite marked reductions in HbA1c and systemic BPs, a >10-fold increase in prescription of statins (with a mean 32 mg/dL reduction in LDL cholesterol), and a nearly fourfold increase in use of renin-angiotensin aldosterone system (RAAS) inhibitors during this period. Thus, these stable rates of diabetic patients developing DKD will continue to translate into increasing patient numbers with nephropathy due to rising rates of diabetes and obesity. The prevalence of DKD in the US population was estimated at 2.2% between 1988 and 1994 (95% CI: 1.8% to 2.6%), with significant increases to 2.8% (95% CI: 2.4% to 3.4%) between 1999 and 2004, and 3.3% (95% CI: 2.8% to 3.7%) between 2005 and 2008 (P < 0.001 for trend) (525). Much of the excess morbidity and mortality in subjects with diabetes appear to relate to the presence of KD (530, 531).
The annual incidence rate of ESKD cases attributed to DKD has also been relatively stable at 152 per million population between 2000 and 2010, although dramatic differences occur based on age and ethnicity (525). The gender-adjusted incident rate for diabetes-attributed ESKD in European Americans aged 30 to 39 years fell by 1% from 2000 to 2010 (to 35.4 cases/million in 2010). In contrast, African Americans, Native Americans, and Asian Americans in this age range saw their incidence rates increase by 69%, 30.1%, and 100% (133.8, 116, and 32.6 million) during this period, respectively. The rate of incident ESKD attributed to diabetes fell by 3.6% between 2000 and 2010 in European Americans 60 to 69 years old, whereas it rose by 29% in those >70 years. Between 2000 and 2010, the incidence rate of diabetes-attributed ESKD in African Americans, Native Americans, and Hispanic Americans aged 60 to 69 fell by 17.2%, 40.4%, and 15.7%, respectively.
Clinical presentation
Our understanding of the natural history of DKD has evolved. Although best evaluated in T1DM with a clear date of disease onset, the histologic and clinical courses of DKD appear similar in T1DM and T2DM. In subjects with hyperglycemia, we do not uniformly see the assumed progression from preglomerular afferent arteriolar vasodilation to high renal blood flows, elevated intraglomerular pressures, and intermittent then fixed microalbuminuria with subsequent macroalbuminuria and declining GFR. Approximately 10% of subjects with T1DM will manifest steadily declining eGFR in the absence of heavy proteinuria (129). Albuminuria and loss of kidney function (declining eGFR) are independent processes with different genetic bases (532).
Current levels of albuminuria divide into three categories: normoalbuminuria (urine albumin:creatinine ratio <30 mg/g), often further divided to high-normal >15 mg/g, which extends into the previously normal range; microalbuminuria (30 to 300 mg/g); and macroalbuminuria (>300 mg/g), also known as overt proteinuria.
Higher risk for CVD events is associated with higher levels of albuminuria (533, 534). UKPDS (195) participants with T2DM and microalbuminuria had equivalent rates of progressing to macroalbuminuria and death (195). In those with macroalbuminuria, the risk of death far exceeded the risk for developing progressive loss of eGFR or initiating renal replacement therapy. The urine albumin:creatinine ratio can vary by up to 40% on repeat testing, and T1DM patients with effective glycemic, lipid, and BP control frequently experience microalbuminuria remission (529, 531). Therefore, an abnormal urine albumin:creatinine ratio may not be reflective of a risk for progression in DKD.
During prolonged follow-up of T1DM subjects with an initially normal eGFR (>60 mL/min), ∼two-thirds of patients with microalbuminuria and one-third with overt proteinuria demonstrated stable renal function with low risk for subsequent progression to ESKD (535, 536). In contrast, one-third of those with microalbuminuria and two-thirds with overt proteinuria had declining kidney function and were at high risk for subsequent ESKD. The rates of decline were variable between patients, but remained relatively consistent in each individual. Early eGFR slope appears predictive of a future rate of progression in DKD (536). In addition, researchers evaluated the relatively frequent failure of ACE inhibitors (ACEi) to halt progression of early T1DM KD. Although precise mechanisms are unclear, poor glycemic control and hypercholesterolemia are most likely involved (535).
These data suggest independence between the development and progression of pathologic changes in the glomerular and interstitial renal compartments in DKD. The glomerulus appears primarily responsible for proteinuria. However, interstitial changes better predict subsequent declines in kidney function, as in other forms of nephropathy. In a study of RAAS-blocking agents in the primary prevention of DKD, glomerular mesangial fractional volumes (and other glomerular parameters) were not appreciably different in normoalbuminuric T1DM patients after 5 years of treatment with an ACEi, ARB, or placebo (100). Additionally, interstitial changes were not different between these treatment groups, suggesting that RAAS blockers are not suitable for the primary prevention of DKD, despite lowering systemic BPs.
Although many patients with progressive DKD and falling eGFR have proteinuria, urine albumin:creatinine ratios better predict CVD events and CVD mortality, relative to the progression of KD (195). Damage to the systemic vasculature, including in the glomerulus, relates to endothelial dysfunction from hyperglycemia and most likely contributes to albumin leakage into the urine. This may not reflect diabetic glomerular changes, but contributes to the high rates of CVD and death. Once on renal replacement therapy, death rates from CVD remain high in subjects with DKD. Adjusted 5-year survival on dialysis was 32% in subjects with DKD through December 2010 (525).
Non-DN is frequently present and often misdiagnosed as DKD in proteinuric patients with T2DM and brief diabetes durations who have severe hypertension or rapid loss of eGFR. Several studies report 50% or more of proteinuric patients with T2DM undergoing renal biopsy had nondiabetic CKD (423, 537, 538). Immunoglobulin A nephropathy frequently coexists with DKD in Asian and American Indian populations, as well (539). These factors contribute to errors in calculating the true incidence and prevalence of DKD and hamper treatment trials in DKD by including cases with non-DKD. This is further supported by the identification of two coding nephropathy risk variants in the apolipoprotein L1 (APOL1) gene that contribute to African-ancestry populations having the majority of nondiabetic CKD cases.
APOL1 is strongly associated with a spectrum of proteinuric KDs related to focal segmental glomerulosclerosis, including HIV-associated nephropathy and focal global glomerulosclerosis, which was erroneously attributed to hypertension in African Americans (540–547). The APOL1 family of nondiabetic KDs accounts for up to 40% of ESKD in African Americans. T2DM and focal segmental glomerulosclerosis are common in African ancestry populations and frequently coexist. It is difficult to accurately determine the cause of nephropathy in those with proteinuria and T2DM without a kidney biopsy, a procedure not commonly performed in patients with longstanding diabetes. APOL1 genotyping may provide a noninvasive tool for identifying CKD that is unrelated to diabetes in individuals of African ancestry (548). As discussed later, partitioning for APOL1 in African Americans with clinically diagnosed DKD replicated the FERM domain-containing 3 (FRMD3) gene association with DKD, an effect not possible prior to accounting for APOL1 (548–550).
A genetic component to diabetic KD risk
In addition to lifestyle and environment, genetic heritage is widely accepted as a contributor to the complex phenotype of DKD. An improved understanding of the genetic contributors to DKD has the potential to play a significant role in early prediction, prevention, and efforts to halt disease progression. For example, if genetic predictions using a combination of genetic variants that are proven to predict higher DKD risk (i.e., a genetic risk score) could identify patients at high risk for DKD, these individuals could undergo active surveillance (with early initiation of antihypertensive, blood sugar, and lipid-lowering treatment) when diabetes is initially diagnosed. The potential of this form of personalized medicine for patients with diabetes has not yet been translated into practice. There is little doubt that genetic variations contribute to DKD risk, supported by a wide range of studies in both T1DM and T2DM in multiple ethnicities. As outlined previously, ethnic disparities in DKD prevalence suggest that the different natural histories of human populations have resulted in genetic architectures that confer different DKD risks. Familial clustering and aggregation of DKD have been documented for discrete definitions of DKD (i.e., ESKD in T1DM-affected European Americans and Europeans) (551–553) and in diverse T2DM populations, such as African Americans (554, 555), European Americans (554), Canadians (556), Native Americans (557), Europeans (558), East Asians (559), Brazilians (560), and South Asians (561). In addition, familial aggregation in the form of heritability of quantitative measures of renal function (e.g., urinary protein excretion and eGFR) has been widely reported (562–564), and segregation analyses in European American (565) and Pima (566) diabetes families suggest that genetics significantly influence variations in urinary protein excretion.
The search for diabetic KD susceptibility genes
The broad acceptance that DKD has a significant genetic component has motivated increasingly sophisticated efforts to identify specific genetic polymorphisms associated with DKD. A widely used approach is the comparison of allele frequencies between DKD cases and non-DKD controls, with KD defined as a dichotomous trait (either affected or unaffected). The simplest approach has been candidate gene analysis, which entails the assessment of genetic variations in one or more genes with plausible physiological links to DKD. Candidate gene studies continue to be reported in large numbers and in diverse ethnicities. These studies are frequently based on small numbers of cases and controls and often on small numbers of genetic polymorphisms. Thus, they have limited power and do not comprehensively test the gene in question. Such studies, however, can contribute to larger, better-powered meta-analysis efforts. Meta-analyses, although better powered, also have limitations, such as focusing on a limited number of genetic variants and including diverse study samples that were not collected in a uniform fashion.
Two large meta-analyses have evaluated the angiotensin 1–converting ACE insertion–deletion polymorphism in diverse samples of T1DM and T2DM cases and control subjects (567, 568). These studies concluded that the ACE D allele was associated with DN risk with ORs in the range of 1.1 to 1.3, an effect similar to many common variant associations with complex diseases. Mooyaart et al. (569) combined bioinformatic and meta-analysis methods to evaluate evidence for genetic associations with DKD. They reported that of 671 genetic association studies investigating DKD, researchers identified 34 replicated genetic variants; 21 of these remained significantly associated with DKD in a random–effects meta-analysis. Genetic variants in the PKCβ1 gene (PRKCB1) were associated with T2DM KD in a carefully performed candidate gene study from Hong Kong, with replication (32). However, results of these studies have not yet been translated into practice. This may prove difficult, given the variations between the many studies of T1DM KD and T2DM KD and variations in sample sizes and ethnic origins. For example, many genes associated with DKD failed to replicate when tested in European-derived samples with T1DM KD (570).
Early studies using classical family-based linkage analysis showed great promise. Vardarli et al. (571) performed a genome linkage scan in Turkish kindreds with multiple DKD-affected individuals. They observed a major linkage peak on chromosome 18 [logarithm of odds score 6.6 (i.e., odds of 106.6:1) for linkage], revealing evidence for a novel DKD gene. Analysis of this locus in the Pima Indian population provided some evidence of confirmation (572). The carnosinase 1 gene (CNDP1) was ultimately implicated as the likely cause of DKD on 18q (573). CNDP1 is expressed in the brain and kidney, and carnosine is a scavenger of oxygen-free radicals and may inhibit the formation of advanced glycosylation end products. A polymorphic trinucleotide repeat in exon 2 of CNDP1 coding for a leucine repeat in the leader peptide of the carnosinase-1 precursor was associated with DKD. There are additional studies in both T1DM and T2DM from multiple ethnic groups that include several study designs (family based and case control). The CNDP1 association was replicated in European Americans with T2DM KD (574), but an analysis of European Americans only nominally associated CNDP1 with T1DM KD (575). Studies have extended the evaluation of CNDP1 to test other genetic variations in the region, including the neighboring CNDP2 gene. McDonough et al. (576) performed a detailed resequencing and analysis of variants in the CNDP1 and CNDP2 genes in European Americans and African Americans. DKD protection was not observed in African Americans, suggesting that the protection afforded by the CNDP1 was masked by additional CNDP1 and CNDP2 risk haplotypes, defined by specific combinations of single-nucleotide polymorphisms (SNPs). Analysis of this locus continues to be of interest (577, 578).
There are a number of other family-based linkage studies that include, in some cases, complementary association analyses (579, 580). The Family Investigation of Nephropathy and Diabetes study (581) included 11 US clinical centers and nearly 10,000 European Americans, African Americans, Mexican Americans, and American Indians with T2DM KD. Initial analyses targeted the relationship of both quantitative albuminuria and GFR with DKD (582–584). In spite of these efforts, the significance and impact of family-based linkage studies remain unclear to human geneticists, with only a few examples of linkage studies leading to the identification of genes underlying complex traits such as DKD. Even in successful cases, such as CNDP1, the overall clinical implications remain uncertain.
Smaller and less comprehensive efforts are now transitioning to larger, better-powered studies with more comprehensive genetic analyses in DKD, including genome-wide association studies (GWAS). In many cases, this new generation of studies has multiple collaborating research groups. For example, McKnight et al. (585) initiated a new level of rigor in study design in their analysis of the Warren 3/UK Genetics of Kidneys in Diabetes Study Group cohort of T1DM patients, with replication testing and meta-analysis of samples from the Finnish Diabetic Nephropathy study. In total, McKnight et al. evaluated >3400 samples with moderate evidence for association (allelic P value 0.006, OR 1.27).
Initial GWAS in Japanese and Pima Indians suggested an association between T2DM KD susceptibility and the engulfment and cell motility 1 gene and the plasmacytoma variant translocation gene, respectively (586, 587).
Recent extensions of the earlier GWAS work reported results for two genes, acetyl-coenzyme A carboxylase β (ACACB) and FRMD3. A report by Maeda et al. (588) identified SNPs in the ACACB gene associated with proteinuria in T2DM, and these explorations have been extended to multiple ethnic populations, with an associated SNP being consistently more frequent in DKD cases compared with controls (589). Additional in vitro functional analysis further supports a role for ACACB (588), and Murea et al. (590) have proposed that genes involved in lipid metabolism, such as ACACB, could influence DKD. Similarly, Pezzolesi et al. (549) performed a GWAS for T1DM KD in a Genetics of Kidneys in Diabetes sample and carried out a replication analysis in DCCT and EDIC participants. Noteworthy was the identification of association between DKD and the FRMD3 gene. Importantly, follow-up analyses in multiple populations, including both T1DM and T2DM KD cases, have also reported evidence for the association of FRMD3 with DKD (548, 591). A recent mechanistic study proposed a pathway by which FRMD3 variants could influence the risk of DKD based on transcriptional regulation of bone morphogenetic protein pathway genes (592).
A new generation of better-powered GWAS with increasingly larger sample sizes is now appearing. One of these is an African American T2DM-ESKD study encompassing >5800 African Americans (550), and another is an analysis of high-density GWAS data from the Family Investigation of Nephropathy and Diabetes consortium with multiethnic samples. Although McDonough et al. (550) detected no genome-wide significant associations with T2DM-ESKD (P ≤5 × 10−8), multiple variants in RNF185, LIMK2, SFI1, APOL3, and MYH9 demonstrated strong evidence of association with all-cause ESKD, including advanced KD attributed to diabetes and nondiabetic etiologies. Strikingly, the majority of these associations were based on the contribution of protection from nephropathy, rather than risk. Sandholm et al. (593) recently performed an analysis of T1DM KD in subjects from both the Genetics of Nephropathy: an International Effort cohort (including subjects from the United Kingdom-Republic of Ireland, Finnish Diabetic Nephropathy study) and the Genetics of Kidneys in Diabetes cohort (including >6500 European DNA samples). The analysis revealed several variants with strong evidence of association with T1DM KD in the AFF3 (AF4/FMR2 family, member 3) gene (P = 1.26 × 10−8, OR = 1.26) and an intergenic SNP on chromosome 15q26 (P = 2.0 × 10−9, OR = 1.80). An important addition to this study was functional data suggesting that AFF3 is involved in renal tubule fibrosis through the TGF-β1 pathway. The strongest genetic association with DKD was in the Genetics of Nephropathy: an International Effort study observed using T1DM-ESKD as the phenotype. With these encouraging new results from GWAS studies, optimism should be guarded; replication in other large studies remains necessary.
Genetics of diabetic KD now and in the future
The search for genes associated with common and complex diseases has been driven by technical developments such as the GWAS method. Even with continuing innovations, researchers have made limited progress in both T1DM KD and T2DM KD in all ethnic groups. Given that DKD is a common disorder with high public health impact, it is surprising that the sample sizes for contemporary studies of DKD are small and powered only to detect major genetic effects. In the future, researchers and funding agencies should consider expanding available study populations to enhance the power for gene detection.
Several research questions remain. Despite shared chronic hyperglycemia, it is uncertain whether shared genetic contributors in DKD exist for T1DM and T2DM. Although there are several studies of DKD in T1DM and T2DM that have identified genes (such as CNDP1, ACACB, FRMD3, and ELMO1) that are shared across populations (586, 594–596), the results are not compelling. It is also unclear whether DKD genes will translate their impact across ethnic differences within human populations. It is striking that mutations in the APOL1 gene are powerfully associated (OR 7.3 to 29) (540, 547) with non-DKD forms of severe nephropathy in African Americans, and yet, these APOL1 risk variants are virtually absent in European-derived populations (540, 597). The discovery of APOL1 is also in striking contrast to the apparent heterogeneous genetic architecture of DKD. Although we do not have a complete picture of DKD, there is clearly no genetic contributor to DKD remotely as powerful as APOL1 is in non-DKD. In sum, the goal of creating genetic risk scores (i.e., combinations of genetic variants that will aid in the prediction of DKD risk) remains a work in progress.
This does not mean that creating genetic tools for DKD that have clinical value will be beyond reach. As outlined previously, the cornerstone of genetic research to date has been the GWAS method, which is limited to common variations and frequently captures information primarily from noncoding variants in the genome. New technical innovations have facilitated the creation of large and growing databases of coding variants (both low frequency and rare) through next-generation sequencing of complete sets of exons from individual DNAs (exome sequencing). Researchers are actively making use of these resources to test for the impact of low-frequency coding variants on DKD, and efforts are underway to perform exome sequencing in DNAs from DKD-affected individuals. For example, the T2DM-Genes Consortium sponsored exome sequencing for >1000 of the DKD cases and controls from the African American T2DM-ESKD GWAS (550). Equally, epigenetic mechanisms (such as posttranslational methylation or demethylation and histone acetylation to alter the expression of genes) represent an attractive potential mechanism by which the hyperglycemic environment could mediate renal failure (83). Initial reports on epigenetic studies are now appearing (598). These many paths of investigation will undoubtedly reveal new insights into the genetic contributors to DKD in the near future.
Current treatment
Therapies to prevent or slow the development of DKD are multifactorial and include lowering blood sugar levels with medications, diet, and exercise, as well as treating hypertension and hyperlipidemia. As previously discussed, an early decline in the eGFR slope best correlated with subsequent risk of ESKD (535, 536). Maintaining eGFR remains the primary focus for preventing advanced DKD and slowing the progression to ESKD. Intensive glycemic control in patients with T1DM and T2DM prevents or delays the development of microvascular complications and reduces the rate of development of overt proteinuria. However, limited data exist on whether improving glycemic control prevents low eGFR and diabetic ESKD. Reducing albuminuria through improved glycemic control and other treatments is expected to lower CVD event rates but requires evaluation regarding how it affects the rate of eGFR loss. It is expected that reducing early-stage DKD, particularly overt proteinuria, will translate into fewer cases of diabetic ESKD in the future (599).
Improving glycemic control remains the mainstay of preventing and delaying DKD and other microvascular complications, as has been shown in longitudinal studies of T1DM and T2DM. The DCCT and subsequent EDIC trial (600) demonstrated that intensive glucose control in T1DM delayed the development and progression of microalbuminuria (601, 602). The UKPDS reached similar conclusions in patients with T2DM, reporting that improved glycemic control (metabolic memory) produced prolonged delays or reductions in microvascular complications, which are potentially linked to epigenetic factors (197, 603). The more recent ADVANCE, ACCORD, and Veterans Affairs Diabetes trials extended this finding, all demonstrating significant reductions in microalbuminuria and overt proteinuria with intensive glycemic control (196, 199, 604). Improving blood sugar control in subjects with the more advanced stages of DKD will most likely reduce the rate of eGFR loss, as has been shown in UKPDS and DCCT/EDIC (197, 600). However, this may be less effective in halting the progression to ESKD once critical reductions in nephron mass develop. Based on these studies, and considering risks of hypoglycemia, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines recommend a target HbA1c of ∼7% (and not treating to <7%) to prevent or delay progression of diabetic microvascular complications, including DKD. A target HbA1c >7% is recommended in those with comorbidities or limited life expectancy and at risk for hypoglycemia (599).
It is important to appreciate that kidney function can impact the metabolism and safety of several blood sugar–lowering medications. National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines suggest avoiding first-generation sulfonylureas when eGFR is <60 mL/min/1.73 m2. Instead, the guidelines prefer second-generation glipizide to reduce the risk of prolonged hypoglycemia (599). Caution is urged when initiating meglitinides when eGFR is <30 mL/min/1.73 m2. US Food and Drug Administration guidelines recommend patients avoid metformin when eGFR is <30 mL/min/1.73 m2 and avoid starting metformin when eGFR is <45 mL/min/1.73 m2. Clinicians should closely follow people on metformin whose GFR is between 30 and 45 mL/min/1.73 m2 and assess them for risk factors for adverse effects of metformin. Japanese and British guidelines also suggest withdrawal when eGFR is <30 mL/min/1.73 m2. Data suggest additional dose adjustments (or avoidance) of diabetes medications in patients with advanced nephropathy for α-glucosidase inhibitors, DPP4 inhibitors, SGLT-2 inhibitors, incretin mimetics, and IAPP analogs (599).
Lowering systemic BPs with antihypertensive medications and dietary modification slows the development and progression of DKD, although patients most likely need to maintain lower BPs to sustain this benefit (603, 605). The Joint National Commission 7 recommends RAAS-blocking agents. However, these agents appear to be most effective at slowing DKD in patients with high levels of proteinuria (606). Although RAAS blockers often slow DKD progression, they do not reliably halt the progression to ESKD. Cessation of RAAS blockers may eventually become necessary in patients with stage-4 and stage-5 CKD because of the excessive lowering of eGFR due to reversible hemodynamic effects and hyperkalemia. Studies attempting greater RAAS blockade by combining two agents (ACEi and ARBs) carry greater risks for adverse events and hyperkalemia and should be avoided (607, 608).
It was disappointing that RAAS blockers proved to be ineffective for the primary prevention of the earliest renal histologic lesions of DKD (100). In a longitudinal kidney biopsy trial, patients receiving an ARB ultimately had higher levels of albuminuria than those on placebo (however, both ACEi and ARBs reduced DR, relative to placebo). Although RAAS blockers are first-line therapies for hypertension in subjects with diabetes, they are not likely to markedly reduce the subsequent development of DKD. National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines do not recommend the routine use of RAAS-blocking agents in normotensive normoalbuminuric subjects with diabetes, although ACEi and ARBs are recommended in normotensive diabetic patients with a urine albumin:creatinine ratio >30 mg/g who are believed to be at risk for future DKD (599).
Anecdotal evidence suggests that statin therapy for hyperlipidemia may slow nephropathy progression in DKD. However, RCTs with statistically significant results are lacking (609). Statins often reduce CVD rates in patients with and at risk for DKD. However, trials using statins to lower LDL cholesterol have not demonstrated reduced mortality in patients with diabetes and ESKD on hemodialysis (610, 611). Difficulties controlling blood sugars and the fact that RAAS inhibition was ineffective at primary prevention led to studies testing novel medications for DKD, including inhibitors of advanced glycation end-product formation and agents to reduce oxidative stress and inflammation. To date, these agents have not proven safe and effective for renal protection (142, 612).
Because glycemic control remains the mainstay for preventing DKD and slowing progression, it is critical to appreciate the effect that advanced stages of DKD have on the accuracy of tests used to assess glycemic control, especially the HbA1c. Because hemoglobin resides in red blood cells, HbA1c assesses glycemic control over the preceding 120 days (the life span of a normal red blood cell). In the late stages of DKD and ESKD, red blood cell survival drops, and clinicians often prescribe medications to treat anemia (erythropoietin). For given degrees of glycemic control, HbA1c levels are markedly reduced in patients with eGFR <30 mL/min/1.73 m2 or those on peritoneal dialysis or hemodialysis, relative to subjects with normal kidney function (613, 614). Inaccurately low HbA1c values provide a false sense of security to clinicians and patients (615). Interpretation of HbA1c in patients with ESKD requires complex statistical adjustment to better reflect ambient blood sugars. Markedly high and low adjusted HbA1c values predict poorer outcomes on dialysis (616, 617). Frequent serum glucose monitoring or novel assays (glycated albumin and continuous glucose monitoring) may more accurately reflect glycemia in patients with advanced DKD.
Summary
Renal microvascular dysfunction, which is common in individuals with diabetes, is characterized by strong but incompletely understood genetic predisposition. Its development and progression are clearly affected by clinical variables, including control of blood glucose and BP. The success in controlling these variables achieved in the past several decades along with other progress with risk factor management are gratifying in that they have lessened progression to ESKD. However, the increased prevalence of diabetes significantly offsets this progress. Consequently, diabetes remains a major contributor to renal failure and the associated increased mortality from CVD seen with ESRD. Developing a more complete understanding of the genetic/molecular factors contributing to initiation and progression of microvascular disease will hopefully lead to even more successful preventive strategies.
The Microvasculature and Diabetic Neuropathy
Introduction
Diabetic neuropathies are very common and troublesome complications of diabetes that lead to morbidity and mortality and a huge economic burden for diabetes care (618, 619). Distal symmetric polyneuropathy (DSPN) is the most common form of neuropathy. It is responsible for 50% to 75% of nontraumatic amputations (619, 620). Diabetic neuropathy is a set of clinical syndromes that affect distinct regions of the nervous system, singly or combined. It may be silent and go undetected while exercising its ravages. Or, it may present with clinical symptoms and signs that, although nonspecific and insidious with slow progression, mimic those of other diseases. Clinicians, therefore, diagnose diabetic neuropathy by exclusion. Unfortunately, diabetic neuropathy is underdiagnosed. Even when symptomatic, less than one third of physicians recognize diabetic neuropathy or discuss it with their patients (621).
Epidemiology
The epidemiology and natural history of diabetic neuropathy remain poorly defined. This is due, in part, to variable criteria for diagnosis, failure of many physicians to recognize and diagnose the disease, and lack of standardized methodologies for the evaluation of these patients (622). It has nonetheless been estimated that 50% of patients with diabetes have diabetic neuropathy, and in the United States, 2.7 million have painful neuropathy. Of 25% of patients attending a diabetes clinic who volunteered symptoms, 50% tested positive for neuropathy after a simple clinical test (such as the ankle jerk or vibration perception test), and almost 90% tested positive to sophisticated tests of autonomic function or peripheral sensation (623). Neurologic complications occur in both T1DM and T2DM and in various forms of acquired diabetes (51). The major morbidity associated with DSPN is foot ulceration, a precursor to gangrene and limb loss. DSPN increases the risk of amputation 1.7-fold. However, that risk jumps to 12-fold if there are deformities (itself a consequence of neuropathy) and 36-fold if there is a history of previous ulceration (624). Each year, 96,000 diabetic patients in the United States undergo amputations. It is estimated that up to 75% of these amputations are preventable (620). Diabetic neuropathy also impacts QOL by causing pain, weakness, ataxia, and incoordination (predisposing to falls and fractures) (625). For patients over the age of 65, diabetes increases fall risk by 17-fold, leading to fractures and traumatic brain injury. Autonomic neuropathy likewise decreases QOL and is associated with mortality rates between 25% and 50% within 5 to 10 years (626, 627).
DSPN causes a variety of syndromes for which there is no universally accepted classification. Operationally, they are subdivided into focal/multifocal neuropathies, including diabetic amyotrophy and symmetric sensorimotor polyneuropathy. The latter is the most common type, affecting ∼30% of diabetic patients in hospital care and 25% of those in the community (628, 629). DSPN is defined as a symmetrical, length-dependent, sensorimotor polyneuropathy attributable to metabolic and microvascular alterations resulting from chronic hyperglycemia exposure (diabetes) and cardiovascular risk covariates (630). Its onset is generally insidious. Without treatment, the course is chronic and progressive. The loss of small-fiber–mediated sensation results in the loss of thermal and pain perception, whereas large-fiber impairment results in loss of touch and vibration perception. Sensory fiber involvement may also result in positive symptoms, such as paresthesias and pain. Nonetheless, up to 50% of neuropathic patients can be asymptomatic.
Diabetic autonomic neuropathy rarely causes severe symptoms (622, 631). However, in its cardiovascular form, it is definitely associated with at least a threefold increased risk for mortality (632–634). More recently, studies have implicated diabetic autonomic neuropathy, or even autonomic imbalance between the sympathetic and the parasympathetic nervous systems, as predictors of cardiovascular risk (633, 634).
Neuropathic pain is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” (635). Diabetic neuropathy pain is a clinical problem that is difficult to manage. It is often associated with mood and sleep disturbances, and patients with diabetic neuropathy pain are more apt to seek medical attention than those with other types of diabetic neuropathy. Recognizing psychological problems early is critical to the management of pain, and physicians need to go beyond the management of pain if they are to achieve success. Patients may also complain of decreased physical activity and mobility, increased fatigue, and negative effects on their social lives. Providing significant pain relief markedly improves QOL measures (636, 637).
Classification of diabetic neuropathies
Figure 7 describes the classification Thomas proposed (638), which was later modified (628, 639–641). It is important to note that different forms of diabetic neuropathy often coexist in the same patient (e.g., distal polyneuropathy and carpal tunnel syndrome).
Figure 7.
Modified diabetic neuropathy classification first proposed by Thomas. N, normal. Reference: Jirousek et al. (25).
Natural history of diabetic neuropathies
The natural history of diabetic neuropathies separates them into two very distinctive entities, namely those that progress gradually with increasing duration of diabetes, and those that remit usually completely. Sensory and autonomic neuropathies generally progress, whereas mononeuropathies, radiculopathies, and acute painful neuropathies (although manifesting severe symptoms) are short-lived and tend to recover (642). The progression of diabetic neuropathy is related to glycemic control in both T1DM and T2DM (1, 643). It appears that the most rapid deterioration of nerve function occurs soon after the onset of T1DM. Within 2 to 3 years, there is a slowing of the progress with a shallower slope to the curve of dysfunction. In T2DM, slowing of NCVs may be one of the earliest neuropathic abnormalities and often is present even at diagnosis (644). After diagnosis, slowing of NCV generally progresses at a steady rate of ∼1 m/s/y, and the level of impairment is positively correlated with the duration of diabetes. Although most studies have documented that symptomatic patients are more likely to have slower NCVs than patients without symptoms, NCVs do not correlate with the severity of symptoms. In a long-term follow-up study of T2DM patients (645), electrophysiologic abnormalities in the lower limb increased from 8% at baseline to 42% after 10 years. In particular, a decrease in sensory and motor amplitudes (indicating axonal destruction) was more pronounced than the slowing of NCVs. Using objective measures of sensory function, such as the vibration perception threshold test, researchers have reported a rate of decline of 1 to 2 vibration units/year. However, this rate of decline now appears to be less severe, most likely due to improvements in general health and nerve nutrition. This is particularly important when doing studies on the treatment of diabetic neuropathy, which have always relied on differences between drug treatment and placebo and have apparently been successful because of the decline in function occurring in placebo-treated patients (646). Recent studies have pointed out the changing natural history of diabetic neuropathy with the advent of therapeutic lifestyle change and the use of statins and ACEi, which have slowed the progression of diabetic neuropathy and drastically changed the requirements for placebo-controlled studies (62). It is also important to recognize that diabetic neuropathy is a disorder wherein the prevailing abnormality is loss of axons, which electrophysiologically translates to a reduction in amplitudes and not conduction velocities; therefore, changes in NCV may not be an appropriate means of monitoring progression or deterioration of nerve function. Small, unmyelinated nerve fibers are affected early in diabetes and are not reflected in NCV studies. Other methods of measuring diabetic neuropathy that do not depend on conduction velocities (such as quantitative sensory testing, autonomic function testing, or skin biopsy with quantification of intraepidermal nerve fibers) are necessary to identify diabetic neuropathy patients (647–649).
Pathogenesis of diabetic neuropathies
Historically, there were two competing hypotheses regarding the origins of diabetic neuropathy. One school of thought held that this was largely secondary to metabolic abnormalities within the nerve and/or Schwann cells, whereas others held that diabetic neuropathy was another manifestation of diabetic microvascular disease. Increasingly, researchers believe that nerve and microvascular injury both contribute to nerve dysfunction. Some of the controversy arose because of the complexity of how diabetic neuropathy presents, as well as the limited ability to study the disease and its pathogenetic mechanisms, particularly early in its course. Research has clearly shown that with DSPN there is progressive axon degeneration of all fiber types, and this is accompanied by demyelination. However, microelectrode polarography has shown sural nerve hypoxemia accompanied by diminished endoneural blood flow (650, 651), indicating that these changes (which are the proximal cause for the nerve dysfunction) are accompanied by changes in the microvasculature.
Causative factors include persistent hyperglycemia, oxidative and nitrosative stress, inflammation, and autoimmune-mediated nerve destruction (see Biochemical Pathways of Microvascular Injury). These factors affect the microvasculature, Schwann cells, and the nerves themselves. However, diabetic neuropathy is a heterogeneous group of conditions with widely varying pathology, suggesting differences in pathogenic mechanisms for the different clinical syndromes. Recognizing the clinical homolog of these pathologic processes is the first step in achieving the appropriate form of intervention. It is also clear that structural changes in the microvasculature within peripheral nerves occur early in the course of diabetes. It has been known for many years that the vessel wall within peripheral nerves becomes thickened in individuals with DPN. This is attributed to increases in BM thickness, which is accompanied by pericyte degeneration and endothelial cell hyperplasia. These changes are, in many ways, similar to what we see within the microvasculature in other tissues. We also see these qualitative changes in individuals with diabetes without clinical peripheral neuropathy, although the changes are quantitatively less marked. These changes are typically more marked in endoneural than in epineurial vessels for reasons that are unclear.
Clinical presentation of diabetic neuropathies
The spectrum of clinical neuropathic syndromes described in patients with diabetes includes dysfunction of almost every segment of the somatic peripheral and autonomic nervous system (38). We can distinguish each syndrome by its pathophysiologic, therapeutic, and prognostic features.
Focal and multifocal neuropathies
Focal neuropathies comprise focal-limb neuropathies and cranial neuropathies. Focal limb neuropathies are usually due to entrapment, and we must distinguish mononeuropathies from these entrapment syndromes (Table 5) (640). Mononeuropathies often occur in the older population; they have an acute onset, are associated with pain, and have a self-limiting course resolving in 6 to 8 weeks. Mononeuropathies can involve the median (5.8% of all diabetic neuropathies), ulnar (2.1%), radial (0.6%), and common peroneal nerves (652). Cranial neuropathies in diabetic patients are extremely rare (0.05%) and occur in older individuals with a long duration of diabetes (653). Entrapment syndromes start slowly, and will progress and persist without intervention. Carpal tunnel syndrome occurs 3 times as frequently in patients with diabetes compared with healthy populations (654) and is found in up to one third of patients with diabetes. Its increased prevalence in diabetes may be related to repeated undetected trauma, metabolic changes, and/or the accumulation of fluid or edema within the confined space of the carpal tunnel (640).
Table 5.
Distinguishing Characteristics of Mononeuropathies, Entrapment Syndromes, and Distal Symmetrical Polyneuropathy
| Feature | Mononeuropathy | Entrapment Syndrome | Distal Symmetric Neuropathy |
|---|---|---|---|
| Onset | Sudden | Gradual | Gradual |
| Pattern | Single nerve but may be multiple | Single nerve exposed to trauma | Distal symmetrical polyneuropathy |
| Nerves involved | CN III, VI, VII, ulnar, median, and peroneal | Median, ulnar, peroneal, medial, and lateral plantar | Mixed, motor, sensory, and autonomic |
Proximal motor neuropathy (diabetic amyotrophy) and chronic demyelinating neuropathies
For many years, clinicians thought that proximal neuropathy was a component of diabetic neuropathy. There was a poor understanding of its pathogenesis (655). As a result, clinicians often left the condition untreated with the anticipation that the patient would eventually recover, albeit over a period of some 1 to 2 years and after suffering considerable pain, weakness, and disability. Proximal neuropathy has a number of synonyms, including diabetic amyotrophy and femoral neuropathy. Its common features include the following: (1) it primarily affects elderly patients (50 to 60 years old) with T2DM; (2) the onset can be gradual or abrupt; (3) it presents with severe pain in the thighs, hips, and buttocks, followed by significant weakness of the proximal muscles of the lower limbs and an inability to rise from the sitting position; (4) it can start unilaterally and then spread bilaterally; (5) it often coexists with DSPN; and (6) it is characterized by muscle fasciculation, either spontaneous or provoked by percussion. Its pathogenesis is not yet clearly understood, although immune-mediated epineurial microvasculitis occurs in some cases. Clinicians generally prescribe immunosuppressive therapy using high-dose steroids or intravenous immunoglobulin (656). Proximal neuropathy can occur secondary to a variety of conditions unrelated to diabetes. However, these unrelated conditions have a greater frequency in patients with diabetes than the general population and include chronic inflammatory demyelinating polyneuropathy, monoclonal gammopathy, circulating GM1 antibodies, and inflammatory vasculitis (653, 654, 657, 658).
In the classic form of diabetic amyotrophy, axonal loss is the predominant process (659) and electrophysiologic evaluation reveals lumbosacral plexopathy (660). In contrast, if demyelination predominates and the motor deficit affects proximal and distal muscle groups, clinicians should consider the diagnoses of chronic inflammatory demyelinating polyneuropathy, monoclonal gammopathy of unknown significance, and vasculitis (661, 662). Clinicians often overlook these demyelinating conditions. However, recognition is very important; unlike diabetic neuropathy, they are sometimes treatable. Furthermore, they occur 11 times more frequently in diabetic than nondiabetic patients (663, 664). A biopsy of the obturator nerve reveals deposits of immunoglobulin, demyelination, and inflammatory cell infiltrate around the vasa nervorum (657, 665). Cerebrospinal fluid protein content is high, and there is an increase in the lymphocyte count. Treatment options include intravenous immunoglobulin for chronic inflammatory demyelinating polyneuropathy (666), plasma exchange for monoclonal gammopathy of unknown significance, steroids and azathioprine for vasculitis, and withdrawal of other drugs or agents that may have caused vasculitis. It is important to divide proximal syndromes into these two subcategories, because the chronic inflammatory demyelinating polyneuropathy variant responds dramatically to intervention (661, 667), whereas proximal neuropathy amyotrophy runs its own course over months to years. Until more evidence is available, clinicians should consider these as separate syndromes.
Diabetic truncal radiculoneuropathy
Diabetic truncal radiculoneuropathy affects middle-aged to elderly patients and has a predilection for the male sex. Pain is the most important symptom, and it occurs in a girdle-like distribution over the lower thoracic or abdominal wall. It can be unilaterally or bilaterally distributed. Motor weakness is rare. Resolution generally occurs within 4 to 6 months.
Generalized symmetric polyneuropathy
Acute sensory neuropathy.
Some consider acute sensory (painful) neuropathy a distinctive variant of distal symmetrical polyneuropathy. The syndrome is characterized by severe pain, cachexia, weight loss, depression, and (in males) erectile dysfunction. It occurs predominantly in male patients and may appear at any time in the course of both T1DM and T2DM. It is self-limiting and invariably responds to simple symptomatic treatment. Conditions such as Fabry’s disease, amyloidosis, HIV infection, heavy metal poisoning (such as arsenic), and excess alcohol consumption should be excluded (638).
Acute sensory neuropathy is usually associated with poor glycemic control but may also appear after a sudden improvement in glycemic control and has been associated with the onset of insulin therapy (occasionally referred to as insulin neuritis) (668). Although the pathologic basis has not been determined, one hypothesis suggests that changes in blood glucose flux produce alterations in epineurial blood flow, leading to ischemia. A study using in vivo epineurial vessel photography and fluorescein angiography demonstrated abnormalities in epineurial vessels, including arteriovenous shunting and new-vessel proliferation in patients with acute sensory neuropathy (669). Some relate this syndrome to diabetic lumbosacral radiculoplexus neuropathy and suggest a possible immune-mediated mechanism (649).
Chronic sensorimotor neuropathy or distal symmetric polyneuropathy
DSPN is seen in both T1DM and T2DM with similar frequency, and it may be already present at the time of T2DM diagnosis (644). A population survey reported that 30% of T1DM and 36% to 40% of T2DM patients experienced neuropathic symptoms (59). Several studies have also suggested that impaired glucose tolerance may lead to polyneuropathy. The studies reported rates of impaired glucose tolerance between 30% and 50% in patients with chronic idiopathic polyneuropathies (670–674). Studies using skin and nerve biopsies have shown a progressive reduction in peripheral nerve fibers from the time of the diagnosis of diabetes or even from earlier prediabetic stages (impaired glucose tolerance and metabolic syndrome) (648, 675, 676). Sensory symptoms are more prominent than motor symptoms and usually involve the lower limbs.
Mild muscle wasting may occur, but severe weakness is rare, which should raise the question of a possible nondiabetic etiology of the neuropathy (51, 622, 630, 649).
Clinical manifestations of small-fiber neuropathies
Clinical manifestations of small-fiber neuropathies (Fig. 7) include symptoms of burning, superficial, or lancinating pain often accompanied by hyperalgesia, dysesthesia, and allodynia; disruption of small thinly myelinated Aδ and unmyelinated C fibers; a progression to numbness; abnormal cold and warm thermal sensations; abnormal autonomic function with decreased sweating, dry skin, cold feet, and impaired vasomotion and skin blood flow; intact motor strength and deep tendon reflexes; negative NCV findings; loss of cutaneous nerve fibers on skin biopsies; and clinical diagnosis by reduced sensitivity to 1.0 g Semmes Weinstein monofilament and prickling pain perception using the Wartenberg wheel or similar instrument.
Clinical manifestations of large-fiber neuropathies
Clinical manifestations of large-fiber neuropathies (Fig. 7) include the following: disruption of large myelinated, rapidly conducting Aα/β fibers, which may involve sensory and/or motor nerves; prominent signs with sensory ataxia (waddling like a duck) and the wasting of small intrinsic muscles of feet and hands with hammertoe deformities and weakness of hands and feet; abnormal deep tendon reflexes; impaired vibration, light touch, and joint position perception; abnormal NCV findings; increased skin blood flow with hot feet; higher risk of falls, fractures, and the development of Charcot neuroarthropathy; and minimal symptoms, which may include a sensation of walking on cotton, floors feeling strange, inability to turn the pages of a book, inability to discriminate among coins, and (in some patients with severe distal muscle weakness) inability to stand on the toes or heels.
Most patients with DPN, however, have a mixed variety of neuropathy with both large and small nerve-fiber damages.
Diagnosing diabetic peripheral neuropathy
In 2010, The Toronto Expert Panel on Diabetic Neuropathy Classification redefined the minimal criteria for the diagnosis of typical DPN (630).
The diagnosis of DPN should rest on the findings from a clinical and neurologic examination. These include the presence of positive and negative neuropathic symptoms and signs (either sensory or motor), such as sensory deficits, allodynia, hyperalgesia, motor weakness, or absence of reflexes (677).
When making a diagnosis of DPN, clinicians should assess both symptoms and signs based on the following guidelines:
-
(1)
Symptoms alone have poor diagnostic accuracy in predicting the presence of polyneuropathy.
-
(2)
Signs are better predictors than symptoms.
-
(3)
Multiple signs are better predictors than a single sign.
-
(4)
Relatively simple examinations are as accurate as complex scoring systems.
Conditions mimicking diabetic neuropathy
Conditions that mimic diabetic neuropathy include neuropathies caused by alcohol abuse, uremia, hypothyroidism, vitamin B12 deficiency, peripheral arterial disease, cancer, inflammatory and infectious diseases, and neurotoxic drugs (70). An atypical pattern of the presentation of symptoms or signs (i.e., the presence of relevant motor deficits, an asymmetrical or proximal distribution, or rapid progression) always requires referral for electrodiagnostic testing.
Clinical assessment tools for diabetic neuropathy
Clinical assessment should be standardized using validated scores for both symptom severity and the degree of reproducible neuropathic deficits. These would include the Michigan Neuropathy Screening Instrument (678), the Neuropathy Symptom Score for neuropathic symptoms, and the Neuropathy Disability Score or the Neuropathy Impairment Score for neuropathic deficits (679).
Objective diagnosis of diabetic neuropathy
The neurologic examination should focus on the lower extremities and include foot inspection for deformities, ulcers, fungal infection, muscle wasting, hair distribution or loss, and the presence or absence of pulses. Clinicians should assess sensory modalities using simple handheld devices (touch by cotton wool or soft brush; vibration by 128 Hz tuning fork; pressure by the Semmes-Weinstein 1 g and 10 g monofilament; pinprick by Wartenberg wheel, Neurotip, or a pin; and temperature by cold and warm objects) (680). Finally, clinicians should test the Achilles reflexes (639, 681) (Table 6).
Table 6.
Examination: Bedside Sensory Tests
| Sensory Modality | Nerve Fiber | Instrument | Associated Sensory Receptors |
|---|---|---|---|
| Vibration | Aβ (large) | 128 Hz | Ruffini corpuscle mechanoreceptors |
| Tuning fork | |||
| Pain (pinprick) | C (small) | Neuro-tips | Nociceptors for pain and warmth |
| Pressure | Aβ, Aα (large) | 1 g and 10 g | Pacinian corpuscle |
| Monofilament | |||
| Light touch | Aβ, Aα (large) | Wisp of cotton | Meissner’s corpuscle |
| Cold | Aδ (small) | Cold tuning fork | Cold thermoreceptors |
Nerve conduction studies
We recommend using electrophysiologic measures for both clinical practice and multicenter clinical trials (682, 683). In a long-term follow-up study of T2DM patients (645), NCV abnormalities in the lower limbs increased from 8% at baseline to 42% after 10 years of disease. The Diabetes Control and Complication trial reported a slow progression of NCV abnormalities. The sural and peroneal NCVs diminished by 2.8 and 2.7 m/s, respectively, over a 5-year period (643). Furthermore, in the same study, patients who were free of neuropathy at baseline had a 40% incidence of abnormal NCV in the conventionally treated group vs 16% in the intensive therapy-treated group after 5 years. However, the neurophysiologic findings vary widely depending on the population tested and the type and distribution of the neuropathy. Patients with painful, predominantly small-fiber neuropathy have normal test results. There is consistent evidence that small, unmyelinated fibers are affected early in diabetes, and routine NCV tests do not diagnose these alterations. Therefore, other methods, such as quantitative sensory testing or skin biopsy with quantification of intraepidermal nerve fibers, are needed to detect these patients (647–649). Nevertheless, electrophysiological testing plays a key role in ruling out other causes of neuropathy and is essential for the identification of focal and multifocal neuropathies (622, 639).
Summary of diagnosis of diabetic polyneuropathies
A detailed clinical examination is the key to diagnosing diabetic polyneuropathies. The last position statement of the American Diabetes Association recommends that clinicians should screen all patients with diabetes for diabetic neuropathies at diagnosis in T2DM and 5 years after diagnosis in T1DM. These screenings should occur annually and must include sensory examinations of feet and ankle reflexes (639).
The diagnosis of diabetic polyneuropathies is mainly clinical and involves specific tests according to the type and severity of the neuropathy. However, depending on the clinical findings, other nondiabetic causes of neuropathy must always be excluded.
Treatment of diabetic polyneuropathies
Diabetic polyneuropathy treatment should target different aspects of the disease in the following order: first, underlying pathogenic mechanisms; second, symptoms and improvement in QOL; and third, the complications of neuropathy and their progression (83). We will review very briefly in this work only those issues related to treating the underlying pathogenetic mechanisms. A more complete approach to clinical management of the consequences of diabetic polyneuropathies is beyond the scope of this review and can be found in other texts.
Treatment of specific underlying pathogenic mechanisms
Glycemic and metabolic control.
Several long-term prospective studies have assessed the effects of intensive diabetes therapy on the prevention and progression of chronic diabetic complications (1, 197). In the DCCT and UKPDS studies, only a minority of subjects had symptomatic DSPN at entry. In the DCCT study, intensive diabetes therapy slowed but did not completely prevent the development of DSPN in T1DM patients. In the DCCT/EDIC cohort, the benefits of former intensive insulin treatment persisted for 13 to 14 years in T1DM patients after DCCT closeout. These included a durable beneficial effect on polyneuropathy and cardiac autonomic neuropathy (hyperglycemic memory) (684, 685).
Conversely, in T2DM patients, the results were largely negative. The UKPDS showed a lower rate of impaired vibration perception thresholds (vibration perception thresholds >25 V) after 15 years for intensive therapy vs conventional therapy (31% vs 52%, respectively). However, the only additional time point at which vibration perception thresholds reached a significant difference was the 9-year follow-up, whereas the rates after 3, 6, and 12 years did not differ between the groups. Likewise, the rates of absent knee and ankle reflexes, as well as the heart rate responses to deep breathing, did not differ between the groups (197). In the ADVANCE study (which included 11,140 patients with T2DM randomly assigned to either standard glucose control or intensive glucose control), the relative risk reduction (95% CI) for new or worsening neuropathy for intensive vs standard glucose control after a median of 5 years of follow-up was −4 (−10 to 2), without a significant difference between groups (604). Likewise, in the Veterans Affairs Diabetes trial [including 1791 military veterans (mean age, 60.4 years) with a suboptimal response to therapy for T2DM], there were no differences between the intensive or standard glucose control groups for DSPN or microvascular complications after a median follow-up of 5.6 years (88). The ACCORD trial (196) halted intensive therapy aimed at HbA1c <6.0% before the study ended (because of a higher mortality in that group) and transitioned patients to standard therapy after 3.7 years, on average. At transition, sensation to light touch was significantly improved on intensive vs standard diabetes therapy. After 5 years (end of study), patients on intensive therapy had a better Michigan Neuropathy Screening Instrument Score and significant improvements in sensation to vibration and light touch vs patients on standard diabetes therapy. However, because of the premature study termination and the aggressive HbA1c goal, the neuropathy outcome in the ACCORD trial is difficult to interpret.
In the Steno 2 study (686), intensified multifactorial risk intervention (including intensive diabetes treatment, ACE inhibitors, antioxidants, statins, aspirin, and smoking cessation) in patients with microalbuminuria showed no effect on DSPN after 7.8 years (range: 6.9 to 8.8) and 13.3 years (patients were subsequently followed for a mean of 5.5 years). However, the progression of cardiac autonomic neuropathy was reduced by 57%. Thus, there is no evidence that intensive diabetes therapy or a target-driven intensified intervention aimed at multiple risk factors favorably influences the development or progression of DSPN, as opposed to cardiac autonomic neuropathy in T2DM patients. However, the Steno study used only vibration detection, which exclusively measures the changes in large-fiber function.
Oxidative stress.
A number of studies have shown that hyperglycemia causes oxidative stress in tissues that are susceptible to diabetes complications, including the microvasculature and peripheral nerves. Therapies that are under investigation include AR inhibitors, ALA, γ-linolenic acid, benfotiamine, Metanx, and PKC inhibitors.
As discussed elsewhere in this review, excess glucose in diabetic patients accelerates AGE generation, which leads to intra- and extracellular protein cross-linking and protein aggregation. RAGE activation alters intracellular signaling and gene expression, releases proinflammatory molecules, and results in an increased production of ROS that contributes to diabetic microvascular complications. Aminoguanidine, an inhibitor of AGE formation, showed good results in animal studies, but trials in humans have been discontinued because of toxicity (687). Benfotiamine is a transketolase activator that reduces tissue AGEs. Several independent pilot studies have demonstrated its effectiveness in diabetic polyneuropathy. In a 3-week placebo-controlled study, subjective improvements in neuropathy scores were seen in the group that received 200 mg daily of benfotiamine tablets, with a pronounced decrease in reported pain levels (688). In a 12-week study, the use of benfotiamine plus vitamin B6/B12 significantly improved NCV in the peroneal nerve along with appreciable improvements in vibratory perception. An alternate combination of benfotiamine (100 mg) and pyridoxine (100 mg) has improved diabetic polyneuropathy in a small number of diabetic patients (689).
Metanx is a natural food product for managing endothelial dysfunction. It contains l-methyl-folate, pyridoxal 5′-phosphate, and methylcobalamin. Metanx counteracts endothelial NOS uncoupling and oxidative stress in vascular endothelium and peripheral nerves. A 24-week, double-blinded, placebo-controlled multisite study concluded that, although there was no significant change in vibration perception threshold, there were significant improvements in both neuropathic symptoms and mental health (690). Metanx significantly improved the Neuropathy Total Symptoms Score-6 (which includes numbness, tingling, aching, burning, lancinating pain, and allodynia) at week 16 (P = 0.013) and week 24 (P = 0.033) vs placebo. Moreover, there were significant improvements on the Mental Health Component of the Short Form-36 Health Survey. In this study, metformin use was a major predictor of a beneficial response. Metformin can cause vitamin B12 deficiency and neuropathy (691). In particular, previously established normal values have grossly underestimated the level at which the peripheral nervous system is at risk (692, 693) (levels >400 pg/mL are required for neuronal integrity). These findings support the use of Metanx as a safe approach for short-term alleviation of diabetic neuropathy symptoms, although we need future studies to further define these effects and their impact on long-term outcomes.
AR inhibitors reduce the flux of glucose through the polyol pathway, inhibiting tissue accumulation of sorbitol and fructose. A 12-month study of zenarestat reported a dose-dependent improvement in nerve-fiber density (694). A 1-year trial of fidarestat in Japanese patients with diabetes reported an improvement of symptoms (695), and a 3-year study of epalrestat showed improved NCV and vibration perception (100). Studies are currently exploring newer ARIs, and some positive results have emerged (696, 697). However, it is becoming clear that these newer ARIs alone may not be sufficient, and combinations of treatments may be needed (640).
Patients have used ALA or thioctic acid, which have antioxidant and thiol-replenishing redox-modulating properties. A number of studies show a favorable influence of these agents on microcirculation and on the reversal of symptoms of neuropathy (698–700). A meta-analysis including 1258 patients from four RCTs concluded that 600 mg intravenous ALA daily significantly reduced symptoms of neuropathy and improved neuropathic deficits (701). The SYDNEY 2 trial showed significant improvement in neuropathic symptoms and neurologic deficits in 181 diabetic patients with three different doses of ALA compared with placebo over a 5-week period (702). The NATHAN 1 trial examined the long-term efficacy and safety of ALA. The trial randomly assigned diabetic patients (n = 460) with mild-to-moderate DSPN to oral treatment with 600 mg ALA once daily (n = 233) or placebo (n = 227) for 4 years. The primary end point was a composite score (NIS-LL and 7 neurophysiologic tests). The study showed that 4-year treatment with ALA in mild-to-moderate DSPN did not influence the primary composite end point. However, ALA did result in a clinically meaningful improvement and prevention of progression of neuropathic impairments, and it was well tolerated. The primary reason the composite score did not improve was that nerve conduction deficits in the placebo-treated group did not progress. Thus, the study was unable to show secondary prevention of progression of the composite end point by treatment with ALA (703).
We need further investigation to clarify ALA’s effect on neuropathic deficits vs nerve conduction parameters and/or quantitative sensory tests. Additionally, we need to address cost-benefit analyses, optimal treatment duration, and delineation of patients with disease characteristics most likely to benefit from ALA supplementation (704).
PKC activation is a critical step in the pathway to diabetic microvascular complications. Both hyperglycemia and disordered FA metabolism activate PKC, resulting in the increased production of vasoconstrictive, angiogenic, and chemotactic cytokines (including TGF-β, VEGF, ET-1, and intercellular adhesion molecules). A multinational, randomized, phase-2, double-blind, placebo-controlled trial with RBX (a PKCβ inhibitor) failed to achieve the primary endpoints, although it reported significant changes in a number of domains (61). Nevertheless, there was a statistically significant improvement in symptoms and vibratory detection thresholds in RBX- vs placebo-treated subjects in a subgroup of patients with clinically significant symptoms but less severe diabetic neuropathy at baseline (sural nerve action potential >0.5 μV) (705). A recent, smaller, single-center study showed improvements in symptom scores, endothelium-dependent skin blood flow measurements, and QOL scores in the RBX-treated group (646). These studies and the NATHAN studies point to a change in the natural history of diabetic neuropathy due to the advent of therapeutic lifestyle change, statins, and ACEi. These factors have slowed the progression of diabetic neuropathy and drastically altered the requirements for placebo-controlled studies.
Growth factors.
There is increasing evidence that there is a deficiency of nerve growth factor in diabetes, as well as a deficiency of dependent neuropeptides substance P and calcitonin gene-related peptide, and that this contributes to the clinical perturbations in small-fiber function (706). Clinical trials with nerve growth factor have not been successful and are subject to certain caveats with regard to design. Nevertheless, nerve growth factor still holds promise for sensory and autonomic neuropathies (623). The pathogenesis of diabetic neuropathy includes loss of vasa nervorum, so it is likely that the appropriate application of VEGF would reverse the dysfunction. Introducing the VEGF gene into the muscle of diabetic animal models improved nerve function (707). There are ongoing VEGF gene studies involving the transfection of the gene into human muscle.
Immune therapy
Several autoantibodies have been found in human sera that are both associated with diabetic neuropathy and can react with epitopes in neuronal cells. One study reported that in patients with diabetes there was a 12% incidence of an association between a predominantly motor form of neuropathy and monosialoganglioside antibodies (anti-GM1 antibodies) (665). Perhaps the clearest link between autoimmunity and neuropathy is the 11-fold increased likelihood of chronic inflammatory demyelinating polyneuropathy, multiple motor polyneuropathy, vasculitis, and monoclonal gammopathies in diabetes (663). New data, however, support a predictive role of the presence of antineuronal antibodies on the later development of neuropathy, suggesting that these antibodies may not be innocent bystanders but neurotoxins (625, 708). There may be selected cases (particularly those with autonomic neuropathy, evidence of antineuronal autoimmunity, and chronic inflammatory demyelinating polyneuropathy) that may benefit from intravenous immunoglobulin or large-dose steroids (661).
Summary
Diabetic neuropathies are some of the most common complications of diabetes that lead to significant morbidity and mortality and higher health care costs. The spectrum of clinical neuropathic syndromes described in patients with diabetes includes dysfunction of almost every segment of the somatic, peripheral, and autonomic nervous system. Focal neuropathies include focal-limb neuropathies due to entrapment syndromes and cranial neuropathies. Proximal muscle weakness from amyotrophy and chronic demyelinating neuropathies both occur with increased frequency in the diabetic population, but require different treatments. Distal neuropathies include DSPN and a distinctive variant known as acute sensory neuropathy, which are diagnosed by history and clinical examination. Specific diagnostic testing (e.g., quantitative sensory testing, skin biopsy and intraepidermal nerve-fiber density analysis, contact heat-evoked potentials, sudomotor function testing, and nerve conduction studies) can aid in the diagnosis and treatment.
Intensive glycemic and metabolic control can significantly influence the development or progression of DSPN, but not reverse established neuropathy. Therapies, including benfotiamine, AR inhibitors, Metanyx, and ALA, to reduce oxidative and nitrosative stress have shown encouraging results.
Conclusion
Increasingly we have learned that the microvasculature within different tissues serves multiple functions beyond being a conduit for exchange of respiratory gases, nutrients, and metabolic waste. Consequently, microvascular injury can express common and unique changes at different sites. The dynamic between microvascular injury and repair determines the manifestation of tissue-specific injury. Diabetes affects both injury and repair processes in a manner distinct from other vascular diseases. We have summarized both the general molecular processes involved in diabetic microvascular disease and many of their tissue-specific expressions. Clearly, there is much we still do not understand, and consequently, our ability to successfully intervene to prevent or reverse microvascular disease is quite limited. Insights gained by the use of newer tools, including genetic, proteomic, metabolomics, and other analyses, will certainly add new insights in the basic functioning of microvascular cells, and these insights will light the way to improved therapies.
Acknowledgments
We acknowledge science writer Eric Vohr for the contribution to this scientific statement.
Financial Support: S.C.’s contributions were in part supported by NIH P30 AG 049638.
Acknowledgments
Disclosure Summary: G.L.K. receives research support from Sanofi. B.I.F. receives research support from Novartis and is a consultant for Ionis and AstraZeneca Pharmaceuticals. B.I.F. and Wake Forest University Health Sciences have filed for a patent related to APOL1 genetic testing. A.I.V. is a consultant for Pfizer, Merck, Astellas, and Alnylam; has received research support from ViroMed, VeroScience, Nestle Health Science-PamLab, Novo Nordisk, MatrixBiomed, Impeto Medical, and the National Institutes of Health; and has served on the education speaker bureau for Merck Pharmaceuticals, Lexicon Pharmaceuticals, and Ipsen. E.J.B., Z.L., M.K., R.K., B.E.K.K., T.M.H., S.C., D.W.B., and C.M.C. have nothing to disclose.
Footnotes
- Aβ
- amyloid β
- ACCORD
- Action to Control Cardiovascular Risk in Diabetes
- ACE
- angiotensin-converting enzyme
- ACEi
- angiotensin-converting enzyme inhibitor
- AD
- Alzheimer’s disease
- ADP
- adenosine 5′-diphosphate
- ADVANCE
- Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- AGE
- advanced glycation end product
- ALA
- α-lipoic acid
- Ang II
- angiotensin II
- APC
- activated protein C
- AR
- aldose reductase
- ARB
- angiotensin II receptor blocker
- ATPase
- adenosine trisphosphatase
- BBB
- blood-brain barrier
- BK
- bradykinin
- BK1R
- BK1 receptor
- BK2R
- BK2 receptor
- BM
- basement membrane
- BP
- blood pressure
- C1-INH
- C1-inhibitor
- CBF
- cerebral blood flow
- CI
- confidence interval
- CKD
- chronic kidney disease
- CMB
- cerebral microbleed
- CMI
- cerebral microinfarct
- CNS
- central nervous system
- CVD
- cardiovascular disease
- DAG
- diacylglycerol
- DCCT
- Diabetes Control and Complications Trial
- DKD
- diabetic kidney disease
- DN
- diabetic nephropathy
- DPN
- diabetic peripheral neuropathy
- DR
- diabetic retinopathy
- DSPN
- distal symmetric polyneuropathy
- DTI
- diffusion tensor imaging
- ECM
- extracellular matrix
- EDIC
- Epidemiology of Diabetes Interventions and Complications
- eGFR
- estimated glomerular filtration rate
- eNOS
- endothelial nitric oxide synthase
- EPC
- endothelial progenitor cell
- EPVS
- enlarged perivascular space
- ER
- endoplasmic reticulum
- ESKD
- end-stage kidney disease
- ET-1
- endothelin 1
- FA
- fatty acid
- FDG
- fludeoxyglucose F18 ligand
- FLT-1
- fms-like tyrosine kinase 1
- GFR
- glomerular filtration rate
- GLP-1
- glucagon-like peptide 1
- GSH
- glutathione
- GWAS
- genome-wide association studies
- HbA1c
- hemoglobin A1c
- IAPP
- islet amyloid polypeptide
- iNOS
- inducible NOS
- IRS 1/2
- insulin receptor substrate 1/2
- KD
- kidney disease
- Keap
- Kelch erythroid cell-derived protein with CNC homolog-associated protein
- KKS
- kallikrein-kinin system
- LDL
- low-density lipoprotein
- MAPK
- mitogen-activated protein kinase
- MRI
- magnetic resonance imaging
- mRNA
- messenger RNA
- MTI
- magnetization transfer imaging
- NAD
- nicotinamide adenine dinucleotide
- NADPH
- nicotinamide adenine dinucleotide phosphate
- NCV
- nerve conduction velocity
- NF-κB
- nuclear factor κ light chain enhancer of activated B cells
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- Nrf2
- NF-E2–related factor 2
- OR
- odds ratio
- PDGF
- platelet-derived growth factor
- PET
- positron emission tomography
- PI3K
- phosphatidylinositol 3-kinase
- PKC
- protein kinase C
- QOL
- quality of life
- RAAS
- renin-angiotensin aldosterone system
- RAGE
- receptor for AGE
- RAS
- renin-angiotensin system
- RBF
- retinal blood flow
- RBX
- ruboxistaurin
- RCT
- randomized controlled trial
- ROS
- reactive oxygen species
- RVP
- retinal vascular permeability
- SHP-1
- Src homology-2 domain-containing phosphatase-1
- SNP
- single-nucleotide polymorphism
- STZ
- streptozotocin
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- TGF-β
- transforming growth factor β
- UKPDS
- United Kingdom Prospective Diabetes Study
- VEGF
- vascular endothelial growth factor
- WESDR
- Wisconsin Epidemiologic Study of Diabetic Retinopathy
- WMH
- white matter hyperintensity.
References
- 1.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 3.Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, Coney J, Orchard TJ, Aiello LP, King GL. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30(8):1995–1997. [DOI] [PubMed] [Google Scholar]
- 4.Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care. 2011;34(4):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–866. [DOI] [PubMed] [Google Scholar]
- 6.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370(Pt 2):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94(21):11233–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–S30. [DOI] [PubMed] [Google Scholar]
- 9.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43(9):1122–1129. [DOI] [PubMed] [Google Scholar]
- 10.Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993;265(5 Pt 1):E783–E793. [DOI] [PubMed] [Google Scholar]
- 11.Craven PA, Davidson CM, DeRubertis FR. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990;39(6):667–674. [DOI] [PubMed] [Google Scholar]
- 12.Ido Y, McHowat J, Chang KC, Arrigoni-Martelli E, Orfalian Z, Kilo C, Corr PB, Williamson JR. Neural dysfunction and metabolic imbalances in diabetic rats: prevention by acetyl-L-carnitine. Diabetes. 1994;43(12):1469–1477. [DOI] [PubMed] [Google Scholar]
- 13.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89(22):11059–11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15(11):1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu XH, Chen X, Zhang SL, Pang L, To C, Wang TT, Hohman TC, Filep JG, Chan JS. Molecular mechanism(s) of insulin action on the expression of the angiotensinogen gene in kidney proximal tubular cells. J Renin Angiotensin Aldosterone Syst. 2000;1(2):166–174. [DOI] [PubMed] [Google Scholar]
- 16.Ayo SH, Radnik R, Garoni JA, Troyer DA, Kreisberg JI. High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am J Physiol. 1991;261(4 Pt 2):F571–F577. [DOI] [PubMed] [Google Scholar]
- 17.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112(7):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52(8):2110–2120. [DOI] [PubMed] [Google Scholar]
- 20.Babazono T, Kapor-Drezgic J, Dlugosz JA, Whiteside C. Altered expression and subcellular localization of diacylglycerol-sensitive protein kinase C isoforms in diabetic rat glomerular cells. Diabetes. 1998;47(4):668–676. [DOI] [PubMed] [Google Scholar]
- 21.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55(11):3112–3120. [DOI] [PubMed] [Google Scholar]
- 22.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57(2):460–469. [DOI] [PubMed] [Google Scholar]
- 23.Menne J, Park JK, Boehne M, Elger M, Lindschau C, Kirsch T, Meier M, Gueler F, Fiebeler A, Bahlmann FH, Leitges M, Haller H. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-alpha-deficient diabetic mice. Diabetes. 2004;53(8):2101–2109. [DOI] [PubMed] [Google Scholar]
- 24.Mima A, Kitada M, Geraldes P, Li Q, Matsumoto M, Mizutani K, Qi W, Li C, Leitges M, Rask-Madsen C, King GL. Glomerular VEGF resistance induced by PKCδ/SHP-1 activation and contribution to diabetic nephropathy. FASEB J. 2012;26(7):2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH III, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A, Baevsky M, Ballas LM, Hall SE, Winneroski LL, Faul MM. (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-d ione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem. 1996;39(14):2664–2671. [DOI] [PubMed] [Google Scholar]
- 26.Parmer TG, Ward MD, Hait WN. Effects of rottlerin, an inhibitor of calmodulin-dependent protein kinase III, on cellular proliferation, viability, and cell cycle distribution in malignant glioma cells. Cell Growth Differ. 1997;8(3):327–334. [PubMed] [Google Scholar]
- 27.Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28(9):453–458. [DOI] [PubMed] [Google Scholar]
- 28.Tuttle KR, Anderson PW. A novel potential therapy for diabetic nephropathy and vascular complications: protein kinase C beta inhibition. Am J Kidney Dis. 2003;42(3):456–465. [DOI] [PubMed] [Google Scholar]
- 29.Kelly DJ, Chanty A, Gow RM, Zhang Y, Gilbert RE. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol. 2005;16(6):1654–1660. [DOI] [PubMed] [Google Scholar]
- 30.Meier M, Menne J, Park JK, Holtz M, Gueler F, Kirsch T, Schiffer M, Mengel M, Lindschau C, Leitges M, Haller H. Deletion of protein kinase C-epsilon signaling pathway induces glomerulosclerosis and tubulointerstitial fibrosis in vivo. J Am Soc Nephrol. 2007;18(4):1190–1198. [DOI] [PubMed] [Google Scholar]
- 31.Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D. Polymorphisms of the protein kinase C-beta gene (PRKCB1) accelerate kidney disease in type 2 diabetes without overt proteinuria. Diabetes Care. 2006;29(4):864–868. [DOI] [PubMed] [Google Scholar]
- 32.Ma RC, Tam CH, Wang Y, Luk AO, Hu C, Yang X, Lam V, Chan AW, Ho JS, Chow CC, Tong PC, Jia W, Ng MC, So WY, Chan JC. Genetic variants of the protein kinase C-beta 1 gene and development of end-stage renal disease in patients with type 2 diabetes. JAMA. 2010;304(8):881–889. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28(11):2686–2690. [DOI] [PubMed] [Google Scholar]
- 34.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. [DOI] [PubMed] [Google Scholar]
- 35.Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44(6):659–673. [DOI] [PubMed] [Google Scholar]
- 36.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272(5262):728–731. [DOI] [PubMed] [Google Scholar]
- 37.Bursell SE, Takagi C, Clermont AC, Takagi H, Mori F, Ishii H, King GL. Specific retinal diacylglycerol and protein kinase C beta isoform modulation mimics abnormal retinal hemodynamics in diabetic rats. Invest Ophthalmol Vis Sci. 1997;38(13):2711–2720. [PubMed] [Google Scholar]
- 38.Matsumoto T, Kobayashi T, Kamata K. Relationships among ET-1, PPARgamma, oxidative stress and endothelial dysfunction in diabetic animals. J Smooth Muscle Res. 2008;44(2):41–55. [DOI] [PubMed] [Google Scholar]
- 39.Kamata K, Sugiura M, Kasuya Y. Decreased Ca2+ influx into the endothelium contributes to the decrease in endothelium-dependent relaxation in the aorta of streptozotocin-induced diabetic mice. Res Commun Mol Pathol Pharmacol. 1995;90(1):69–74. [PubMed] [Google Scholar]
- 40.Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes. 2003;52(3):838–845. [DOI] [PubMed] [Google Scholar]
- 41.Aiello LM, Cavallerano J. Diabetic retinopathy. Curr Ther Endocrinol Metab. 1997;6:475–485. [PubMed] [Google Scholar]
- 42.Murakami T, Frey T, Lin C, Antonetti DA. Protein kinase cβ phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 2012;61(6):1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feener EP, Zhou Q, Fickweiler W. Role of plasma kallikrein in diabetes and metabolism. Thromb Haemost. 2013;110(3):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81(3):363–371. [DOI] [PubMed] [Google Scholar]
- 45.Suzuma K, Takahara N, Suzuma I, Isshiki K, Ueki K, Leitges M, Aiello LP, King GL. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA. 2002;99(2):721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danis RP, Bingaman DP, Jirousek M, Yang Y. Inhibition of intraocular neovascularization caused by retinal ischemia in pigs by PKCbeta inhibition with LY333531. Invest Ophthalmol Vis Sci. 1998;39(1):171–179. [PubMed] [Google Scholar]
- 47.Nakamura S, Chikaraishi Y, Tsuruma K, Shimazawa M, Hara H. Ruboxistaurin, a PKCbeta inhibitor, inhibits retinal neovascularization via suppression of phosphorylation of ERK1/2 and Akt. Exp Eye Res. 2010;90(1):137–145. [DOI] [PubMed] [Google Scholar]
- 48.Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD, Wolka AM, Shahri N, Milton RC; PKC-DRS and PKC-DRS2 Study Groups . Oral protein kinase c β inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C β Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C β Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31(10):2084–2094. [DOI] [PubMed] [Google Scholar]
- 49.Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE; PKC-DRS2 Group . Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113(12):2221–2230. [DOI] [PubMed] [Google Scholar]
- 50.PKC-DRS Study Group The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54(7):2188–2197. [DOI] [PubMed] [Google Scholar]
- 51.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O’Brien PC, Melton LJ III, Service FJ. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. [DOI] [PubMed] [Google Scholar]
- 52.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44(11):1973–1988. [DOI] [PubMed] [Google Scholar]
- 53.Sytze Van Dam P, Cotter MA, Bravenboer B, Cameron NE. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur J Pharmacol. 2013;719(1-3):180–186. [DOI] [PubMed] [Google Scholar]
- 54.Stevens MJ, Dananberg J, Feldman EL, Lattimer SA, Kamijo M, Thomas TP, Shindo H, Sima AA, Greene DA. The linked roles of nitric oxide, aldose reductase and, (Na+,K+)-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J Clin Invest. 1994;94(2):853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eichberg J. Protein kinase C changes in diabetes: is the concept relevant to neuropathy? Int Rev Neurobiol. 2002;50:61–82. [DOI] [PubMed] [Google Scholar]
- 56.Borghini I, Ania-Lahuerta A, Regazzi R, Ferrari G, Gjinovci A, Wollheim CB, Pralong WF. Alpha, beta I, beta II, delta, and epsilon protein kinase C isoforms and compound activity in the sciatic nerve of normal and diabetic rats. J Neurochem. 1994;62(2):686–696. [PubMed] [Google Scholar]
- 57.Kim J, Rushovich EH, Thomas TP, Ueda T, Agranoff BW, Greene DA. Diminished specific activity of cytosolic protein kinase C in sciatic nerve of streptozocin-induced diabetic rats and its correction by dietary myo-inositol. Diabetes. 1991;40(11):1545–1554. [DOI] [PubMed] [Google Scholar]
- 58.Yamagishi S, Uehara K, Otsuki S, Yagihashi S. Differential influence of increased polyol pathway on protein kinase C expressions between endoneurial and epineurial tissues in diabetic mice. J Neurochem. 2003;87(2):497–507. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura J, Kato K, Hamada Y, Nakayama M, Chaya S, Nakashima E, Naruse K, Kasuya Y, Mizubayashi R, Miwa K, Yasuda Y, Kamiya H, Ienaga K, Sakakibara F, Koh N, Hotta N. A protein kinase C-beta-selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes. 1999;48(10):2090–2095. [DOI] [PubMed] [Google Scholar]
- 60.Cameron NE, Cotter MA. Effects of protein kinase Cbeta inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev. 2002;18(4):315–323. [DOI] [PubMed] [Google Scholar]
- 61.Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ III; MBBQ Study Group . Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005;27(8):1164–1180. [DOI] [PubMed] [Google Scholar]
- 62.Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ III, Wolka AM, Vinik AI. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30(4):896–902. [DOI] [PubMed] [Google Scholar]
- 63.Boyd A, Casselini C, Vinik E, Vinik A. Quality of life and objective measures of diabetic neuropathy in a prospective placebo-controlled trial of ruboxistaurin and topiramate. J Diabetes Sci Technol. 2011;5(3):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. [DOI] [PubMed] [Google Scholar]
- 65.Fu ZJ, Li SY, Kociok N, Wong D, Chung SK, Lo AC. Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53(9):5698–5712. [DOI] [PubMed] [Google Scholar]
- 66.Yeung CM, Lo AC, Cheung AK, Chung SS, Wong D, Chung SK. More severe type 2 diabetes-associated ischemic stroke injury is alleviated in aldose reductase-deficient mice. J Neurosci Res. 2010;88(9):2026–2034. [DOI] [PubMed] [Google Scholar]
- 67.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301(2):E252–E263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, Chung SK. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55(7):1946–1953. [DOI] [PubMed] [Google Scholar]
- 69.Demaine AG. Polymorphisms of the aldose reductase gene and susceptibility to diabetic microvascular complications. Curr Med Chem. 2003;10(15):1389–1398. [DOI] [PubMed] [Google Scholar]
- 70.Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110(10):1364–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88(2):E14–E22. [DOI] [PubMed] [Google Scholar]
- 72.Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes. 2002;51(2):522–527. [DOI] [PubMed] [Google Scholar]
- 73.Garcia Soriano F, Virág L, Jagtap P, Szabó E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabó C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7(1):108–113. [DOI] [PubMed] [Google Scholar]
- 74.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. [DOI] [PubMed] [Google Scholar]
- 75.Othman A, Ahmad S, Megyerdi S, Mussell R, Choksi K, Maddipati KR, Elmarakby A, Rizk N, Al-Shabrawey M. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS One. 2013;8(2):e57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.You YH, Okada S, Ly S, Jandeleit-Dahm K, Barit D, Namikoshi T, Sharma K. Role of Nox2 in diabetic kidney disease. Am J Physiol Renal Physiol. 2013;304(7):F840–F848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73(14):1813–1824. [DOI] [PubMed] [Google Scholar]
- 78.Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong Y, Dai Y, Mazloom AR, Chen EY, D’Agati V, Xiong H, Ross MJ, Chen N, Ma’ayan A, He JC. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med. 2012;18(4):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taniguchi K, Xia L, Goldberg HJ, Lee KW, Shah A, Stavar L, Masson EA, Momen A, Shikatani EA, John R, Husain M, Fantus IG. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes. 2013;62(11):3874–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol. 2013;305(5):F691–F700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fallahzadeh MK, Dormanesh B, Sagheb MM, Roozbeh J, Vessal G, Pakfetrat M, Daneshbod Y, Kamali-Sarvestani E, Lankarani KB. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60(6):896–903. [DOI] [PubMed] [Google Scholar]
- 82.Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(6):3941–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kowluru RA, Zhong Q. Beyond AREDS: is there a place for antioxidant therapy in the prevention/treatment of eye disease? Invest Ophthalmol Vis Sci. 2011;52(12):8665–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC; HOPE Study; MICRO-HOPE Study . Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25(11):1919–1927. [DOI] [PubMed] [Google Scholar]
- 85.Haritoglou C, Gerss J, Hammes HP, Kampik A, Ulbig MW; RETIPON Study Group . Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica. 2011;226(3):127–137. [DOI] [PubMed] [Google Scholar]
- 86.Hamilton RT, Bhattacharya A, Walsh ME, Shi Y, Wei R, Zhang Y, Rodriguez KA, Buffenstein R, Chaudhuri AR, Van Remmen H. Elevated protein carbonylation, and misfolding in sciatic nerve from db/db and Sod1(-/-) mice: plausible link between oxidative stress and demyelination. PLoS One. 2013;8(6):e65725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uzar E, Tamam Y, Evliyaoglu O, Tuzcu A, Beyaz C, Acar A, Aydın B, Tasdemir N. Serum prolidase activity and oxidative status in patients with diabetic neuropathy. Neurol Sci. 2012;33(4):875–880. [DOI] [PubMed] [Google Scholar]
- 88.Tütüncü NB, Bayraktar M, Varli K. Reversal of defective nerve conduction with vitamin E supplementation in type 2 diabetes: a preliminary study. Diabetes Care. 1998;21(11):1915–1918. [DOI] [PubMed] [Google Scholar]
- 89.Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167(4):465–471. [DOI] [PubMed] [Google Scholar]
- 90.Reddy VP, Obrenovich ME, Atwood CS, Perry G, Smith MA. Involvement of Maillard reactions in Alzheimer disease. Neurotox Res. 2002;4(3):191–209. [DOI] [PubMed] [Google Scholar]
- 91.Chen CY, Abell AM, Moon YS, Kim KH. An advanced glycation end product (AGE)-receptor for AGEs (RAGE) axis restores adipogenic potential of senescent preadipocytes through modulation of p53 protein function. J Biol Chem. 2012;287(53):44498–44507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14(10):1521–1528. [DOI] [PubMed] [Google Scholar]
- 93.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108(2):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4(9):1025–1031. [DOI] [PubMed] [Google Scholar]
- 96.Li G, Tang J, Du Y, Lee CA, Kern TS. Beneficial effects of a novel RAGE inhibitor on early diabetic retinopathy and tactile allodynia. Mol Vis. 2011;17:3156–3165. [PMC free article] [PubMed] [Google Scholar]
- 97.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79(10):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jandeleit-Dahm KA, Lassila M, Allen TJ. Advanced glycation end products in diabetes-associated atherosclerosis and renal disease: interventional studies. Ann N Y Acad Sci. 2005;1043:759–766. [DOI] [PubMed] [Google Scholar]
- 99.Burnier M, Zanchi A. Blockade of the renin-angiotensin-aldosterone system: a key therapeutic strategy to reduce renal and cardiovascular events in patients with diabetes. J Hypertens. 2006;24(1):11–25. [DOI] [PubMed] [Google Scholar]
- 100.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93(6):2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou Y, Komuro I, Yamazaki T, Kudoh S, Aikawa R, Zhu W, Shiojima I, Hiroi Y, Tobe K, Kadowaki T, Yazaki Y. Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gbetagamma subunit, Src family, and Ras in cardiac fibroblasts. Circ Res. 1998;82(3):337–345. [DOI] [PubMed] [Google Scholar]
- 103.Wang N, Zheng Z, Jin HY, Xu X. Treatment effects of captopril on non-proliferative diabetic retinopathy. Chin Med J (Engl). 2012;125(2):287–292. [PubMed] [Google Scholar]
- 104.Harindhanavudhi T, Mauer M, Klein R, Zinman B, Sinaiko A, Caramori ML; Renin Angiotensin System Study (RASS) Group . Benefits of renin-angiotensin blockade on retinopathy in type 1 diabetes vary with glycemic control. Diabetes Care. 2011;34(8):1838–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilkinson-Berka JL, Tan G, Binger KJ, Sutton L, McMaster K, Deliyanti D, Perera G, Campbell DJ, Miller AG. Aliskiren reduces vascular pathology in diabetic retinopathy and oxygen-induced retinopathy in the transgenic (mRen-2)27 rat. Diabetologia. 2011;54(10):2724–2735. [DOI] [PubMed] [Google Scholar]
- 106.Wu X, He Y, Jing Y, Li K, Zhang J. Albumin overload induces apoptosis in renal tubular epithelial cells through a CHOP-dependent pathway. OMICS. 2010;14(1):61–73. [DOI] [PubMed] [Google Scholar]
- 107.Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res 2012;2012:589589 10.1155/2012/589589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu YB, Li HQ, Ren MS, Li WT, Lv XY, Wang L. CHOP/ORP150 ratio in endoplasmic reticulum stress: a new mechanism for diabetic peripheral neuropathy. Cell Physiol Biochem. 2013;32(2):367–379. [DOI] [PubMed] [Google Scholar]
- 109.Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb Haemost. 2007;98(1):77–83. [PubMed] [Google Scholar]
- 110.Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3(10):845–852. [DOI] [PubMed] [Google Scholar]
- 111.Clermont A, Chilcote TJ, Kita T, Liu J, Riva P, Sinha S, Feener EP. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes. 2011;60(5):1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phipps JA, Clermont AC, Sinha S, Chilcote TJ, Bursell SE, Feener EP. Plasma kallikrein mediates angiotensin II type 1 receptor-stimulated retinal vascular permeability. Hypertension. 2009;53(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abdouh M, Talbot S, Couture R, Hasséssian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. Br J Pharmacol. 2008;154(1):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lawson SR, Gabra BH, Nantel F, Battistini B, Sirois P. Effects of a selective bradykinin B1 receptor antagonist on increased plasma extravasation in streptozotocin-induced diabetic rats: distinct vasculopathic profile of major key organs. Eur J Pharmacol. 2005;514(1):69–78. [DOI] [PubMed] [Google Scholar]
- 115.Sogawa K, Nagaoka T, Izumi N, Nakabayashi S, Yoshida A. Acute hyperglycemia-induced endothelial dysfunction in retinal arterioles in cats. Invest Ophthalmol Vis Sci. 2010;51(5):2648–2655. [DOI] [PubMed] [Google Scholar]
- 116.Kojima N, Saito M, Mori A, Sakamoto K, Nakahara T, Ishii K. Role of cyclooxygenase in vasodilation of retinal blood vessels induced by bradykinin in Brown Norway rats. Vascul Pharmacol. 2009;51(2-3):119–124. [DOI] [PubMed] [Google Scholar]
- 117.Abdouh M, Khanjari A, Abdelazziz N, Ongali B, Couture R, Hasséssian HM. Early upregulation of kinin B1 receptors in retinal microvessels of the streptozotocin-diabetic rat. Br J Pharmacol. 2003;140(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeppesen P, Aalkjaer C, Bek T. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2002;43(6):1891–1896. [PubMed] [Google Scholar]
- 119.Busse R, Fleming I. Molecular responses of endothelial tissue to kinins. Diabetes. 1996;45(Suppl 1):S8–S13. [DOI] [PubMed] [Google Scholar]
- 120.Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kolte D, Bryant J, Holsworth D, Wang J, Akbari P, Gibson G, Shariat-Madar Z. Biochemical characterization of a novel high-affinity and specific plasma kallikrein inhibitor. Br J Pharmacol. 2011;162(7):1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brovkovych V, Zhang Y, Brovkovych S, Minshall RD, Skidgel RA. A novel pathway for receptor-mediated post-translational activation of inducible nitric oxide synthase. J Cell Mol Med. 2011;15(2):258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yayama K, Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: role of bradykinin and nitric oxide. Int Immunopharmacol. 2008;8(2):312–318. [DOI] [PubMed] [Google Scholar]
- 124.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H, Reshef A, Zuraw B; HAWK (Hereditary Angioedema International Working Group) . Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67(2):147–157. [DOI] [PubMed] [Google Scholar]
- 126.Catanzaro O, Labal E, Andornino A, Capponi JA, Di Martino I, Sirois P. Blockade of early and late retinal biochemical alterations associated with diabetes development by the selective bradykinin B1 receptor antagonist R-954. Peptides. 2012;34(2):349–352. [DOI] [PubMed] [Google Scholar]
- 127.Pruneau D, Bélichard P, Sahel JA, Combal JP. Targeting the kallikrein-kinin system as a new therapeutic approach to diabetic retinopathy. Curr Opin Investig Drugs. 2010;11(5):507–514. [PubMed] [Google Scholar]
- 128.Pouliot M, Talbot S, Sénécal J, Dotigny F, Vaucher E, Couture R. Ocular application of the kinin B1 receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One. 2012;7(3):e33864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348(23):2285–2293. [DOI] [PubMed] [Google Scholar]
- 130.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hale LJ, Hurcombe J, Lay A, Santamaría B, Valverde AM, Saleem MA, Mathieson PW, Welsh GI, Coward RJ. Insulin directly stimulates VEGF-A production in the glomerular podocyte. Am J Physiol Renal Physiol. 2013;305(2):F182–F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hermann C, Assmus B, Urbich C, Zeiher AM, Dimmeler S. Insulin-mediated stimulation of protein kinase Akt: a potent survival signaling cascade for endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(2):402–409. [DOI] [PubMed] [Google Scholar]
- 134.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15(3):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Artwohl M, Brunmair B, Fürnsinn C, Hölzenbein T, Rainer G, Freudenthaler A, Porod EM, Huttary N, Baumgartner-Parzer SM. Insulin does not regulate glucose transport and metabolism in human endothelium. Eur J Clin Invest. 2007;37(8):643–650. [DOI] [PubMed] [Google Scholar]
- 136.Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol Endocrinol. 2009;23(10):1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oliver FJ, de la Rubia G, Feener EP, Lee ME, Loeken MR, Shiba T, Quertermous T, King GL. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem. 1991;266(34):23251–23256. [PubMed] [Google Scholar]
- 138.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55(3):691–698. [DOI] [PubMed] [Google Scholar]
- 139.Maeno Y, Li Q, Park K, Rask-Madsen C, Gao B, Matsumoto M, Liu Y, Wu IH, White MF, Feener EP, King GL. Inhibition of insulin signaling in endothelial cells by protein kinase C-induced phosphorylation of p85 subunit of phosphatidylinositol 3-kinase (PI3K). J Biol Chem. 2012;287(7):4518–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park K, Li Q, Rask-Madsen C, Mima A, Mizutani K, Winnay J, Maeda Y, D’Aquino K, White MF, Feener EP, King GL. Serine phosphorylation sites on IRS2 activated by angiotensin II and protein kinase C to induce selective insulin resistance in endothelial cells. Mol Cell Biol. 2013;33(16):3227–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Motawi TK, Rizk SM, Ibrahim IA, El-Emady YF. Alterations in circulating angiogenic and anti-angiogenic factors in type 2 diabetic patients with neuropathy. Cell Biochem Funct. 2014;32(2):155–163. [DOI] [PubMed] [Google Scholar]
- 142.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de Zeeuw D, Akizawa T, Agarwal R, Audhya P, Bakris GL, Chin M, Krauth M, Lambers Heerspink HJ, Meyer CJ, McMurray JJ, Parving HH, Pergola PE, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Warnock DG, Wittes J, Chertow GM. Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: the occurrence of renal events (BEACON). Am J Nephrol. 2013;37(3):212–222. [DOI] [PubMed] [Google Scholar]
- 144.Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011;51(1):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lei H, Rheaume MA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res. 2010;90(3):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. [DOI] [PubMed] [Google Scholar]
- 147.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–3112. [DOI] [PubMed] [Google Scholar]
- 148.Liu SH, Sheu WH, Lee MR, Lee WJ, Yi YC, Yang TJ, Jen JF, Pan HC, Shen CC, Chen WB, Tien HR, Sheu ML. Advanced glycation end product Nε-carboxymethyllysine induces endothelial cell injury: the involvement of SHP-1-regulated VEGFR-2 dephosphorylation. J Pathol. 2013;230(2):215–227. [DOI] [PubMed] [Google Scholar]
- 149.Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: potential therapeutic approaches. Exp Biol Med (Maywood). 2013;238(5):461–481. [DOI] [PubMed] [Google Scholar]
- 150.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97(14):8015–8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petersen M, Thorikay M, Deckers M, van Dinther M, Grygielko ET, Gellibert F, de Gouville AC, Huet S, ten Dijke P, Laping NJ. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73(6):705–715. [DOI] [PubMed] [Google Scholar]
- 152.Chen S, Ziyadeh FN. Vascular endothelial growth factor and diabetic nephropathy. Curr Diab Rep. 2008;8(6):470–476. [DOI] [PubMed] [Google Scholar]
- 153.Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010;58(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61(11):2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, Velazquez H, Kashgarian M, Moeckel GW, Tufro A. Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2011;54(5):1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frank RN. On the pathogenesis of diabetic retinopathy: a 1990 update. Ophthalmology. 1991;98(5):586–593. [DOI] [PubMed] [Google Scholar]
- 158.Dhondt J, Peeraer E, Verheyen A, Nuydens R, Buysschaert I, Poesen K, Van Geyte K, Beerens M, Shibuya M, Haigh JJ, Meert T, Carmeliet P, Lambrechts D. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. 2011;25(5):1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gomes E, Papa L, Hao T, Rockwell P. The VEGFR2 and PKA pathways converge at MEK/ERK1/2 to promote survival in serum deprived neuronal cells. Mol Cell Biochem. 2007;305(1-2):179–190. [DOI] [PubMed] [Google Scholar]
- 160.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13(11):1349–1358. [DOI] [PubMed] [Google Scholar]
- 161.Li Calzi S, Neu MB, Shaw LC, Grant MB. Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits. EPMA J. 2010;1(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gupta A, Gerlitz B, Richardson MA, Bull C, Berg DT, Syed S, Galbreath EJ, Swanson BA, Jones BE, Grinnell BW. Distinct functions of activated protein C differentially attenuate acute kidney injury. J Am Soc Nephrol. 2009;20(2):267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17(11):1437–1443. [DOI] [PubMed] [Google Scholar]
- 164.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53(1):195–199. [DOI] [PubMed] [Google Scholar]
- 165.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res. 2010;106(5):854–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, Duriez M, Tobelem G, Lévy BI. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164(2):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107(16):2134–2139. [DOI] [PubMed] [Google Scholar]
- 168.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. [DOI] [PubMed] [Google Scholar]
- 169.Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206(13):2897–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yan J, Tie G, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg. 2009;50(6):1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim BJ, Lee JK, Schuchman EH, Jin HK, Bae JS. Synergistic vasculogenic effects of AMD3100 and stromal-cell-derived factor-1α in vasa nervorum of the sciatic nerve of mice with diabetic peripheral neuropathy. Cell Tissue Res. 2013;354(2):395–407. [DOI] [PubMed] [Google Scholar]
- 172.Chen YT, Cheng BC, Ko SF, Chen CH, Tsai TH, Leu S, Chang HW, Chung SY, Chua S, Yeh KH, Chen YL, Yip HK. Value and level of circulating endothelial progenitor cells, angiogenesis factors and mononuclear cell apoptosis in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(1):83–91. [DOI] [PubMed] [Google Scholar]
- 173.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–1544. [DOI] [PubMed] [Google Scholar]
- 175.Klein R, Myers CE, Lee KE, Gangnon R, Klein BE. Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch Ophthalmol. 2012;130(6):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Klein R, Klein BE, Moss SE, Wang Q. Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Arch Ophthalmol. 1994;112(1):92–98. [DOI] [PubMed] [Google Scholar]
- 177.Mackenzie S, Schmermer C, Charnley A, Sim D, Vikas Tah, Dumskyj M, Nussey S, Egan C. SDOCT imaging to identify macular pathology in patients diagnosed with diabetic maculopathy by a digital photographic retinal screening programme. PLoS One. 2011;6(5):e14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. [DOI] [PubMed] [Google Scholar]
- 179.Gardner TW, Abcouwer SF, Barber AJ, Jackson GR. An integrated approach to diabetic retinopathy research. Arch Ophthalmol. 2011;129(2):230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bresnick GH. Diabetic retinopathy viewed as a neurosensory disorder. Arch Ophthalmol. 1986;104(7):989–990. [DOI] [PubMed] [Google Scholar]
- 181.VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci. 2008;28(1):1–11. [DOI] [PubMed] [Google Scholar]
- 182.Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49(6):2635–2642. [DOI] [PubMed] [Google Scholar]
- 183.Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27(3):284–330. [DOI] [PubMed] [Google Scholar]
- 184.Lott ME, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D, Gardner TW, Bettermann K. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol. 2012;90(6):e434–e441. [DOI] [PubMed] [Google Scholar]
- 185.Lasta M, Pemp B, Schmidl D, Boltz A, Kaya S, Palkovits S, Werkmeister R, Howorka K, Popa-Cherecheanu A, Garhöfer G, Schmetterer L. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2013;54(1):842–847. [DOI] [PubMed] [Google Scholar]
- 186.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tilton RG, Chang K, Hasan KS, Smith SR, Petrash JM, Misko TP, Moore WM, Currie MG, Corbett JA, McDaniel ML, Williamson JR. Prevention of diabetic vascular dysfunction by guanidines: inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993;42(2):221–232. [DOI] [PubMed] [Google Scholar]
- 188.Kohner EM, Porta M, Hyer SL. The pathogenesis of diabetic retinopathy and cataract In: Pickup J, Williams G, eds. Textbook of Diabetes. Vol 2 Oxford, UK: Blackwell Scientific Publications; 1991:564–574. [Google Scholar]
- 189.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. [DOI] [PubMed] [Google Scholar]
- 190.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy? Arch Intern Med. 1989;149(11):2427–2432. [PubMed] [Google Scholar]
- 191.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–243. [DOI] [PubMed] [Google Scholar]
- 192.Varma R, Torres M, Peña F, Klein R, Azen SP; Los Angeles Latino Eye Study Group . Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(7):1298–1306. [DOI] [PubMed] [Google Scholar]
- 193.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP; Los Angeles Latino Eye Study Group . Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752–761.e1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS Group . Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–232. [DOI] [PubMed] [Google Scholar]
- 196.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I; ACCORD Trial Group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 198.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260(19):2864–2871. [PubMed] [Google Scholar]
- 199.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. [DOI] [PubMed] [Google Scholar]
- 200.Mann DM, Woodward M, Ye F, Krousel-Wood M, Muntner P. Trends in medication use among US adults with diabetes mellitus: glycemic control at the expense of controlling cardiovascular risk factors. Arch Intern Med. 2009;169(18):1718–1720. [DOI] [PubMed] [Google Scholar]
- 201.Klein R, Klein BE. Vision disorders in diabetes In: Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, eds. Diabetes in America. Vol 2. Bethesda, MD: National Institutes of Health; 1995:293–338. [Google Scholar]
- 202.Nelson RG, Knowler WC, Pettitt DJ, Bennett PH. Kidney diseases in diabetes In: Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, eds. Diabetes in America. Vol 2. Bethesda, MD: National Institutes of Health; 1995:349–400. [Google Scholar]
- 203.Kohner EM. Diabetic retinopathy. Br Med Bull. 1989;45(1):148–173. [DOI] [PubMed] [Google Scholar]
- 204.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 205.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–1576. [DOI] [PubMed] [Google Scholar]
- 206.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ; ACCORD Study Group; ACCORD Eye Study Group . Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes: The EUCLID Study Group: EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351(9095):28–31. [DOI] [PubMed] [Google Scholar]
- 208.Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N; DIRECT Programme Study Group . Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372(9647):1385–1393. [DOI] [PubMed] [Google Scholar]
- 209.Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, Bilous R, Sjølie AK; DIRECT Programme Study Group . Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372(9647):1394–1402. [DOI] [PubMed] [Google Scholar]
- 210.Executive summary: standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dornan TL, Carter RD, Bron AJ, Turner RC, Mann JI. Low density lipoprotein cholesterol: an association with the severity of diabetic retinopathy. Diabetologia. 1982;22(3):167–170. [DOI] [PubMed] [Google Scholar]
- 212.Miccoli R, Odello G, Giampietro O, Marchetti P, Cristofani R, Penno G, Meucci G, Navalesi R. Circulating lipid levels and severity of diabetic retinopathy in type I diabetes mellitus. Ophthalmic Res. 1987;19(1):52–56. [DOI] [PubMed] [Google Scholar]
- 213.Mouton DP, Gill AJ. Prevalence of diabetic retinopathy and evaluation of risk factors: a review of 1,005 diabetic clinic patients. S Afr Med J. 1988;74(8):399–402. [PubMed] [Google Scholar]
- 214.Weber B, Burger W, Hartmann R, Hövener G, Malchus R, Oberdisse U. Risk factors for the development of retinopathy in children and adolescents with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1986;29(1):23–29. [DOI] [PubMed] [Google Scholar]
- 215.Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53(11):2883–2892. [DOI] [PubMed] [Google Scholar]
- 216.Dale J, Farmer J, Jones AF, Gibson JM, Dodson PM. Diabetic ischaemic and exudative maculopathy: are their risk factors different? Dia Med. 2000;17:47. [Google Scholar]
- 217.Freyberger H, Schifferdecker E, Schatz H. Regression of hard exudates in diabetic background retinopathy in therapy with etofibrate antilipemic agent [in German] Med Klin (Munich). 1994;89(11):594–597, 633. [PubMed] [Google Scholar]
- 218.Gordon B, Chang S, Kavanagh M, Berrocal M, Yannuzzi L, Robertson C, Drexler A. The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol. 1991;112(4):385–391. [DOI] [PubMed] [Google Scholar]
- 219.Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG; FIELD Study Investigators . Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. [DOI] [PubMed] [Google Scholar]
- 220.Toth PP, Simko RJ, Palli SR, Koselleck D, Quimbo RA, Cziraky MJ. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2012;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Friedman EA. Advanced glycosylated end products and hyperglycemia in the pathogenesis of diabetic complications. Diabetes Care. 1999;22(Suppl 2):B65–B71. [PubMed] [Google Scholar]
- 222.McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, Lyons TJ. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91(6):2470–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nakamura Y, Horii Y, Nishino T, Shiiki H, Sakaguchi Y, Kagoshima T, Dohi K, Makita Z, Vlassara H, Bucala R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol. 1993;143(6):1649–1656. [PMC free article] [PubMed] [Google Scholar]
- 224.Sugiyama S, Miyata T, Horie K, Iida Y, Tsuyuki M, Tanaka H, Maeda K. Advanced glycation end-products in diabetic nephropathy. Nephrol Dial Transplant. 1996;11(Suppl 5):91–94. [DOI] [PubMed] [Google Scholar]
- 225.Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75(1):95–108. [DOI] [PubMed] [Google Scholar]
- 226.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review [published correction appears in Diabetologia. 2002;45(2):293]. Diabetologia. 2001;44(2):129–146. [DOI] [PubMed] [Google Scholar]
- 227.Vlassara H. Advanced glycation end-products and atherosclerosis. Ann Med. 1996;28(5):419–426. [DOI] [PubMed] [Google Scholar]
- 228.Vlassara H, Bucala R. Recent progress in advanced glycation and diabetic vascular disease: role of advanced glycation end product receptors. Diabetes. 1996;45(Suppl 3):S65–S66. [DOI] [PubMed] [Google Scholar]
- 229.Nath KA, Grande J, Croatt A, Haugen J, Kim Y, Rosenberg ME. Redox regulation of renal DNA synthesis, transforming growth factor-beta1 and collagen gene expression. Kidney Int. 1998;53(2):367–381. [DOI] [PubMed] [Google Scholar]
- 230.Katusic ZS. Superoxide anion and endothelial regulation of arterial tone. Free Radic Biol Med. 1996;20(3):443–448. [DOI] [PubMed] [Google Scholar]
- 231.Lin J, Bierhaus A, Bugert P, Dietrich N, Feng Y, Vom Hagen F, Nawroth P, Brownlee M, Hammes HP. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006;49(5):1089–1096. [DOI] [PubMed] [Google Scholar]
- 232.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50(8):1938–1942. [DOI] [PubMed] [Google Scholar]
- 233.Hammes HP, Bartmann A, Engel L, Wülfroth P. Antioxidant treatment of experimental diabetic retinopathy in rats with nicanartine. Diabetologia. 1997;40(6):629–634. [DOI] [PubMed] [Google Scholar]
- 234.Nowak M, Wielkoszyński T, Marek B, Kos-Kudła B, Swietochowska E, Siemińska L, Karpe J, Kajdaniuk D, Głogowska-Szelag J, Nowak K. Antioxidant potential, paraoxonase 1, ceruloplasmin activity and C-reactive protein concentration in diabetic retinopathy. Clin Exp Med. 2010;10(3):185–192. [DOI] [PubMed] [Google Scholar]
- 235.Konat GW, Kraszpulski M, James I, Zhang HT, Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008;23(3):325–333. [DOI] [PubMed] [Google Scholar]
- 236.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9(4):315–327. [DOI] [PubMed] [Google Scholar]
- 237.Calabrese V, Mancuso C, Sapienza M, Puleo E, Calafato S, Cornelius C, Finocchiaro M, Mangiameli A, Di Mauro M, Stella AM, Castellino P. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12(4):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Altomare E, Grattagliano I, Vendemaile G, Micelli-Ferrari T, Signorile A, Cardia L. Oxidative protein damage in human diabetic eye: evidence of a retinal participation. Eur J Clin Invest. 1997;27(2):141–147. [DOI] [PubMed] [Google Scholar]
- 239.Armstrong D, al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radic Biol Med. 1991;11(4):433–436. [DOI] [PubMed] [Google Scholar]
- 240.Anderson RE, Rapp LM, Wiegand RD. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984;3(1):223–227. [DOI] [PubMed] [Google Scholar]
- 241.Flores L, Rodela S, Abian J, Clària J, Esmatjes E. F2 isoprostane is already increased at the onset of type 1 diabetes mellitus: effect of glycemic control. Metabolism. 2004;53(9):1118–1120. [DOI] [PubMed] [Google Scholar]
- 242.The Diabetes Control and Complications Trial Research Group Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes. 1997;46(11):1829–1839. [PubMed] [Google Scholar]
- 243.Rema M, Saravanan G, Deepa R, Mohan V. Familial clustering of diabetic retinopathy in South Indian type 2 diabetic patients. Diabet Med. 2002;19(11):910–916. [DOI] [PubMed] [Google Scholar]
- 244.Paterson AD, Bull SB. Does familial clustering of risk factors for long-term diabetic complications leave any place for genes that act independently? J Cardiovasc Transl Res. 2012;5(4):388–398. [DOI] [PubMed] [Google Scholar]
- 245.Paterson AD, Waggott D, Boright AP, Hosseini SM, Shen E, Sylvestre MP, Wong I, Bharaj B, Cleary PA, Lachin JM, Below JE, Nicolae D, Cox NJ, Canty AJ, Sun L, Bull SB; MAGIC (Meta-Analyses of Glucose and Insulin-Related Traits Consortium); Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . A genome-wide association study identifies a novel major locus for glycemic control in type 1 diabetes, as measured by both A1C and glucose. Diabetes. 2010;59(2):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hallman DM, Boerwinkle E, Gonzalez VH, Klein BE, Klein R, Hanis CL. A genome-wide linkage scan for diabetic retinopathy susceptibility genes in Mexican Americans with type 2 diabetes from Starr County, Texas. Diabetes. 2007;56(4):1167–1173. [DOI] [PubMed] [Google Scholar]
- 247.Fukuda M, Nakano S, Imaizumi N, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Mitochondrial DNA mutations are associated with both decreased insulin secretion and advanced microvascular complications in Japanese diabetic subjects. J Diabetes Complications. 1999;13(5-6):277–283. [DOI] [PubMed] [Google Scholar]
- 248.Yamamoto T, Sato T, Hosoi M, Yoshioka K, Tanaka S, Tahara H, Nishizawa Y, Fujii S. Aldose reductase gene polymorphism is associated with progression of diabetic nephropathy in Japanese patients with type 1 diabetes mellitus. Diabetes Obes Metab. 2003;5(1):51–57. [DOI] [PubMed] [Google Scholar]
- 249.Taverna MJ, Sola A, Guyot-Argenton C, Pacher N, Bruzzo F, Chevalier A, Slama G, Reach G, Selam JL. eNOS4 polymorphism of the endothelial nitric oxide synthase predicts risk for severe diabetic retinopathy. Diabet Med. 2002;19(3):240–245. [DOI] [PubMed] [Google Scholar]
- 250.Kao Y, Donaghue KC, Chan A, Bennetts BH, Knight J, Silink M. Paraoxonase gene cluster is a genetic marker for early microvascular complications in type 1 diabetes. Diabet Med. 2002;19(3):212–215. [DOI] [PubMed] [Google Scholar]
- 251.Kanková K, Muzík J, Karásková J, Beránek M, Hájek D, Znojil V, Vlková E, Vácha J. Duration of non-insulin-dependent diabetes mellitus and the TNF-beta NcoI genotype as predictive factors in proliferative diabetic retinopathy. Ophthalmologica. 2001;215(4):294–298. [DOI] [PubMed] [Google Scholar]
- 252.Santos A, Salguero ML, Gurrola C, Muñoz F, Roig-Melo E, Panduro A. The epsilon4 allele of apolipoprotein E gene is a potential risk factor for the severity of macular edema in type 2 diabetic Mexican patients. Ophthalmic Genet. 2002;23(1):13–19. [DOI] [PubMed] [Google Scholar]
- 253.Kamiuchi K, Hasegawa G, Obayashi H, Kitamura A, Ishii M, Yano M, Kanatsuna T, Yoshikawa T, Nakamura N. Intercellular adhesion molecule-1 (ICAM-1) polymorphism is associated with diabetic retinopathy in type 2 diabetes mellitus. Diabet Med. 2002;19(5):371–376. [DOI] [PubMed] [Google Scholar]
- 254.Matsubara Y, Murata M, Maruyama T, Handa M, Yamagata N, Watanabe G, Saruta T, Ikeda Y. Association between diabetic retinopathy and genetic variations in alpha2beta1 integrin, a platelet receptor for collagen. Blood. 2000;95(5):1560–1564. [PubMed] [Google Scholar]
- 255.Liew G, Klein R, Wong TY. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin. 2009;49(2):35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yang B, Cross DF, Ollerenshaw M, Millward BA, Demaine AG. Polymorphisms of the vascular endothelial growth factor and susceptibility to diabetic microvascular complications in patients with type 1 diabetes mellitus. J Diabetes Complications. 2003;17(1):1–6. [DOI] [PubMed] [Google Scholar]
- 257.Qiu M, Xiong W, Liao H, Li F. VEGF -634G>C polymorphism and diabetic retinopathy risk: a meta-analysis. Gene. 2013;518(2):310–315. [DOI] [PubMed] [Google Scholar]
- 258.Bonnefond A, Saulnier PJ, Stathopoulou MG, Grarup N, Ndiaye NC, Roussel R, Nezhad MA, Dechaume A, Lantieri O, Hercberg S, Lauritzen T, Balkau B, El-Sayed Moustafa JS, Hansen T, Pedersen O, Froguel P, Charpentier G, Marre M, Hadjadj S, Visvikis-Siest S. What is the contribution of two genetic variants regulating VEGF levels to type 2 diabetes risk and to microvascular complications? PLoS One. 2013;8(2):e55921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992;15(12):1875–1891. [DOI] [PubMed] [Google Scholar]
- 260.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117(11):1487–1495. [DOI] [PubMed] [Google Scholar]
- 261.Davis MD, Hiller R, Magli YL, Podgor MJ, Ederer F, Harris WA, Long JW, Haug GA. Prognosis for life in patients with diabetes: relation to severity of retinopathy. Trans Am Ophthalmol Soc. 1979;77:144–170. [PMC free article] [PubMed] [Google Scholar]
- 262.Hanis CL, Chu HH, Lawson K, Hewett-Emmett D, Barton SA, Schull WJ, Garcia CA. Mortality of Mexican Americans with NIDDM: retinopathy and other predictors in Starr County, Texas. Diabetes Care. 1993;16(1):82–89. [DOI] [PubMed] [Google Scholar]
- 263.Neil A, Hawkins M, Potok M, Thorogood M, Cohen D, Mann J. A prospective population-based study of microalbuminuria as a predictor of mortality in NIDDM. Diabetes Care. 1993;16(7):996–1003. [DOI] [PubMed] [Google Scholar]
- 264.Sorbinil Retinopathy Trial Research Group A randomized trial of sorbinil, an aldose reductase inhibitor, in diabetic retinopathy. Arch Ophthalmol. 1990;108(9):1234–1244. [DOI] [PubMed] [Google Scholar]
- 265.The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583–600. [PubMed] [Google Scholar]
- 266.Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C; Diabetic Retinopathy Clinical Research Network (DRCR.net) . Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127(3):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695.e1–1695.e15. [DOI] [PubMed] [Google Scholar]
- 268.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL III, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK; Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112(4):593–598. [DOI] [PubMed] [Google Scholar]
- 270.Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010;21(3):178–183. [DOI] [PubMed] [Google Scholar]
- 271.Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: the past, the present, and the future. Surv Ophthalmol. 2008;53(2):139–149. [DOI] [PubMed] [Google Scholar]
- 272.Machemer R, Blankenship G. Vitrectomy for proliferative diabetic retinopathy associated with vitreous hemorrhage. Ophthalmology. 1981;88(7):643–646. [DOI] [PubMed] [Google Scholar]
- 273.Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy: four-year results of a randomized trial: Diabetic Retinopathy Vitrectomy Study Report 5 [published correction appears in Arch Ophthalmol. 1990;108(10):1452]. Arch Ophthalmol. 1990;108(7):958–964. [DOI] [PubMed] [Google Scholar]
- 274.Genuth S, Ismail-Beigi F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab. 2012;97(1):41–48. [DOI] [PubMed] [Google Scholar]
- 275.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. [DOI] [PubMed] [Google Scholar]
- 276.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–491. [DOI] [PubMed] [Google Scholar]
- 277.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–2469. [DOI] [PubMed] [Google Scholar]
- 278.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7(2):108–114. [DOI] [PubMed] [Google Scholar]
- 279.Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11(3):261–271. [DOI] [PubMed] [Google Scholar]
- 280.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Exalto LG, Whitmer RA, Kappele LJ, Biessels GJ. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp Gerontol. 2012;47(11):858–864. [DOI] [PubMed] [Google Scholar]
- 282.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006;37(1):82–86. [DOI] [PubMed] [Google Scholar]
- 283.Qiu C, Cotch MF, Sigurdsson S, Garcia M, Klein R, Jonasson F, Klein BE, Eiriksdottir G, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility-Reykjavik study. Diabetes. 2008;57(6):1645–1650. [DOI] [PubMed] [Google Scholar]
- 284.Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, Eiriksdottir G, Klein R, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75(24):2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, De Jong PT. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129(Pt 1):182–188. [DOI] [PubMed] [Google Scholar]
- 286.Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens. 2013;31(8):1502–1516. [DOI] [PubMed] [Google Scholar]
- 287.Verhaaren BF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, Ikram MA. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61(6):1354–1359. [DOI] [PubMed] [Google Scholar]
- 288.King KS, Chen KX, Hulsey KM, McColl RW, Weiner MF, Nakonezny PA, Peshock RM. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology. 2013;267(3):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Loitfelder M, Seiler S, Schwingenschuh P, Schmidt R. Cerebral microbleeds: a review. Panminerva Med. 2012;54(3):149–160. [PubMed] [Google Scholar]
- 290.Rajagopalan P, Refsum H, Hua X, Toga AW, Jack CR Jr, Weiner MW, Thompson PM; Alzheimer’s Disease Neuroimaging Initiative . Mapping creatinine- and cystatin C-related white matter brain deficits in the elderly. Neurobiol Aging. 2013;34(4):1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Vogels SC, Emmelot-Vonk MH, Verhaar HJ, Koek HL. The association of chronic kidney disease with brain lesions on MRI or CT: a systematic review. Maturitas. 2012;71(4):331–336. [DOI] [PubMed] [Google Scholar]
- 292.Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R, DeKosky ST. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70(19):1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Umemura T, Kawamura T, Umegaki H, Kawano N, Mashita S, Sakakibara T, Hotta N, Sobue G. Association of chronic kidney disease and cerebral small vessel disease with cognitive impairment in elderly patients with type 2 diabetes. Dement Geriatr Cogn Dis Extra. 2013;3(1):212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann Neurol. 2013;74(4):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, Sullivan M, Horowitz KR, Ding J, Marcovina S, Lovato LC, Lovato J, Margolis KL, O’Connor P, Lipkin EW, Hirsch J, Coker L, Maldjian J, Sunshine JL, Truwit C, Davatzikos C, Bryan RN; ACCORD MIND Investigators . Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ursache A, Wedin W, Tirsi A, Convit A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology. 2012;37(8):1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GE, van der Grond J, Kappelle LJ; Utrecht Diabetic Encephalopathy Study Group . Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50(11):2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hudson BI, Moon YP, Kalea AZ, Khatri M, Marquez C, Schmidt AM, Paik MC, Yoshita M, Sacco RL, DeCarli C, Wright CB, Elkind MS. Association of serum soluble receptor for advanced glycation end-products with subclinical cerebrovascular disease: the Northern Manhattan Study (NOMAS). Atherosclerosis. 2011;216(1):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tangney CC, Aggarwal NT, Li H, Wilson RS, Decarli C, Evans DA, Morris MC. Vitamin B12, cognition, and brain MRI measures: a cross-sectional examination. Neurology. 2011;77(13):1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Feng L, Isaac V, Sim S, Ng TP, Krishnan KR, Chee MW. Associations between elevated homocysteine, cognitive impairment, and reduced white matter volume in healthy old adults. Am J Geriatr Psychiatry. 2013;21(2):164–172. [DOI] [PubMed] [Google Scholar]
- 303.de Lau LM, Smith AD, Refsum H, Johnston C, Breteler MM. Plasma vitamin B12 status and cerebral white-matter lesions. J Neurol Neurosurg Psychiatry. 2009;80(2):149–157. [DOI] [PubMed] [Google Scholar]
- 304.Kealey SM, Provenzale JM. Tensor diffusion imaging in B12 leukoencephalopathy. J Comput Assist Tomogr. 2002;26(6):952–955. [DOI] [PubMed] [Google Scholar]
- 305.Craft S, Cholerton B, Baker LD. Insulin and Alzheimer’s disease: untangling the web. J Alzheimers Dis. 2013;33(Suppl 1):S263–S275. [DOI] [PubMed] [Google Scholar]
- 306.Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18(10):1577–1584. [DOI] [PubMed] [Google Scholar]
- 307.Baskin DG, Figlewicz DP, Woods SC, Porte D Jr, Dorsa DM. Insulin in the brain. Annu Rev Physiol. 1987;49:335–347. [DOI] [PubMed] [Google Scholar]
- 308.Baura GD, Foster DM, Porte D Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92(4):1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49(9):1525–1533. [DOI] [PubMed] [Google Scholar]
- 310.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177(1-2):125–134. [DOI] [PubMed] [Google Scholar]
- 312.Figlewicz DP, Szot P, Israel PA, Payne C, Dorsa DM. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Res. 1993;602(1):161–164. [DOI] [PubMed] [Google Scholar]
- 313.Kopf SR, Baratti CM. Effects of posttraining administration of insulin on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiol Learn Mem. 1999;71(1):50–61. [DOI] [PubMed] [Google Scholar]
- 314.Dandona P. Endothelium, inflammation, and diabetes. Curr Diab Rep. 2002;2(4):311–315. [DOI] [PubMed] [Google Scholar]
- 315.Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62(10):1539–1544. [DOI] [PubMed] [Google Scholar]
- 316.Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lahiri DK, Maloney B. Beyond the signaling effect role of amyloid-ß42 on the processing of APP, and its clinical implications. Exp Neurol. 2010;225(1):51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282(46):33305–33312. [DOI] [PubMed] [Google Scholar]
- 319.Westermark GT, Westermark P. Localized amyloids important in diseases outside the brain--lessons from the islets of Langerhans and the thoracic aorta. FEBS J. 2011;278(20):3918–3929. [DOI] [PubMed] [Google Scholar]
- 320.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101(4):527–537. [DOI] [PubMed] [Google Scholar]
- 321.Strachan MWR. R D Lawrence Lecture 2010: the brain as a target organ in type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med. 2011;28(2):141–147. [DOI] [PubMed] [Google Scholar]
- 322.Pandini G, Pace V, Copani A, Squatrito S, Milardi D, Vigneri R. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology. 2013;154(1):375–387. [DOI] [PubMed] [Google Scholar]
- 323.Bartl J, Meyer A, Brendler S, Riederer P, Grünblatt E. Different effects of soluble and aggregated amyloid β42 on gene/protein expression and enzyme activity involved in insulin and APP pathways. J Neural Transm (Vienna). 2013;120(1):113–120. [DOI] [PubMed] [Google Scholar]
- 324.Candeias E, Duarte AI, Carvalho C, Correia SC, Cardoso S, Santos RX, Plácido AI, Perry G, Moreira PI. The impairment of insulin signaling in Alzheimer’s disease. IUBMB Life. 2012;64(12):951–957. [DOI] [PubMed] [Google Scholar]
- 325.Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, Oliveira CR, Moreira PI. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation. Brain Res. 2012;1441:64–78. [DOI] [PubMed] [Google Scholar]
- 326.Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheimers Dis. 2009;17(2):245–257. [DOI] [PubMed] [Google Scholar]
- 327.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–360. [DOI] [PubMed] [Google Scholar]
- 328.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol (1985). 2006;100(2):739–743. [DOI] [PubMed] [Google Scholar]
- 329.Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97(10):1055–1062. [DOI] [PubMed] [Google Scholar]
- 330.Massaad CA, Amin SK, Hu L, Mei Y, Klann E, Pautler RG. Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer’s disease. PLoS One. 2010;5(5):e10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012;322(1-2):141–147. [DOI] [PubMed] [Google Scholar]
- 332.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65(9):963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, Founds H, Atwood CS, Perry G, Smith MA. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic Biol Med. 2001;31(2):175–180. [DOI] [PubMed] [Google Scholar]
- 334.Southern L, Williams J, Esiri MM. Immunohistochemical study of N-epsilon-carboxymethyl lysine (CML) in human brain: relation to vascular dementia. BMC Neurol. 2007;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105(7):816–822. [DOI] [PubMed] [Google Scholar]
- 336.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87(3):181–194. [DOI] [PubMed] [Google Scholar]
- 337.Takeda S, Sato N, Rakugi H, Morishita R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: beta-amyloid peptide, insulin signaling, and neuronal function. Mol Biosyst. 2011;7(6):1822–1827. [DOI] [PubMed] [Google Scholar]
- 338.Valente T, Gella A, Fernàndez-Busquets X, Unzeta M, Durany N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol Dis. 2010;37(1):67–76. [DOI] [PubMed] [Google Scholar]
- 339.Wegiel J, Imaki H, Wang KC, Wegiel J, Rubenstein R. Cells of monocyte/microglial lineage are involved in both microvessel amyloidosis and fibrillar plaque formation in APPsw tg mice. Brain Res. 2004;1022(1-2):19–29. [DOI] [PubMed] [Google Scholar]
- 340.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M; STandards for ReportIng Vascular Changes on nEuroimaging (STRIVE v1) . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33(8):1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Carne RP, Vogrin S, Litewka L, Cook MJ. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci. 2006;13(1):60–72. [DOI] [PubMed] [Google Scholar]
- 343.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65(1):113–120. [DOI] [PubMed] [Google Scholar]
- 344.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, Schmidt R. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64(10):1704–1711. [DOI] [PubMed] [Google Scholar]
- 345.Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–2068. [DOI] [PubMed] [Google Scholar]
- 346.Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 2013;23(11):2521–2530. [DOI] [PubMed] [Google Scholar]
- 347.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29(11):2539–2548. [DOI] [PubMed] [Google Scholar]
- 348.Falvey CM, Rosano C, Simonsick EM, Harris T, Strotmeyer ES, Satterfield S, Yaffe K; Health ABC Study . Macro- and microstructural magnetic resonance imaging indices associated with diabetes among community-dwelling older adults. Diabetes Care. 2013;36(3):677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group . Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299(1-2):126–130. [DOI] [PubMed] [Google Scholar]
- 350.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30(5):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Korf ES, van Straaten EC, de Leeuw FE, van der Flier WM, Barkhof F, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund LO, Rostrup E, Schmidt R, Fazekas F, Scheltens P; LADIS Study Group . Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet Med. 2007;24(2):166–171. [DOI] [PubMed] [Google Scholar]
- 352.Kumar A, Haroon E, Darwin C, Pham D, Ajilore O, Rodriguez G, Mintz J. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J Magn Reson Imaging. 2008;27(1):14–19. [DOI] [PubMed] [Google Scholar]
- 353.Hoogendam YY, van der Geest JN, van der Lijn F, van der Lugt A, Niessen WJ, Krestin GP, Hofman A, Vernooij MW, Breteler MM, Ikram MA. Determinants of cerebellar and cerebral volume in the general elderly population. Neurobiol Aging. 2012;33(12):2774–2781. [DOI] [PubMed] [Google Scholar]
- 354.Kooistra M, Geerlings MI, Mali WP, Vincken KL, van der Graaf Y, Biessels GJ; SMART-MR Study Group. Diabetes mellitus and progression of vascular brain lesions and brain atrophy in patients with symptomatic atherosclerotic disease: the SMART-MR study. J Neurol Sci. 2013;332(1–2):69–74. [DOI] [PubMed] [Google Scholar]
- 355.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, Hermann BP, La Rue A, Asthana S, Bendlin BB. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36(2):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging. 2011;32(11):1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, Decarli C, Vasan RS, Wolf PA, Seshadri S. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care. 2011;34(8):1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Benedict C, Brooks SJ, Kullberg J, Burgos J, Kempton MJ, Nordenskjöld R, Nylander R, Kilander L, Craft S, Larsson EM, Johansson L, Ahlström H, Lind L, Schiöth HB. Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care. 2012;35(3):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, de Leon MJ. Current challenges for the early detection of Alzheimer’s disease: brain imaging and CSF studies. J Clin Neurol. 2009;5(4):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. [DOI] [PubMed] [Google Scholar]
- 362.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Burns CM, Chen K, Kaszniak AW, Lee W, Alexander GE, Bandy D, Fleisher AS, Caselli RJ, Reiman EM. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology. 2013;80(17):1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Thambisetty M, Beason-Held LL, An Y, Kraut M, Metter J, Egan J, Ferrucci L, O’Brien R, Resnick SM. Impaired glucose tolerance in midlife and longitudinal changes in brain function during aging. Neurobiol Aging. 2013;34(10):2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Tiehuis AM, Vincken KL, van den Berg E, Hendrikse J, Manschot SM, Mali WP, Kappelle LJ, Biessels GJ. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51(7):1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Beason-Held LL, Thambisetty M, Deib G, Sojkova J, Landman BA, Zonderman AB, Ferrucci L, Kraut MA, Resnick SM. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke. 2012;43(6):1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Brundel M, van den Berg E, Reijmer YD, de Bresser J, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group . Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J Diabetes Complications. 2012;26(3):205–209. [DOI] [PubMed] [Google Scholar]
- 368.Musen G, Jacobson AM, Bolo NR, Simonson DC, Shenton ME, McCartney RL, Flores VL, Hoogenboom WS. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61(9):2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: the Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, Steinling M, Valk J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114(1):7–12. [DOI] [PubMed] [Google Scholar]
- 371.Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group . Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55(4):1106–1113. [DOI] [PubMed] [Google Scholar]
- 372.Jongen C, van der Grond J, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JP; Utrecht Diabetic Encephalopathy Study Group . Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50(7):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.van Harten B, Oosterman JM, Potter van Loon BJ, Scheltens P, Weinstein HC. Brain lesions on MRI in elderly patients with type 2 diabetes mellitus. Eur Neurol. 2007;57(2):70–74. [DOI] [PubMed] [Google Scholar]
- 374.Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR Jr, Weiner M, DeCarli C; Alzheimer’s Disease Neuroimaging Initiative . Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67(11):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Ropele S, Enzinger C, Söllinger M, Langkammer C, Wallner-Blazek M, Schmidt R, Fazekas F. The impact of sex and vascular risk factors on brain tissue changes with aging: magnetization transfer imaging results of the Austrian stroke prevention study. AJNR Am J Neuroradiol. 2010;31(7):1297–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57(11):3083–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Aye T, Barnea-Goraly N, Ambler C, Hoang S, Schleifer K, Park Y, Drobny J, Wilson DM, Reiss AL, Buckingham BA. White matter structural differences in young children with type 1 diabetes: a diffusion tensor imaging study. Diabetes Care. 2012;35(11):2167–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Reijmer YD, Brundel M, de Bresser J, Kappelle LJ, Leemans A, Biessels GJ; Utrecht Vascular Cognitive Impairment Study Group . Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care. 2013;36(1):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Hsu JL, Chen YL, Leu JG, Jaw FS, Lee CH, Tsai YF, Hsu CY, Bai CH, Leemans A. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage. 2012;59(2):1098–1105. [DOI] [PubMed] [Google Scholar]
- 381.Shimoji K, Abe O, Uka T, Yasmin H, Kamagata K, Asahi K, Hori M, Nakanishi A, Tamura Y, Watada H, Kawamori R, Aoki S. White matter alteration in metabolic syndrome: diffusion tensor analysis. Diabetes Care. 2013;36(3):696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Zhang A, Ajilore O, Zhan L, Gadelkarim J, Korthauer L, Yang S, Leow A, Kumar A. White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology. 2013;38(8):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sacco S, Marini C, Totaro R, Russo T, Cerone D, Carolei A. A population-based study of the incidence and prognosis of lacunar stroke. Neurology. 2006;66(9):1335–1338. [DOI] [PubMed] [Google Scholar]
- 384.Hart RG, Pearce LA, Bakheet MF, Benavente OR, Conwit RA, McClure LA, Talbert RL, Anderson DC. Predictors of stroke recurrence in patients with recent lacunar stroke and response to interventions according to risk status: secondary prevention of small subcortical strokes trial. J Stroke Cerebrovasc Dis. 2014;23(4):618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH Jr, Coresh J, Szklo M, Carvalho MS, Selvin E. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78(2):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11(3):272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32(3):425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. [DOI] [PubMed] [Google Scholar]
- 389.van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, Spliet WG, Hendrikse J, Luijten PR, Biessels GJ. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2013;33(3):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sonnen JA, Larson EB, Haneuse S, Woltjer R, Li G, Crane PK, Craft S, Montine TJ. Neuropathology in the adult changes in thought study: a review. J Alzheimers Dis. 2009;18(3):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study Group . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, Lopez OL, van Buchem MA, Jonsson PV, Gudnason V, Launer LJ. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79(9):1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam Scan Study. Stroke. 2010;41(10, Suppl):S103–S106. [DOI] [PubMed] [Google Scholar]
- 394.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42(3):656–661. [DOI] [PubMed] [Google Scholar]
- 395.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35(8):1831–1835. [DOI] [PubMed] [Google Scholar]
- 396.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130(Pt 8):1988–2003. [DOI] [PubMed] [Google Scholar]
- 397.Cullen KM, Kócsi Z, Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging. 2006;27(12):1786–1796. [DOI] [PubMed] [Google Scholar]
- 398.Tomita N, Furukawa K, Okamura N, Tashiro M, Une K, Furumoto S, Iwata R, Yanai K, Kudo Y, Arai H. Brain accumulation of amyloid β protein visualized by positron emission tomography and BF-227 in Alzheimer’s disease patients with or without diabetes mellitus. Geriatr Gerontol Int. 2013;13(1):215–221. [DOI] [PubMed] [Google Scholar]
- 399.Thambisetty M, Jeffrey Metter E, Yang A, Dolan H, Marano C, Zonderman AB, Troncoso JC, Zhou Y, Wong DF, Ferrucci L, Egan J, Resnick SM, O’Brien RJ. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70(9):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114(Pt 2):761–774. [DOI] [PubMed] [Google Scholar]
- 401.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48(10):745–754. [DOI] [PubMed] [Google Scholar]
- 402.Gutierrez J, Rundek T, Ekind MS, Sacco RL, Wright CB. Perivascular spaces are associated with atherosclerosis: an insight from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2013;34(9):1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450–454. [DOI] [PubMed] [Google Scholar]
- 404.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52(1):149–156. [DOI] [PubMed] [Google Scholar]
- 406.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 2005;7(4):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Herman IM, D’Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985;101(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Liu S, Agalliu D, Yu C, Fisher M. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18(25):3653–3662. [DOI] [PubMed] [Google Scholar]
- 409.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122(1):1–9. [DOI] [PubMed] [Google Scholar]
- 411.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45(4):545–552. [DOI] [PubMed] [Google Scholar]
- 412.Kanal E. Gadolinium-based magnetic resonance contrast agents for neuroradiology: an overview. Magn Reson Imaging Clin N Am. 2012;20(4):625–631. [DOI] [PubMed] [Google Scholar]
- 413.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Siperstein MD, Unger RH, Madison LL. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J Clin Invest. 1968;47(9):1973–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Williamson JR, Tilton RG, Chang K, Kilo C. Basement membrane abnormalities in diabetes mellitus: relationship to clinical microangiopathy. Diabetes Metab Rev. 1988;4(4):339–370. [DOI] [PubMed] [Google Scholar]
- 416.Williamson JR, Rowold E, Hoffman P, Kilo C. Influence of fixation and morphometric technics on capillary basement-membrane thickening prevalence data in diabetes. Diabetes. 1976;25(7):604–613. [DOI] [PubMed] [Google Scholar]
- 417.Ganda OP, Williamson JR, Soeldner JS, Gleason RE, Kilo C, Kaldany A, Miller JP, Garovoy MR, Carpenter CB. Muscle capillary basement membrane width and its relationship to diabetes mellitus in monozygotic twins. Diabetes. 1983;32(6):549–556. [DOI] [PubMed] [Google Scholar]
- 418.Tilton RG, Faller AM, Burkhardt JK, Hoffmann PL, Kilo C, Williamson JR. Pericyte degeneration and acellular capillaries are increased in the feet of human diabetic patients. Diabetologia. 1985;28(12):895–900. [DOI] [PubMed] [Google Scholar]
- 419.Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Lond). 1995;88(6):629–633. [DOI] [PubMed] [Google Scholar]
- 420.Parving HH, Rossing N, Sander E. Increased metabolic turnover rate and transcapillary escape rate of albumin in long-term juvenile diabetics. Scand J Clin Lab Invest. 1975;35(1):59–66. [PubMed] [Google Scholar]
- 421.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med (Maywood). 2007;232(9):1121–1129. [DOI] [PubMed] [Google Scholar]
- 422.Haitoglou CS, Tsilibary EC, Brownlee M, Charonis AS. Altered cellular interactions between endothelial cells and nonenzymatically glucosylated laminin/type IV collagen. J Biol Chem. 1992;267(18):12404–12407. [PubMed] [Google Scholar]
- 423.Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31(3):343–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Tilton RG, Hoffmann PL, Kilo C, Williamson JR. Pericyte degeneration and basement membrane thickening in skeletal muscle capillaries of human diabetics. Diabetes. 1981;30(4):326–334. [DOI] [PubMed] [Google Scholar]
- 425.Mårin P, Andersson B, Krotkiewski M, Björntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care. 1994;17(5):382–386. [DOI] [PubMed] [Google Scholar]
- 426.Levy BI, Schiffrin EL, Mourad J-J, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HAJ. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118(9):968–976. [DOI] [PubMed] [Google Scholar]
- 427.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R307–R316. [DOI] [PubMed] [Google Scholar]
- 428.Benedict KF, Coffin GS, Barrett EJ, Skalak TC. Hemodynamic systems analysis of capillary network remodeling during the progression of type 2 diabetes. Microcirculation. 2011;18(1):63–73. [DOI] [PubMed] [Google Scholar]
- 429.Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol. 2011;214(Pt 2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13(3):294–307. [DOI] [PubMed] [Google Scholar]
- 431.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man: a novel mechanism for insulin resistance. J Clin Invest. 1990;85(6):1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab. 1991;73(3):637–643. [DOI] [PubMed] [Google Scholar]
- 433.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41(9):1076–1083. [DOI] [PubMed] [Google Scholar]
- 434.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46(9):1381–1388. [DOI] [PubMed] [Google Scholar]
- 435.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50(12):2682–2690. [DOI] [PubMed] [Google Scholar]
- 436.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285(1):E123–E129. [DOI] [PubMed] [Google Scholar]
- 437.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94(3):1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Inyard AC, Chong DG, Klibanov AL, Barrett EJ. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes. 2009;58(11):2457–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293(6):E1804–E1809. [DOI] [PubMed] [Google Scholar]
- 440.Rattigan S, Clark MG, Barrett EJ. Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes. 1999;48(3):564–569. [DOI] [PubMed] [Google Scholar]
- 441.Eggleston EM, Jahn LA, Barrett EJ. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care. 2013;36(1):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Chan A, Barrett EJ, Anderson SM, Kovatchev BP, Breton MD. Muscle microvascular recruitment predicts insulin sensitivity in middle-aged patients with type 1 diabetes mellitus. Diabetologia. 2012;55(3):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab. 2011;96(2):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care. 2009;32(9):1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55(5):1436–1442. [DOI] [PubMed] [Google Scholar]
- 446.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290(6):E1191–E1197. [DOI] [PubMed] [Google Scholar]
- 447.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28(2):385–390. [DOI] [PubMed] [Google Scholar]
- 448.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Kassab GS, Lin DH, Fung YC. Morphometry of pig coronary venous system. Am J Physiol. 1994;267(6 Pt 2):H2100–H2113. [DOI] [PubMed] [Google Scholar]
- 451.Wei K, Kaul S. The coronary microcirculation in health and disease. Cardiol Clin. 2004;22(2):221–231. [DOI] [PubMed] [Google Scholar]
- 452.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83(1):59–115. [DOI] [PubMed] [Google Scholar]
- 453.Jayaweera AR, Wei K, Coggins M, Bin JP, Goodman C, Kaul S. Role of capillaries in determining CBF reserve: new insights using myocardial contrast echocardiography. Am J Physiol. 1999;277(6 Pt 2):H2363–H2372. [DOI] [PubMed] [Google Scholar]
- 454.Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59(5):483–495. [DOI] [PubMed] [Google Scholar]
- 455.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. [DOI] [PubMed] [Google Scholar]
- 456.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251(4 Pt 2):H779–H788. [DOI] [PubMed] [Google Scholar]
- 457.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88(3):1009–1086. [DOI] [PubMed] [Google Scholar]
- 458.Laine H, Nuutila P, Luotolahti M, Meyer C, Elomaa T, Koskinen P, Rönnemaa T, Knuuti J. Insulin-induced increment of coronary flow reserve is not abolished by dexamethasone in healthy young men. J Clin Endocrinol Metab. 2000;85(5):1868–1873. [DOI] [PubMed] [Google Scholar]
- 459.Laine H, Sundell J, Nuutila P, Raitakari OT, Luotolahti M, Rönnemaa T, Elomaa T, Koskinen P, Knuuti J. Insulin induced increase in coronary flow reserve is abolished by dexamethasone in young men with uncomplicated type 1 diabetes. Heart. 2004;90(3):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Jagasia D, Whiting JM, Concato J, Pfau S, McNulty PH. Effect of non-insulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation. 2001;103(13):1734–1739. [DOI] [PubMed] [Google Scholar]
- 461.Sundell J, Laine H, Nuutila P, Rönnemaa T, Luotolahti M, Raitakari O, Knuuti J. The effects of insulin and short-term hyperglycaemia on myocardial blood flow in young men with uncomplicated type I diabetes. Diabetologia. 2002;45(6):775–782. [DOI] [PubMed] [Google Scholar]
- 462.Sundell J, Nuutila P, Laine H, Luotolahti M, Kalliokoski K, Raitakari O, Knuuti J. Dose-dependent vasodilating effects of insulin on adenosine-stimulated myocardial blood flow. Diabetes. 2002;51(4):1125–1130. [DOI] [PubMed] [Google Scholar]
- 463.Iozzo P, Chareonthaitawee P, Di Terlizzi M, Betteridge DJ, Ferrannini E, Camici PG. Regional myocardial blood flow and glucose utilization during fasting and physiological hyperinsulinemia in humans. Am J Physiol Endocrinol Metab. 2002;282(5):E1163–E1171. [DOI] [PubMed] [Google Scholar]
- 464.Lautamäki R, Airaksinen KEJ, Seppänen M, Toikka J, Härkönen R, Luotolahti M, Borra R, Sundell J, Knuuti J, Nuutila P. Insulin improves myocardial blood flow in patients with type 2 diabetes and coronary artery disease. Diabetes. 2006;55(2):511–516. [DOI] [PubMed] [Google Scholar]
- 465.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483. [DOI] [PubMed] [Google Scholar]
- 466.Wei K, Kaul S. Recent advances in myocardial contrast echocardiography. Curr Opin Cardiol. 1997;12(6):539–546. [DOI] [PubMed] [Google Scholar]
- 467.Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol. 1997;29(5):1081–1088. [DOI] [PubMed] [Google Scholar]
- 468.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab. 2007;293(5):E1250–E1255. [DOI] [PubMed] [Google Scholar]
- 469.Chai W, Liu J, Jahn LA, Fowler DE, Barrett EJ, Liu Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care. 2011;34(7):1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation. 2005;112(2):179–184. [DOI] [PubMed] [Google Scholar]
- 471.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317(3):1106–1113. [DOI] [PubMed] [Google Scholar]
- 472.Nyström T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res. 2008;40(9):593–606. [DOI] [PubMed] [Google Scholar]
- 473.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61(4):888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W, Liu Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304(2):E222–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52(5):752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Barrett EJ, Liu Z. The endothelial cell: an “early responder” in the development of insulin resistance. Rev Endocr Metab Disord. 2013;14(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes. 2007;56(9):2194–2200. [DOI] [PubMed] [Google Scholar]
- 478.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–567. [DOI] [PubMed] [Google Scholar]
- 479.Fischer VW, Barner HB, Leskiw ML. Capillary basal laminar thichness in diabetic human myocardium. Diabetes. 1979;28(8):713–719. [DOI] [PubMed] [Google Scholar]
- 480.Strauer BE, Motz W, Vogt M, Schwartzkopff B. Impaired coronary flow reserve in NIDDM: a possible role for diabetic cardiopathy in humans. Diabetes. 1997;46(Suppl 2):S119–S124. [DOI] [PubMed] [Google Scholar]
- 481.Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali J-R. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42(7):1017–1025. [DOI] [PubMed] [Google Scholar]
- 482.Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30(6):1472–1477. [DOI] [PubMed] [Google Scholar]
- 483.Pitkänen OP, Nuutila P, Raitakari OT, Rönnemaa T, Koskinen PJ, Iida H, Lehtimäki TJ, Laine HK, Takala T, Viikari JS, Knuuti J. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47(2):248–254. [DOI] [PubMed] [Google Scholar]
- 484.Nitenberg A, Ledoux S, Valensi P, Sachs R, Attali J-R, Antony I. Impairment of coronary microvascular dilation in response to cold pressor--induced sympathetic stimulation in type 2 diabetic patients with abnormal stress thallium imaging. Diabetes. 2001;50(5):1180–1185. [DOI] [PubMed] [Google Scholar]
- 485.Sundell J, Laine H, Luotolahti M, Kalliokoski K, Raitakari O, Nuutila P, Knuuti J. Obesity affects myocardial vasoreactivity and coronary flow response to insulin. Obes Res. 2002;10(7):617–624. [DOI] [PubMed] [Google Scholar]
- 486.Scognamiglio R, Negut C, de Kreutzenberg SV, Tiengo A, Avogaro A. Effects of different insulin regimes on postprandial myocardial perfusion defects in type 2 diabetic patients. Diabetes Care. 2006;29(1):95–100. [PubMed] [Google Scholar]
- 487.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25(5):989–994. [DOI] [PubMed] [Google Scholar]
- 488.Rim S-J, Leong-Poi H, Lindner JR, Wei K, Fisher NG, Kaul S. Decrease in coronary blood flow reserve during hyperlipidemia is secondary to an increase in blood viscosity. Circulation. 2001;104(22):2704–2709. [DOI] [PubMed] [Google Scholar]
- 489.Bin J-P, Le E, Pelberg RA, Coggins MP, Wei K, Kaul S. Mechanism of inducible regional dysfunction during dipyridamole stress. Circulation. 2002;106(1):112–117. [DOI] [PubMed] [Google Scholar]
- 490.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: bolus or continuous infusion? J Am Coll Cardiol. 1998;32(1):252–260. [DOI] [PubMed] [Google Scholar]
- 491.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34(1):48–55. [DOI] [PubMed] [Google Scholar]
- 492.Pacella JJ, Villanueva FS. Effect of coronary stenosis on adjacent bed flow reserve: assessment of microvascular mechanisms using myocardial contrast echocardiography. Circulation. 2006;114(18):1940–1947. [DOI] [PubMed] [Google Scholar]
- 493.Rosenbloom AL, Silverstein JH, Riley WJ, Maclaren NK. Limited joint mobility in childhood diabetes: family studies. Diabetes Care. 1983;6(4):370–373. [DOI] [PubMed] [Google Scholar]
- 494.Rosenbloom AL, Malone JI, Yucha J, Van Cader TC. Limited joint mobility and diabetic retinopathy demonstrated by fluorescein angiography. Eur J Pediatr. 1984;141(3):163–164. [DOI] [PubMed] [Google Scholar]
- 495.Greenman RL, Panasyuk S, Wang X, Lyons TE, Dinh T, Longoria L, Giurini JM, Freeman J, Khaodhiar L, Veves A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366(9498):1711–1717. [DOI] [PubMed] [Google Scholar]
- 496.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27(9):503–508. [DOI] [PubMed] [Google Scholar]
- 497.Khan F, Elhadd TA, Greene SA, Belch JJ. Impaired skin microvascular function in children, adolescents, and young adults with type 1 diabetes. Diabetes Care. 2000;23(2):215–220. [DOI] [PubMed] [Google Scholar]
- 498.Koïtka A, Abraham P, Bouhanick B, Sigaudo-Roussel D, Demiot C, Saumet JL. Impaired pressure-induced vasodilation at the foot in young adults with type 1 diabetes. Diabetes. 2004;53(3):721–725. [DOI] [PubMed] [Google Scholar]
- 499.Forst T, Pfützner A, Kunt T, Pohlmann T, Schenk U, Bauersachs R, Küstner E, Beyer J. Skin microcirculation in patients with type I diabetes with and without neuropathy after neurovascular stimulation. Clin Sci (Lond). 1998;94(3):255–261. [DOI] [PubMed] [Google Scholar]
- 500.Chang CH, Tsai RK, Wu WC, Kuo SL, Yu HS. Use of dynamic capillaroscopy for studying cutaneous microcirculation in patients with diabetes mellitus. Microvasc Res. 1997;53(2):121–127. [DOI] [PubMed] [Google Scholar]
- 501.Tooke JE, Lins PE, Ostergren J, Fagrell B. Skin microvascular autoregulatory responses in type I diabetes: the influence of duration and control. Int J Microcirc Clin Exp. 1985;4(3):249–256. [PubMed] [Google Scholar]
- 502.Pazos-Moura CC, Moura EG, Bouskela E, Torres Filho IP, Breitenbach MM. Nailfold capillaroscopy in non-insulin dependent diabetes mellitus: blood flow velocity during rest and post-occlusive reactive hyperaemia. Clin Physiol. 1990;10(5):451–461. [DOI] [PubMed] [Google Scholar]
- 503.de Jongh RT, Serné EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109(21):2529–2535. [DOI] [PubMed] [Google Scholar]
- 504.Serné EH, de Jongh RT, Eringa EC, IJzerman RG, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50(1):204–211. [DOI] [PubMed] [Google Scholar]
- 505.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Pham HT, Economides PA, Veves A. The role of endothelial function on the foot: microcirculation and wound healing in patients with diabetes. Clin Podiatr Med Surg. 1998;15(1):85–93. [PubMed] [Google Scholar]
- 507.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117(5):1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clément K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124(1):67–76. [DOI] [PubMed] [Google Scholar]
- 509.Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric oxide and beta-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation. 2004;109(1):47–52. [DOI] [PubMed] [Google Scholar]
- 510.Tobin L, Simonsen L, Bülow J. The dynamics of the microcirculation in the subcutaneous adipose tissue is impaired in the postprandial state in type 2 diabetes. Clin Physiol Funct Imaging. 2011;31(6):458–463. [DOI] [PubMed] [Google Scholar]
- 511.Summers LK, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond). 1996;91(6):679–683. [DOI] [PubMed] [Google Scholar]
- 512.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293(4):E1118–E1128. [DOI] [PubMed] [Google Scholar]
- 514.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32(3):451–463. [DOI] [PubMed] [Google Scholar]
- 515.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci. 2002;99:10730–10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10(6):625–632. [DOI] [PubMed] [Google Scholar]
- 517.Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59(4):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61(7):1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Lu X, Ji Y, Zhang L, Zhang Y, Zhang S, An Y, Liu P, Zheng Y. Resistance to obesity by repression of VEGF gene expression through induction of brown-like adipocyte differentiation. Endocrinology. 2012;153(7):3123–3132. [DOI] [PubMed] [Google Scholar]
- 520.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, Scotney P, Nyqvist D, Samén E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjöholm A, Nash A, Eriksson U. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490(7420):426–430. [DOI] [PubMed] [Google Scholar]
- 521.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62(2):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365(9473):1817–1820. [DOI] [PubMed] [Google Scholar]
- 523.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210(2):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U, Fox CS. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assoc. 2012;1(6):e004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.NIH. Atlas of chronic kidney disease and end-stage renal disease in the United States. Vol. 1. U.S. Renal Data System, USRDS 2012 Annual Data Report 2012. Available at: http://www.usrds.org/atlas12.aspx. Accessed 30 March 2016.
- 526.Fox CS, Muntner P. Trends in diabetes, high cholesterol, and hypertension in chronic kidney disease among U.S. adults: 1988-1994 to 1999-2004. Diabetes Care. 2008;31(7):1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Plantinga LC, Crews DC, Coresh J, Miller ER III, Saran R, Yee J, Hedgeman E, Pavkov M, Eberhardt MS, Williams DE, Powe NR; CDC CKD Surveillance Team . Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5(4):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16(7):2134–2140. [DOI] [PubMed] [Google Scholar]
- 529.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K, Heikkilä O, Forsblom C; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Placha G, Canani LH, Warram JH, Krolewski AS. Evidence for different susceptibility genes for proteinuria and ESRD in type 2 diabetes. Adv Chronic Kidney Dis. 2005;12(2):155–169. [DOI] [PubMed] [Google Scholar]
- 533.Ficociello LH, Perkins BA, Roshan B, Weinberg JM, Aschengrau A, Warram JH, Krolewski AS. Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care. 2009;32(5):889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Perkins BA, Krolewski AS. Early nephropathy in type 1 diabetes: the importance of early renal function decline. Curr Opin Nephrol Hypertens. 2009;18(3):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. [DOI] [PubMed] [Google Scholar]
- 536.Skupien J, Warram JH, Smiles AM, Niewczas MA, Gohda T, Pezzolesi MG, Cantarovich D, Stanton R, Krolewski AS. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int. 2012;82(5):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Pham TT, Sim JJ, Kujubu DA, Liu I-LA, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27(3):322–328. [DOI] [PubMed] [Google Scholar]
- 538.Mou S, Wang Q, Liu J, Che X, Zhang M, Cao L, Zhou W, Ni Z. Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;87(3):354–359. [DOI] [PubMed] [Google Scholar]
- 539.Shah VO, Scavini M, Stidley CA, Tentori F, Welty TK, MacCluer JW, Narva AS, Bobelu A, Albert CP, Kessler DS, Harford AM, Wong CS, Harris AA, Paine S, Zager PG. Epidemic of diabetic and nondiabetic renal disease among the Zuni Indians: the Zuni Kidney Project. J Am Soc Nephrol. 2003;14(5):1320–1329. [DOI] [PubMed] [Google Scholar]
- 540.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, Orringer EP, Eckman JR, Telen MJ. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol. 2011;155(3):386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, Divers J, Kopp JB, Winkler CA, Nelson GW, Langefeld CD, Bowden DW. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75(7):736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB; SK Investigators . Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Skorecki KL, Wasser WG. Hypertension-misattributed kidney disease in African Americans. Kidney Int. 2013;83(1):6–9. [DOI] [PubMed] [Google Scholar]
- 546.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Gopalakrishnan I, Iskandar SS, Daeihagh P, Divers J, Langefeld CD, Bowden DW, Hicks PJ, Rocco MV, Freedman BI. Coincident idiopathic focal segmental glomerulosclerosis collapsing variant and diabetic nephropathy in an African American homozygous for MYH9 risk variants. Hum Pathol. 2011;42(2):291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Pezzolesi MG, Poznik GD, Mychaleckyj JC, Paterson AD, Barati MT, Klein JB, Ng DPK, Placha G, Canani LH, Bochenski J, Waggott D, Merchant ML, Krolewski B, Mirea L, Wanic K, Katavetin P, Kure M, Wolkow P, Dunn JS, Smiles A, Walker WH, Boright AP, Bull SB, Doria A, Rogus JJ, Rich SS, Warram JH, Krolewski AS; DCCT/EDIC Research Group . Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes. 2009;58(6):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.McDonough CW, Palmer ND, Hicks PJ, Roh BH, An SS, Cooke JN, Hester JM, Wing MR, Bostrom MA, Rudock ME, Lewis JP, Talbert ME, Blevins RA, Lu L, Ng MCY, Sale MM, Divers J, Langefeld CD, Freedman BI, Bowden DW. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int. 2011;79(5):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320(18):1161–1165. [DOI] [PubMed] [Google Scholar]
- 552.Quinn M, Angelico MC, Warram JH, Krolewski AS. Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia. 1996;39(8):940–945. [DOI] [PubMed] [Google Scholar]
- 553.Borch-Johnsen K, Nørgaard K, Hommel E, Mathiesen ER, Jensen JS, Deckert T, Parving H-H. Is diabetic nephropathy an inherited complication? Kidney Int. 1992;41(4):719–722. [DOI] [PubMed] [Google Scholar]
- 554.Freedman BI, Tuttle AB, Spray BJ. Familial predisposition to nephropathy in African-Americans with non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1995;25(5):710–713. [DOI] [PubMed] [Google Scholar]
- 555.Freedman BI, Volkova NV, Satko SG, Krisher J, Jurkovitz C, Soucie JM, McClellan WM. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005;25(6):529–535. [DOI] [PubMed] [Google Scholar]
- 556.O’Dea DF, Murphy SW, Hefferton D, Parfrey PS. Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: a population-based study. Am J Kidney Dis. 1998;32(5):794–801. [DOI] [PubMed] [Google Scholar]
- 557.Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1990;33(7):438–443. [DOI] [PubMed] [Google Scholar]
- 558.Forsblom CM, Kanninen T, Lehtovirta M, Saloranta C, Groop LC. Heritability of albumin excretion rate in families of patients with type II diabetes. Diabetologia. 1999;42(11):1359–1366. [DOI] [PubMed] [Google Scholar]
- 559.Ramirez SPB, McClellan W, Port FK, Hsu SI. Risk factors for proteinuria in a large, multiracial, southeast Asian population. J Am Soc Nephrol. 2002;13(7):1907–1917. [DOI] [PubMed] [Google Scholar]
- 560.Canani LH, Gerchman F, Gross JL. Familial clustering of diabetic nephropathy in Brazilian type 2 diabetic patients. Diabetes. 1999;48(4):909–913. [DOI] [PubMed] [Google Scholar]
- 561.Vijay V, Snehalatha C, Shina K, Lalitha S, Ramachandran A. Familial aggregation of diabetic kidney disease in type 2 diabetes in south India. Diabetes Res Clin Pract. 1999;43(3):167–171. [DOI] [PubMed] [Google Scholar]
- 562.Langefeld CD, Beck SR, Bowden DW, Rich SS, Wagenknecht LE, Freedman BI. Heritability of GFR and albuminuria in Caucasians with type 2 diabetes mellitus. Am J Kidney Dis. 2004;43(5):796–800. [DOI] [PubMed] [Google Scholar]
- 563.Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9(7):1270–1276. [DOI] [PubMed] [Google Scholar]
- 564.Satko SG, Sedor JR, Iyengar SK, Freedman BI. Familial clustering of chronic kidney disease. Semin Dial. 2007;20(3):229–236. [DOI] [PubMed] [Google Scholar]
- 565.Fogarty DG, Rich SS, Hanna L, Warram JH, Krolewski AS. Urinary albumin excretion in families with type 2 diabetes is heritable and genetically correlated to blood pressure. Kidney Int. 2000;57(1):250–257. [DOI] [PubMed] [Google Scholar]
- 566.Imperatore G, Knowler WC, Pettitt DJ, Kobes S, Bennett PH, Hanson RL. Segregation analysis of diabetic nephropathy in Pima Indians. Diabetes. 2000;49(6):1049–1056. [DOI] [PubMed] [Google Scholar]
- 567.Ng DPK, Tai BC, Koh D, Tan KW, Chia KS. Angiotensin-I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: a meta-analysis of studies reported between 1994 and 2004 and comprising 14,727 subjects. Diabetologia. 2005;48(5):1008–1016. [DOI] [PubMed] [Google Scholar]
- 568.Wang F, Fang Q, Yu N, Zhao D, Zhang Y, Wang J, Wang Q, Zhou X, Cao X, Fan X. Association between genetic polymorphism of the angiotensin-converting enzyme and diabetic nephropathy: a meta-analysis comprising 26,580 subjects. J Renin Angiotensin Aldosterone Syst. 2012;13(1):161–174. [DOI] [PubMed] [Google Scholar]
- 569.Mooyaart AL, Valk EJJ, van Es LA, Bruijn JA, de Heer E, Freedman BI, Dekkers OM, Baelde HJ. Genetic associations in diabetic nephropathy: a meta-analysis [published correction appears in Diabetologia. 2014;57(3):650]. Diabetologia. 2011;54(3):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Williams WW, Salem RM, McKnight AJ, Sandholm N, Forsblom C, Taylor A, Guiducci C, McAteer JB, McKay GJ, Isakova T, Brennan EP, Sadlier DM, Palmer C, Söderlund J, Fagerholm E, Harjutsalo V, Lithovius R, Gordin D, Hietala K, Kytö J, Parkkonen M, Rosengård-Bärlund M, Thorn L, Syreeni A, Tolonen N, Saraheimo M, Wadén J, Pitkäniemi J, Sarti C, Tuomilehto J, Tryggvason K, Österholm AM, He B, Bain S, Martin F, Godson C, Hirschhorn JN, Maxwell AP, Groop PH, Florez JC; GENIE Consortium . Association testing of previously reported variants in a large case-control meta-analysis of diabetic nephropathy. Diabetes. 2012;61(8):2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Vardarli I, Baier LJ, Hanson RL, Akkoyun I, Fischer C, Rohmeiss P, Basci A, Bartram CR, Van Der Woude FJ, Janssen B. Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3-23. Kidney Int. 2002;62(6):2176–2183. [DOI] [PubMed] [Google Scholar]
- 572.Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC; Pima Diabetes Genes Group . Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Diabetes. 1998;47(5):821–830. [DOI] [PubMed] [Google Scholar]
- 573.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJL, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Köppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54(8):2320–2327. [DOI] [PubMed] [Google Scholar]
- 574.Freedman BI, Hicks PJ, Sale MM, Pierson ED, Langefeld CD, Rich SS, Xu J, McDonough C, Janssen B, Yard BA, van der Woude FJ, Bowden DW. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant. 2007;22(4):1131–1135. [DOI] [PubMed] [Google Scholar]
- 575.Wanic K, Placha G, Dunn J, Smiles A, Warram JH, Krolewski AS. Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case-control and follow-up studies. Diabetes. 2008;57(9):2547–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.McDonough CW, Hicks PJ, Lu L, Langefeld CD, Freedman BI, Bowden DW. The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum Genet. 2009;126(2):265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Ahluwalia TS, Lindholm E, Groop LC. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia. 2011;54(9):2295–2302. [DOI] [PubMed] [Google Scholar]
- 578.Mooyaart AL, Zutinic A, Bakker SJL, Grootendorst DC, Kleefstra N, van Valkengoed IGM, Böhringer S, Bilo HJG, Dekker FW, Bruijn JA, Navis G, Janssen B, Baelde HJ, De Heer E. Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes. 2010;59(6):1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.McDonough CW, Bostrom MA, Lu L, Hicks PJ, Langefeld CD, Divers J, Mychaleckyj JC, Freedman BI, Bowden DW. Genetic analysis of diabetic nephropathy on chromosome 18 in African Americans: linkage analysis and dense SNP mapping. Hum Genet. 2009;126(6):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Bowden DW, Colicigno CJ, Langefeld CD, Sale MM, Williams A, Anderson PJ, Rich SS, Freedman BI. A genome scan for diabetic nephropathy in African Americans. Kidney Int. 2004;66(4):1517–1526. [DOI] [PubMed] [Google Scholar]
- 581.Knowler WC, Coresh J, Elston RC, Freedman BI, Iyengar SK, Kimmel PL, Olson JM, Plaetke R, Sedor JR, Seldin MF; Family Investigation of Nephropathy and Diabetes Research Group . The Family Investigation of Nephropathy and Diabetes (FIND): design and methods. J Diabetes Complications. 2005;19(1):1–9. [DOI] [PubMed] [Google Scholar]
- 582.Iyengar SK, Abboud HE, Goddard KA, Saad MF, Adler SG, Arar NH, Bowden DW, Duggirala R, Elston RC, Hanson RL, Ipp E, Kao WH, Kimmel PL, Klag MJ, Knowler WC, Meoni LA, Nelson RG, Nicholas SB, Pahl MV, Parekh RS, Quade SR, Rich SS, Rotter JI, Scavini M, Schelling JR, Sedor JR, Sehgal AR, Shah VO, Smith MW, Taylor KD, Winkler CA, Zager PG, Freedman BI; Family Investigation of Nephropathy and Diabetes Research Group . Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: the family investigation of nephropathy and diabetes (FIND). Diabetes. 2007;56(6):1577–1585. [DOI] [PubMed] [Google Scholar]
- 583.Igo RP Jr, Iyengar SK, Nicholas SB, Goddard KA, Langefeld CD, Hanson RL, Duggirala R, Divers J, Abboud H, Adler SG, Arar NH, Horvath A, Elston RC, Bowden DW, Guo X, Ipp E, Kao WH, Kimmel PL, Knowler WC, Meoni LA, Molineros J, Nelson RG, Pahl MV, Parekh RS, Rasooly RS, Schelling JR, Shah VO, Smith MW, Winkler CA, Zager PG, Sedor JR, Freedman BI; Family Investigation of Nephropathy and Diabetes Research Group . Genomewide linkage scan for diabetic renal failure and albuminuria: the FIND study. Am J Nephrol. 2011;33(5):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Schelling JR, Abboud HE, Nicholas SB, Pahl MV, Sedor JR, Adler SG, Arar NH, Bowden DW, Elston RC, Freedman BI, Goddard KA, Guo X, Hanson RL, Ipp E, Iyengar SK, Jun G, Kao WH, Kasinath BS, Kimmel PL, Klag MJ, Knowler WC, Nelson RG, Parekh RS, Quade SR, Rich SS, Saad MF, Scavini M, Smith MW, Taylor K, Winkler CA, Zager PG, Shah VO; Family Investigation of Nephropathy and Diabetes Research Group . Genome-wide scan for estimated glomerular filtration rate in multi-ethnic diabetic populations: the Family Investigation of Nephropathy and Diabetes (FIND). Diabetes. 2008;57(1):235–243. [DOI] [PubMed] [Google Scholar]
- 585.McKnight AJ, Patterson CC, Sandholm N, Kilner J, Buckham TA, Parkkonen M, Forsblom C, Sadlier DM, Groop PH, Maxwell AP; Warren 3/UK GoKinD Study Group . Genetic polymorphisms in nitric oxide synthase 3 gene and implications for kidney disease: a meta-analysis. Am J Nephrol. 2010;32(5):476–481. [DOI] [PubMed] [Google Scholar]
- 586.Shimazaki A, Kawamura Y, Kanazawa A, Sekine A, Saito S, Tsunoda T, Koya D, Babazono T, Tanaka Y, Matsuda M, Kawai K, Iiizumi T, Imanishi M, Shinosaki T, Yanagimoto T, Ikeda M, Omachi S, Kashiwagi A, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakajima M, Nakamura Y, Maeda S. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54(4):1171–1178. [DOI] [PubMed] [Google Scholar]
- 587.Hanson RL, Craig DW, Millis MP, Yeatts KA, Kobes S, Pearson JV, Lee AM, Knowler WC, Nelson RG, Wolford JK. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes. 2007;56(4):975–983. [DOI] [PubMed] [Google Scholar]
- 588.Maeda S, Kobayashi MA, Araki S, Babazono T, Freedman BI, Bostrom MA, Cooke JN, Toyoda M, Umezono T, Tarnow L, Hansen T, Gaede P, Jorsal A, Ng DPK, Ikeda M, Yanagimoto T, Tsunoda T, Unoki H, Kawai K, Imanishi M, Suzuki D, Shin HD, Park KS, Kashiwagi A, Iwamoto Y, Kaku K, Kawamori R, Parving H-H, Bowden DW, Pedersen O, Nakamura Y. A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet. 2010;6(2):e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Tang SCW, Leung VTM, Chan LYY, Wong SSH, Chu DWS, Leung JCK, Ho YW, Lai KN, Ma L, Elbein SC, Bowden DW, Hicks PJ, Comeau ME, Langefeld CD, Freedman BI. The acetyl-coenzyme A carboxylase beta (ACACB) gene is associated with nephropathy in Chinese patients with type 2 diabetes. Nephrol Dial Transplant. 2010;25(12):3931–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5(12):2373–2379. [DOI] [PubMed] [Google Scholar]
- 591.Pezzolesi MG, Jeong J, Smiles AM, Skupien J, Mychaleckyj JC, Rich SS, Warram JH, Krolewski AS. Family-based association analysis confirms the role of the chromosome 9q21.32 locus in the susceptibility of diabetic nephropathy. PLoS One. 2013;8(3):e60301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Martini S, Nair V, Patel SR, Eichinger F, Nelson RG, Weil EJ, Pezzolesi MG, Krolewski AS, Randolph A, Keller BJ, Werner T, Kretzler M. From single nucleotide polymorphism to transcriptional mechanism: a model for FRMD3 in diabetic nephropathy. Diabetes. 2013;62(7):2605–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, McKay GJ, Williams WW, Sadlier DM, Mäkinen VP, Swan EJ, Palmer C, Boright AP, Ahlqvist E, Deshmukh HA, Keller BJ, Huang H, Ahola AJ, Fagerholm E, Gordin D, Harjutsalo V, He B, Heikkilä O, Hietala K, Kytö J, Lahermo P, Lehto M, Lithovius R, Osterholm AM, Parkkonen M, Pitkäniemi J, Rosengård-Bärlund M, Saraheimo M, Sarti C, Söderlund J, Soro-Paavonen A, Syreeni A, Thorn LM, Tikkanen H, Tolonen N, Tryggvason K, Tuomilehto J, Wadén J, Gill GV, Prior S, Guiducci C, Mirel DB, Taylor A, Hosseini SM, Parving HH, Rossing P, Tarnow L, Ladenvall C, Alhenc-Gelas F, Lefebvre P, Rigalleau V, Roussel R, Tregouet DA, Maestroni A, Maestroni S, Falhammar H, Gu T, Möllsten A, Cimponeriu D, Ioana M, Mota M, Mota E, Serafinceanu C, Stavarachi M, Hanson RL, Nelson RG, Kretzler M, Colhoun HM, Panduru NM, Gu HF, Brismar K, Zerbini G, Hadjadj S, Marre M, Groop L, Lajer M, Bull SB, Waggott D, Paterson AD, Savage DA, Bain SC, Martin F, Hirschhorn JN, Godson C, Florez JC, Groop PH, Maxwell AP; DCCT/EDIC Research Group . New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 2012;8(9):e1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Hanson RL, Millis MP, Young NJ, Kobes S, Nelson RG, Knowler WC, DiStefano JK. ELMO1 variants and susceptibility to diabetic nephropathy in American Indians. Mol Genet Metab. 2010;101(4):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Leak TS, Perlegas PS, Smith SG, Keene KL, Hicks PJ, Langefeld CD, Mychaleckyj JC, Rich SS, Kirk JK, Freedman BI, Bowden DW, Sale MM. Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann Hum Genet. 2009;73(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Pezzolesi MG, Katavetin P, Kure M, Poznik GD, Skupien J, Mychaleckyj JC, Rich SS, Warram JH, Krolewski AS. Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes. 2009;58(11):2698–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Cooke JN, Bostrom MA, Hicks PJ, Ng MCY, Hellwege JN, Comeau ME, Divers J, Langefeld CD, Freedman BI, Bowden DW. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant. 2012;27(4):1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Sapienza C, Lee J, Powell J, Erinle O, Yafai F, Reichert J, Siraj ES, Madaio M. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics. 2011;6(1):20–28. [DOI] [PubMed] [Google Scholar]
- 599.National Kidney Foundation 2012 update. Am J Kidney Dis. 2012;60(5):850–886. [DOI] [PubMed] [Google Scholar]
- 600.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B; DCCT/EDIC Research Group . Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.The Diabetes Control and Complications (DCCT) Research Group Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47(6):1703–1720. [DOI] [PubMed] [Google Scholar]
- 602.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. JAMA. 2003;290(16):2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. [DOI] [PubMed] [Google Scholar]
- 604.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 605.de Boer IH. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Cohort: microalbuminuria outcomes in type 1 diabetes. Arch Intern Med. 2011;171:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560– 2572. [DOI] [PubMed] [Google Scholar]
- 607.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148(1):30–48. [DOI] [PubMed] [Google Scholar]
- 608.Mann JFE, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET Investigators . Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553. [DOI] [PubMed] [Google Scholar]
- 609.Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Coombes JS. Effect of atorvastatin on kidney function in chronic kidney disease: a randomised double-blind placebo-controlled trial. Atherosclerosis. 2010;213(1):218–224. [DOI] [PubMed] [Google Scholar]
- 610.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Wanner C, Krane V, März W, Olschewski M, Mann JFE, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. [DOI] [PubMed] [Google Scholar]
- 612.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth J-P; ACTION I Investigator Group . Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24(1):32–40. [DOI] [PubMed] [Google Scholar]
- 613.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y; Osaka CKD Expert Research Group . Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18(3):896–903. [DOI] [PubMed] [Google Scholar]
- 614.Peacock TP, Shihabi ZK, Bleyer AJ, Dolbare EL, Byers JR, Knovich MA, Calles-Escandon J, Russell GB, Freedman BI. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9):1062–1068. [DOI] [PubMed] [Google Scholar]
- 615.Freedman BI. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: time to dispense with the hemoglobin A1C in end-stage kidney disease. Diabetes Care. 2012;35(7):1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: A1C remains the gold standard outcome predictor in diabetic dialysis patients: counterpoint. Diabetes Care. 2012;35(7):1625–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Ricks J, Molnar MZ, Kovesdy CP, Shah A, Nissenson AR, Williams M, Kalantar-Zadeh K. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61(3):708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Leslie RDGRD, ed. Diabetes: Clinical Science in Practice. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 619.Holzer SE, Camerota A, Martens L, Cuerdon T, Crystal-Peters J, Zagari M. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20(1):169–181. [DOI] [PubMed] [Google Scholar]
- 620.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331(13):854–860. [DOI] [PubMed] [Google Scholar]
- 621.Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28(6):1480–1481. [DOI] [PubMed] [Google Scholar]
- 622.Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. [DOI] [PubMed] [Google Scholar]
- 623.Vinik AI. Diabetic neuropathy: pathogenesis and therapy. Am J Med. 1999;107(2B):17S–26S. [DOI] [PubMed] [Google Scholar]
- 624.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859. [DOI] [PubMed] [Google Scholar]
- 625.Vinik AI, Anandacoomaraswamy D, Ullal J. Antibodies to neuronal structures: innocent bystanders or neurotoxins? Diabetes Care. 2005;28(8):2067–2072. [DOI] [PubMed] [Google Scholar]
- 626.Levitt NS, Stansberry KB, Wynchank S, Vinik AI. The natural progression of autonomic neuropathy and autonomic function tests in a cohort of people with IDDM. Diabetes Care. 1996;19(7):751–754. [DOI] [PubMed] [Google Scholar]
- 627.Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med. 1993;10(9):820–824. [DOI] [PubMed] [Google Scholar]
- 628.Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab. 2006;2(5):269–281. [DOI] [PubMed] [Google Scholar]
- 629.Sadosky A, McDermott AM, Brandenburg NA, Strauss M. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008;8(1):45–56. [DOI] [PubMed] [Google Scholar]
- 630.Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments [published correction appears in Diabetes Care. 2010;33(12):2725]. Diabetes Care. 2010;33(10):2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev. 2008;24(Suppl 1):S52–S57. [DOI] [PubMed] [Google Scholar]
- 632.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. [DOI] [PubMed] [Google Scholar]
- 633.Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28(6):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Vinik AI, Maser RE, Ziegler D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care. 2010;33(7):1688–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. [DOI] [PubMed] [Google Scholar]
- 636.Vinik EJ, Hayes RP, Oglesby A, Bastyr E, Barlow P, Ford-Molvik SL, Vinik AI. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7(3):497–508. [DOI] [PubMed] [Google Scholar]
- 637.Vinik EJ, Paulson JF, Ford-Molvik SL, Vinik AI. German-translated Norfolk quality of life (QOL-DN) identifies the same factors as the English version of the tool and discriminates different levels of neuropathy severity. J Diabetes Sci Technol. 2008;2(6):1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes. 1997;46(Suppl 2):S54–S57. [DOI] [PubMed] [Google Scholar]
- 639.Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D; American Diabetes Association . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. [DOI] [PubMed] [Google Scholar]
- 640.Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am. 2004;88(4):947–999, xi. [DOI] [PubMed] [Google Scholar]
- 641.Vinik AI, Nevoret M-L, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42(4):747–787. [DOI] [PubMed] [Google Scholar]
- 642.Watkins PJ. Progression of diabetic autonomic neuropathy. Diabet Med. 1993;10(Suppl 2):77S–78S. [DOI] [PubMed] [Google Scholar]
- 643.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122(8):561–568. [DOI] [PubMed] [Google Scholar]
- 644.Ziegler D, Cicmir I, Mayer P, Wiefels K, Gries FA. The natural course of peripheral and autonomic neural function during the first two years after diagnosis of type 1 diabetes. Klin Wochenschr. 1988;66(21):1085–1092. [DOI] [PubMed] [Google Scholar]
- 645.Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(2):89–94. [DOI] [PubMed] [Google Scholar]
- 646.Dyck PJ, Kratz KM, Lehman KA, Karnes JL, Melton LJ III, O’Brien PC, Litchy WJ, Windebank AJ, Smith BE, Low PA, Service FJ, Rizza RA, Zimmerman BR. The Rochester Diabetic Neuropathy Study: design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology. 1991;41(6):799–807. [DOI] [PubMed] [Google Scholar]
- 647.Pittenger GL, Mehrabyan A, Simmons K, Amandarice, Dublin C, Barlow P, Vinik AI. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord. 2005;3(2):113–121. doi:10.1089/met.2005.3.113 [DOI] [PubMed] [Google Scholar]
- 648.Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. 2004;27(8):1974–1979. [DOI] [PubMed] [Google Scholar]
- 649.Sinnreich M, Taylor BV, Dyck PJB. Diabetic neuropathies: classification, clinical features, and pathophysiological basis. Neurologist. 2005;11(2):63–79. [DOI] [PubMed] [Google Scholar]
- 650.Tesfaye S, Harris N, Jakubowski JJ, Mody C, Wilson RM, Rennie IG, Ward JD. Impaired blood flow and arterio-venous shunting in human diabetic neuropathy: a novel technique of nerve photography and fluorescein angiography. Diabetologia. 1993;36(12):1266–1274. [DOI] [PubMed] [Google Scholar]
- 651.Vinik AI, Nevoret M, Casellini C, Parson H. Neurovascular function and sudorimetry in health and disease. Curr Diab Rep. 2013;13(4):517–532. [DOI] [PubMed] [Google Scholar]
- 652.Dyck PJTP, ed. Diabetic Neuropathy. Philadelphia, PA: W.B. Saunders; 1999. [Google Scholar]
- 653.Watanabe K, Hagura R, Akanuma Y, Takasu T, Kajinuma H, Kuzuya N, Irie M. Characteristics of cranial nerve palsies in diabetic patients. Diabetes Res Clin Pract. 1990;10(1):19–27. [DOI] [PubMed] [Google Scholar]
- 654.Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care. 2002;25(3):565–569. [DOI] [PubMed] [Google Scholar]
- 655.Llewelyn JG, Thomas PK, King RHM. Epineurial microvasculitis in proximal diabetic neuropathy. J Neurol. 1998;245(3):159–165. [DOI] [PubMed] [Google Scholar]
- 656.Dyck PJB, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–491. [DOI] [PubMed] [Google Scholar]
- 657.Rg E, ed. Molecular Mechanisms of Endocrine and Organ Specific Autoimmunity. 1st ed. Georgetown, Washington DC: Landes Company; 1998. [Google Scholar]
- 658.Steck AJ, Kappos L. Gangliosides and autoimmune neuropathies: classification and clinical aspects of autoimmune neuropathies. J Neurol Neurosurg Psychiatry. 1994;57(Suppl):26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Said G, Goulon-Goeau C, Lacroix C, Moulonguet A. Nerve biopsy findings in different patterns of proximal diabetic neuropathy. Ann Neurol. 1994;35(5):559–569. [DOI] [PubMed] [Google Scholar]
- 660.Sander HW, Chokroverty S. Diabetic amyotrophy: current concepts. Semin Neurol. 1996;16(2):173–178. [DOI] [PubMed] [Google Scholar]
- 661.Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol. 1995;52(11):1053–1061. [DOI] [PubMed] [Google Scholar]
- 662.Britland ST, Young RJ, Sharma AK, Clarke BF. Acute and remitting painful diabetic polyneuropathy: a comparison of peripheral nerve fibre pathology. Pain. 1992;48(3):361–370. [DOI] [PubMed] [Google Scholar]
- 663.Sharma KR, Cross J, Farronay O, Ayyar DR, Shebert RT, Bradley WG. Demyelinating neuropathy in diabetes mellitus. Arch Neurol. 2002;59(5):758–765. [DOI] [PubMed] [Google Scholar]
- 664.Krendel DA, Zacharias A, Younger DS. Autoimmune diabetic neuropathy. Neurol Clin. 1997;15(4):959–971. [DOI] [PubMed] [Google Scholar]
- 665.Milicevic Z, Newlon PG, Pittenger GL, Stansberry KB, Vinik AI. Anti-ganglioside GM1 antibody and distal symmetric “diabetic polyneuropathy” with dominant motor features. Diabetologia. 1997;40(11):1364–1365. [DOI] [PubMed] [Google Scholar]
- 666.Ayyar DR, Sharma KR. Chronic inflammatory demyelinating polyradiculoneuropathy in diabetes mellitus. Curr Diab Rep. 2004;4(6):409–412. [DOI] [PubMed] [Google Scholar]
- 667.Reljanovic M, Barada A, Bilic R, Kovjanic J. One year follow-up in diabetic patients after surgical treatment of carpal tunnel syndrome. J Peripheral Nervous System. 2000;5:188. [Google Scholar]
- 668.Oyibo SO, Prasad YDM, Jackson NJ, Jude EB, Boulton AJM. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet Med. 2002;19(10):870–873. [DOI] [PubMed] [Google Scholar]
- 669.Tesfaye S, Malik R, Harris N, Jakubowski JJ, Mody C, Rennie IG, Ward JD. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis). Diabetologia. 1996;39(3):329–335. [DOI] [PubMed] [Google Scholar]
- 670.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7(11):682–690. [DOI] [PubMed] [Google Scholar]
- 671.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24(8):1448–1453. [DOI] [PubMed] [Google Scholar]
- 672.Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve. 2001;24(9):1225–1228. [DOI] [PubMed] [Google Scholar]
- 673.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001;24(9):1229–1231. [DOI] [PubMed] [Google Scholar]
- 674.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108–111. [DOI] [PubMed] [Google Scholar]
- 675.Dyck PJ, Lais A, Karnes JL, O’Brien P, Rizza R. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol. 1986;19(5):425–439. [DOI] [PubMed] [Google Scholar]
- 676.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies ISJ, Polydefkis M, Smith AG, Sommer C, Valls-Solé J; European Federation of Neurological Societies; Peripheral Nerve Society . European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903–912, e44–e49. [DOI] [PubMed] [Google Scholar]
- 677.England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ; American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199–207. [DOI] [PubMed] [Google Scholar]
- 678.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. [DOI] [PubMed] [Google Scholar]
- 679.Young MJ, Boulton AJM, MacLeod AF, Williams DRR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–154. [DOI] [PubMed] [Google Scholar]
- 680.Haanpää ML, Backonja M-M, Bennett MI, Bouhassira D, Cruccu G, Hansson PT, Jensen TS, Kauppila T, Rice ASC, Smith BH, Treede R-D, Baron R. Assessment of neuropathic pain in primary care. Am J Med. 2009;122(10, Suppl):S13–S21. [DOI] [PubMed] [Google Scholar]
- 681.Boulton AJM, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15(6):508–514. [DOI] [PubMed] [Google Scholar]
- 682.American Diabetes Association American Academy of Neurology Consensus statement: report and recommendations of the San Antonio conference on diabetic neuropathy. Diabetes Care. 1988;11(7):592–597. [DOI] [PubMed] [Google Scholar]
- 683.Diabetic polyneuropathy in controlled clinical trials: Consensus Report of the Peripheral Nerve Society. Ann Neurol. 1995;38(3):478–482. [DOI] [PubMed] [Google Scholar]
- 684.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM; Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group . Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH; DCCT/EDIC Research Group . Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC). Circulation. 2009;119(22):2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. [DOI] [PubMed] [Google Scholar]
- 687.Miyauchi Y, Shikama H, Takasu T, Okamiya H, Umeda M, Hirasaki E, Ohhata I, Nakayama H, Nakagawa S. Slowing of peripheral motor nerve conduction was ameliorated by aminoguanidine in streptozocin-induced diabetic rats. Eur J Endocrinol. 1996;134(4):467–473. [DOI] [PubMed] [Google Scholar]
- 688.Haupt E, Ledermann H, Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy--a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther. 2005;43(2):71–77. [DOI] [PubMed] [Google Scholar]
- 689.Stracke H, Lindemann A, Federlin K. A benfotiamine-vitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes. 1996;104(4):311–316. [DOI] [PubMed] [Google Scholar]
- 690.Fonseca VA, Lavery LA, Thethi TK, Daoud Y, DeSouza C, Ovalle F, Denham DS, Bottiglieri T, Sheehan P, Rosenstock J. Metanx in type 2 diabetes with peripheral neuropathy: a randomized trial. Am J Med. 2013;126(2):141–149. [DOI] [PubMed] [Google Scholar]
- 691.Ting RZ-W, Szeto CC, Chan MH, Ma KK, Chow KM. Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch Intern Med. 2006;166(18):1975–1979. [DOI] [PubMed] [Google Scholar]
- 692.Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, Satterfield S, Kritchevsky SB, Schwartz AV, Vinik AI, Cauley JA, Simonsick EM, Newman AB, Strotmeyer ES; Health, Aging and Body Composition Study . Sensory and motor peripheral nerve function and incident mobility disability [published correction appears in J Am Geriatr Soc. 2015;63(3):625]. J Am Geriatr Soc. 2014;62(12):2273–2279. 10.1111/jgs.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Leishear K, Boudreau R, Studenski S, Ferrucci L, Rosano C, de Rekeneire N, Houston D, Kritchevsky S, Schwartz A, Vinik A, Hogervorst E, Yaffe K, Harris T, Newman A, Strotmeyer E. Vitamin B12 and neurological function: is there a threshold level? (P02.059). Neurology 2012;78:P02.059–P002.059. [Google Scholar]
- 694.Greene DA, Arezzo JC, Brown MB; Zenarestat Study Group . Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Neurology. 1999;53(3):580–591. [DOI] [PubMed] [Google Scholar]
- 695.Hotta N, Toyota T, Matsuoka K, Shigeta Y, Kikkawa R, Kaneko T, Takahashi A, Sugimura K, Koike Y, Ishii J, Sakamoto N; SNK-860 Diabetic Neuropathy Study Group . Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001;24(10):1776–1782. [DOI] [PubMed] [Google Scholar]
- 696.Bril V, Buchanan RA. Long-term effects of ranirestat (AS-3201) on peripheral nerve function in patients with diabetic sensorimotor polyneuropathy. Diabetes Care. 2006;29(1):68–72. [DOI] [PubMed] [Google Scholar]
- 697.Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, Toyota T, Nakashima M, Yoshimura I, Sakamoto N, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care. 2006;29(7):1538–1544. [DOI] [PubMed] [Google Scholar]
- 698.Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, Low PA, Nehrdich D, Novosadova M, O’Brien PC, Reljanovic M, Samigullin R, Schuette K, Strokov I, Tritschler HJ, Wessel K, Yakhno N, Ziegler D; SYDNEY Trial Study Group . The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial [published correction appears in Diabetes Care. 2003;26(7):2227]. Diabetes Care. 2003;26(3):770–776. [DOI] [PubMed] [Google Scholar]
- 699.Ruhnau KJ, Meissner HP, Finn JR, Reljanovic M, Lobisch M, Schütte K, Nehrdich D, Tritschler HJ, Mehnert H, Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet Med. 1999;16(12):1040–1043. [DOI] [PubMed] [Google Scholar]
- 700.Ziegler D, Hanefeld M, Ruhnau KJ. Meissner HP, Lobisch M, Schütte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid: a 3-week multicenter randomized controlled trial (ALADIN Study). Diabetologia. 1995;38:1425–1433. [DOI] [PubMed] [Google Scholar]
- 701.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21(2):114–121. [DOI] [PubMed] [Google Scholar]
- 702.Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365–2370. [DOI] [PubMed] [Google Scholar]
- 703.Ziegler D, Low PA, Litchy WJ, Boulton AJM, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schütte K, Dyck PJ. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Papanas N, Maltezos E. α-Lipoic acid, diabetic neuropathy, and Nathan’s prophecy. Angiology. 2012;63(2):81–83. [DOI] [PubMed] [Google Scholar]
- 705.Vinik AI, Bril V, Litchy WJ, Price KL, Bastyr EJ III; MBBQ Study Group . Sural sensory action potential identifies diabetic peripheral neuropathy responders to therapy. Muscle Nerve. 2005;32(5):619–625. [DOI] [PubMed] [Google Scholar]
- 706.Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4(4):271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154(2):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Granberg V, Ejskjaer N, Peakman M, Sundkvist G. Autoantibodies to autonomic nerves associated with cardiac and peripheral autonomic neuropathy. Diabetes Care. 2005;28(8):1959–1964. [DOI] [PubMed] [Google Scholar]