Abstract
Context:
The pregnancy-associated plasma protein A2 (PAPP-A2) cleaves insulinlike growth factor binding proteins 3 and 5, releasing free insulinlike growth factor 1 (IGF-1). Homozygous mutations in PAPP-A2 result in growth failure with elevated total but low free IGF-1.
Objective:
To determine the 24-hour pharmacokinetic (PK) profile of free and total IGF-1 after a dose of recombinant human insulinlike growth factor 1 (rhIGF-1). We describe the growth response and effects on glucose metabolism and bone mineral density (BMD) after 1 year of rhIGF-1 therapy.
Design and Patients:
Three affected siblings, their heterozygous parents, and two healthy controls participated. The subjects received a dose of rhIGF-1, followed by serial blood samples collected over 24 hours. The two younger siblings were started on rhIGF-1 treatment. An oral glucose tolerance test and dual-energy X-ray absorptiometry scans were obtained at baseline and after 1 year of treatment.
Results:
Subcutaneous administration of rhIGF-1 increased the concentration of free and total IGF-1 in patients with PAPP-A2 deficiency. The PK profile was comparable in all participants. At baseline, all three subjects demonstrated insulin resistance and below-average BMD. Treatment with rhIGF-1 is ongoing in the youngest patient but was discontinued in his brother because of the development of pseudotumor cerebri. The treated patient had an increase in height velocity from 3.0 to 6.2 cm/y, resolution of insulin resistance, and an increase in total body BMD.
Conclusions:
rhIGF-1 at standard dosages resulted in similar PK characteristics in patients with PAPP-A2 deficiency, heterozygous relatives, and healthy controls. The youngest affected patient experienced a modest growth response to therapy with rhIGF-1, as well as beneficial effects on glucose metabolism and bone mass.
Homozygous mutations in PAPP-A2 result in a syndrome of significant growth failure. rhIGF-I therapy results in improved growth, glucose metabolism, and bone mass.
The pregnancy-associated plasma protein A2 (PAPP-A2) is a metalloproteinase that specifically cleaves insulinlike growth factor binding protein 3 (IGFBP-3) and 5 (IGFBP-5), thereby freeing insulinlike growth factor 1 (IGF-1) from its ternary complex, allowing it to bind its receptor and activate signaling cascades that ultimately promote growth (1–4). Our group recently described five patients from two unrelated families with homozygous loss-of-function mutations in PAPPA2 (p.Ala1033Val and p.D643fs25*) resulting in a syndrome of progressive growth failure, with markedly elevated serum concentrations of total IGF-1, IGFBP-3, IGFBP-5, and acid labile subunit (ALS) but decreased concentrations of free IGF-1 (5).
In addition to growth failure, patients with PAPP-A2 deficiency exhibit discrete skeletal features including thin, long bones, a small chin, delayed dental eruption, and low bone mineral density (BMD) at the lumbar spine. Although glucose metabolism has not been extensively studied in these patients, we previously reported mild to moderate fasting hyperinsulinemia in some affected patients (5). Furthermore, various animal models recapitulate the biochemical and clinical phenotype of patients with PAPP-A2 deficiency. In zebrafish, lack of PAPP-A2 leads to severely reduced cranial cartilages (6). Similarly, PAPP-A2 knockout mice have elevated total IGF-1 with low free IGF-1 concentrations, leading to marked postnatal growth retardation and decreased length of the mandible, skull, femur, and tailbone (7, 8).
Recombinant human insulinlike growth factor 1 (rhIGF-1) has been proposed as a potential treatment to improve growth in children with PAPP-A2 deficiency, based on the idea that administering exogenous rhIGF-1 will transiently increase the levels of free IGF-1 in affected patients. Muñoz-Calvo et al. (9) recently reported their favorable experience in treating two Spanish siblings from one of the previously described families. rhIGF-1 led to moderate increases in the patients’ growth velocity and improved their height standard deviation score (SDS) after 1 year of treatment (9). They noted improvement in insulin resistance but no significant change in BMD. Additionally, they performed a limited pharmacokinetic (PK) study looking at total IGF-1 levels and serum insulinlike growth factor bioactivity in the two affected siblings.
In the current study, we sought to expand our knowledge about the PK of rhIGF-1 in the setting of PAPP-A2 deficiency by directly assessing free IGF-1 levels and comparing them with those of the heterozygous parents and two healthy controls. Furthermore, we provide detailed descriptions of the baseline glycemic status and BMD results, as well as the response to therapy in the second family with PAPP-A2 deficiency, who presented with a more severe growth phenotype.
Subjects and Methods
Family description
The studied family is of Middle Eastern descent with three children [patient 1 (P1), patient 2 (P2), and patient 3 (P3)] known to have PAPP-A2 deficiency due to the homozygous missense mutation p.Ala1033Val in PAPPA2 (5). P1 is a 19-year-old woman who has completed growth. P2 and P3 are affected brothers aged 14 and 10 years. Their parents [mother (M) and father (D)] are heterozygous carriers of this mutation and are clinically unaffected. Of note, P1 had previously received a trial of rhIGF-1 during childhood. Therapy was discontinued shortly after initiation because of the development of headaches. No medical evaluation for these headaches was performed at that time.
The research study protocol was approved by the institutional review board of Cincinnati Children’s Hospital Medical Center, and written informed consent or assent was obtained from all participants or their parent or guardian. This study was registered at clinicaltrials.gov (NCT02636270) and was conducted under an investigator-initiated investigational new drug application from the US Food and Drug Administration (IND #128611).
PK study design
rhIGF-1 is a potential treatment option to improve growth in patients with PAPP-A2 deficiency. The theoretical mechanism of action of this intervention is that when additional free IGF-1 is administered, the concentration of IGF-1 will exceed the IGF binding protein capacity and thereby increase circulating free IGF-1 concentrations, resulting in increased growth. However, because of the absence of PAPP-A2 proteolytic activity, it is also possible that the administered rhIGF-1 will form additional ternary complexes, thus impeding the increase in the free IGF-1 concentration. To understand the PK of rhIGF-1 in the setting of PAPP-A2 deficiency, we performed a 24-hour PK study comparing the response to 120 µg/kg rhIGF-1 in patients with PAPP-A2 deficiency (P1, P2, and P2) compared with their heterozygous parents (M and D) and two healthy adult controls (Con 1 and Con 2).
All participants presented for a study visit after a 10-hour overnight fast. Baseline blood samples were collected for laboratory analysis, including IGF-1, free IGF-1, IGFBP-3, growth hormone (GH), insulin, and blood glucose. A subcutaneous (SC) injection of rhIGF-1 was administered in a standard dose of 120 µg/kg at 9 am. Breakfast was provided immediately after the administration of rhIGF-1. Blood samples for IGF-1, free IGF-1, IGFBP-3, GH, and glucose were collected at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 10, 12, and 24 hours postdose. All the participants received three meals, and they had free access to blood glucose monitoring. PK parameter estimates were generated by noncompartmental analysis in Phoenix WinNonlin version 6.4 (Certara LP, Princeton, NJ). To isolate the effect of the injected rhIGF-1 as opposed to the endogenous IGF-1, we used baseline-corrected concentrations in the analysis by subtracting the baseline concentration from the measured concentrations. This method was applied to both total and free IGF-1 analyses.
Pilot rhIGF-1 trial design
This was a prospective, open-label, pilot clinical trial of rhIGF-1 in the two affected younger brothers (P2 and P3) with growth failure due to PAPP-A2 deficiency, focusing on the ability of rhIGF-1 to stimulate longitudinal growth. P2 and P3 were started on treatment with rhIGF-1 at an SC dose of 60 µg/kg given twice daily. To avoid hypoglycemia, the doses were administered within 20 minutes of breakfast and dinner. Tolerability was determined with frequent blood glucose monitoring. After 1 week, the dosage was increased to SC 120 µg/kg twice daily, and regular blood glucose monitoring was continued for another week after the dosage increase. The patients underwent clinical, laboratory, and anthropometric evaluation at baseline and 3, 6, 9, and 12 months of treatment. Height in triplicate (centimeters) and weight (kilograms) were measured on a calibrated, wall-mounted stadiometer and electronic scale, respectively. z Scores for age and sex were calculated based on the 2000 Centers for Disease Control and Prevention National Standards. Serum levels of GH, IGF-1, IGFBP-3, ALS, free IGF-1, and PAPP-A2 were obtained at each visit. Additionally, bone age, osteocalcin, C-telopeptide, renal, and spleen ultrasounds were obtained at baseline and 12 months, and echocardiograms were obtained at 6 and 12 months.
Serum GH was measured with the Beckman Access Ultrasensitive Human GH Assay (Beckman Coulter, Chaska, MN). Serum IGF-1 and IGFBP-3 were determined by a chemiluminescent immunometric assay (IDS-iSYS; Immunodiagnostic Systems Ltd., Boldon, UK) (10, 11). ALS was measured by double antibody radioimmunoassay (Laboratory Corporation of America, Burlington, NC), and PAPP-A2 concentration was measured by enzyme-linked immunosorbent assay with commercially available kits (Ansh Laboratories, Webster, TX). Free IGF-1 for the PK study was determined by ultracentrifugation as previously described (12). Free IGF-1 for the pilot rhIGF-1 trial was measured by enzyme-linked immunosorbent assay with commercially available kits (Ansh Laboratories).
Glucose metabolism
An oral glucose tolerance test (OGTT) was performed on the three affected patients (P1, P2, and P3) at baseline, and it was repeated for P2 and P3 at 12 months. After a 10-hour overnight fast, 1.75 g glucose per kilogram of body weight (maximum 75 g) was administered, and blood samples were obtained before and at 30, 60, 90, and 120 minutes after glucose administration for measurement of plasma glucose and insulin. A hemoglobin A1c (HbA1c) level was obtained at baseline for all patients and at 12 months for P2 and P3. Glucose and insulin were measured locally with Roche COBAS 311 and 411 clinical analyzers, respectively. HbA1c was determined with a Siemens DCA Vantage Analyzer.
Bone density and body composition
Dual-energy X-ray absorptiometry (DXA) scans (Hologic Discovery) were performed at baseline on P1, P2, and P3 and at 12 months on P2 and P3 to evaluate BMD, bone mineral content, body fat, and lean body mass of the whole body minus the head, lumbar spine, total hip, and forearm. Scans were analyzed with Apex 4.0 software. The BMD results were evaluated and expressed as z scores with reference to the Bone Mineral Density in Childhood pediatric reference data (13). Age- and sex-specific z score results were corrected for body size (height-adjusted Z-score) with an online tool (https://bmdcs.nichd.nih.gov/zscore.htm).
Results
PK study
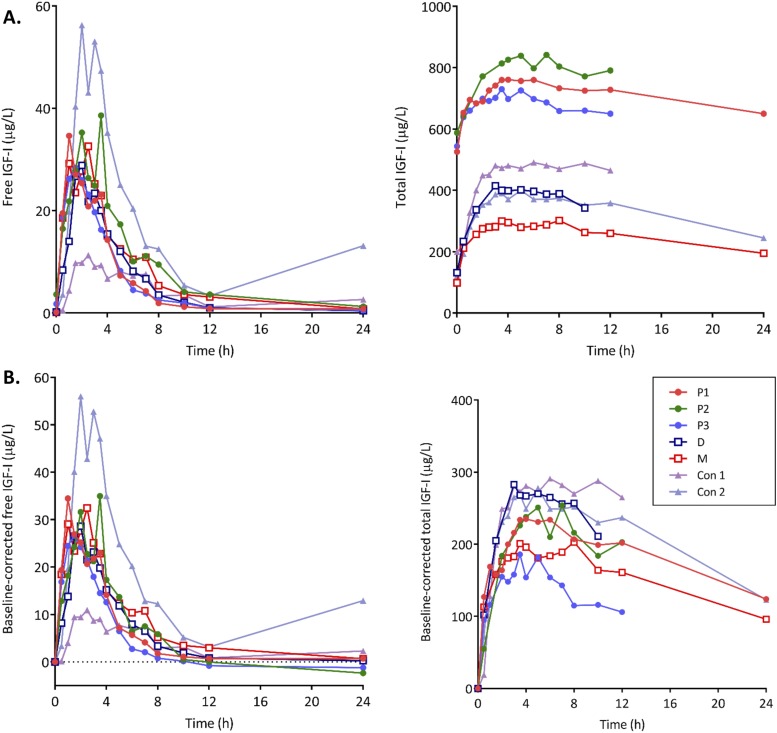
Figure 1 illustrates the total and free IGF-1 response in all subjects. rhIGF-1 at a dosage of 120 µg/kg resulted in comparable PK profile and parameter estimates in all participants. Postinjection free and total IGF-1 concentrations remained above baseline for 10 to 12 hours, with total IGF-1 remaining elevated up to 24 hours in some subjects. The IGFBP-3 concentrations remained relatively stable (Supplemental Fig. 1 (229.4KB, pdf) ). GH levels were low throughout the study, with no significant difference in patterns between the patients and controls. Table 1 summarizes the descriptive PK parameters for free and total IGF-1.
Figure 1.
Free and total IGF-1 response to an SC injection of 120 µg/kg rhIGF-1 in PAPP-A2-deficient patients (P1, P3, and P3), their heterozygous parents (M and D), and healthy controls (Con 1 and Con 2). (A) Uncorrected serum concentration–time profile. (B) Baseline-corrected serum concentration–time profile.
Table 1.
Baseline-Corrected PK Study Parameters
| P1 | P2 | P3 | D | M | Con 1 | Con 2 | |
|---|---|---|---|---|---|---|---|
| Age, y | 19 | 14 | 10 | 47 | 40 | 20 | 29 |
| Sex | Female | Male | Male | Male | Female | Male | Female |
| Weight, kg | 41 | 55 | 22 | 78 | 72 | 74 | 54 |
| Dose, µg | 5000 | 6600 | 2700 | 9400 | 8600 | 8800 | 6500 |
| Total IGF-1, µg/L | |||||||
| Cmax, µg/L | 235 | 254 | 186 | 283 | 203 | 291 | 278 |
| Tmax, h | 4 | 5 (7)a | 3.5 (5)a | 3 | 3.5 (8)a | 6 | 5 |
| AUC0–12h, µg·h/L | 2406 | 2346 | 1602 | 2742 | 2068 | 3002 | 2755 |
| Free IGF-1 | |||||||
| Cmax, µg/L | 34.5 | 35.0 | 26.6 | 28.6 | 32.4 | 10.9 | 56.0 |
| Tmax, h | 1 | 3.5 (2)b | 1.5 | 2 | 2.5 | 2.5 | 2 |
| AUC0–12h, µg·h/L | 119 | 133 | 95 | 118 | 152 | 64 | 247 |
| Half-life, h | 1.8 | 2.9 | 2.0 | 2.1 | 2.8 | 3.5 | 2.4 |
P1, P2, and P3 represent the PAPP-A2-deficient patients, D and M their heterozygous parents, and Con 1 and Con 2, two healthy unrelated controls. Estimation of half-life for total IGF-1 was not possible because of missing later data points (12 and 24 h).
Abbreviations: AUC, area under the curve; Cmax, maximum concentration; Tmax, time to reach maximum concentration.
Profiles with multiple peaks with the second peak shown in parentheses; for Tmax estimation the time of first peak was used.
Profile with multiple peaks. The 3.5-h level was unexpectedly high. The initial peak is shown in parentheses.
Pilot rhIGF-1 trial
Growth response
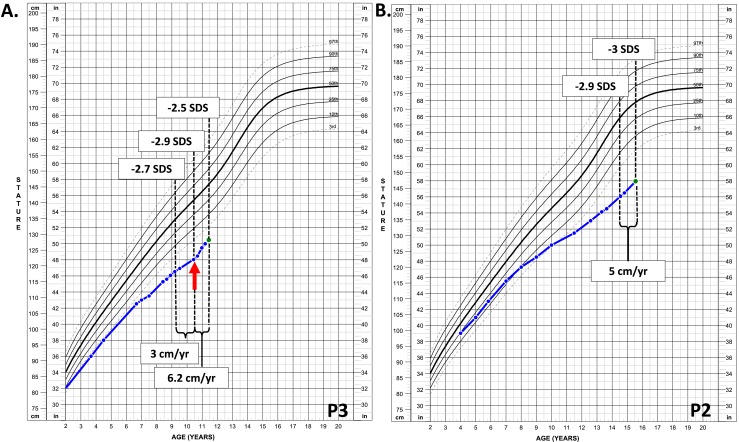
The two younger patients (P2 and P3) were started on treatment with rhIGF-1 at standard dosage as described in the Methods section. The anthropometric measurements, pubertal stage, and laboratory values are summarized in Table 2. On day 51 after initiation of therapy, P2 developed severe headache and was diagnosed with intracranial hypertension (bilateral papilledema and lumbar puncture opening pressure of 52 mm H2O), leading to therapy discontinuation. A brain magnetic resonance imaging scan was obtained and found to be unremarkable except for findings consistent with elevated intracranial pressure. His symptoms resolved soon after therapy discontinuation. At 1 year, P2’s height SDS remained stable [decreased by 0.1 standard deviation (SD)], and his height velocity did not significantly increase (4.8 cm/y vs 4.3 cm/y at baseline) despite an increase in his testicular volumes from 6 to 12 mL (Fig. 2B). P3 was temporarily suspended from therapy for 54 days because of regulatory concerns when his brother experienced the adverse event. Treatment was subsequently restarted and continued for the remainder of the year. Over this 1-year period, treatment with rhIGF-1 in P3 resulted in accelerated height velocity from 3.0 to 6.2 cm/y, which improved his height SDS from −2.9 to −2.5 in the first year of treatment (Fig. 2A). P3 remained prepubertal throughout the year. He did not experience any adverse effects related to rhIGF-1 therapy except for mild coarsening of his facial features.
Table 2.
Anthropometric and Laboratory Measurements at Baseline and 3, 6, 9, and 12 Months After rhIGF-1 Pilot Trial
| Study Visit | Age, y | Height, cm (SDS) | HV, cm/y (SDS) | BMI, kg/m2 (SDS) | Bone Age, y | Test. Vol., mL | Total IGF-1, ng/mL | Free IGF-1, µg/L | PAPP-A2, µg/L | IGFBP-3, ng/mL | ALS, mg/L | GH, ng/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P3a baseline | 10.4 | 122 (−2.9) | 3 (−2.8) | 14 (−1.5) | 9 | 3 | 546.8 | 0.79 | 0.11 | >10,000 | 18 | 0.2 |
| 3 mob | 10.7 | 123.1 (−2.8) | — | 14.7 (−1.4) | — | 3 | 524.0 | 0.35 | 0.133 | >10,000 | 22 | 0.8 |
| 6 mo | 11 | 125.7 (−2.6) | — | 15.7 (−0.7) | — | 3 | 688.9 | 1.05 | 0.176 | 6141 | 14 | 0.1 |
| 9 mo | 11.2 | 127 (−2.5) | — | 16 (−0.6) | — | 3 | 739.2 | 1.67 | 0.157 | 6492 | 11 | 0.1 |
| 12 mo | 11.4 | 128.2 (−2.5) | 6.2 (+1.2) | 16.4 (−0.5) | 10 | 3 | 709.1 | 1.17 | 0.19 | 6362 | 14 | 0.1 |
| P2 baseline | 14.5 | 142.4 (−2.9) | 4.3 (−1.6) | 27 (+1.7) | 13.5 | 6 | 573.2 | 5.59 | 0.064 | 8997 | 23 | 0.1 |
| 3 mo | 14.8 | 143.5 (−2.9) | — | 27 (+1.7) | — | — | 656.3 | 5.93 | 0.088 | >10,000 | 24 | 0.1 |
| 12 mo | 15.5 | 147.2 (−3.0) | 4.8 (+1.5) | 27 (+1.7) | 14.5 | 12 | 756.2 | 6.07 | 0.135 | 8983 | 17 | 0.1 |
Reference values for P3 (10-year-old boy): Total IGF-1, 104–430 ng/mL; IGFBP-3, 2343–5879 ng/mL; ALS, 5.6–16 mg/L.
Reference values for P2 (14-year-old boy): Total IGF-1, 150–554 ng/mL; IGFBP-3, 2599–6294 ng/mL; ALS, 5.6–16 mg/L.
Reference values for PAPP-A2: Tanner I, 0.16–2.69 µg/L; Tanner V, 0.23–0.80 µg/L.
Reference values for free IGF-1: Tanner I, 1.58–3.15 µg/L; Tanner V, 4.89–9.37 µg/L.
Abbreviations: BMI, body mass index; HV, height velocity; Test. Vol., testicular volume.
P3 was treated with rhIGF-1.
P3 was not receiving rhIGF-1 treatment at the time of the study visit.
Figure 2.
(A) Growth chart of the male patient with PAPP-A2 deficiency who was treated with rhIGF-1 at a dosage of 120 µg/kg. The red arrow points to the initiation of treatment. (B) Growth chart of the male patient with PAPP-A2 deficiency who was not treated with rhIGF-1. Therapy was discontinued because of the development of intracranial hypertension.
Glucose metabolism
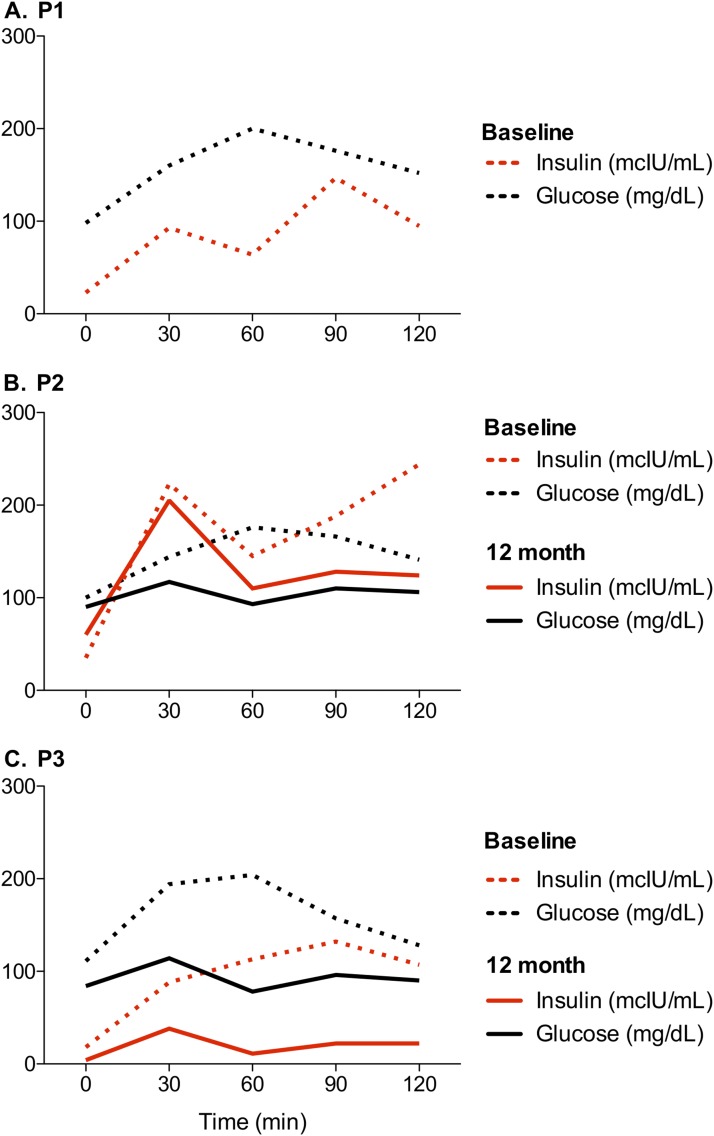
The OGTT results are presented in Fig. 3. At baseline, the three patients had marked hyperinsulinemia. P1 and P2 had impaired glucose tolerance, with 120-minute blood glucose levels of 152 and 141 mg/dL, respectively. P2 and P3 demonstrated impaired fasting glucose, with values of 100 and 111 mg/dL, respectively. The OGTT was repeated at 12 months by P2 and P3. P2, who was not treated with rhIGF-1, had persistent hyperinsulinemia, but his fasting and 120-minute blood glucose values normalized. After 12 months of treatment with rhIGF-1, P3’s OGTT was normal, with complete resolution of his hyperinsulinemia. All participants had normal HbA1c levels.
Figure 3.
OGTT results at baseline (dotted lines) and at 12 months (solid lines). P3 was treated with rhIGF-1.
Bone density and body composition
DXA and bone biomarker results are summarized in Table 3. P1 and P3 had normal BMD at baseline, with height-adjusted z scores ranging from −1 to 0. However, P2 had low bone density at the whole body minus head (BMD z score of −2.5 SD) and low-normal bone density at the forearm (−1.7 SD), hip (−1.6 SD), and lumbar spine (−1.3 SD). Of note, none of the patients had a prior history of fractures. After 12 months of treatment with rhIGF-1, P3 had marked improvement in bone mass at the whole body less head (13.6% gain) and lumbar spine (12.9% gain), with more modest improvement at the hip (6.1% gain) and forearm (8.5% gain). Again, these improvements in bone mass occurred in the absence of pubertal development. Likewise, his untreated brother (P2) also had modest improvement at all sites, which may be attributable to his pubertal progression. The concentration of C-telopeptides increased in P3 and remained relatively stable in P2. The osteocalcin concentration increased similarly in both patients. These results demonstrate an increase in bone turnover in P3, consistent with his increase in bone density acquisition during rhIGF-1 therapy. At 12 months, the body composition of both P2 and P3 improved, with increases in lean body mass and decreases in percentage body fat.
Table 3.
Bone Density and Body Composition at Baseline and 12 Months
| Baseline |
12 mo |
Change |
|||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3a | P2 | P3a | P2 | P3 | |
| DXA | |||||||
| Age, y | 19 | 14 | 10 | 15 | 11 | ||
| Height SDS | −3.8 | −2.9 | −2.9 | −3.0 | −2.5 | −1 | +0.4 |
| Whole body minus head | |||||||
| BMD, g/cm2 | 0.764 | 0.652 | 0.569 | 0.716 | 0.646 | 9.9% | 13.6% |
| z Score | −2.9 | −3.6 | −2.5 | −3.2 | −1.8 | +0.4 | +0.7 |
| HA z score | −0.5 | −2.5 | −0.7 | −2.2 | −0.1 | +0.3 | +0.6 |
| Lumbar spine | |||||||
| BMD, g/cm2 | 0.749 | 0.504 | 0.497 | 0.531 | 0.561 | 5.4% | 12.9% |
| z Score | −2.7 | −3.2 | −1.6 | −3.9 | −1.0 | −0.7 | +0.6 |
| HA z score | −1 | −1.3 | −0.1 | −1.9 | 0.3 | −0.6 | +0.4 |
| Total hip | |||||||
| BMD, g/cm2 | 0.726 | 0.567 | 0.593 | 0.600 | 0.629 | 5.9% | 6.1% |
| z Score | −1.8 | −3.8 | −2.1 | −3.5 | −2.0 | +0.3 | +0.1 |
| HA z score | −0.03 | −1.6 | −0.5 | −2.0 | −0.4 | −0.4 | +0.1 |
| Forearm | |||||||
| BMD, g/cm2 | 0.627 | 0.499 | 0.448 | 0.538 | 0.486 | 7.6% | 8.5% |
| z Score | −1.1 | −2.8 | −1.9 | −2.8 | −1.3 | 0 | +0.6 |
| HA z score | 0 | −1.7 | −0.5 | −1.8 | 0 | −0.1 | +0.5 |
| Total lean body mass, g | 23092 | 27286 | 15274 | 32964 | 18961 | 20.8% | 24.1% |
| Total fat mass, g | 17809 | 27380 | 6543 | 26969 | 7390 | −1.5% | 12.9% |
| Total body fat % | 42.3 | 49.2 | 29 | 44.2 | 27.1 | −5 | −1.9 |
| Bone markers | |||||||
| C-telopeptides, ng/mL | 726 | 1974 | 1744 | 1855 | 2262 | −6% | 29% |
| Osteocalcin, ng/mL | 28 | 101 | 86 | 138 | 123 | 38% | 43% |
Abbreviation: HA, height-adjusted.
Patient treated with rhIGF-1.
Discussion
Herein, we present a detailed clinical characterization of a family with three children with homozygous loss-of-function mutations in PAPP-A2, focusing on glucose and bone metabolism at baseline and after 1 year of therapy with rhIGF-1. Additionally, we provide a detailed analysis examining the PK of rhIGF-1 in affected patients compared with heterozygous relatives and healthy controls.
The SC administration of rhIGF-1 resulted in comparable PK parameters in PAPP-A2-deficient patients, their heterozygous relatives, and healthy adult controls. The free IGF-1 concentration remained above baseline for ~12 hours in all participants, achieving peak concentrations between 1 and 3.5 hours after the injection. Based on these observations, we determined that rhIGF-1 is a feasible approach to treat PAPP-A2 deficiency because it successfully increases the circulating free IGF-1 concentration responsible for the growth failure observed in these patients. Our data suggest that the administered rhIGF-1 is not immediately captured into a ternary complex and thus made inaccessible by the PAPP-A2 deficiency. Furthermore, twice-daily administration of rhIGF-1 is an appropriate dosing strategy for this patient population.
This study adds to the sparse evidence that short-term treatment with rhIGF-1 might improve linear growth in PAPP-A2-deficient patients, as previously reported (9). However, it does raise a note of caution given that two of the three affected members had to discontinue rhIGF-1 therapy because of side effects. As noted earlier, P2 developed intracranial hypertension [which is a known side effect of rhIGF-1 (14)] on treatment day 51, resulting in therapy discontinuation. Intriguingly, their elder affected sister (P1) was given a trial of rhIGF-1 at age 10 years and self-discontinued therapy because of headaches. Because no formal evaluation for papilledema or intracranial hypertension was performed at that time, we are unable to be certain whether she had pseudotumor cerebri. Regardless, it is possible that patients with PAPP-A2 deficiency may be at increased risk for elevated intracranial pressure, but additional studies are needed to determine this risk. This concern must be balanced with the mild increase in linear growth seen in our one successfully treated patient. Of note, no adverse effects of rhIGF-1 therapy were reported in the Spanish patients (9).
Nonetheless, the occurrence of the adverse event in P2 allowed us to simultaneously compare the treated and the untreated clinical courses of the two brothers with PAPP-A2 deficiency. In P3, who remained prepubertal during the study period, treatment with rhIGF-1 resulted in accelerated height velocity from 3.0 to 6.2 cm/y, which improved his height SDS from −2.9 to −2.5 in the first year of treatment. The growth response was similar to that observed in patients with GH insensitivity treated with rhIGF-1 (14). In contrast, P2’s height velocity did not significantly increase despite his being in midpuberty, and a pubertal growth spurt was not observed (Fig. 2). These results are consistent with those in the previous study, which also showed improved linear growth with rhIGF-1 therapy in the two Spanish patients with PAPP-A2 deficiency (9). Interestingly, we observed that P3’s IGFBP-3 and ALS concentrations decreased progressively during treatment, as did his unstimulated fasting GH levels (Table 2). Our hypothesis is that as the free IGF-1 concentration increases, the negative feedback mechanism is restored at the pituitary gland, which decreases the production of GH and all other GH-derived factors (i.e., IGFBP-3 and ALS). Consistent with this conclusion, the Spanish group demonstrated a decrease in overnight GH secretion in their patients after treatment with rhIGF-1 (9). This premise is further supported by the fact that the concentration of IGFBP-3 and ALS remained stable in the untreated patient. This decrease in binding proteins may result in a further increase in free IGF-1 levels, although it is difficult to interpret P3’s total IGF-1 and free IGF-1 levels at 6, 9, and 12 months because these were drawn during rhIGF-1 therapy (although they were drawn before the morning dose).
Interestingly, the three patients with PAPP-A2 deficiency had abnormal glucose metabolism and marked hyperinsulinemia at baseline. This finding was somewhat unexpected for P1 and P3, considering that they have normal body mass index (BMI). However, P2 is obese (BMI >95th percentile), which probably contributed to his particularly severe insulin resistance when compared with his siblings (Fig. 3). The mechanism underlying the abnormal glucose metabolism observed in these patients has not been fully determined, and our observations contrast with metabolic studies of PAPP-A2 knockout mice, which appear able to maintain normal glucose tolerance and adiposity despite the lack of PAPP-A2 (15). We speculate that the abnormal glucose metabolism may be related to the increased secretion of GH along with reduced insulinlike effects resulting from low circulating free IGF-1. Likewise, insulin resistance has also been reported in other genetic defects in the GH–IGF-1 axis, resulting in IGF-1 deficiency and GH insensitivity, including growth hormone receptor (GHR) defects (16), IGF1 gene mutations or deletions (17), and ALS deficiency (18). Additionally, it has been reported that insulin hypersecretion increases the fraction of circulating free IGF-1 by downregulating hepatic synthesis of IGFBP-1 in obese patients (19). Consequently, it is possible that PAPP-A2 deficiency also leads to insulin resistance as a compensatory mechanism in an attempt to restore the circulating concentrations of free IGF-1. These hypotheses are further supported by the observation that treatment with rhIGF-1 resulted in complete resolution of P3’s impaired fasting glucose and insulin resistance, whereas it persisted in the untreated patient (P2). These findings are consistent with the Spanish report, which showed resolution of the mild hyperinsulinemia after rhIGF-1 treatment in the two PAPP-A2-deficient patients from the other reported family (9). Furthermore, rhIGF-1 therapy also results in resolution of insulin resistance in patients with GHR defects and IGF1 gene mutations (16, 17).
In the current study we found that P1 and P3 had normal BMD, whereas P2 had low total body BMD and low-normal BMD at other sites as baseline. Clearly, treatment with rhIGF-1 induced positive changes in P3 because his whole body minus head and lumbar spine BMD increased by ~13%, which led to an increase in his height-adjusted BMD z score. His untreated brother, who had low to low-normal bone density at baseline, also had modest improvement in his BMD that resembled the accrual of bone mass during puberty (20). However, his height-adjusted BMD z score remained nearly unchanged. Similarly, the serum level of C-telopeptides, a marker of bone resorption and turnover, increased significantly in the treated patient, probably as a consequence of improved linear growth, whereas it remained stable in the untreated patient. The osteocalcin level, a marker of osteoblast function and bone formation, increased equally in both patients. Multiple studies have shown that IGF-1 signaling plays an important role in the acquisition and maintenance of bone. Sophisticated animal models demonstrated that igf1 knockout mice have decreased bone formation rates and reduced cortical thickness, whereas trabecular bone structure and density are preserved (21, 22). Recombinant IGF-1 treatment in GHR-null mice eliminated all effects on bone growth and remodeling, indicating that the main defect may relate to reduced IGF-1 levels in the absence of GHR (23). In human studies of GH deficiency, GH has been shown to primarily play a role in bone growth (i.e., mass accrual) and thus secondarily to increase cortical thickness without a major effect on BMD (22, 24). Similar findings were noted in patients with ALS deficiency, which results in low circulating IGF-1 levels (25). These patients also had smaller bones but normal size-adjusted bone mineralization (25). Notably, in patients with IGF-1 deficiency due to mutations either in the GHR gene (26) or in the IGF-1 gene itself (17), BMD has been within the normal range and has shown only modest increases with rhIGF-1 treatment, as seen in our patient.
Treatment with rhIGF-1 also resulted in improved body composition in P3. After 1 year of treatment, we noticed an increase in his BMI secondary to a marked gain in lean body mass, with a mild decrease in his fat percentage. These changes can certainly be explained by the anabolic effects of IGF-1 because it is known to induce muscle cell proliferation, differentiation, and repair (27, 28). It is possible that this increase in lean body mass led to the favorable effect on bone mass because muscle enlargement is accompanied by increased muscle strength, and the mechanical strain imposed on the bone tissue by muscle action results in periosteal expansion (22, 29–31). Likewise, his untreated brother also demonstrated an important gain in lean body mass, possibly due to a combination of healthy lifestyle changes and increased muscle mass associated with a normal pubertal progression.
Conclusions
Our results emphasize that PAPP-A2 deficiency not only results in significant growth failure but also has important physiologic consequences for bone mass, body composition, and glucose homeostasis. In the current study, we demonstrate that rhIGF-1 at standard dosages is a reasonable treatment approach to improve linear growth in patients with PAPP-A2 deficiency. Moreover, rhIGF-1 therapy also appears to have beneficial effects on bone density, body composition, and glucose metabolism. Careful monitoring for adverse events, including elevated intracranial pressure, is critical.
Acknowledgments
The authors thank Ron Rosenfeld for his helpful thoughts about the study design.
Financial Support: This work was supported by Grant K23HD07335 (to A.D.) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH). Additional support was provided by Ipsen Biopharmaceuticals Inc. and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Clinical Trial Information: ClinicalTrials.gov no. NCT02636270 (registered 10 November 2015).
Acknowledgments
Disclosure Summary: A.D. and P.B. have served as consultants for Ipsen Biopharmaceuticals Inc. A.D. and V.H. have a patent application pending involving the therapeutic use of PAPP-A2. The remaining authors have nothing to disclose. Ipsen Biopharmaceuticals Inc. provided Increlex for use in this protocol and is providing ongoing research funding for this project. Ipsen Biopharmaceuticals Inc. was not involved in the design, conduct, or analysis of data from this study, nor was it involved with the preparation of the manuscript.
Footnotes
- ALS
- acid labile subunit
- BMD
- bone mineral density
- BMI
- body mass index
- Con 1
- control subject 1
- Con 2
- control subject 2
- D
- father
- DXA
- dual-energy X-ray absorptiometry
- GH
- growth hormone
- GHR
- growth hormone receptor
- HbA1c
- hemoglobin A1c
- IGF-1
- insulinlike growth factor 1
- IGFBP-3
- insulinlike growth factor binding protein 3
- IGFBP-5
- insulinlike growth factor binding protein 5
- M
- mother
- OGTT
- oral glucose tolerance test
- P1
- patient 1
- P2
- patient 2
- P3
- patient 3
- PAPP-A2
- pregnancy-associated plasma protein A2
- PK
- pharmacokinetic
- rhIGF-1
- recombinant human insulinlike growth factor 1
- SC
- subcutaneous
- SD
- standard deviation
- SDS
- standard deviation score.
References
- 1.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxvig C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal. 2015;9(2):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor–binding protein-5 proteinase. J Biol Chem. 2001;276(24):21849–21853. [DOI] [PubMed] [Google Scholar]
- 4.Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol. 2007;21(5):1246–1257. [DOI] [PubMed] [Google Scholar]
- 5.Dauber A, Muñoz-Calvo MT, Barrios V, Domené HM, Kloverpris S, Serra-Juhé C, Desikan V, Pozo J, Muzumdar R, Martos-Moreno GA, Hawkins F, Jasper HG, Conover CA, Frystyk J, Yakar S, Hwa V, Chowen JA, Oxvig C, Rosenfeld RG, Pérez-Jurado LA, Argente J. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med. 2016;8(4):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjaer-Sorensen K, Engholm DH, Jepsen MR, Morch MG, Weyer K, Hefting LL, Skov LL, Laursen LS, Oxvig C. Papp-a2 modulates development of cranial cartilage and angiogenesis in zebrafish embryos. J Cell Sci. 2014;127(Pt 23):5027–5037. [DOI] [PubMed] [Google Scholar]
- 7.Christians JK, de Zwaan DR, Fung SH. Pregnancy associated plasma protein A2 (PAPP-A2) affects bone size and shape and contributes to natural variation in postnatal growth in mice. PLoS One. 2013;8(2):e56260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conover CA, Boldt HB, Bale LK, Clifton KB, Grell JA, Mader JR, Mason EJ, Powell DR. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology. 2011;152(7):2837–2844. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz-Calvo MT, Barrios V, Pozo J, Chowen JA, Martos-Moreno GA, Hawkins F, Dauber A, Domené HM, Yakar S, Rosenfeld RG, Pérez-Jurado LA, Oxvig C, Frystyk J, Argente J. Treatment with recombinant human insulin-like growth factor-I improves growth in patients with PAPP-A2 deficiency. J Clin Endocrinol Metab. 2016;101(11):3879–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Körner A, Obermayer-Pietsch B, Hübener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712–1721. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich N, Wolthers OD, Arafat AM, Emeny RT, Spranger J, Roswall J, Kratzsch J, Grabe HJ, Hübener C, Pfeiffer AF, Döring A, Bielohuby M, Dahlgren J, Frystyk J, Wallaschofski H, Bidlingmaier M. Age- and sex-specific reference intervals across life span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab. 2014;99(5):1675–1686. [DOI] [PubMed] [Google Scholar]
- 12.Frystyk J, Skjaerbaek C, Dinesen B, Orskov H. Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Lett. 1994;348(2):185–191. [DOI] [PubMed] [Google Scholar]
- 13.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chernausek SD, Backeljauw PF, Frane J, Kuntze J, Underwood LE, GH Insensitivity Syndrome Collaborative Group . Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. J Clin Endocrinol Metab. 2007;92(3):902–910. [DOI] [PubMed] [Google Scholar]
- 15.Christians JK, Bath AK, Amiri N. Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity. Growth Horm IGF Res. 2015;25(5):232–239. [DOI] [PubMed] [Google Scholar]
- 16.Laron Z, Avitzur Y, Klinger B. Carbohydrate metabolism in primary growth hormone resistance (Laron syndrome) before and during insulin-like growth factor-I treatment. Metabolism. 1995;44(10, Suppl 4):113–118. [DOI] [PubMed] [Google Scholar]
- 17.Woods KA, Camacho-Hübner C, Bergman RN, Barter D, Clark AJ, Savage MO. Effects of insulin-like growth factor I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J Clin Endocrinol Metab. 2000;85(4):1407–1411. [DOI] [PubMed] [Google Scholar]
- 18.Domené HM, Hwa V, Argente J, Wit JM, Camacho-Hübner C, Jasper HG, Pozo J, van Duyvenvoorde HA, Yakar S, Fofanova-Gambetti OV, Rosenfeld RG; International ALS Collaborative Group . Human acid-labile subunit deficiency: clinical, endocrine and metabolic consequences [published correction appears in Horm Res. 2010;73(1):80] Horm Res. 2009;72(3):129–141. [DOI] [PubMed] [Google Scholar]
- 19.Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44(10, suppl 4):37–44. [DOI] [PubMed] [Google Scholar]
- 20.Saggese G, Baroncelli GI, Bertelloni S. Puberty and bone development. Best Pract Res Clin Endocrinol Metab. 2002;16(1):53–64. [DOI] [PubMed] [Google Scholar]
- 21.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16(12):2320–2329. [DOI] [PubMed] [Google Scholar]
- 22.Högler W, Shaw N. Childhood growth hormone deficiency, bone density, structures and fractures: scrutinizing the evidence. Clin Endocrinol (Oxf). 2010;72(3):281–289. [DOI] [PubMed] [Google Scholar]
- 23.Sims NA, Clément-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106(9):1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyldstrup L, Conway GS, Racz K, Keller A, Chanson P, Zacharin M, Lysgaard AL, Andreasen AH, Kappelgaard AM. Growth hormone effects on cortical bone dimensions in young adults with childhood-onset growth hormone deficiency. Osteoporos Int. 2012;23(8):2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Högler W, Martin DD, Crabtree N, Nightingale P, Tomlinson J, Metherell L, Rosenfeld R, Hwa V, Rose S, Walker J, Shaw N, Barrett T, Frystyk J. IGFALS gene dosage effects on serum IGF-I and glucose metabolism, body composition, bone growth in length and width, and the pharmacokinetics of recombinant human IGF-I administration. J Clin Endocrinol Metab. 2014;99(4):E703–E712. [DOI] [PubMed] [Google Scholar]
- 26.Shaw NJ, Fraser NC, Rose S, Crabtree NJ, Boivin CM. Bone density and body composition in children with growth hormone insensitivity syndrome receiving recombinant IGF-I. Clin Endocrinol (Oxf). 2003;59(4):487–491. [DOI] [PubMed] [Google Scholar]
- 27.Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135(2):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009;20(7):349–356. [DOI] [PubMed] [Google Scholar]
- 29.Frost HM, Schönau E. The “muscle–bone unit” in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000;13(6):571–590. [DOI] [PubMed] [Google Scholar]
- 30.Schoenau E, Neu CM, Beck B, Manz F, Rauch F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle–bone unit. J Bone Miner Res. 2002;17(6):1095–1101. [DOI] [PubMed] [Google Scholar]
- 31.Högler W, Briody J, Woodhead HJ, Chan A, Cowell CT. Importance of lean mass in the interpretation of total body densitometry in children and adolescents. J Pediatr. 2003;143(1):81–88. [DOI] [PubMed] [Google Scholar]