Abstract
Context:
Postmenopausal estradiol therapy (ET) can reduce the stress response. However, it remains unclear whether such reductions can mitigate effects of stress on cognition.
Objective:
Investigate effects of ET on cortisol response to a physical stressor, cold pressor test (CPT), and whether ET attenuates stress effects on working memory.
Design:
Women completed the CPT or control condition across two sessions and subsequently completed a sentence span task.
Setting:
General community: Participants were recruited from the Early vs Late Intervention Trial with Estradiol (ELITE).
Participants:
ELITE participants (mean age = 66, standard deviation age = 6.8) in this study did not suffer from any major chronic illness or use medications known to affect the stress response or cognition.
Interventions:
Participants had received a median of randomized 4.7 years of estradiol (n = 21) or placebo (n = 21) treatment at time of participation in this study.
Main Outcome Measures:
Salivary cortisol and sentence span task performance.
Results:
Women assigned to estradiol exhibited blunted cortisol responses to CPT compared with placebo (P = 0.017) and lesser negative effects of stress on working memory (P = 0.048).
Conclusions:
We present evidence suggesting ET may protect certain types of cognition in the presence of stress. Such estrogenic protection against stress hormone exposure may prove beneficial to both cognition and the neural circuitry that maintains and propagates cognitive faculties.
An average of nearly 5 years of postmenopause estradiol treatment reduced cortisol response to the cold pressor and mitigated effects of stress on sentence span task performance.
Estradiol therapy (ET) after menopause may protect women from the deleterious effects of stress exposure (1), via the hormone’s ability to reduce hypothalamic-pituitary-adrenal (HPA) responses to mental and immune stressors (2–4). One such deleterious effect of stress is interference with prefrontal cognitive processes such as working memory (5). Because ET can reduce the HPA response to stress, the hormone may also mitigate the effects of stress on working memory by limiting the cortisol response to the stressor.
Evidence from the double-blinded, placebo (PL)-controlled, randomized Early vs Late Intervention Trial with Estradiol (ELITE; ClinicalTrials.gov no. NCT00114517) suggests that estradiol treatment, in the absence of stress threat, does not exert an effect on cognition (6). However, the ability of ET to reduce the stress response might benefit some types of cognitive processes when under stress. In particular, cortisol impairs working memory (7–9). Thus, the ability of ET to dampen the cortisol response to acute stressors may mitigate the impairing effect of stress on working memory due to the attenuated cortisol release. If this is the case, then maintenance of estradiol levels after menopause with ET may improve working memory performance under stress.
In this study, we examined how ET affected the stress response and modulated the effects of acute stress on working memory. We hypothesized that ET would decrease the bioavailable cortisol response to stress, thereby limiting the effects of stress on working memory performance. In addition, we examined whether there were differences according to whether ET was initiated within 6 years of menopause (early initiation) or beyond 10 years of menopause (late initiation). A possible critical window for hormone therapy (10, 11), related to the theory of a healthy cell bias for estradiol action (12), may result in different effects of ET when treatment is initiated years later past menopause, such as abolishment of the attenuating effect of HPA reactivity, or even potentiation of a stress response.
Materials and Methods
Participants
This study was approved by the University of Southern California Institutional Review Board. Forty-nine postmenopausal women were recruited from ELITE, where participants received either 1 mg oral micronized 17β-estradiol (E2) daily, or PL, for a median of 5 years. Participants were enrolled within 6 years of menopause (early initiation) or beyond 10 years of menopause (late initiation), creating four groups: early initiation-E2, early initiation-PL, late initiation-E2, and late initiation-PL. The primary outcome of ELITE was the rate of change in intima-media thickness of the right carotid artery. Secondary outcomes were (1) cognitive measures, including verbal memory, executive function, and global cognition, and (2) development/progression of coronary atherosclerosis measured by cardiac computed tomography (13).
ELITE participants in this study had received E2 or PL for a median of 4.7 years at time of participation (see Table 1 for exclusionary criteria). Women with an intact uterus received sequential progesterone (45 mg delivered via 4% vaginal gel), or a PL-matched gel if assigned to PL, daily for 10 days in each 30-day cycle. Women were instructed to take their medication upon awakening with brushing teeth or breakfast. Our study sample included three women with unilateral oophorectomies (one with accompanying hysterectomy) and two women with a hysterectomy without oophorectomy.
Table 1.
Exclusionary Criteria for Participation in This Study and Dropped/Withdrawn Participants
| Exclusionary Criteria | |
|---|---|
| Medical conditions | Heart disease |
| Peripheral vascular disease | |
| Diabetes | |
| Reynaud phenomenon | |
| Cryoglobulinemia | |
| Vasculitis | |
| Lupus | |
| Tingling or numbness in the hands and/or feet or any other serious chronic illness contraindicated for exposure to our stressor | |
| Medications | Beta-blockers |
| Corticosteroid-based medications | |
| Psychoactive medications or drugs (e.g., antidepressants, anxiolytics, Adderall, marijuana) | |
| Smoking status | Current smoker |
| Vision and hearing | Noncorrected vision or hearing |
| Language | Lacking fluency in English |
| Cognitive status | Score ≤ 29 on the TICS-m |
| Reason for participant attrition/withdrawal (number of participants) | Discomfort providing saliva samples (1) |
| Computer failure during session (1) | |
| Failure to complete the CPT (2) | |
| Failure to return for second session (3) | |
Sessions
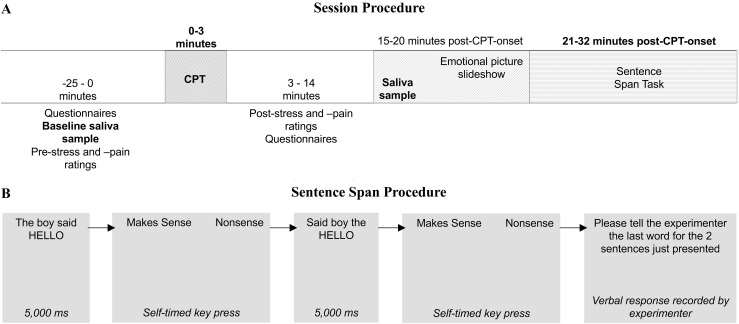
Participants completed one stress and one control session, order counterbalanced. All women provided written informed consent at the beginning of their first session. Of 49 women enrolled in the study, 42 (n = 21 E2; n = 21 PL) completed both sessions and were included in the analyses (participant attrition/withdrawal explained in Table 1). At both sessions, participants provided saliva samples, completed questionnaires, and completed a working memory task (see Fig. 1A for session timing protocol).
Figure 1.
(A) General protocol and timing of all sessions, relative to cold pressor test (CPT) onset. Order of first CPT condition (cold or warm water) counterbalanced. A subset of negative, positive, and neutral pictures from the International Affective Picture System (16) was used in the emotional picture slideshow. Emotional picture slideshow occurred between the CPT and the working memory task. It was expected that the emotional picture slideshow would not affect the magnitude of the free cortisol response to CPT, as viewing International Affective Picture System does not elicit increases in salivary cortisol levels (17). (B) Sentence span protocol and timing. Sentences were collected from various sources and have been used in similar tasks (18–20). Nonsense sentences were created by inverting the four words just before the final word of a sentence as done in Turner and Engle (21). Sentences were presented on the center of the screen with the last word in all capital letters (e.g., The boy said HELLO) for 5 seconds. Immediately after sentence presentation, participants made “Makes sense” and “Nonsense” judgments before seeing the next sentence. Participants completed 13 blocks. Blocks 1 and 2 were practice and consisted of one-sentence and two-sentence loads, respectively. At the end of each block, the participant was prompted to tell the experimenter the last word of the one or two sentences just viewed, in the order they were presented. The remainder of the task proceeded in the same fashion and included four blocks of two-sentence load, three blocks of three-sentence load, and two blocks each of four-, five-, and six-sentence loads.
Sessions for this study took place when women attended their ELITE research clinic sessions between the hours 7:00 to 14:00. Although desirable to conduct stress studies in the afternoons to control for the diurnal cortisol rhythm, it has been shown that although baseline free cortisol levels are affected by time of day, the magnitude of the cortisol response to laboratory stress does not differ in the morning or afternoon (22).
Hormone sampling
Bioavailable levels of free cortisol, estradiol, and progesterone were measured in saliva (23, 24). Participants refrained from exercise and food/drink (except water) within 1 hour, sleep within 2 hours, and caffeine and alcohol within 3 hours of their session start time.
Baseline cortisol levels follow a diurnal cycle (25), with peak baseline levels occurring 30 minutes after waking (26). To avoid seeing women during the acrophase, women were told to awaken at least 2 hours prior to their appointment start time. To verify participants had been awake for the minimum amount of time, at the beginning of each session, they were asked what time they woke up.
The baseline sample was collected via passive drool and was processed for free cortisol and sex hormone levels. The poststress sample was processed for free cortisol only and was collected using two sponge sorbettes (bvi Visitec, Wallham, MA). Sorbettes were placed in participants’ mouths, one at a time, and remained in the mouth until the sponge was adequately moist. Participants were told to not chew or suck on the sponge. Sorbettes were then placed in a tube for storage and frozen. Samples were packaged and transported frozen in dry ice to Clinical Laboratory Improvement Amendments–certified analytical laboratories (Salimetrics, LLC, State College, PA), where samples were processed using enzyme-linked immunosorbent assay. Lower limits of detection were <0.007 μg/dL for cortisol, 0.1 pg/mL for estradiol, and 5 pg/mL for progesterone. All samples were processed in duplicate. Interassay and intra-assay variations were 4.4% and 5.0% for cortisol, 3.9% and 6.9% for estradiol, and 5.0% and 9.1% for progesterone.
Stress manipulation
Participants completed the cold pressor test (CPT) or control across the two sessions. The CPT is a physical stressor that has been shown to induce cortisol secretion (27). Participants submerged their nondominant hand, up to the wrist, in ice water (0°C to 5°C immediately before hand immersion) for as long as possible up to 3 minutes. The control condition used warm water (37°C to 40°C immediately before hand immersion). Order of the CPT and control sessions was randomly counterbalanced.
Subjective measures of stress and pain before and after CPT
Participants completed pre– and post–hand-immersion pain and stress ratings using visual analog scales. Immediately before immersing their hand in water, participants rated how much pain they were currently feeling and how much stress they were currently feeling, from none to most possible. Immediately after removing their hand from the water, participants completed two additional ratings for the peak amount of stress and pain felt while their hand was in the water, again from none to most possible.
Working memory: sentence span task
The working memory task began ~21.5 minutes after stress onset. Sentences were presented one at a time on a computer screen, via PsyScope (28). Participants were told to remember the last word of each sentence. After presentation of a sentence, participants reported whether the sentence made semantic and syntactic sense. At the end of each load, participants were asked to recall the last word of each sentence in that load. “Makes sense” and “Nonsense” judgments were recorded by key press on a computer keyboard, while word recall was recorded on a paper scoring sheet by the experimenter (see Fig. 1B for sentence span timing and protocol). Loads spanned from two to six sentences.
A lenient scoring criterion was used; women were given one point for each word they remembered whether or not they recalled the words in the order presented. This task took ~11 minutes to complete. Participants saw different sentences at each session.
Statistical analysis
To examine subjective effects of CPT, we conducted separate 2 (stress: CPT vs control) × 2 (treatment: estradiol vs PL) × 2 (initiation: early vs late) mixed-model analyses of variance (ANOVAs) on post–hand-immersion minus pre–hand-immersion difference scores for stress and pain ratings. To examine cortisol response, we conducted a 2 (stress: CPT vs control) × 2 (time: baseline vs 15-minute poststress onset) × 2 (treatment: estradiol vs PL) × 2 (initiation: early vs late) mixed-model ANOVA on free cortisol response to stress. Sentence span loads were divided into low and high loads: The average proportion of words recalled in two- and three-sentence loads (low load) and in the four-, five-, and six-sentence loads (high load) were dependent variables in a 2 (stress: CPT vs control) × 2 (load: low load vs high load) × 2 (treatment: estradiol vs PL) × 2 (initiation: early vs late) mixed-model ANOVA. Additional post hoc independent and paired t tests were conducted where appropriate. Boxplots did uncover outliers; however, removal of these data did not change the overall presented results. For this reason, all data have been included in the analyses. Outliers have been depicted in the figures, and any differences in results have been described in the respective figure caption.
Results
Hormone levels and demographics
As expected, women receiving ET had significantly higher E2 levels than women receiving PL [mean difference (Mdiff) = 46.52 pg/mL; 95% confidence interval (CI), 9.37 to 83.66]. Groups did not differ in their progesterone levels [Mdiff = –12.85 pg/mL; 95% CI, –77.63 to 51.92]. ET and PL did not differ in any demographic information, verbal intelligence, negative affect, positive affect, or depression scores during the sessions (Table 2).
Table 2.
Hormone, Demographic, and Psychological Measures by Randomized Treatment and Time of Initiation Relative to Menopause
| ET (n = 21) Mean ± SD | PL (n = 21) Mean ± SD | P Value | Early (n = 21) Mean ± SD | Late (n = 21) Mean ± SD | P Value | |
|---|---|---|---|---|---|---|
| Salivary estradiol (pg/mL) | 48.9 ± 84.2 | 2.4 ± 1.6 | 0.015 | 31.0 ± 76.4 | 20.4 ± 48.4 | 0.59 |
| Salivary progesterone (pg/mL) | 41.5 ± 43.6 | 54.3 ± 140.2 | 0.69 | 34.5 ± 45.6 | 61.3 ± 138.6 | 0.41 |
| Age at menopause | 52 ± 4.3 | 51 ± 4.1 | 0.61 | 52.9 ± 3.5 | 50.2 ± 4.6 | 0.047 |
| Age at time of this study | 66 ± 7.5 | 65 ± 6.3 | 0.89 | 61.3 ± 4.2 | 70.6 ± 5.6 | <0.001 |
| Age at randomization in ELITE | 61 ± 7.5 | 60 ± 6.6 | 0.80 | 56.4 ± 4.3 | 65.9 ± 5.7 | <0.001 |
| Years of education | 17 ± 1.8 | 16 ± 2.1 | 0.10 | 17 ± 1.5 | 16 ± 2.4 | 0.10 |
| WTAR | 45 ± 5.0 | 42 ± 6.5 | 0.12 | 45 ± 5.7 | 43 ± 6.0 | 0.20 |
| Positive affect (PANAS) | ||||||
| Control session | 31.8 ± 5.3 | 36.1 ± 8.4 | 0.052 | 35.2 ± 8.4 | 32.8 ± 5.8 | 0.28 |
| CPT session | 31.9 ± 5.6 | 35.9 ± 8.4 | 0.08 | 35.0 ± 8.2 | 32.8 ± 6.3 | 0.35 |
| Negative affect (PANAS) | ||||||
| Control session | 11.0 ± 1.5 | 11.3 ± 2.4 | 0.55 | 11.1 ± 1.5 | 11.2 ± 2.4 | 0.88 |
| CPT session | 11.3 ± 1.7 | 11.2 ± 1.5 | 0.85 | 11.1 ± 1.7 | 11.3 ± 1.5 | 0.70 |
| Depression (CES-D) | ||||||
| Control session | 5.8 ± 8.6 | 7.1 ± 5.0 | 0.54 | 7.9 ± 8.8 | 5.0 ± 4.1 | 0.17 |
| CPT session | 9.1 ± 10.1 | 5.4 ± 3.6 | 0.12 | 9.1 ± 9.8 | 5.5 ± 4.2 | 0.13 |
| Subjective stress level today (1 = very low, 9 = very high) | ||||||
| Control session | 2.4 ± 1.6 | 2.9 ± 1.8 | 0.33 | 2.4 ± 1.4 | 2.9 ± 2.0 | 0.42 |
| CPT session | 2.6 ± 1.9 | 3.6 ± 2.2 | 0.14 | 3.0 ± 2.9 | 3.2 ± 2.1 | 0.77 |
Participants completed several questionnaires, including a health, demographics, and daily event form (e.g., amount of sleep the night before, time food or caffeine was last consumed, etc.); the Daily Stress Inventory (29); the Positive and Negative Affective Scale (30); the Center for Epidemiological Studies Depression Scale (31); the Wechsler Test of Adult Reading (32) as a measure of verbal intelligence; and subjective ratings for how stressed women felt at the beginning of each session.
Abbreviations: CES-D, Center for Epidemiological Studies Depression Scale; early, early-initiation women (within 6 years of menopause); late, late-initiation women (beyond 10 years since menopause); PANAS, Positive and Negative Affective Scale; SD, standard deviation; WTAR, Wechsler Test of Adult Reading.
Pre- and post-CPT stress and pain ratings
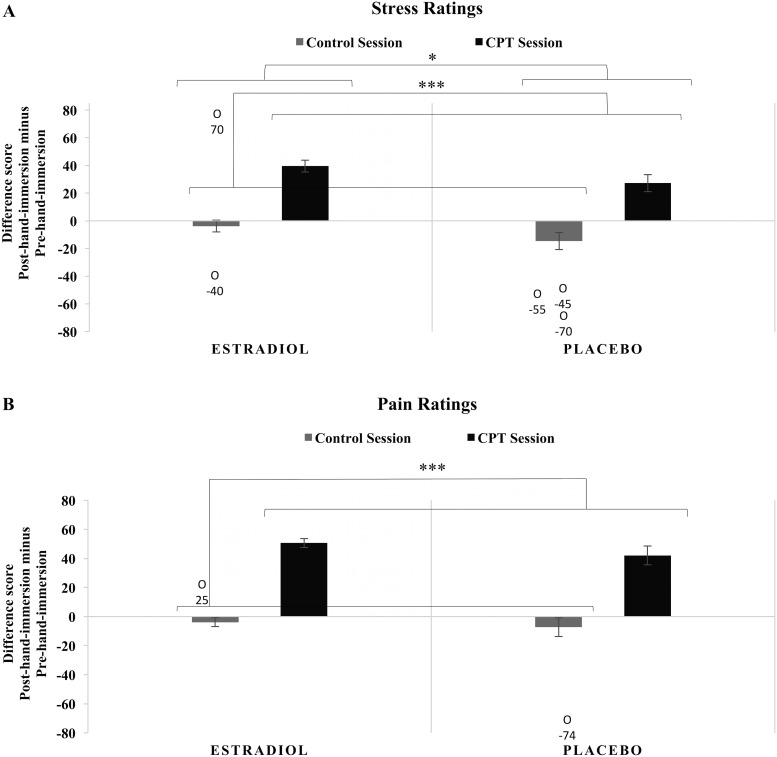
Participants found the CPT more stressful than the control task as shown by higher stress rating difference scores (post–hand-immersion rating minus pre–hand-immersion rating) in the CPT than the control condition [F(1,38) = 53.255; P < 0.001; ηp2 = 0.584; Mdiff (CPT-control) = 42.6; 95% CI, 30.8 to 54.4]. With this same measure, ET participants reported larger increases in subjective stress than PL participants [F(1,38) = 6.170; P = 0.018; ηp2 = 0.140; Mdiff = 11.6; 95% CI, 2.1 to 21.1]. Timing of initiation of randomized treatment relative to menopause had no effect on the stress ratings difference scores in the control or CPT sessions (P > 0.05; see Fig. 2A).
Figure 2.
(A) Difference scores for subjective stress ratings immediately before and immediately after hand immersion in the control and CPT sessions. Women completed visual analog scales for subjective stress ratings immediately before and immediately after immersing their hand in water in both sessions. Difference scores were calculated by subtracting pre–hand-immersion ratings from post–hand-immersion ratings, thus positive values indicate an increase in stress and negative values indicate a decrease in stress. There were main effects of stress condition and treatment, with no other main effects or interactions. Five outliers were detected for stress rating difference scores during the control session. When these outliers were removed, there was no longer a main effect of treatment. ***P < 0.001, *P < 0.05. (B) Difference scores for subjective pain ratings immediately before and immediately after CPT exposure in the control and CPT sessions. Women completed visual analog scales for subjective pain ratings immediately before and immediately after immersing their hand in water in both sessions. Difference scores were calculated in the same manner as stress difference scores. There was a main effect of stress condition, but no other main effects or interactions. Two outliers were detected for pain rating differences scores during the control session. Results remained the same when these outliers were removed. ***P < 0.001. O, outlier with accompanying data point.
Compared with control, CPT also led to significantly higher pain rating difference scores (post–hand-immersion rating minus pre–hand-immersion rating) [F(1,38) = 94.852; P < 0.001; ηp2 = 0.714; Mdiff (CPT-control) = 51.9; 95% CI, 41.1 to 62.6] with increases during the CPT session and decreases in the control session. Randomized treatment and time of initiation relative to menopause had no effect on the pain ratings in either the control or CPT sessions (P > 0.05; Fig. 2B).
Cortisol response to CPT
The working memory task occurred ~21 minutes after stress onset, when free cortisol levels typically begin to peak (33). Saliva was collected immediately prior to behavioral tasks. Cortisol analyses focused on this time frame to test differences in approximate peak levels.
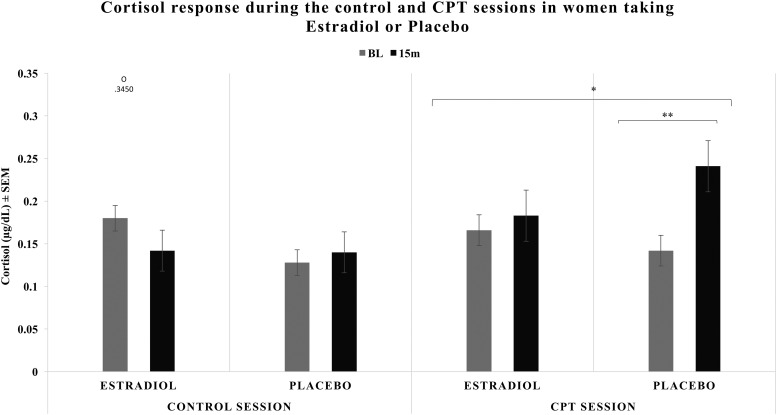
Free cortisol levels significantly increased in the CPT session, not the control session [F(1,38) = 4.358; P = 0.044; ηp2 = 0.103; Mdiff = 0.035 μg/dL; 95% CI, 0.001 to 0.070]. A time by stress interaction [F(1,38) = 11.486; P = 0.002; ηp2 = 0.232] revealed cortisol levels increased only in response to CPT. There was a significant interaction of time and treatment [F(1,38) = 6.266; P = 0.017; ηp2 = 0.142] with an increase in cortisol over time only observed in PL. Neither treatment nor initiation factors significantly affected free cortisol levels during the CPT or control sessions.
Analysis of the CPT session alone, collapsed across initiation groups, confirmed the previous patterns. Free cortisol levels increased from pre- to post-CPT [F(1,40) = 8.960; P = 0.005; ηp2 = 0.183; Mdiff = 0.058 μg/dL; 95% CI, 0.019 to 0.097]. A time-by-treatment interaction [F(1,40) = 4.368; P = 0.043; ηp2 = 0.098] was driven by PL participants experiencing significant increases in free cortisol [t(20) = 2.921; P = 0.008; Mdiff = 0.098 μg/dL; 95% CI, 0.028 to 0.169] and ET participants experiencing no change in cortisol levels [t(20) = 0.916; P > 0.05; Mdiff = 0.018 μg/dL; 95% CI, –0.022 to 0.057] (Fig. 3). The same analysis testing the control session revealed no main effects or interactions (Fig. 3).
Figure 3.
Free cortisol response during control and CPT sessions in women assigned to daily ET or PL. There was a main effect of stress condition and a significant time × stress condition interaction, with an increase over time in the CPT session, and no change in the control session. When the CPT session was analyzed alone (right panel), there was a main effect of time and a time × treatment interaction, where PL experienced significant increases in free cortisol after CPT and ET experienced no significant change in free cortisol levels after CPT. One outlier was detected for baseline cortisol during the control session. Results remained the same when this outlier was removed. *P < 0.05, **P < 0.01. 15M, 15 minutes poststress onset; BL, baseline; O, outlier with accompanying data point; SEM, standard error of the mean.
Working memory
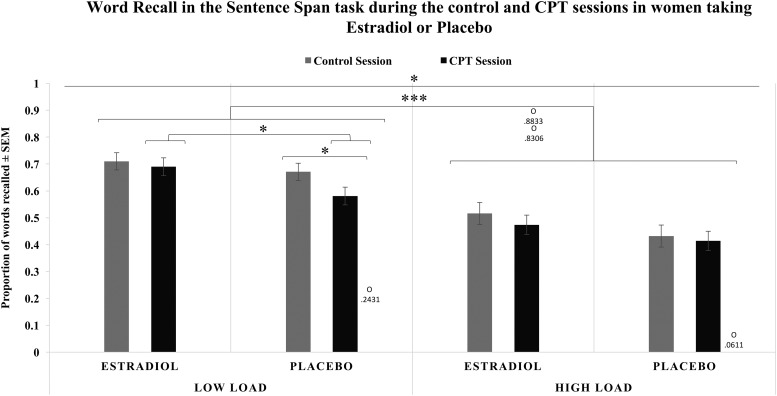
CPT decreased word recall working memory performance compared with control [F(1,38) = 7.083; P = 0.011; ηp2 = 0.157; Mdiff = –0.043; 95% CI, –0.075 to –0.010], as did increasing loads [F(1,38) = 231.648; P < 0.001; ηp2 = 0.859; Mdiff = –0.204; 95% CI, –0.231 to –0.177] (Fig. 4). An initiation by load interaction [F(1,38) = 4.981; P = 0.032; ηp2 = 0.116] revealed a pattern of larger decreases in performance as load increased in late-initiation than early-initiation groups. A three-way interaction of stress, load, and treatment [F(1,38) = 4.168; P = 0.048; ηp2 = 0.099] showed that although both ET and PL had poorer performance on high loads than low loads, ET participants did not differ in their performance after CPT or the control condition in either load, whereas PL participants showed poorer performance on low loads after CPT compared with the control condition (Fig. 4). Neither treatment nor initiation exerted main effects.
Figure 4.
Average proportion of words recalled in the sentence span task for low-load blocks (two- and three-sentence blocks) and high-load blocks (four-, five-, and six-sentence blocks) during the control and CPT sessions by women assigned to either ET or PL. There were main effects of stress condition and load, as well as a load × initiation interaction and stress condition × load × treatment interaction. All women performed worse on high-load blocks than low-load blocks regardless of session. ET and PL performed similarly on low-load blocks during the control session; however, ET performed significantly better than PL during the CPT session. ET also performed similarly in both the control and CPT sessions, whereas PL performed worse in the CPT session than the control session. ET and PL performed similarly on high-load blocks during both the control and CPT sessions. Four outliers were detected for working memory performance during the stress session: one in the low load and three in the high load. Removal of these outliers resulted in the following differences: (1) in the overall ANOVA, the load by time-of-initiation interaction is no longer significant; (2) the main effect of stress was no longer significant in the PL-only analyses, although all other interactions and follow-up analyses remained the same; and (3) the significant follow-up independent t test showing differences between ET and PL women in the low load during the stress session was no longer significant. *P < 0.05, ***P < 0.001. O, outlier with accompanying data point.
When ET participants were tested alone, decreased performance as load increased was confirmed in both CPT and control sessions [F(1,20) = 66.046; P < 0.001; ηp2 = 0.768; Mdiff = –0.203; 95% CI, –0.255 to –0.151]. However, CPT did not significantly affect performance [F(1,20) = 2.581; P > 0.05; ηp2 = 0.114; Mdiff = –0.032; 95% CI, –0.074 to 0.010]. In contrast, when PL participants were tested alone, CPT impaired performance [F(1,20) = 4.856; P = 0.039; ηp2 = 0.195; Mdiff = –0.054; 95% CI, –0.105 to –0.003], as did increases in load [F(1,20) = 233.605; P < 0.001; ηp2 = 0.921; Mdiff = –0.203; 95% CI, –0.231 to –0.176]. A stress by load interaction [F(1,20) = 5.770; P = 0.026; ηp2 = 0.224] indicated that CPT exerted its effect on the low-load blocks, with performance on low-load blocks worse after CPT [t(20)= –3.219; P = 0.004; Mdiff = –0.09; 95% CI, –0.15 to –0.03], whereas performance on high-load blocks remained the same across CPT and control sessions [t(20) = –0.595; P > 0.05; Mdiff = –0.02; 95% CI, –0.08 to 0.04].
t tests showed ET and PL groups performed similarly on low-load blocks during the control session, but that ET performed significantly better than PL participants during the CPT session [t(40) = 2.350; P = 0.024; Mdiff = 0.11; 95% CI, 0.02 to 0.20] (Fig. 4). Similar analyses comparing performance on high-load blocks revealed no differences between ET and PL participants during the control session [t(40) = 1.514; P > 0.05; Mdiff = 0.09; 95% CI, –0.03 to 0.21] or CPT [t(40) = 1.232; P > 0.05; Mdiff = 0.06; 95% CI, –0.04 to 0.17].
Discussion
Previous research among women has shown that short-term random assignment to estrogen therapy decreases cortisol responses to stressors (34, 35). Our study extends these findings, providing evidence that long-term ET after menopause can reduce the free cortisol response to a physical stressor, with similar effects regardless as to whether treatment is initiated within or beyond 6 years of menopause. Our findings also extend the cognitive findings reported from ELITE (6). In ELITE, when women were tested in the absence of threat, ET failed to influence cognitive performance regardless of when ET was initiated. Results in the control session of our study support this finding. However, our study also shows that ET can exert a beneficial effect on cognition after an episode of acute stress.
Although our primary hypotheses were supported, an unexpected finding was the larger increase in subjective stress ratings in the ET as compared with the PL condition, as reflected in the post– minus pre–hand-immersion difference scores (Fig. 2A). This finding may speak to the robust nature of the E2 effect on the free cortisol response. Despite ET women reporting higher levels of subjective stress, these women still showed significantly lower free cortisol responses to the physical stressor than did their PL counterparts.
We also tested effects of ET on working memory during stress. Both hydrocortisone administration (8) and psychological stressors (5) impair working memory performance. We hypothesized that by decreasing free cortisol responses to stress, ET should also protect working memory from stress-induced impairment. Indeed, random assignment to ET reduced the stress response and prevented stress-induced decrements in working memory performance, whereas assignment to PL did not prevent stress-induced decrements. This ET protection was limited to the low-load working memory blocks, which may have been due to a floor effect reducing our ability to see stress impairments in the high-load blocks, as women generally performed worse on the high-load blocks regardless of treatment group, initiation group, or CPT vs control session.
One interpretation for the pattern of higher pre– to post–hand-immersion stress ratings with lower cortisol responses to CPT could be that ET women were experiencing chronic stress, which has been shown to blunt cortisol responses to acute stressors (36). Chronic stress leading to blunted cortisol responses also is associated with elevated baseline cortisol levels (36), which may contribute to the blunted stress responses through a ceiling effect. However, because ET and PL women did not differ in their baseline levels and PL women reached numerically higher levels of cortisol post hand immersion in the CPT session, we do not believe our observation is driven by this mechanism. Further, ET and PL women did not differ in their perceived stress at the beginning of each session (Table 2). Not only did ET and PL women not differ in their subjective rating for stress level that day, both groups reported stress levels on the lower end at each session, with average ratings for each group at each session ranging from 2.4 to 3.6 (maximum of 9).
Instead, it is possible that corticosteroid binding globulin (CBG) can explain the blunting effect of E2 on the stress response and the subsequent prevention of stress-induced decrements in working memory. Cortisol primarily binds to CBG, leaving only 5% to 10% available to act on tissue (37). Estradiol has been shown to increase CBG in plasma (38), which could account for the lower free cortisol levels in response to stress in ET, despite reporting larger changes in subjective stress from immediately before to immediately after CPT exposure. Further, as E2-induced CBG upregulation binds more of the released cortisol, less cortisol is left available to act on tissue, including prefrontal cortical regions integral to working memory (39).
The ability of ET to reduce the HPA response to a variety of stressors also has important implications beyond attenuating acute stress-induced decrements in cognitive performance. Aging has been associated with hyperactive dysfunction of the HPA axis in response to stress (40). The dysfunction is attributed to a reduced ability of the primary negative feedback source in the brain, the hippocampus, to dampen the HPA response to acute stressors. This failure leads to prolonged glucocorticoid secretion and exposure, causing additional receptor loss in the hippocampus and further inability to effectively dampen the HPA response to future stressors. In addition to prolonged exposure of glucocorticoids leading to receptor loss, prolonged stress exposure leads to hippocampal degeneration (41), as well as prefrontal cortical degeneration (42). Importantly, due to the heavy involvement of these regions in cognition, it is possible that degradation resulting from age-related hyperactivation of the HPA axis may impair cognitive processes.
However, the ability of ET to reduce cortisol response to acute stressors may prevent this age-related hyperactivation of the HPA axis (43) by preventing bouts of prolonged HPA responses to acute stressors. Protection of the brain via ET may be twofold, as E2 can directly protect neuronal tissue from various neurotoxic insults, including glucocorticoids (44). Thus, whether via upregulation of CBG, protecting brain regions in the face of HPA dysregulation, or other mechanisms, ET-induced reductions to cortisol exposure may delay or minimize the age-related dysfunction of the axis and any potential cognitive effects related to hyperactivation-induced neuronal damage. On the other hand, the rapid and dramatic decline in E2 levels during menopause may leave women more vulnerable to the detrimental effects of stress hormone exposure on HPA, neural, and cognitive integrity.
Although our hypotheses regarding effects of treatment on stress response and stress effects on cognition were supported, we found time-of-treatment initiation relative to menopause only affected performance on high-load blocks. Because we found no interaction between E2 and time of initiation on load, it appears that the effect of initiation on load simply reflected the age difference between groups (Table 2) rather than an effect of time of ET initiation. Such failure to find any treatment by initiation interactions could indicate that a critical window for ET administration does not apply to all physiological and cognitive domains; however, the lack of interaction may also result from one of the limitations of this study. Eligibility criteria for this study were quite stringent, requiring women recruited from ELITE to be free from cardiovascular diseases and cognitive impairment and not using β-adrenergic, corticosteroid, or psychoactive (e.g., antidepressants, antianxiolytics, or Adderall) medications. Thus, despite older age in the late-initiation treatment group, these women lacked serious illness, including cardiovascular conditions associated with aging, such as prior myocardial infarction. Based on the premise of the healthy cell bias theory (12), the good health of our sample may have translated to reduced age-related brain changes and placed them in a category above the threshold of decline or damage, protecting them from the potentially deleterious effects of E2 on cognitive function observed in at-risk populations (45).
Replication with a larger sample is important to confirm these effects. It also is unclear if other executive functions, or cognitive domains not encompassing executive function, are offered the same protection under stress. A prior study examined a set of cognitive functions such as vigilance and episodic memory and found that older women (average age 65) who had 3 months of E2 supplementation as well as social stress before testing showed worse performance than those who experienced social stress without prior E2 supplementation. It is possible that performance on these tasks benefits from stress, and by reducing the stress response, E2 attenuated those benefits. However, there was no stress-free comparison group, which limits conclusions (46). We also included no measures of total (bound and unbound) cortisol levels, which would be beneficial in elucidating which mechanisms might be involved in E2-induced reductions of free cortisol levels after stress exposure.
Despite these limitations, this study suggests there are other roles of ET besides relief from menopause-related symptoms, including limiting effects of stress on working memory and perhaps aiding in maintenance of proper HPA reactivity. With the growing interest in estrogens that limit the negative effects of ET, such as selective estrogen receptor modulators (47) and the tissue-selective estrogen complex (48), ET after menopause may become a more feasible treatment of symptoms and a preventative strategy against a host of other health-related declines observed to increase after menopause, including age-related dysfunction of the HPA axis and the associated neural damage caused by such dysfunction.
Acknowledgments
We thank the Atherosclerosis Research Unit for providing space and resources to conduct experimental sessions and recruiting and scheduling participants.
Financial Support: This work was supported by the National Institute on Aging Grants R01AG-024154, R01AG-038043, and R21AG-048463.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- CBG
- corticosteroid binding globulin
- CI
- confidence interval
- CPT
- cold pressor test
- E2
- 17β-estradiol
- ELITE
- Early vs Late Intervention Trial with Estradiol
- ET
- estradiol therapy
- HPA
- hypothalamic-pituitary-adrenal
- Mdiff
- mean difference
- PL
- placebo.
References
- 1.Ycaza Herrera A, Mather M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: implications for aging women. Neurosci Biobehav Rev. 2015;55:36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. [DOI] [PubMed] [Google Scholar]
- 3.Ceresini G, Freddi M, Morganti S, Rebecchi I, Modena AB, Rinaldi M, Manca C, Amaducci A, Del Rio G, Valenti G. The effects of transdermal estradiol on the response to mental stress in postmenopausal women: a randomized trial. Am J Med. 2000;109(6):463–468. [DOI] [PubMed] [Google Scholar]
- 4.Puder JJ, Freda PU, Goland RS, Wardlaw SL. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine responses to endotoxin in women. J Clin Endocrinol Metab. 2001;86(6):2403–2408. [DOI] [PubMed] [Google Scholar]
- 5.Oei NYL, Everaerd WTAM, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006;9(3):133–141. [DOI] [PubMed] [Google Scholar]
- 6.Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young AH, Sahakian BJ, Robbins TW, Cowen PJ. The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology (Berl). 1999;145(3):260–266. [DOI] [PubMed] [Google Scholar]
- 8.Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113(3):420–430. [DOI] [PubMed] [Google Scholar]
- 9.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–237. [DOI] [PubMed] [Google Scholar]
- 10.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20(6):695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288(17):2170–2172. [DOI] [PubMed] [Google Scholar]
- 12.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052(1):57–74. [DOI] [PubMed] [Google Scholar]
- 13.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP, Group ER; ELITE Research Group . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 15.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 16.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- 17.van Stegeren AH, Wolf OT, Kindt M. Salivary alpha amylase and cortisol responses to different stress tasks: impact of sex. Int J Psychophysiol. 2008;69(1):33–40. [DOI] [PubMed] [Google Scholar]
- 18.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learn Verbal Behav. 1980;19(4):450–466. [Google Scholar]
- 19.Copeland DE, Radvansky GA. Phonological similarity in working memory. Mem Cognit. 2001;29(5):774–776. [DOI] [PubMed] [Google Scholar]
- 20.Friedman NP, Miyake A. The reading span test and its predictive power for reading comprehension ability. J Mem Lang. 2004;51(1):136–158. [Google Scholar]
- 21.Turner ML, Engle RW. Is working memory capacity task dependent? J Mem Lang. 1989;28(2):127–154. [Google Scholar]
- 22.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–992. [DOI] [PubMed] [Google Scholar]
- 23.Tunn S, Möllmann H, Barth J, Derendorf H, Krieg M. Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin Chem. 1992;38(8 Pt 1):1491–1494. [PubMed] [Google Scholar]
- 24.Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29(10):1752–1756. [PubMed] [Google Scholar]
- 25.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468–2473. [DOI] [PubMed] [Google Scholar]
- 26.Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]
- 27.Herrera AY, Nielsen SE, Mather M. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol Stress. 2016;3:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JD. PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- 29.Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment. 2002;9(1):41–55. [DOI] [PubMed] [Google Scholar]
- 30.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 33.Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–657. [DOI] [PubMed] [Google Scholar]
- 34.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84(2):606–610. [DOI] [PubMed] [Google Scholar]
- 35.Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167(6):1831–1836. [DOI] [PubMed] [Google Scholar]
- 36.Teixeira RR, Díaz MM, Santos TV, Bernardes JT, Peixoto LG, Bocanegra OL, Neto MB, Espindola FS. Chronic stress induces a hyporeactivity of the autonomic nervous system in response to acute mental stressor and impairs cognitive performance in business executives. PLoS One. 2015;10(3):e0119025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability--effects on hormone measurement and action. Nat Rev Endocrinol. 2012;8(12):717–727. [DOI] [PubMed] [Google Scholar]
- 38.Brien TG. Human corticosteroid binding globulin. Clin Endocrinol (Oxf). 1981;14(2):193–212. [DOI] [PubMed] [Google Scholar]
- 39.Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. [DOI] [PubMed] [Google Scholar]
- 40.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301. [DOI] [PubMed] [Google Scholar]
- 41.MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194(2):376–383. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nater UM, Hoppmann CA, Scott SB. Diurnal profiles of salivary cortisol and alpha-amylase change across the adult lifespan: evidence from repeated daily life assessments. Psychoneuroendocrinology. 2013;38(12):3167–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17β-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20(6):768–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA. 2000;283(8):1007–1015. [DOI] [PubMed] [Google Scholar]
- 46.Newhouse PA, Dumas J, Wilkins H, Coderre E, Sites CK, Naylor M, Benkelfat C, Young SN. Estrogen treatment impairs cognitive performance after psychosocial stress and monoamine depletion in postmenopausal women. Menopause. 2010;17(4):860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newhouse P, Albert K, Astur R, Johnson J, Naylor M, Dumas J. Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsychopharmacology. 2013;38(13):2632–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pazhekattu R, Lau AN, Adachi JD. The tissue-selective estrogen complex: a review of current evidence. Rheumatol Ther. 2015;2(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]