Abstract
Context:
Mineral and bone disorders (MBDs) might be relevant in the etiology of infection.
Objective:
To determine whether MBD biomarkers were associated with the incidence of hospitalization with infection. We also assessed the cross-sectional association between MBD biomarker levels and kidney function.
Design, Setting, Participants:
Community-based cohort study of 11,218 participants with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73m2 in the Atherosclerosis Risk in Communities study. We assessed the cross-sectional associations of five MBD markers—fibroblast growth factor 23 (FGF23), 25-hydroxyvitamin D [25(OH)D], parathyroid hormone (PTH), calcium corrected for hypoalbuminemia, and phosphorus—with eGFR from 1990 to 1992 and their longitudinal associations with incident hospitalization with infection in 1990 to 2013.
Main Outcome:
Incident hospitalization with infection.
Results:
In age-, sex-, and race-adjusted models, lower eGFRs were significantly associated with greater levels of FGF23, PTH, and corrected calcium but not 25(OH)D or phosphorus. During follow-up, 5078 hospitalizations with infection occurred. In fully adjusted Cox models, with the second quartile as the reference, the hazard ratio (HR) was significantly greater in the highest quartile of FGF23 [HR, 1.12; 95% confidence interval (CI), 1.03 to 1.21], PTH (HR, 1.09; 95% CI, 1.01 to 1.18), and corrected calcium (HR, 1.11; 95% CI, 1.03 to 1.20), and lowest quartile for 25(OH)D (HR, 1.11; 95% CI, 1.03 to 1.21). The association with phosphorus was significant only when the outcome was restricted to primary diagnosis of infection. These findings were consistent across subgroups of age, sex, race, and eGFR (<60 vs ≥60 mL/min/1.73 m2).
Conclusions:
MBD biomarkers were associated with eGFR and the subsequent risk of infection, supporting MBD involvement in the etiology of infection.
We studied a community-based cohort and found that high levels of FGF23, PTH, and calcium and low levels of 25(OH)D were each associated with incident hospitalization with infection.
Mineral and bone disorders (MBDs) are common complications of chronic kidney disease (CKD) (1), and we have recently shown an increased risk of infection in those with CKD, even at mild to moderate stages (2). Although the underlying mechanisms of an elevated risk of infection in CKD are not fully understood, MBD biomarkers might mediate this association. The results from animal studies have suggested that derangements in some MBD biomarkers, such as fibroblast growth factor 23 (FGF23) and vitamin D, disrupt aspects of the immune system, including leukocyte and innate immune function (3–5).
Previous epidemiological studies reported an increased risk of infection associated with some MBD biomarkers (6–11); however, their generalizability might have been limited by their cross-sectional design (6, 9), small sample size (11), and selected high-risk populations (7, 8, 10). Also, the association between the full spectrum of MBD biomarkers and infection risk has not been well characterized in the general population. Such knowledge will be important to understanding the pathophysiology of mineral and bone metabolism in infection and could provide preventive and therapeutic targets because MBD biomarkers are potentially modifiable.
The aims of the present study were to assess the association of the serum levels of five measured MBD biomarkers—FGF23, 25-hydroxyvitamin D [25(OH)D], parathyroid hormone (PTH), calcium, and phosphorus—with risk of incident hospitalization with infection in a well-established community-based cohort, the Atherosclerosis Risk in Communities study. Because MBDs are common complications of CKD, we also explored the cross-sectional association between the serums levels of MBD biomarkers and kidney function at baseline.
Methods
Study population
The Atherosclerosis Risk in Communities study is a prospective cohort study of 15,792 individuals aged 45 to 64 years who were enrolled from 1987 to 1989 from four US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. MBD biomarkers were measured from serum samples drawn at visit 2 (1990 to 1992), which was set as the baseline for the present study. Of 14,348 participants who attended visit 2, we excluded persons with a history of hospitalization with infection (n = 582), missing MBD biomarkers (n = 1039), missing eGFR values (n = 1061), race other than white or African-American (n = 42), no provision of informed consent for noncardiovascular disease research (n = 37), and missing covariates (n = 331). We also excluded persons with end-stage renal disease (ESRD) or an eGFR <30 mL/min/1.73 m2 (n = 38) to account for potentially influential outlying levels of MBD biomarkers in those with advanced kidney failure (12). After the exclusions, 11,218 participants were included in the present analysis. All the participants provided written informed consent, and the institutional review board at each study site approved the study (approval no. H.34.99.07.02.A1 at Johns Hopkins University).
MBD biomarkers
The blood samples collected at visit 2 were processed, shipped, and stored at −70°C in accordance with the standard protocol. Using these samples, the assays for the MBD biomarkers were performed from 2012 to 2013 at the Advanced Research and Diagnostic Laboratory and the Molecular Epidemiology and Biomarker Research Laboratory, both at the University of Minnesota (Minneapolis, MN). The serum level of FGF23 was measured using a two-site enzyme-linked immunosorbent assay (FGF23 ELISA Kit; Kainos Laboratories, Inc., Tokyo, Japan). The serum level of 25(OH)D was measured using liquid chromatography–mass spectrometry/mass spectrometry instrumentation and was calibrated to account for seasonality using the residual approach (13). The serum level of PTH was measured using a sandwich immunoassay method (Roche Diagnostics, Indianapolis, IN) on a Roche Elecsys 2010 Analyzer (Roche Diagnostics). Serum calcium and phosphorus levels were measured using a colorimetric method using a Roche Modular P Chemistry Analyzer (Roche Diagnostics). The calcium levels were corrected to account for hypoalbuminemia, defined as serum albumin <4 g/dL, using the following formula: corrected calcium = measured serum calcium in mg/dL + 0.8 × (4 − serum albumin in g/dL) (14). No correction for calcium was applied if the serum albumin levels were normal (i.e. ≥4 g/L).
Estimated glomerular filtration rate
The estimated glomerular filtration rate (eGFR) was calculated using the 2012 Chronic Kidney Disease Epidemiology Collaboration equation and accounting for serum creatinine and cystatin C (15). Serum creatinine was measured using a modified kinetic Jaffé method, calibrated to the Cleveland Clinic laboratory measurements (16) and then standardized to the isotope-dilution mass spectrometry traceable method (17). Serum cystatin C was measured using an enhanced immunonephelometric assay (Siemens Health Care Diagnostics, Newark, DE).
Infection-related hospitalization and mortality
The primary outcome of interest was incident hospitalization with infection. Hospitalizations were identified through annual telephone calls to the participants or their proxies and active surveillance of the local hospital discharge lists. Additionally, the participant information was linked to the state and national death records. All hospital discharge records were reviewed, and the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), codes were extracted. Hospitalizations with infection were identified using the same list of ICD-9-CM codes for defining infection in the US Renal Data System (18) (details provided in Supplemental Table 1 (715.3KB, docx) ). For primary analysis, we included all the ICD-9-CM codes, regardless of their diagnostic position. Participants who did not develop the primary outcome were censored when they had died, were lost to follow-up, or had been administratively censored on 31 December 2013. The secondary outcome was infection-related mortality, which was defined as subsequent death during or within 30 days after hospitalization with infection (19).
Covariates
All covariates were assessed at visit 2 when the participants were aged 45 to 64 years, except for the years of education, which was assessed at visit 1. Smoking status and alcohol consumption were determined from self-reported questionnaires. Medication use was also determined by subject self-report and examination of medication containers, which were brought to the clinic visit. Hypertension was defined as the use of an antihypertensive drug within the previous 2 weeks, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Diabetes was defined as antidiabetic drug use within the previous 2 weeks, self-reported physician diagnosis, a fasting blood glucose level of ≥126 mg/dL, or a random blood glucose level of ≥200 mg/dL. A history of cancer and chronic obstructive pulmonary disease (COPD) were determined from the relevant ICD-9-CM codes between visits 1 and 2 (ICD-9-CM codes 140 to 165, 170 to 176, 179 to 209, and 235 to 239 for cancer and 490 to 492, 494, and 496 for COPD). A history of stroke or coronary heart disease was defined by self-reported history at visit 1 or adjudicated cases between visits 1 and 2. The serum level of C-reactive protein (CRP) was measured using the immunoturbidimetric CRP-Latex (II) high-sensitivity assay (Denka Seiken, Tokyo, Japan) using a Hitachi 911 analyzer. The serum level of albumin was measured using the Coulter's bromocresol green colorimetric assay and a Coulter DACOS instrument (Coulter Diagnostics, Hialeah, FL).
Statistical analysis
The baseline characteristics were compared across quartiles of FGF23 levels using the Kruskal-Wallis test and χ2 tests, because FGF23 demonstrated the most consistent results in our analysis. The baseline characteristics were also compared across eGFR categories (30 to 59, 60 to 89, and ≥90 mL/min/1.73 m2). In addition, to assess the cross-sectional relationship between eGFR levels and MBD biomarkers as continuous variables, each MBD marker was regressed on eGFR as restricted cubic splines, with knots at 60, 75, and 90 mL/min/1.73 m2. The FGF23 and PTH levels were log-transformed to account for their right-skewed distribution.
The incidence rates (IRs) and 95% confidence intervals (CIs) were estimated using Poisson regression models. Hazard ratios (HRs) were estimated using Cox proportional hazards models. The MBD biomarker levels were categorized according to their quartiles and also modeled as continuous variables using restricted cubic splines with knots at values corresponding to the 25th, 50th, and 75th percentiles. Because the level of MBD biomarkers is regulated within a certain range (1), both low and high levels can have clinical relevance. Thus, we a priori chose the median and second quartile as the reference for the continuous analysis and categorical analysis, respectively. Multivariable models were adjusted for age, sex, race, body mass index, smoking, alcohol consumption, years of education, serum albumin, high-sensitivity CRP (hsCRP), medication use (vitamin D, calcium, steroids, and antineoplastic agent), diabetes, hypertension, a history of cancer, COPD, coronary heart disease and stroke, and eGFR as restricted cubic splines with knots at 60, 75, and 90 mL/min/1.73 m2.
Several sensitivity analyses were performed. First, we restricted the outcome to cases with a primary diagnosis of infection. Second, we censored those with incident ESRD during follow-up to account for the potential outlying levels of MBD biomarkers in advanced kidney failure (12). Incident ESRD was ascertained through the linkage to the US Renal Data System. Interaction was assessed by age (<65 vs ≥65 years), sex (male vs female), race (white vs African American), and kidney function (eGFR <60 vs ≥60 mL/min/1.73 m2) using log-likelihood tests. Two-sided P values < 0.05 were considered statistically significant. All the statistical analyses were performed using STATA, version 13 (StataCorp).
Results
Baseline characteristics
The median age was 56 years (interquartile range, 52 to 62), 24% (n = 3134) were African American, and 56% (n = 7095) were women. Of the study participants, 2.5% (n = 321) had an eGFR of <60 mL/min/1.73 m2. In the entire study population, the median value was 41.7 pg/mL (interquartile range, 33.8 to 51.4) for FGF23, 23.9 ng/mL (interquartile range, 18.5 to 29.6) for 25(OH)D, 39.2 pg/mL (interquartile range, 31.2 to 49.1) for PTH, 9.4 mg/dL (interquartile range, 9.1 to 9.6) for corrected calcium, and 3.5 mg/dL (interquartile range, 3.2 to 3.8) for phosphorus. The baseline characteristics by FGF23 quartiles are listed in Table 1. Individuals with higher FGF23 levels were more likely to be older and African American, to have a higher body mass index, to have a medical history of hypertension, diabetes, coronary heart disease, and stroke, and to have lower eGFR levels and higher levels of hsCRP. The prevalence of ever smoking tended to be lower in those with higher FGF23 levels. When the baseline characteristics were compared by eGFR category, individuals with a low eGFR (<60 mL/min/1.73 m2) were more likely to be current or former smokers and to have a medical history of hypertension, diabetes, cancer, COPD, coronary heart disease, and stroke (Supplemental Table 2 (715.3KB, docx) ).
Table 1.
Baseline Characteristics Stratified by FGF23 Category: the Atherosclerosis Risk in Communities Study, 1990 to 1992
| Characteristic | Total (n = 11,218) | FGF23 Category (pg/mL) |
P Valuea | |||
|---|---|---|---|---|---|---|
| <33.8 (n = 2802) | 33.8–41.6 (n = 2808) | 41.7–51.4 (n = 2816) | ≥51.5 (n = 2792) | |||
| Age, y, median (IQR) | 56 (52–62) | 56 (51–61) | 56 (51–61) | 57 (52–62) | 57 (52–62) | < 0.001 |
| Black race, n (%) | 2719 (24) | 665 (24) | 649 (23) | 658 (23) | 747 (27) | 0.004 |
| Female sex, n (%) | 6197 (55) | 1643 (59) | 1530 (54) | 1497 (53) | 1527 (55) | < 0.001 |
| Body mass index, kg/m2, median (IQR) | 27.1 (24.2–30.6) | 26.2 (23.4–29.4) | 27 (24.1–30.4) | 27.4 (24.5–30.8) | 27.9 (25–31.6) | < 0.001 |
| History of smoking, n (%) | 6696 (60) | 1757 (63) | 1664 (59) | 1670 (59) | 1605 (57) | < 0.001 |
| Current/former alcohol consumer, n (%) | 8711 (78) | 2206 (79) | 2209 (79) | 2187 (78) | 2109 (76) | 0.013 |
| Education, ≥12 y, n (%) | 8870 (79) | 2267 (81) | 2237 (80) | 2221 (79) | 2145 (77) | 0.002 |
| Medication use, n (%) | ||||||
| Vitamin D | 131 (1.2) | 26 (0.9) | 34 (1.2) | 35 (1.2) | 36 (1.3) | 0.586 |
| Calcium | 761 (6.8) | 195 (7.0) | 186 (6.6) | 205 (7.3) | 175 (6.3) | 0.472 |
| Steroids | 130 (1.2) | 28 (1.0) | 24 (0.9) | 35 (1.2) | 43 (1.5) | 0.086 |
| Antineoplastic agents | 66 (0.6) | 11 (0.4) | 17 (0.6) | 20 (0.7) | 18 (0.6) | 0.437 |
| Medical history, n (%) | ||||||
| Hypertension | 3241 (29) | 647 (23) | 700 (25) | 841 (30) | 1053 (38) | < 0.001 |
| Diabetes | 1179 (11) | 281 (10) | 240 (8.5) | 297 (11) | 361 (13) | < 0.001 |
| Cancer | 158 (1.4) | 40 (1.4) | 33 (1.2) | 38 (1.3) | 47 (1.7) | 0.441 |
| COPD | 48 (0.4) | 12 (0.4) | 12 (0.4) | 12 (0.4) | 12 (0.4) | 1.000 |
| Coronary heart disease | 730 (6.5) | 154 (5.5) | 169 (6.0) | 195 (6.9) | 212 (7.6) | 0.007 |
| Stroke | 184 (1.6) | 31 (1.1) | 35 (1.2) | 46 (1.6) | 72 (2.6) | < 0.001 |
| Laboratory data, median (IQR) | ||||||
| eGFR, mL/min/1.73 m2 | 97.0 (85.6–107.0) | 100.7 (90.7–110.4) | 98.6 (88.6–107.7) | 96.2 (85.1–105.7) | 91.5 (79.6–102.6) | < 0.001 |
| Serum albumin, g/dL | 4.2 (4.0–4.4) | 4.2 (4.0–4.4) | 4.2 (4.0–4.4) | 4.2 (4.0–4.4) | 4.2 (4.0–4.4) | 0.013 |
| hsCRP, mg/L | 2.2 (1.0–4.7) | 2.0 (0.9–4.2) | 2.0 (0.9–4.3) | 2.2 (1.1–4.8) | 2.5 (1.2–5.3) | < 0.001 |
| MBD biomarkers, median (IQR) | ||||||
| 25(OH)D, ng/mL | 23.9 (18.5–29.6) | 23.0 (17.7–29.2) | 23.9 (18.5–29.7) | 24.4 (18.9–29.7) | 24.5 (18.9–30) | < 0.001 |
| PTH, pg/mL | 39.2 (31.2–49.1) | 37.9 (30.2–47.1) | 38.7 (30.7–48.8) | 39.4 (31.7–49.8) | 40.9 (32.4–50.6) | < 0.001 |
| Corrected calcium, mg/dL | 9.4 (9.1–9.6) | 9.3 (9.1–9.6) | 9.3 (9.1–9.6) | 9.4 (9.1–9.6) | 9.4 (9.2–9.7) | < 0.001 |
| Serum calcium, mg/dL | 9.3 (9.1–9.6) | 9.3 (9–9.5) | 9.3 (9.1–9.6) | 9.4 (9.1–9.6) | 9.4 (9.1–9.7) | < 0.001 |
| Phosphorus, mg/dL | 3.5 (3.2–3.8) | 3.5 (3.1–3.8) | 3.5 (3.2–3.8) | 3.5 (3.2–3.9) | 3.6 (3.3–3.9) | < 0.001 |
Abbreviation: IQR, interquartile range.
P values based on χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Cross-sectional association of eGFR with MBD biomarkers
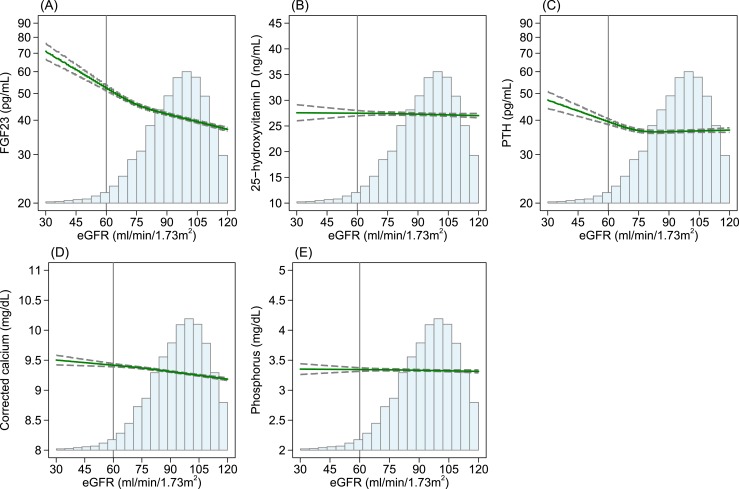
Figure 1 shows the level of each MBD marker regressed on eGFR and adjusted for age, sex, and race. A lower eGFR was associated with higher levels of FGF23, PTH, and corrected calcium. Specifically, the level of FGF23 and PTH was higher with a lower eGFR, especially at <75 mL/min/1.73 m2, with an apparently steeper slope for FGF23 than for PTH. The level of corrected calcium was slightly higher with a lower eGFR but was less evident than for FGF23 or PTH. The level of 25(OH)D and phosphorus did not differ in the observed range of eGFR ≥30 mL/min/1.73 m2.
Figure 1.
Estimated levels of MBD biomarkers regressed on eGFR in the age-, sex-, and race-adjusted restricted cubic spline model: the Atherosclerosis Risk in Communities Study, 1990 to 1992. (A) FGF23, (B) 25(OH)D, (C) PTH, (D) corrected calcium, and (E) phosphorus. Cubic splines models for eGFR included knots at 60, 75, and 90 mL/min/1.73 m2. The vertical reference line indicates 60 mL/min/1.73 m2, the solid green line indicates point estimates, and dashed lines indicate corresponding upper and lower limits for 95% CIs. The histogram represents the distribution of eGFR.
Incident hospitalization with infection across levels of MBD biomarkers
During the follow-up period (median, 19.3 years), 5078 incident hospitalizations with infection occurred (crude IR per 1000 person-years, 27.8; 95% CI, 27.1 to 28.6). The crude IRs and adjusted HRs across the quartiles of each MBD marker are listed in Table 2. The crude IR was highest with the highest quartile for FGF23, PTH, and corrected calcium. In contrast, the crude IR was highest with the lowest quartile for 25(OH)D and phosphorus.
Table 2.
IR per 1000 Person-Years and HR of Hospitalization With Infection: the Atherosclerosis Risk in Communities Study, 1990 to 2013
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| FGF23, pg/mL | ||||
| Range | <33.8 | 33.8–41.7 | 41.7–51.4 | ≥51.4 |
| Events/persons | 1206/2802 | 1191/2808 | 1296/2816 | 1385/2792 |
| Crude IR per 1000 person-years | 26.1 (24.6–27.6) | 25.4 (24.0–26.9) | 28.1 (26.6–29.6) | 32.1 (30.5–33.9) |
| Age-, sex-, and race-adjusted HR | 1.04 (0.96–1.13) | 1 (Reference) | 1.09 (1.01–1.18) | 1.24 (1.15–1.34) |
| Fully adjusted HR | 1.03 (0.95–1.11) | 1 (Reference) | 1.04 (0.96–1.13) | 1.12 (1.03–1.21) |
| 25(OH)D, ng/mL | ||||
| Range | <18.5 | 18.5–23.8 | 23.8–29.6 | ≥29.6 |
| Events/persons | 1318/2768 | 1245/2779 | 1311/2835 | 1204/2836 |
| Crude IR per 1000 person-years | 30.3 (28.7–32.0) | 27.5 (26.0–29.0) | 28.3 (26.8–29.9) | 25.4 (24.0–26.9) |
| Age-, sex-, and race-adjusted HR | 1.18 (1.09–1.28) | 1 (Reference) | 1.02 (0.94–1.10) | 0.89 (0.82–0.97) |
| Fully adjusted HR | 1.11 (1.03–1.21) | 1 (Reference) | 1.04 (0.96–1.12) | 0.93 (0.86–1.01) |
| PTH, pg/mL | ||||
| Range | <31.2 | 31.2–39.2 | 39.2–49.1 | ≥49.1 |
| Events/persons | 1240/2785 | 1218/2811 | 1265/2836 | 1355/2786 |
| Crude IR per 1000 person-years | 27.5 (26.0–29.1) | 26.0 (24.6–27.5) | 27.2 (25.7–28.7) | 30.8 (29.2–32.4) |
| Age-, sex-, and race-adjusted HR | 1.09 (1.00–1.18) | 1 (Reference) | 1.03 (0.96–1.12) | 1.15 (1.07–1.25) |
| Fully adjusted HR | 1.07 (0.99–1.16) | 1 (Reference) | 1.02 (0.94–1.10) | 1.09 (1.01–1.18) |
| Corrected calcium, mg/dL | ||||
| Range | <9.1 | 9.1–9.4 | 9.4–9.6 | ≥9.6 |
| Events/persons | 988/2281 | 1417/3209 | 989/2214 | 1684/3514 |
| Crude IR per 1000 person-years | 26.1 (24.5–27.8) | 26.9 (25.5–28.3) | 26.9 (25.3–28.7) | 30.5 (29.1–32.0) |
| Age-, sex-, and race-adjusted HR | 1.00 (0.92–1.08) | 1 (Reference) | 0.99 (0.91–1.08) | 1.11 (1.04–1.19) |
| Fully adjusted HR | 1.02 (0.94–1.11) | 1 (Reference) | 1.00 (0.92–1.09) | 1.11 (1.03–1.20) |
| Phosphorus, mg/dL | ||||
| Range | <3.2 | 3.2–3.5 | 3.5–3.8 | ≥3.8 |
| Events/persons | 1135/2392 | 1153/2585 | 1512/3456 | 1278/2785 |
| Crude IR per 1000 person-years | 29.7 (28.1–31.5) | 27.8 (26.2–29.4) | 26.4 (25.1–27.7) | 28.2 (26.7–29.7) |
| Age-, sex-, and race-adjusted HR | 1.04 (0.96–1.13) | 1 (Reference) | 0.95 (0.88–1.02) | 1.04 (0.96–1.13) |
| Fully adjusted HR | 1.02 (0.94–1.11) | 1 (Reference) | 0.98 (0.90–1.05) | 1.05 (0.97–1.14) |
Data in parentheses are interquartile ranges.
Fully adjusted models adjusted for age, sex, race, body mass index, smoking, alcohol consumption, years of education, serum albumin, and hsCRP, medication use (vitamin D, calcium, steroids, and antineoplastic agents), diabetes, hypertension, history of cancer, COPD, coronary heart disease, stroke, and eGFR. The levels of 25(OH)D were adjusted to account for seasonality. Corrected calcium (mg/dL) = serum calcium (mg/dL) + 0.8 × (4.0 − serum albumin in g/dL) if serum albumin <4.0 g/dL.
In the fully adjusted Cox regression analyses, including adjustment for eGFR, the HR was significantly greater for the highest quartile of FGF23 (HR, 1.12; 95% CI, 1.03 to 1.21), PTH (HR, 1.09; 95% CI, 1.01 to 1.18), and corrected calcium (HR, 1.11; 95% CI, 1.03 to 1.20) compared with the second quartile. The HR was also slightly greater in the lowest quartile of PTH (HR, 1.07; 95% CI, 0.99 to 1.16). For 25(OH)D, an inverse linear trend (P-for-linear-trend < 0.001) was seen, with the risk 11% greater in the lowest quartile (HR, 1.11; 95% CI, 1.03 to 1.21) compared with the second quartile.
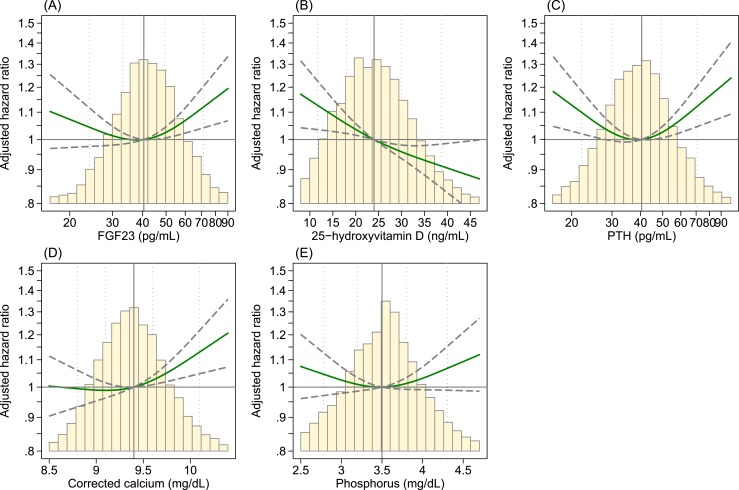
When assessed in restricted cubic spline models, the association was nonlinear for FGF23, PTH, and corrected calcium (Fig. 2). For FGF23 and corrected calcium, the risk gradient was not relevant at low levels; however, the infection risk was substantially greater at higher levels. For PTH, the risk was substantially greater at both higher and lower levels. The association was nearly linear for 25(OH)D, with a greater risk of infection at its lower levels. No significant association for phosphorus was found either at low or high levels.
Figure 2.
HR of hospitalization with infection in cubic spline model: the Atherosclerosis Risk in Communities Study, 1990 to 2013. (A) FGF23, (B), 25(OH)D, (C) PTH, (D) corrected calcium, and (E) phosphorus. The vertical reference line indicates the median; the dotted lines indicate values corresponding to the 5th, 25th, 75th, and 95th percentiles; the solid green line indicates point estimates; and the dashed lines indicate the corresponding upper and lower limits for the 95% CIs. The histograms represent the distribution of each MBD marker. Models were adjusted for age, sex, race, body mass index, smoking, alcohol consumption, years of education, serum albumin, and hsCRP, medication use (vitamin D, calcium, steroids, and antineoplastic agents), diabetes, hypertension, and history of cancer, COPD, coronary heart disease, stroke, and eGFR.
The associations were consistent in the sensitivity analyses when we censored individuals with incident ESRD during the follow-up period (Supplemental Fig. 1 (715.3KB, docx) ). When restricting the analysis to the 2321 cases with the primary diagnosis of infection (43% of all cases of hospitalization with infection), the results were generally consistent with the significant associations for high FGF23, low 25(OH)D, and low PTH. However, the associations of high PTH and high corrected calcium were attenuated to no statistical significance (Supplemental Fig. 2 (715.3KB, docx) ). In contrast, a weak, but relevant, greater risk of infection was found at higher levels of phosphorus.
Because FGF23 and 25(OH)D showed the significant association in both primary and sensitivity analyses, we assessed whether the association of FGF23 or 25(OH)D was modified by the level of the other marker. To obtain reliable estimates, the level of FGF23 and 25(OH)D was dichotomized at the median [FGF23 <41.7 vs ≥41.7 pg/mL and 25(OH)D <23.8 vs ≥23.8 ng/mL]. Multivariable Cox models revealed the overall multiplicative association, without a significant interaction (Supplemental Table 3 (715.3KB, docx) ).
In subgroup analyses, no statistically significant interaction was found by age (<65 vs ≥65 years; P-for-interaction > 0.2 for all five markers), sex (male vs female; P-for-interaction > 0.05 for all five markers), race (white vs African American; P-for-interaction > 0.4 for all five markers), or kidney function (eGFR <60 vs ≥60 mL/min/1.73 m2; P-for-interaction > 0.1 for all five markers).
Mortality risk related to hospitalization with infection
During the follow-up period, 985 subjects died during or within 30 days of hospitalization with infection (Table 3). Consistent with the primary analysis, the risk was significantly greater in the highest quartile for FGF23 and corrected calcium and in the lowest quartile for 25(OH)D. In the fully adjusted models with the second quartile as the reference, the HR in the highest quartile was 1.25 (95% CI, 1.04 to 1.50) for FGF23 and 1.20 (95% CI, 1.02 to 1.42) for corrected calcium. The HR in the lowest quartile was 1.36 (95% CI, 1.14 to 1.63) for 25(OH)D. The levels of PTH and phosphorus were not significantly associated statistically with infection-related mortality.
Table 3.
HR of Infection-Related Mortality: the Atherosclerosis Risk in Communities Study, 1990 to 2013
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| FGF23, pg/mL | ||||
| Range | <33.8 | 33.8–41.7 | 41.7–51.4 | ≥51.4 |
| Deaths/persons | 219/2802 | 204/2808 | 256/2816 | 306/2792 |
| Mortality rate per 1000 person-years | 4.0 (3.5–4.6) | 3.7 (3.2–4.3) | 4.7 (4.1–5.3) | 5.9 (5.2–6.6) |
| Age-, sex-, and race-adjusted HR | 1.12 (0.92–1.35) | 1 (Reference) | 1.21 (1.01–1.46) | 1.48 (1.24–1.77) |
| Fully adjusted HR | 1.08 (0.89–1.31) | 1 (Reference) | 1.16 (0.96–1.39) | 1.25 (1.04–1.50) |
| 25(OH)D, ng/mL | ||||
| Range | <18.5 | 18.5–23.8 | 23.8–29.6 | ≥29.6 |
| Deaths/persons | 284/2768 | 222/2779 | 243/2835 | 236/2836 |
| Mortality rate per 1000 person-years | 5.5 (4.9–6.2) | 4.1 (3.6–4.7) | 4.4 (3.9–5.0) | 4.2 (3.7–4.8) |
| Age-, sex-, and race-adjusted HR | 1.51 (1.26–1.80) | 1 (Reference) | 1.03 (0.86–1.24) | 0.96 (0.80–1.16) |
| Fully adjusted HR | 1.36 (1.14–1.63) | 1 (Reference) | 1.03 (0.86–1.24) | 0.95 (0.79–1.15) |
| PTH, pg/mL | ||||
| Range | <31.2 | 31.2–39.2 | 39.2–49.1 | ≥49.1 |
| Deaths/persons | 245/2785 | 234/2811 | 256/2836 | 250/2786 |
| Mortality rate per 1000 person-years | 4.6 (4.1–5.2) | 4.2 (3.7–4.8) | 4.7 (4.1–5.3) | 4.7 (4.1–5.3) |
| Age-, sex-, and race-adjusted HR | 1.15 (0.96–1.38) | 1 (Reference) | 1.09 (0.91–1.30) | 1.03 (0.86–1.24) |
| Fully adjusted HR | 1.12 (0.93–1.34) | 1 (Reference) | 1.08 (0.90–1.29) | 0.97 (0.81–1.16) |
| Corrected calcium (mg/dL) | ||||
| Range | <9.1 | 9.1–9.4 | 9.4–9.6 | ≥9.6 |
| Deaths/persons | 172/2281 | 268/3209 | 189/2214 | 356/3514 |
| Mortality rate per 1000 person-years | 3.9 (3.3–4.5) | 4.3 (3.8–4.9) | 4.4 (3.8–5.1) | 5.3 (4.8–5.9) |
| Age-, sex-, and race-adjusted HR | 0.94 (0.77–1.14) | 1 (Reference) | 1.01 (0.84–1.22) | 1.21 (1.03–1.42) |
| Fully adjusted HR | 0.96 (0.80–1.17) | 1 (Reference) | 1.04 (0.86–1.25) | 1.20 (1.02–1.42) |
| Phosphorus, mg/dL | ||||
| Range | <3.2 | 3.2–3.5 | 3.5–3.8 | ≥3.8 |
| Deaths/persons | 218/2392 | 240/2585 | 281/3456 | 246/2785 |
| Mortality rate per 1000 person-years | 4.8 (4.2–5.4) | 4.9 (4.3–5.5) | 4.2 (3.7–4.7) | 4.6 (4.0–5.2) |
| Age-, sex-, and race-adjusted HR | 0.90 (0.75–1.08) | 1 (Reference) | 0.90 (0.76–1.07) | 1.04 (0.87–1.26) |
| Fully adjusted HR | 0.86 (0.72–1.04) | 1 (Reference) | 0.94 (0.79–1.12) | 1.04 (0.86–1.25) |
Analysis was based on 985 infection-related deaths, defined as death occurring during or within 30 days of hospitalization with infection. Fully adjusted models adjusted for age, sex, race, body mass index, smoking, alcohol consumption, years of education, serum albumin, hsCRP, medication use (vitamin D, calcium, steroids, and antineoplastic agents), diabetes, hypertension, history of cancer, COPD, coronary heart disease, stroke, and eGFR. Levels of 25(OH)D were adjusted to account for seasonality. Corrected calcium (mg/dL) = serum calcium (mg/dL) + 0.8 × (4.0 − serum albumin in g/dL) if serum albumin <4.0 g/dL.
Discussion
In this community cohort of middle-age adults, lower eGFR was associated with greater levels of FGF23, PTH, and corrected calcium but not 25(OH)D and phosphorus. In the longitudinal analysis, elevated levels of FGF23, PTH, and corrected calcium and decreased levels of 25(OH)D were each associated with an increased risk of hospitalization with infection. These findings were generally consistent across subgroups defined by age, sex, race, and kidney function and in sensitivity analyses when restricting to primary diagnosis of infection or accounting for incident ESRD. Finally, similar associations were observed for infection-related mortality.
Our finding of the positive association between FGF23 and the risk of infection is consistent with a few previous reports of patients requiring dialysis (8) or of older adults (10). Our study extended this association to the middle-age general population. Importantly, the association of FGF23 with the risk of infection was consistent for infection-related mortality in both sensitivity analyses and subgroup analyses. Although the underlying mechanisms are not fully understood, experimental studies have revealed that FGF23 might have a role in immune function, including the release of inflammatory cytokines (20) and neutrophil chemotaxis (21). In addition, FGF23 has been reported to directly impair host defenses by interfering with chemokine signaling and integrin activation on polymorphonuclear leukocytes (22).
The association of low 25(OH)D levels with an increased risk of infection was consistent with a few previous reports assessing the risk of respiratory infections (6, 9, 11). The unique aspects of our study included the prospective design, treating 25(OH)D as a continuous variable with splines, and a large sample, which allowed for subgroup analysis. Activated vitamin D [1,25(OH)2D] is known to interact with the innate and adaptive immunity, including macrophage maturation (23) and lymphocyte proliferation and differentiation (24). Furthermore, decreased 1,25(OH)2D was shown to impair the activation of neutrophils and monocytes through its act on cathelicidins in animal models (25). Conversion from 25(OH)D to 1,25(OH)2D is impaired in those with reduced kidney function owing to the reduced 1𝛼-hydroxylase activity (26, 27). Thus, although the association of eGFR with 25(OH)D was not relevant, those with a low eGFR might still have an increased susceptibility to infection owing to the low levels of 1,25(OH)2D.
To the best of our knowledge, this is the first epidemiological study showing significant associations of PTH and corrected calcium with the risk of infection. In most of our analyses, the association of PTH was U-shaped. Although PTH and calcium are known to interact with the immune system, the underlying mechanisms of increased susceptibility to infection are not well understood. PTH stimulates the production of CD8+ T cells in mice (28); however, human studies examining the effect of PTH on T cells were inconsistent (29). For calcium, intracellular calcium has essential roles in signaling in immune cells (30); however, the role of extracellular calcium is not well understood.
Finally, in the present study, the level of phosphorus did not differ across eGFR levels, and its association with infection risk was only significant when the outcome was restricted to persons with a primary diagnosis of infection. These overall weak associations might be related to our inclusion criterion of an eGFR ≥30 mL/min/1.73 m2. Studies of dialysis populations reported that elevated levels of phosphorus (e.g., >5.0 mg/dL) were associated with diminished lymphocyte counts (3) and an increased risk of infection (7). The lack of an association in the present, generally healthy, population suggests that phosphorus might be relevant in infection etiology, primarily in populations with advanced CKD.
The results of the present study have several clinical and research implications. The cross-sectional association of MBD biomarker levels with eGFR and their longitudinal independent associations with the risk of infection support our hypothesis of the involvement of mineral and bone metabolism in infection etiology in CKD. In addition, the modest size (10% to 20% increased HRs) of associations might indicate that the MBD biomarker levels can only explain a part of the increased risk of infection. In contrast, unlike a reduced eGFR, MBD biomarkers are potentially modifiable through diet (31) and medication (1) and, thus, could become therapeutic targets. A recent meta-analysis suggested the beneficial effect of vitamin D supplementation on preventing acute respiratory tract infection (32). Nevertheless, whether interventions targeting MBD biomarkers could reduce the risk of infection remains largely unanswered. Therefore, future studies should be directed toward the causality, including a better understanding of the pathophysiological role of MBD biomarkers on infection, replication studies in populations with advanced CKD, and clinical trials to assess the efficacy of interventions targeting MBD biomarkers.
Several limitations should be acknowledged. First, the levels of MBD biomarkers were measured using stored samples and assessed only one time at baseline, which could have resulted in some measurement error (33), leading to dilution bias. Second, we focused on hospitalizations with infection and did not capture less severe cases treated in the outpatient setting. However, hospitalizations with infection are clinically relevant, with the most costs and morbidities (34). Third, although a previous study reported the positive predictive value of ICD-9-CM infection codes was ≥80% (35), our outcome ascertainment might have led to misclassification. Fourth, our participants were white and African-American subjects; thus, confirmatory studies of other racial groups could be needed. Fifth, the nature of our observational study could not exclude the possibility of residual confounding, although we accounted for many key potential confounders, including kidney function (eGFR) (2) and inflammatory biomarkers [serum albumin (36) and hsCRP (37)]. Finally, although we primarily focused on the association of each MBD marker with the risk of infection, the MBD abnormalities could correlate with each other with complex complementary feedback. Thus, future studies are needed to explore the pathophysiological pathways in the association between MBD biomarkers and the risk of infection.
In conclusion, among community-based middle-age individuals, a lower eGFR was associated with higher levels of FGF23, PTH, and corrected calcium. On longitudinal analysis, elevated levels of FGF23, PTH, and corrected calcium and lower 25(OH)D levels were each associated with an increased risk of hospitalization with infection. These findings support the involvement of mineral and bone metabolism in the etiology of infection.
Acknowledgments
We thank the staff and participants of the Atherosclerosis Risk in Communities Study for their important contributions. We thank Dr. Kevin Abbott for supplying data of the United States Renal System (USRDS).
Financial Support: J.I. was supported by the National Heart, Lung, and Blood Institute (Grant T32HL007024). C.M.R. is supported by a Mentored Research Scientist Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases (Grant K01 DK107782). The Atherosclerosis Risk in Communities Study is performed as a collaborative study supported by National Heart, Lung, and Blood Institute (Grants HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C,HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was supported in part by the National Heart, Lung, and Blood Institute (Grant R01HL103706; principal investigator, P.L.L.). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
Disclosure Summary: C.P.K. received honoraria from Abbott, Abbvie, Amgen, Bayer, Keryx, and Sanofi-aventis. The remaining authors have nothing to disclose.
Footnotes
- 1,25(OH)2D
- 1,25-dihydroxy vitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- PTH
- parathyroid hormone
- CI
- confidence interval
- CKD
- chronic kidney disease
- COPD
- chronic obstructive pulmonary disease
- CRP
- C-reactive protein
- eGFR
- estimated glomerular filtration rate
- ESRD
- end-stage renal disease
- FGF23
- fibroblast growth factor 23
- HR
- hazard ratio
- hsCRP
- high-sensitivity CRP
- ICD-9-CM
- International Classification of Diseases, Ninth Revision, Clinical Modification
- IR
- incidence rate
- MBD
- mineral and bone disorder.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–S130. [DOI] [PubMed]
- 2.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K. CKD and risk for hospitalization with infection: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2016;69(6):752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70(2):371–376. [DOI] [PubMed] [Google Scholar]
- 4.Sterling KA, Eftekhari P, Girndt M, Kimmel PL, Raj DS. The immunoregulatory function of vitamin D: implications in chronic kidney disease. Nat Rev Nephrol. 2012;8(7):403–412. [DOI] [PubMed] [Google Scholar]
- 5.Bacchetta J, Salusky IB, Hewison M. Beyond mineral metabolism, is there an interplay between FGF23 and vitamin D in innate immunity? Pediatr Nephrol. 2013;28(4):577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginde AA, Mansbach JM, Camargo CA Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plantinga LC, Fink NE, Melamed ML, Briggs WA, Powe NR, Jaar BG. Serum phosphate levels and risk of infection in incident dialysis patients. Clin J Am Soc Nephrol. 2008;3(5):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol. 2016;27(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempker JA, Magee MJ, Cegielski JP, Martin GS. Associations between vitamin D level and hospitalizations with and without an infection in a national cohort of Medicare beneficiaries. Am J Epidemiol. 2016;183(10):920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak KL, Bartz TM, Dalrymple L, de Boer IH, Kestenbaum B, Shlipak MG, Garimella PS, Ix JH, Chonchol M. Fibroblast growth factor 23 and the risk of infection-related hospitalization in older adults. J Am Soc Nephrol. 2016; 28(4):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutsey PL, Eckfeldt JH, Ogagarue ER, Folsom AR, Michos ED, Gross M. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC). Clin Chim Acta. 2015;442:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. BMJ. 1973;4(5893):643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. [DOI] [PubMed] [Google Scholar]
- 18.United States Renal Data System. ESRD analytical methods. Available at: http://www.usrds.org/2014/view/v2_00_appx.aspx. Accessed 14 Feb 2016.
- 19.Metersky ML, Tate JP, Fine MJ, Petrillo MK, Meehan TP. Temporal trends in outcomes of older patients with pneumonia. Arch Intern Med. 2000;160(22):3385–3391. [DOI] [PubMed] [Google Scholar]
- 20.Masuda Y, Ohta H, Morita Y, Nakayama Y, Miyake A, Itoh N, Konishi M. Expression of Fgf23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol Pharm Bull. 2015;38(5):687–693. [DOI] [PubMed] [Google Scholar]
- 21.Haddad LE, Khzam LB, Hajjar F, Merhi Y, Sirois MG. Characterization of FGF receptor expression in human neutrophils and their contribution to chemotaxis. Am J Physiol Cell Physiol. 2011;301(5):C1036–C1045. [DOI] [PubMed] [Google Scholar]
- 22.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstädt HJ, Meersch M, Unruh M, Zarbock A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126(3):962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe E, Miyaura C, Tanaka H, Shiina Y, Kuribayashi T, Suda S, Nishii Y, DeLuca HF, Suda T. 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci USA. 1983;80(18):5583–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76(3):315–325. [DOI] [PubMed] [Google Scholar]
- 25.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. [DOI] [PubMed] [Google Scholar]
- 26.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–38. [DOI] [PubMed] [Google Scholar]
- 27.Nakano C, Hamano T, Fujii N, Matsui I, Tomida K, Mikami S, Inoue K, Obi Y, Okada N, Tsubakihara Y, Isaka Y, Rakugi H. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7(5):810–819. [DOI] [PubMed] [Google Scholar]
- 28.Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, Gilbert L, Nanes MS, Zayzafoon M, Guldberg R, Lamar DL, Singer MA, Lane TF, Kronenberg HM, Weitzmann MN, Pacifici R. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10(3):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geara AS, Castellanos MR, Bassil C, Schuller-Levis G, Park E, Smith M, Goldman M, Elsayegh S. Effects of parathyroid hormone on immune function. Clin Developmental Immunol. 2010;2010:418695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutiérrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha SW, Beck GR Jr. Impact of phosphorus-based food additives on bone and mineral metabolism. J Clin Endocrinol Metab. 2015;100(11):4264–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, Loehr LR, Eckfeldt JH, Coresh J. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3(3):e000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49(7):1025–1035. [DOI] [PubMed] [Google Scholar]
- 35.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran’s Affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. [DOI] [PubMed] [Google Scholar]
- 36.Dalrymple LS, Johansen KL, Chertow GM, Cheng SC, Grimes B, Gold EB, Kaysen GA. Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis. 2010;56(3):522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HE, Shapiro NI, Safford MM, Griffin R, Judd S, Rodgers JB, Warnock DG, Cushman M, Howard G. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7):e69232. [DOI] [PMC free article] [PubMed] [Google Scholar]