Abstract
Objective:
We investigated the daily dose of vitamin D needed to achieve serum 25-hydroxyvitamin D [25(OH)D] sufficiency among schoolchildren at risk for deficiency.
Study Design:
The Daily D Health Study was a randomized double-blind vitamin D supplementation trial among racially/ethnically diverse schoolchildren (n = 685) in the northeastern United States. Children were supplemented with vitamin D3 at 600, 1000, or 2000 IU/d for 6 months. Measurements included serum 25(OH)D at baseline (October to December), 3 months (January to March), 6 months (April to June), and 12 months (6 months after supplementation).
Results:
At baseline, mean ± standard deviation serum 25(OH)D level was 22.0 ± 6.8 ng/mL, with 5.5% severely vitamin D deficient (<12 ng/mL), 34.1% deficient (12 to 19 ng/mL), 49.0% insufficient (20 to 29 ng/mL), and 11.4% sufficient (≥30 ng/mL). The lowest levels of serum 25(OH)D were found among black (17.9 ± 6.7 ng/mL) and Asian children (18.9 ± 4.8 ng/mL), with no baseline differences by weight status. Serum 25(OH)D increased over 6 months in all three dose groups. The 2000 IU/d group achieved a higher mean serum 25(OH)D level than the other two dose groups (33.1 vs 26.3 and 27.5 ng/mL; P < 0.001), with 59.9% of this group attaining sufficiency at 3 months and only 5.3% remaining severely deficient/deficient at 6 months. All dose groups demonstrated a fall in 25(OH)D at 12 months.
Conclusions:
Children at risk for vitamin D deficiency benefited from daily sustained supplementation of 2000 IU/d compared with lower doses closer to the current recommended daily allowance for vitamin D intake. This benefit occurred over the winter months, when serum 25(OH)D level tend to fall.
Children at risk for vitamin D deficiency and insufficiency benefited from 6 months of supplementation with 2000 IU/d of vitamin D3 compared with lower doses closer to the current RDA of 600 IU/d.
The optimal “healthy” blood concentration of 25-hydroxyvitamin D [25(OH)D] is under active debate in the medical and nutrition communities. Currently, the American Academy of Pediatrics and the Institute of Medicine contend that children should achieve a serum 25(OH)D concentration of at least 20 ng/mL, a “threshold” based primarily on the literature base for prevention or treatment of rickets (1). However, longitudinal studies in adults (2, 3) and limited and primarily cross-sectional studies in pediatric populations (4–6) are demonstrating that a serum 25(OH)D concentration >30 ng/mL corresponds to decreased health risks. On the basis of this evidence, the Endocrine Society has recommended striving for serum 25(OH)D concentration ≥30 ng/mL for prevention of osteoporosis or diseases that may derive benefit from an improved vitamin D status among both children and adults, defining concentrations between 20 and 29 ng/mL as “insufficiency” and <20 ng/mL as “deficiency” (7). Of note, although the focus of this study was not prevention of rickets, a recent international consensus statement set a 25(OH)D threshold for vitamin D deficiency as <12 ng/mL, further illustrating the controversy (8).
Nonetheless, children are consuming less than half the Institute of Medicine’s current vitamin D intake recommendation of 600 IU/d (1, 9), in part contributing to 21% of healthy weight children and 34% of obese children falling below the 25(OH)D target of 20 ng/mL (10). Furthermore, more than two-thirds of children have concentrations below 30 ng/mL, including 80% of Hispanic children and 92% of non-Hispanic black children (10, 11). Compounding the problem, both obese children/adolescents (12–14) and minority racial/ethnic groups (15, 16) have shown blunted 25(OH)D responses to supplementation. Furthermore, children living in the northeastern part of the United States are at particular risk because of living at northern latitudes where the skin is unable to synthesize vitamin D from exposure to the sun during the winter months (17).
Currently, there is a research gap regarding the impact of different supplemental doses of vitamin D on vitamin D status among diverse children at high risk for deficiency. It may be that the current recommendation of 600 IU/d, although adequate to raise or maintain serum 25(OH)D concentrations at ≥20 ng/mL, may not be effective in raising serum 25(OH)D concentrations to ≥30 ng/mL, especially over the winter months. Therefore, the overall objective of the current study was to determine the appropriate vitamin D supplementation requirements for children living at northern latitudes to achieve 25(OH)D sufficiency. Our secondary goal was to investigate in a randomized controlled trial what happens to serum 25(OH)D concentrations after supplementation is discontinued. We examined these aims in healthy schoolchildren from socioeconomically disadvantaged and racially diverse communities with high obesity rates in the northeastern United States, for whom our previous data showed an extremely high prevalence of vitamin D deficiency (18, 19).
Methods
Study design
The Daily D Health Study was a randomized double-blind clinical trial examining the impact of three supplemental doses of vitamin D3 (600, 1000, or 2000 IU/d) on serum concentrations of 25(OH)D over 6 months in urban schoolchildren. As previously reported, children were recruited from schools and upon study entry were randomly assigned to a dose and given a corresponding bottle with a 3-month supply of vitamin D pills. A restricted block randomization schedule was used by nonstudy staff to ensure balance at the study start, which allocated participant identification numbers to one of three concealed treatments (dose A, dose B, or dose C) with corresponding supplement containers labeled with the same letters (A, B, or C). The letter-to-actual dose map was held in a sealed envelope under lock and key by the statistician.
Children were subsequently followed up for another 6 months after supplementation to measure changes in serum 25(OH)D levels (20). Children were randomly assigned at the baseline study visit (October to December) and completed follow-up visits at 3 (January to March), 6 (April to June), and 12 (September to November) months, which included blood draws and anthropometric, dietary, and skin color measures. All study visits occurred in the child’s school before the school day. The study protocol and study documents were approved by Tufts University’s institutional review board, and both parental informed consent and child assent were obtained.
Study subjects
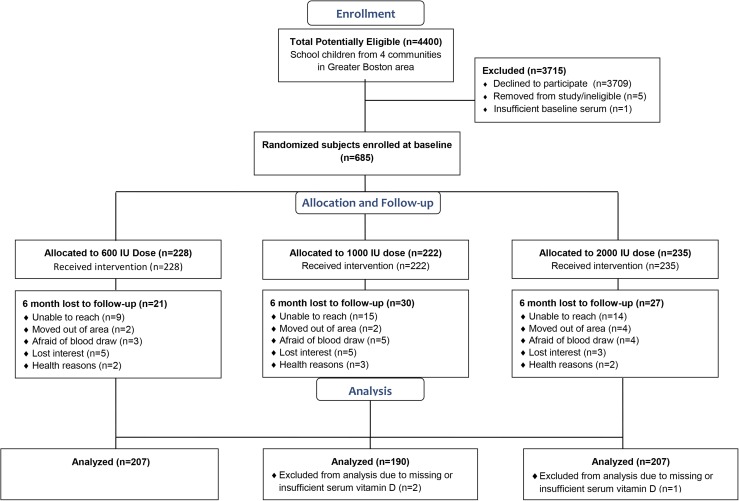
Schoolchildren in the fourth through eighth grades (aged 8 to 15 years) during the 2011-2012 and 2012-2013 school years were recruited from four urban school districts in the surrounding Boston, Massachusetts (42°N), area (20). Exclusion criteria included vitamin D supplement use and having rickets, cystic fibrosis, kidney disease, sarcoidosis, irritable bowel syndrome, epilepsy, or HIV/AIDS. Birth date, race/ethnicity, and data on socioeconomic status were reported by parental questionnaire. Parents identified their child’s racial/ethnic background, and children were subsequently aggregated as white/Caucasian, Hispanic/Latino, black/African American, Asian, and multiracial/other. Parents also reported their child’s eligibility for free or reduced-price lunch and annual household income. Enrollment, randomization scheme, and final sample distributions by dose group are presented in the CONSORT diagram (Fig. 1).
Figure 1.
CONSORT diagram.
Anthropometric measures
Height and weight were measured in triplicate as previously described (19–21). Body mass index (BMI) was calculated and converted into a percentile and z score according to the Centers for Disease Control and Prevention age- and sex-specific growth charts (22). Pubertal status was assessed at baseline using a self-administered questionnaire (23).
Dietary intake and supplement use
Daily vitamin D intake from dietary sources was captured using the Block Kid 2004 Food Frequency Questionnaire (NutritionQuest, Berkeley, CA) as described previously (19, 21, 24). Subjects were asked to refrain from any vitamin D supplement use for the month before study start and for the 6-month supplementation period. Supplements were manufactured specifically for the study as small grape-flavored chewable tablets and bottled in 3-month supplies at 600, 1000, or 2000 IU (Tishcon Corporation, Westbury, NY). Compliance to study supplement use was evaluated by returned pill bottle counts as previously reported (24). At the 12-month study visit, subjects were asked if they had taken any vitamin D supplements (including multivitamins) after the study stopped providing them and, if so, how often they had taken them in the past week.
Skin color
Constitutive and facultative skin colors were measured at anatomical sites on the upper inner arm and posterior forearm, respectively, with reflectance colorimetry using the Chroma Meter 400 (CR-400; Konica Minolta, Ramsey, NJ) by trained research assistants following an established protocol, as reported previously (21, 25), where tanning (changes in facultative skin color) was significantly associated with serum 25(OH)D concentration independent of race/ethnicity. Skin color was classified into six categories using individual typology angle: very light > 55 > light > 41 > intermediate > 28 > tanned > 10 > brown > −30 > dark (26).
Biochemical markers
Blood was drawn after an overnight fast as previously reported (21). Total serum 25(OH)D concentration was measured at Boston University Medical Center using the validated liquid chromatography-mass spectrometry method, including fractionation of 25(OH)D3 and 25(OH)D2 in serum (27). 25(OH)D3 and 25(OH)D2 calibration control solutions were generated from National Institute of Standards and Technology reference standards provided by Calbiochem (San Diego, CA). The analysis was performed using a TSQ Quantum Ultra triple mass-spectrometer (Thermo Finnigan Corp., San Jose, CA). Serum 25(OH)D status was classified as severely deficient (<12 ng/mL), deficient (12 to 19 ng/mL), insufficient (20 to 29 ng/mL), and sufficient (≥30 ng/mL) (7).
Intact parathyroid hormone (PTH) was measured using IMMULITE Intact PTH chemiluminescent assay (Diagnostic Products, Los Angeles, CA). Serum calcium, phosphorus, and alkaline phosphatase were measured using an end point assay in a multichannel analyzer (Roche Diagnostics, Indianapolis, IN). Safety of vitamin D supplement use was assessed by the concentration of serum calcium at the 3- and 6-month study visits.
Statistical analyses
Sample size calculations based on 90% power for a difference in 25(OH)D as low as 2 to 6 ng/mL yielded an approximate sample size of 182 per group. In this intention-to-treat analysis, differences in serum 25(OH)D concentrations and other blood biomarkers among the three dose groups (600, 1000, or 2000 IU/d) over time were analyzed using linear mixed models with repeated measurements at three time points (3, 6, and 12 months) and an unstructured covariance structure. Because linear mixed models directly account for the correlation among the multiple measurements taken over time on the same participant, they yield valid inferences between groups and within time points (28). A P value <0.05 was considered statistically significant in all analyses. Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Of the 685 participants who were eligible, enrolled, and randomly assigned at baseline, 604 had follow-up visits at 3 and/or 6 months and constituted the final study sample (Fig. 1). Table 1 presents characteristics of this sample (n = 604; mean age, 11.7 years) at baseline by vitamin D dose group. On the basis of BMI percentiles for age and sex, almost half (46.4%) of children were considered overweight or obese, whereas 59.6% were classified as racial/ethnic minorities, and 68.2% were eligible for free or reduced-price school meals.
Table 1.
Baseline Characteristics of Daily D Health Study Participants (n = 604)
| 600 IU (n = 207) | 1000 IU (n = 190) | 2000 IU (n = 207) | |
|---|---|---|---|
| Age, y | 11.7 (1.4) | 11.7 (1.3) | 11.7 (1.5) |
| Female sex, no. (%) | 109 (52.7) | 89 (46.8) | 109 (52.7) |
| Race/ethnicity, no. (%) | |||
| White | 92 (44.4) | 81 (42.6) | 71 (34.3) |
| Black | 23 (11.1) | 28 (14.7) | 34 (16.4) |
| Hispanic or Latino | 42 (20.3) | 43 (22.6) | 50 (24.2) |
| Asian | 20 (9.7) | 17 (9.0) | 16 (7.7) |
| Multiracial or other | 30 (14.5) | 21 (11.1) | 36 (17.4) |
| Constitutive skin colora | 33.1 (25.1) | 30.9 (25.6) | 29.7 (25.8) |
| Facultative skin colora | 13.2 (23.8) | 11.2 (24.3) | 9.8 (24.6) |
| Height, cm | 149.5 (11.3) | 150.9 (11.2) | 151.1 (10.8) |
| Weight, kg | 48.7 (16.0) | 50.6 (15.9) | 50.0 (15.8) |
| Body mass index, kg/m2 | 21.3 (4.6) | 21.8 (5.0) | 21.6 (5.0) |
| Body mass index, z score | 0.78 (1.05) | 0.82 (1.14) | 0.79 (1.09) |
| Weight status, no. (%) | |||
| Normal | 107 (51.7) | 103 (54.2) | 114 (55.1) |
| Overweightb | 51 (24.6) | 33 (17.4) | 39 (18.8) |
| Obeseb | 49 (23.7) | 54 (28.4) | 54 (26.1) |
| Puberty, no. (%) | 67 (32.4) | 66 (34.7) | 78 (37.7) |
| Household income, no. (%) | |||
| <$30,000 | 92 (44.4) | 94 (49.5) | 103 (49.8) |
| ≥$30,000 | 102 (49.3) | 87 (45.8) | 88 (42.5) |
| Missing | 13 (6.3) | 9 (4.7) | 16 (7.7) |
| Free or reduced-price lunch eligible, no. (%) | 135 (65.2) | 131 (68.9) | 146 (70.5) |
| Dietary vitamin D, IU/d | 123.1 (80.9) | 141.0 (109.2) | 127.6 (87.1) |
| Vitamin D or multivitamin in past month, no. (%) | 17 (8.2) | 27 (14.2) | 27 (13.0) |
| Serum 25(OH)D status,c no. (%) | |||
| <12 ng/mL | 16 (7.7) | 7 (3.7) | 10 (4.8) |
| 12−19 ng/mL | 70 (33.8) | 65 (34.2) | 71 (34.3) |
| 20−29 ng/mL | 92 (44.4) | 104 (54.7) | 100 (48.3) |
| ≥30 ng/mL | 29 (14.0) | 14 (7.4) | 26 (12.6) |
Data are presented as mean (standard deviation) unless otherwise indicated.
Skin color was classified using individual typology angle: very light > 55 > light > 41 > intermediate > 28 > tanned > 10 > brown > −30 > dark (26).
Overweight was defined as 85th to 94th BMI percentile; obese was defined as ≥95th BMI percentile.
Serum 25(OH)D status was defined as follows: <12 ng/mL = severe deficiency; 12 to 19 ng/mL = deficiency; 20 to 29 ng/mL = insufficiency; ≥30 ng/mL = sufficiency.
At baseline, mean ± standard deviation serum 25(OH)D concentration was 22.0 ± 6.8 ng/mL, with 5.5% of children being severely deficient (<12 ng/mL) and 88.6% being less than sufficient (<30 ng/mL). Few reported having taken a supplement containing vitamin D before the start of the study (11.7%), and vitamin D intake was well below the recommended 600 IU/d, averaging 130.3 ± 92.9 IU/d.
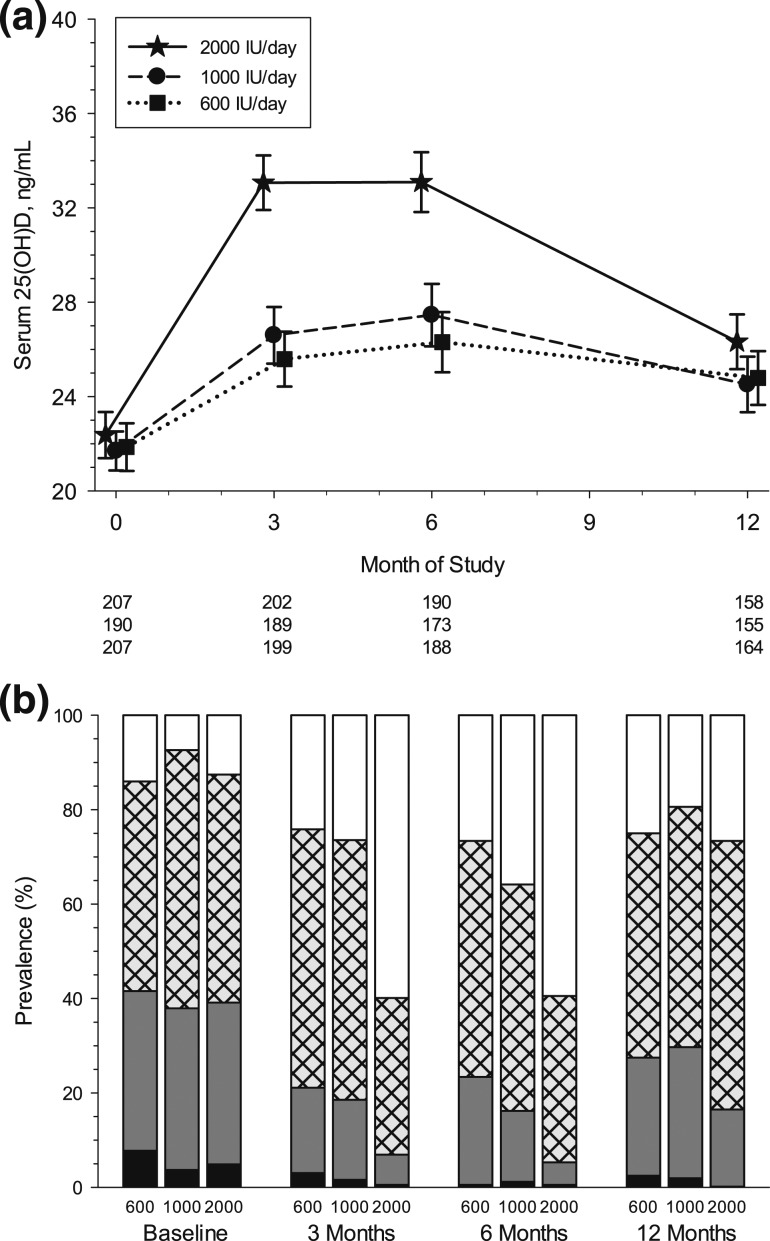
Mean serum 25(OH)D concentrations were similar among the three dose groups at baseline [Fig. 2(a); Table 2]. Compliance in taking the vitamin D supplement appeared to be high and did not differ by dose group at 3 months or 6 months (24). Across doses and time points, the median percentage of vitamin D pills taken ranged from 87% to 92%. Compliance also did not differ significantly by race/ethnicity at either 3 or 6 months (data not shown). Mean serum 25(OH)D concentration increased significantly by 3 months in all dose groups, with the 2000 IU/d group achieving significantly higher 25(OH)D concentrations than the 600 IU/d or 1000 IU/d group (P < 0.001). At the 6-month visit, all three dose groups had not changed in comparison with the 3-month visit, and the 2000 IU/d group continued to have significantly higher mean serum 25(OH)D concentrations than the 600 IU/d and 1000 IU/d dose groups (33.1 vs 26.3 and 27.5 ng/mL; P < 0.001; Table 2). The highest levels of 25(OH)D attained in the 600, 1000, and 2000 IU/d groups were 53.0, 51.8, and 66.5 ng/mL, respectively.
Figure 2.
Serum 25(OH)D concentrations over time by dose group. (a) Means and 95% confidence intervals are shown at baseline and 3, 6, and 12 months by dose group, with number of subjects below the horizontal axis. Symbols are staggered at each time point to better discern overlapping means and confidence intervals. Estimates are from a linear mixed model with repeated measurements at three time points (3, 6, and 12 months), three dose groups (600, 1000, and 2000 IU), and unstructured covariance structure. (b) Prevalence of serum 25(OH)D status is shown at baseline and 3, 6, and 12 months by dose group. Severely deficient (<12 ng/mL) = black shading; deficient (12 to 19 ng/mL) = gray shading; insufficient (20 to 29 ng/mL) = cross-hatching; and sufficient (≥30 ng/mL) = white shading.
Table 2.
Serum 25(OH)D by Daily D Dose Group During 6 Months’ Supplementation With 600, 1000, or 2000 IU/d of Vitamin D3 and 6 Months After Supplementation (12 Months)a
| Baseline |
3 Months |
6 Months |
12 Months |
|
|---|---|---|---|---|
| Serum 25(OH)D, ng/mL | ||||
| 600 IUb | 21.9 (0.5) | 25.6 (0.6) | 26.3 (0.6) | 24.8 (0.6) |
| 1000 IUc | 21.7 (0.5) | 26.6 (0.6) | 27.5 (0.7) | 24.5 (0.6) |
| 2000 IUd | 22.4 (0.5) | 33.1 (0.6) | 33.1 (0.6) | 26.3 (0.6) |
| P value among dose groups | 0.59 | <0.001 | <0.001 | 0.07 |
Values are means (standard errors). Estimates are from a linear mixed model with repeated measurements at three time points (3, 6, and 12 months), three dose groups (600, 1000, and 2000 IU), and unstructured covariance structure.
n = 207, 199, 188, and 188 at baseline, 3, 6, and 12 months, respectively.
n = 190, 189, 173, and 173 at baseline, 3, 6, and 12 months, respectively.
n = 207, 202, 190, and 190 at baseline, 3, 6, and 12 months, respectively.
Although the 600 IU/d and 1000 IU/d groups achieved similar mean concentrations of 25(OH)D at 6 months, it is noteworthy that a lower percentage of children were deficient (12 to 19 ng/mL) in the 1000 IU/d group compared with the 600 IU/d group (15.0% vs 22.9%) and more were sufficient at 6 months in the 1000 IU/d group than in the 600 IU/d group [35.8% vs 26.6%; Fig. 2(b)]. Even more striking is that 59.9% of children in the 2000 IU/d group attained sufficient levels (≥30 ng/mL) at 3 months and only 4.7% remained deficient (12 to 19 ng/mL) at 6 months compared with 22.9% and 15.0% deficient in the 600 and 1000 IU/d dose groups, respectively. Severe deficiency (<12 ng/mL) was virtually eliminated in all three dose groups at 6 months.
Laboratory values for alkaline phosphatase, calcium, phosphorus, and PTH are presented in Table 3. There were no significant changes in any of these values over the 6-month supplementation period and no significant changes in these biomarkers across dose groups.
Table 3.
Measures of Biological Safety Biomarkers (Mean and Standard Error) by Daily D Dose Group During 6 Months’ Supplementation With 600, 1000, or 2000 IU/d of Vitamin D3a
| Baseline |
3 Months |
6 Months |
|
|---|---|---|---|
| Alkaline phosphatase, μg/L | |||
| 600 IUb | 228.2 (5.9) | — | 237.7 (7.9) |
| 1000 IUc | 234.9 (6.2) | — | 249.5 (8.3) |
| 2000 IUd | 245.9 (5.9) | — | 239.9 (7.9) |
| P value among dose groups | 0.10 | — | 0.55 |
| Calcium, mg/dL | |||
| 600 IUb | 9.6 (0.03) | 9.7 (0.02) | 9.7 (0.03) |
| 1000 IUc | 9.6 (0.03) | 9.7 (0.02) | 9.6 (0.03) |
| 2000 IUd | 9.6 (0.03) | 9.7 (0.02) | 9.6 (0.03) |
| P value among dose groups | 0.55 | 0.86 | 0.22 |
| Phosphorus, mg/dL | |||
| 600 IUb | 4.8 (0.04) | 4.9 (0.03) | 4.9 (0.04) |
| 1000 IUc | 4.8 (0.04) | 4.9 (0.03) | 4.9 (0.04) |
| 2000 IUd | 4.8 (0.04) | 4.8 (0.03) | 4.9 (0.04) |
| P value among dose groups | 0.69 | 0.31 | 0.79 |
| PTH, pg/mL | |||
| 600 IUb | 35.2 (1.1) | — | 36.7 (1.3) |
| 1000 IUc | 35.0 (1.2) | — | 34.7 (1.3) |
| 2000 IUd | 33.9 (1.1) | — | 32.6 (1.3) |
| P value among dose groups | 0.71 | — | 0.08 |
Estimates are from linear mixed models with repeated measurements at two time points (3 and 6 months), three dose groups (600, 1000, and 2000 IU), and unstructured covariance structure.
n = 207, 199, and 188 at baseline, 3 and 6 months, respectively.
n = 190, 189, and 173 at baseline, 3 and 6 months, respectively.
n = 207, 202, and 190 at baseline, 3 and 6 months, respectively.
All three dose groups demonstrated a significant fall in mean serum 25(OH)D concentration at the 12-month visit [Fig. 2(a); Table 2], but serum concentrations remained significantly elevated compared with baseline values (P < 0.001). The 2000 IU/d group, however, did not have significantly higher mean 25(OH)D concentration at 12 months compared with the other two dose groups [26.3 (2000 IU) vs 24.8 (1000 IU) and 24.5 (600 IU/d) ng/mL; P = 0.07]. Of note, prevalence of severe deficiency and deficiency returned (albeit not to baseline levels) in the 600 and 1000 IU/d groups (27.4% and 29.7%, respectively), but less so in the 2000 IU/d group (16.5%). At the 12-month visit, similar percentages of children across dose groups reported taking a supplement containing vitamin D since the conclusion of the supplementation period (13.5%, 12.5%, and 16.8% in the 600, 1000, and 2000 IU/d dose groups, respectively). Facultative skin color was different between baseline and 12 months (11.4 at baseline vs 9.2 at 12 months; P < 0.01), although this difference was small and not likely clinically meaningful.
In subgroup analyses, white children had significantly higher mean baseline serum 25(OH)D concentrations than children in all other racial/ethnic groups (whites, 24.8 ng/mL vs 17.9 ng/mL for blacks, 20.8 ng/mL for Hispanics/Latinos, 18.8 ng/mL for Asians, and 21.7 ng/mL for multiracial/other; P < 0.001); however, there were no baseline differences in serum 25(OH)D concentration by weight status (22.4, 21.9, and 21.1 ng/mL for normal weight, overweight, and obese children, respectively; P = 0.15).
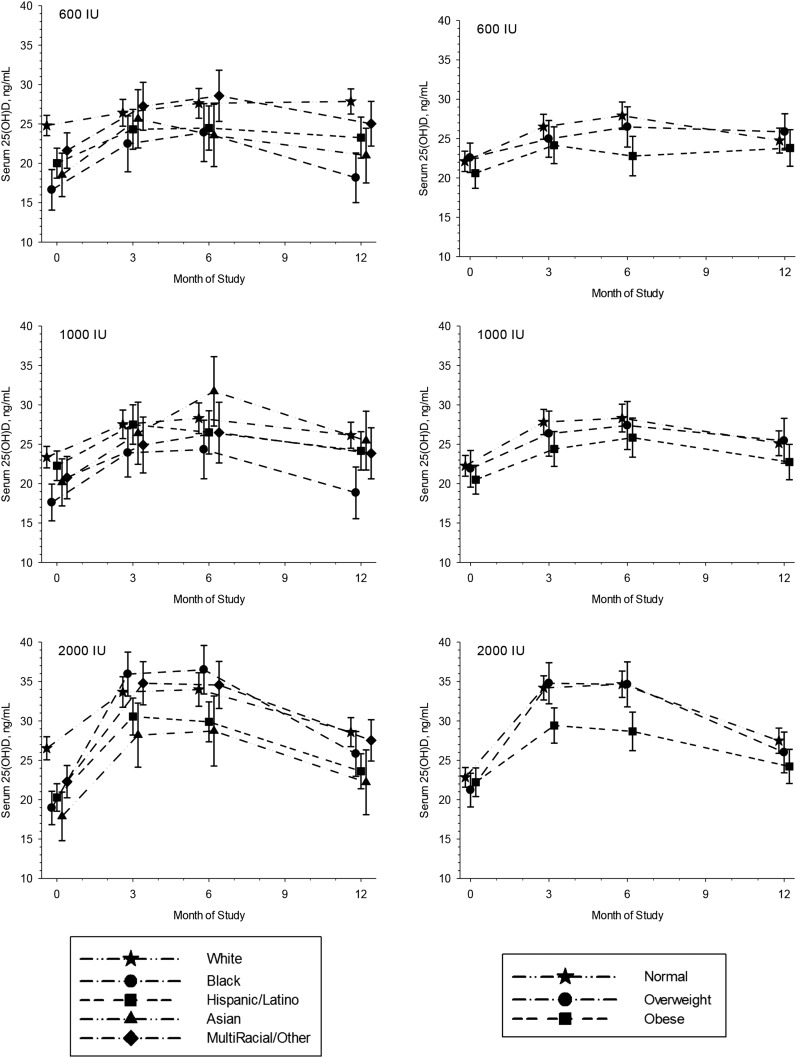
There were similar responses to supplementation across races/ethnicities for the 600 IU/d and 1000 IU/d dose groups (Fig. 3). In the 2000 IU/d group, black children had the greatest response to supplementation (mean ± standard error increase of 17.1 ± 2.2 ng/mL over 6 months), whereas the Asian group had the lowest response over the 6-month supplementation period (11.0 ± 1.7 ng/mL over 6 months). There were only small differences in serum 25(OH)D concentrations among weight status categories in the 600 IU/d and 1000 IU/d dose groups (P = 0.05 and 0.04, respectively; Fig. 3). However, the response to supplementation was lowest among obese children supplemented with 2000 IU/d (mean increase of 6.7 ± 1.3 ng/mL vs 11.8 ± 1.1 and 13.1 ± 1.7 ng/mL for normal weight and overweight children, respectively, over 6 months). Six months after supplementation, all subgroups experienced a fall in serum 25(OH)D concentration. There were no differences in serum 25(OH)D concentrations among weight status categories at 12 months, whereas serum 25(OH)D differences among racial/ethnic groups mirrored baseline differences.
Figure 3.
Serum 25(OH)D concentrations over time by dose group, stratified by race/ethnicity (left panels) and by weight status (right panels). Means and 95% confidence intervals are shown at baseline and 3, 6, and 12 months. Symbols are staggered at each time point to better discern overlapping means and confidence intervals.
Discussion
Supplemental doses of vitamin D3 at 600, 1000, or 2000 IU per day significantly increased serum 25(OH)D concentrations over 6 months among schoolchildren with mean baseline concentrations near deficiency. It is particularly noteworthy that this occurred over the winter months at 42°N, when serum 25(OH)D levels tend to fall, and it occurred among all racial/ethnic subgroups and weight status categories. However, serum vitamin D sufficiency, defined as ≥30 ng/mL, was reached on average by only those children in the 2000 IU/d group. After 6 months of supplementation, serum 25(OH)D concentrations surprisingly had not returned to baseline values in any of the dose groups at the 12-month study visit.
The recommended daily allowance (RDA) for vitamin D in children has been debated in recent years and was increased from 400 to 600 IU/d in 2012 (1). Researchers recognized that the RDA most likely needed to be higher and emphasized the need for additional studies on different groups of children to determine responses to supplementation. Therefore, we chose to measure the effect of the current RDA of 600 IU/d on serum 25(OH)D concentrations as well as two higher, reasonable, and safe doses (29) to determine whether the RDA or a higher dose could transition children from deficiency and insufficiency to sufficiency. In fact, only 2000 IU/d increased mean serum 25(OH)D concentrations to sufficiency (59.5% sufficiency at 6 months), whereas 600 and 1000 IU/d increased 25(OH)D on average, but many children remained deficient or insufficient (73.4% and 64.2% in the 600 IU/d and 1000 IU/d groups at 6 months). However, all supplemental doses resulted in few children remaining severely deficient at 6 months.
Others have similarly found that supplementation with 1000 IU/d over 6 months was effective in increasing serum 25(OH)D concentrations to ≥20 ng/mL but not to sufficiency in African American and white children with vitamin D deficiency (30). Also in children with vitamin D insufficiency (mean concentration of 27.7 ng/mL), 1000 IU/d was an adequate dose to raise serum 25(OH)D concentrations to sufficiency among nonobese children of different races/ethnicities (31). For our diverse population starting at a relatively low baseline 25(OH)D concentration (∼22 ng/mL) before the winter months, it is logical that a 2000 IU/d dose was necessary to increase serum 25(OH)D concentrations to sufficiency.
Unique responses to the 2000 IU/d dose were found for both black and obese schoolchildren. Black children had a significantly greater response to supplementation than other racial/ethnic groups for the 2000 IU/d dose only. This is important given that black (and Asian) children started at the lowest serum 25(OH)D concentrations, although Asian children did not have the same response to 2000 IU/d and, in fact, had the lowest response of all racial/ethnic groups. Others have found that children starting at lower serum concentrations typically have the greatest response to supplementation (13, 30, 32), which supports our findings in black but not in Asian children. Moreover, a recent study found the converse, with African American children being less responsive than white children to 4000 IU/d, although the authors attributed this finding to compliance issues (16). We did not observe any differences in supplement compliance across racial/ethnic groups.
The opposite response to 2000 IU/d occurred for obese children in our study, who had a suppressed serum 25(OH)D response compared with those of normal weight and overweight children despite no differences in serum 25(OH)D concentrations at baseline across weight status categories. No differences in baseline 25(OH)D concentrations among a small sample of obese and nonobese African American children was also reported by Rajakumar et al. (12), whereas national data demonstrate a higher prevalence of deficiency among obese children. The few studies that have examined the response of obese and nonobese children to supplementation found blunted responses to supplementation (12, 13, 33). Notably, our study is the only one that evaluated three supplemental doses, and the disparity occurred only with the highest dose. This may be due to differences in metabolism or more sequestration of vitamin D in adipose tissue in response to higher doses in obese children (34).
It is striking that 6 months after supplementation, mean serum 25(OH)D concentrations did not return to baseline values in any of the dose groups, nor were there differences among children from different racial/ethnic groups or between weight status categories. There was also no meaningful difference in facultative skin color (sun exposure) between baseline and the 12-month visit, and sun exposure during the summer months was similar among racial/ethnic groups (21). Although only 14% of children self-reported taking some form of vitamin D supplement during the summer, more may have done so and offset the return to baseline 25(OH)D concentrations. A more interesting possibility is that serum 25(OH)D may take longer to return to presupplementation values, which would necessitate further investigation (35).
The study is not without its limitations. Given our prior research on the relatively high prevalence of vitamin D deficiency and insufficiency in this population (18, 19), schoolchildren were randomly assigned to a supplemental dose without knowledge of their baseline 25(OH)D status, which would have been extremely difficult given the community-/school-based nature of this research. Similarly, we did not include a placebo group for ethical reasons. Dietary intake of vitamin D was by child self-report from a food frequency questionnaire. Vitamin D supplement use between the 6- and 12-month visits was by parent self-report and did not include data on the exact dose consumed. BMI percentile was used as a measure of adiposity because other body composition measures, such as waist circumference, could not be performed in the school setting.
However, the strengths of our study include a unique strategy to reach a large, diverse sample of healthy children at risk for vitamin D deficiency due to diet, northern latitude, high prevalence of overweight/obesity, and the inclusion of several racial/ethnic groups. Our supplementation with three different doses spanned a 6-month period that included the winter months, when vitamin D synthesis does not occur from sunlight exposure in this region of the United States. We had a high level of participant retention and supplement compliance (20, 21, 24). We also examined serum 25(OH)D 6 months after supplementation.
Conclusion
Schoolchildren who are at higher risk for vitamin D deficiency and insufficiency appeared to benefit from daily supplementation of 2000 IU/d compared with lower doses closer to the current RDA for dietary vitamin D intake to achieve sufficiency, as defined by a 25(OH)D concentration of ≥30 ng/mL. Severe deficiency (<12 ng/mL) was nearly eliminated after 6 months of supplementation of 600, 1000, or 2000 IU/d. Notably, these benefits of supplementation occurred over the winter months in the northeastern United States, when serum 25(OH)D levels tend to fall. Obese children were less responsive to the higher 2000 IU/d dose, whereas children of certain races/ethnicities responded better than others to supplementation, findings that warrant further investigation.
Acknowledgments
The authors thank the administrators, staff, teachers, and nurses at the Everett, Malden, Medford, and Somerville, Massachusetts, public schools for allowing us to conduct this research within their schools. In addition, we gratefully acknowledge Peter Bakun at the Friedman School of Nutrition Science and Policy at Tufts University for his assistance with data management; Elizabeth Olson, Charlette Steed, MSN, and Lauren Au, RD, for project management; and the rest of the Daily D Health Study staff and team who helped with data collection.
Financial Support: This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL106160.
Clinical Trial Information: ClinicalTrials.gov no. NCT01537809 (registered 23 February 2012).
Author Contributions: J.M.S., V.R.C., E.G., C.M.G., M.F.H., and C.D.E. designed the research. J.M.S., M.I.V.R., V.R.C., E.G., C.M.G., and M.F.H. conducted the research. M.I.V.R. and M.E. analyzed the data. J.M.S. wrote the article and had final responsibility for the content. All authors read and approved the final manuscript.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25(OH)D
- 25-hydroxyvitamin D
- BMI
- body mass index
- PTH
- parathyroid hormone
- RDA
- recommended daily allowance.
References
- 1.Institute of Medicine Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59(2):381–391. [DOI] [PubMed] [Google Scholar]
- 3.Husemoen LL, Skaaby T, Thuesen BH, Jørgensen T, Fenger RV, Linneberg A. Serum 25(OH)D and incident type 2 diabetes: a cohort study. Eur J Clin Nutr. 2012;66(12):1309–1314. [DOI] [PubMed] [Google Scholar]
- 4.Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab. 2012;97(1):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nsiah-Kumi PA, Erickson JM, Beals JL, Ogle EA, Whiting M, Brushbreaker C, Borgeson CD, Qiu F, Yu F, Larsen JL. Vitamin D insufficiency is associated with diabetes risk in Native American children. Clin Pediatr (Phila). 2012;51(2):146–153. [DOI] [PubMed] [Google Scholar]
- 6.Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr. 2011;94(1):225–233. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 8.Munns CFSN, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au LE, Rogers GT, Harris SS, Dwyer JT, Jacques PF, Sacheck JM. Associations of vitamin D intake with 25-hydroxyvitamin D in overweight and racially/ethnically diverse US children. J Acad Nutr Diet. 2013;113(11):1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131(1):e152–e161. [DOI] [PubMed] [Google Scholar]
- 11.Mansbach JM, Ginde AA, Camargo CA Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring). 2008;16(1):90–95. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, Pierce GL, White J, Holick MF, Zhu H. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95(10):4584–4591. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre Castaneda R, Nader N, Weaver A, Singh R, Kumar S. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78(4):226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratton-Loeffler MJ, Lo JC, Hui RL, Coates A, Minkoff JR, Budayr A. Treatment of vitamin D deficiency within a large integrated health care delivery system. J Manag Care Pharm. 2012;18(7):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis RD, Laing EM, Hill Gallant KM, Hall DB, McCabe GP, Hausman DB, Martin BR, Warden SJ, Peacock M, Weaver CM. A randomized trial of vitamin D3 supplementation in children: dose-response effects on vitamin D metabolites and calcium absorption. J Clin Endocrinol Metab. 2013;98(12):4816–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 18.Sacheck J, Goodman E, Chui K, Chomitz V, Must A, Economos C. Vitamin D deficiency, adiposity, and cardiometabolic risk in urban schoolchildren. J Pediatr. 2011;159(6):945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Au LE, Economos CD, Goodman E, Must A, Chomitz VR, Sacheck JM. Vitamin D intake and serum vitamin D in ethnically diverse urban schoolchildren. Public Health Nutr. 2012;15(11):2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacheck JM, Van Rompay MI, Olson EM, Chomitz VR, Goodman E, Gordon CM, Eliasziw M, Holick MF, Economos CD. Recruitment and retention of urban schoolchildren into a randomized double-blind vitamin D supplementation trial. Clin Trials. 2015;12(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawicki CM, Van Rompay MI, Au LE, Gordon CM, Sacheck JM. Sun-exposed skin color is associated with changes in serum 25-hydroxyvitamin D in racially/ethnically diverse children. J Nutr. 2016;146(4):751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention BMI percentile calculator for child and teen: English version. https://nccd.cdc.gov/dnpabmi/calculator.aspx. Accessed June 2015.
- 23.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. [DOI] [PubMed] [Google Scholar]
- 24.Au LE, Harris SS, Jacques PF, Dwyer JT, Sacheck JM. Adherence to a vitamin D supplement intervention in urban schoolchildren. J Acad Nutr Diet. 2014;114(1):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au LE, Harris SS, Dwyer JT, Jacques PF, Sacheck JM. Association of serum 25-hydroxyvitamin D with race/ethnicity and constitutive skin color in urban schoolchildren. J Pediatr Endocrinol Metab. 2014;27(11-12):1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Bino S, Sok J, Bessac E, Bernerd F. Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res. 2006;19(6):606–614. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19(13):1793–1819. [DOI] [PubMed] [Google Scholar]
- 29.Maalouf J, Nabulsi M, Vieth R, Kimball S, El-Rassi R, Mahfoud Z, El-Hajj Fuleihan G. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93(7):2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajakumar K, Moore CG, Yabes J, Olabopo F, Haralam MA, Comer D, Bogusz J, Nucci A, Sereika S, Dunbar-Jacob J, Holick MF, Greenspan SL. Effect of vitamin D3 supplementation in black and in white children: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2015;100(8):3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrams SA, Hawthorne KM, Chen Z. Supplementation with 1000 IU vitamin D/d leads to parathyroid hormone suppression, but not increased fractional calcium absorption, in 4-8-y-old children: a double-blind randomized controlled trial. Am J Clin Nutr. 2013;97(1):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putman MS, Pitts SA, Milliren CE, Feldman HA, Reinold K, Gordon CM. A randomized clinical trial of vitamin D supplementation in healthy adolescents. J Adolesc Health. 2013;52(5):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harel Z, Flanagan P, Forcier M, Harel D. Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health. 2011;48(5):448–452. [DOI] [PubMed] [Google Scholar]
- 34.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. [DOI] [PubMed] [Google Scholar]
- 35.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S–586S. [DOI] [PubMed] [Google Scholar]