Abstract
There is increasing evidence of a connection between hearing function and myasthenia gravis (MG). Studies of the pathophysiological basis of this relationship suggest that acetylcholine receptors (AChRs) on outer hair cells (OHCs) play a central role. In patients with MG, autoantibodies against AChRs induce a progressive loss of AChRs on OHCs, decreasing their electromotility. The stapedial reflex decay test can be altered in MG patients, and can be used as an additional tool for diagnosis and monitoring. Transient evoked and distortion product otoacoustic emissions are the main diagnostic tool for monitoring OHC functionality in MG patients, and can be used to record subclinical hearing alterations before the onset of clinically evident hearing loss. Understanding the association between MG and hearing dysfunction requires a multidisciplinary approach. Otolaryngologists should take this relationship into account when approaching patients with a diagnosis of myasthenia gravis and “in patients with MG” with ã in MG patients, and the progress of hearing alterations should always be monitored in patients with MG.
Keywords: Myasthenia gravis, tinnitus, hearing loss, otoacustic emissions, contralateral acoustic stimulation
Abbreviations
- MG
myasthenia gravis
- ACh
acetylcholine
- AChR
acetylcholine receptor
- MuSK
muscle-specific kinase
- OHCs
outer hair cells
- TEOAEs
transient evoked otoacoustic emissions
- DPOAEs
distortion product otoacoustic emissions
Introduction
Myasthenia gravis (MG) is a condition causing muscle weakness and fatigue, and is the most common autoimmune disorder affecting the neuromuscular junction. The prevalence of MG in the USA is estimated at 14 to 20 per 100,000 people, with approximately 36,000 to 60,000 affected individuals.1,2 Deenen3 conducted a comprehensive review of 24 studies published between 1990 and 2014, reporting that the worldwide prevalence of MG ranged from 5.35 to 35 per 100,000 people. Although MG can occur at any age, the age of onset in women is typically between 20 and 40 years, and the typical age of onset for men is between 40 to 60 years of age. Epidemiological studies from Canada and Japan have reported an increasing frequency of MG among elderly people in recent decades, possibly because of increased awareness among medical doctors.4,5
Typical clinical symptoms of MG include weakness of the facial, ocular, bulbar, respiratory or limb muscles, worsening after prolonged muscle usage.6 The primary mechanism of MG is an antibody-mediated attack on the proteins of the postsynaptic membrane, resulting in decreased signal transmission at the neuromuscular junction. Antibody mediated attack on acetylcholine receptors (AChRs) is reported to be involved in approximately 85% of cases, while in 5–8% of the cases, muscle-specific kinase (MuSK) is affected. Lipoprotein receptor-related protein 4 (LRP-4), also known as multiple epidermal growth factor-like domains 7 (MEGF7), is involved in 5–7% of MG cases.7
A number of studies have investigated the connection between hearing function and MG, increasing understanding of the pathophysiological mechanisms underlying this relationship.9–11,22 Hearing loss in MG patients typically presents at a subclinical stage with abnormal otoacoustic emissions and pure tone audiometry (PTA) often within the normal range. One study reported clinically evident hearing loss with auditory thresholds higher than 25 dBHL in 30% of MG patients examined, while all patients in the sample exhibited abnormal distortion product otoacoustic emissions (DPOAEs) and transient evoked otoacoustic emissions (TEOAEs).10 These techniques have been found to exhibit greater sensitivity to incipient cochlear damage than PTA, particularly for high frequencies.11
A correct diagnosis and management of hearing disorders in patients with MG requires a multidisciplinary approach. Awareness of this connection among neurologists, otolaryngologists and other specialists involved in the clinical and research aspects of MG can aid investigation of hearing function at first admission and follow up, facilitating the management of possible hearing dysfunction. In the current review, we discuss evidence from studies of hearing function alterations in patients with MG, focusing on research into the pathophysiological basis of the effects of MG on hearing, as well as the clinical diagnosis and management of hearing disorder in these patients.
MG and hearing function
Hearing function and MG: The role of AChRs
The relationship between MG and hearing function has been investigated, indicating that AChRs on outer hair cells (OHCs) play a central role.10,12,22 In an early immunohistochemical study, Plinkert8 used nicotinic acetylcholine receptor (nAChR) antibodies in the cochlea to visualize nAChRs, examining the development of efferent fibres and postsynaptic OHC receptors in 13 cochleae from fetal guinea pigs. The results revealed that maturation of postsynaptic nAChRs coincided with the development of motile properties of OHCs.8 In healthy subjects, acetylcholine (ACh), the primary neurotransmitter of the efferent auditory system, has been found to enhance the electromotility of OHC binding to AChRs, which are localized on the post-synaptic membrane of OHCs.9 In patients with MG, autoantibodies against AChRs were reported to bind to AChRs on OHCs, resulting in a progressive loss of AChRs that eventually affected their functionality and decreased OHC electromotility.10 This cascade of events has been found to induce apoptosis in all three rows of OHCs, evolving into clinically evident hearing loss in some cases.10
Hearing function and MG: The role of the efferent auditory system
The functional aspects of the efferent auditory system in MG patients have previously been investigated using contralateral acoustic stimulation (CAS).12 CAS produces physiological suppression of otoacoustic emissions13 by activating the medial olivocochlear complex, providing efferent feedback by releasing ACh from the olivocochlear nerve fibres onto OHCs and thus protecting the hair cells from noise of moderate to high intensity.14,15 A reduced CAS effect has been reported in patients with MG compared with control subjects, suggesting a possible role of progressive reduction of the beta subunits of nicotinic AChRs, which has been associated with the destruction of the basal membrane and OHCs due to prolonged exposure to autoantibodies.10,12
Evaluating hearing function in MG
Evaluation of hearing function in MG: The role of the stapedial reflex
A number of studies between the mid 1970s and early 1990s examined the stapedial reflex (SR) in MG patients as an additional tool for diagnosis and monitoring of the disorder. The use of SR in MG was initially examined by Blom,16 and the SR decay test (SRDT) with a 1-minute sound stimulus of 500 Hz was subsequently proposed by Warren.17 Kramer18 measured the pattern of SR fatigue in response to pulsed acoustic stimulation in 89 MG patients, compared with the effects of repetitive nerve stimulation and single-fibre electromyography. The results revealed that SR fatigue exceeded normal control values in 84% of MG patients, particularly in subjects with mild forms of myasthenia.18 Yamane19 investigated the threshold and latency parameters and the response pattern of the reflex in 11 patients with MG, reporting an unstable response pattern to 10-second tone stimuli and a lengthened mean reflex latency. Bischoff20 studied the decay and recovery of SR amplitude in 19 patients with MG and reported that the continuous prolonged stimulation of the stapedial muscle could be considered a practical and valid method for the evaluation of MG patients. Smith20 studied the SRDT in 30 patients with suspected MG, successfully measuring the reflex in 17 patients. Of these, 41% exhibited abnormal values.21 Tóth22 reported a series of 16 MG cases, finding that SR values were increased in 93% of patients, while stapedial exhaustion was observed in 71% of patients. Moreover, after the administration of a reversible cholinesterase inhibitor (Mestinon), the reflex threshold decreased and exhaustion occurred in only 50% of cases.22 Taken together, these findings suggest that examination of the stapedial reflex in patients with MG may represent a useful supplementary diagnostic and monitoring tool.
Evaluation of hearing function in MG: The role of otoacoustic emissions
While alteration of the SR has been extensively documented in MG, there is increasing evidence that OHCs play a central role in auditory alterations in the disorder. Several studies have produced evidence of a reduction of both TEOAEs9 and DPOAEs10,12,23 in MG patients compared with normal controls. Furthermore, treatment with reversible ACh inhibitors has been shown to increase amplitudes of TEOAEs and DPOAEs, although not always to the levels observed for healthy controls.12,23
Tóth9 measured TEOAEs in 29 ears of MG patients, reporting significantly lower TEOAE values in MG patients compared with healthy controls. The administration of a reversible cholinesterase inhibitor (pyridostigmine bromide; Mestinon) resulted in a significant increase in TEOAE amplitude, although the amplitudes remained lower than those of controls.9 Paludetti23 recorded DPOAEs in 25 patients with MG, with normal hearing, before and 1 h after administration of 60 mg pyridostigmine bromide, and compared the results with those of 25 control subjects. DPOAE amplitudes were found to be significantly lower in MG patients before the administration of the drug, and were increased after drug administration, particularly for middle to high frequencies. In accord with the findings reported by Tóth9, even after drug administration, DPOAE values remained lower among MG patients compared with controls.23 As mentioned above, CAS is considered a useful additional test for monitoring OHC in MG patients. Di Girolamo12 recorded DPOAEs in 20 MG subjects with and without CAS before and 1 h after administration of a reversible ACh inhibitor. The results revealed no significant DPOAE amplitude changes upon exposure to contralateral noise before drug administration. However, after drug administration, CAS induced a significant decrease of DPOAE amplitudes for middle frequencies.12 Hamed10 investigated OHC function in 16 MG patients 1 week and 2 months after a myasthenic crisis or acute oropharyngeal dysfunction. Comparison with healthy controls revealed that MG patients exhibited a significant reduction in TEOAE amplitude at 1–2 kHz and a reduction of DPOAE amplitude at 1–6 kHz. No changes were observed among control subjects.10 In all of the studies discussed above, substantial variation was observed in the response amplitudes for middle and high frequencies, possibly due to a higher concentration of ACh receptors in the basal and middle cochlear turns.
The role of otolaryngologists
MG is a condition that requires a multidisciplinary approach. The pathophysiological evidence described in the current review typically precedes clinically evident hearing loss, and MG is often associated with hearing loss that is not noticed by the patient. Thus, otolaryngologists play a central role in identifying possible hearing dysfunction and monitoring the progress of hearing alterations in patients with a diagnosis of MG. Due to the involvement of OHCs in MG, otoacoustic emissions represent the most relevant test for monitoring hearing function, even if other examination methods, such as PTA and Auditory Brainstem Response (ABR) produce results within the normal range. Besides a complete clinical and audiological examination, transient evoked and distortion product OAE should always be used at MG diagnosis, and should be repeated over time. The onset of associated symptoms such as tinnitus may follow progressive hearing loss, and should therefore always be investigated.24 The studies reviewed above also support the use of CAS, and evaluation of the responsiveness of the OHCs to the administration of an acetylcholinesterase inhibitor by monitoring OAE amplitude changes.
The role of otolaryngologists: Our experience
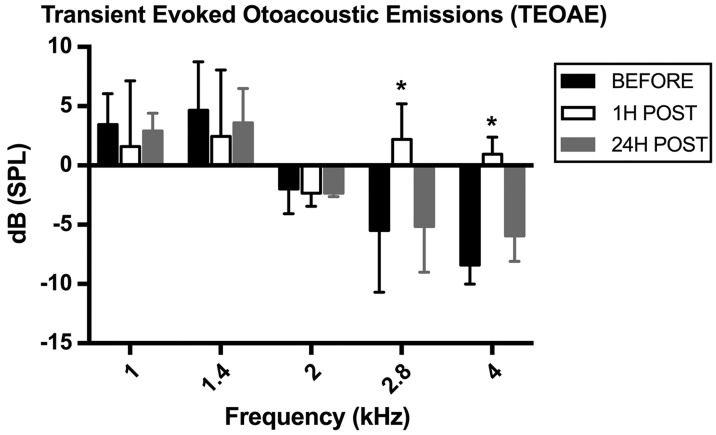
The Tinnitus Unit at the Sapienza State University, Policlinico Umberto I in Rome has treated approximately 4,300 tinnitus patients over the past 10 years.25 A case study of a patient with MG and tinnitus produced results that are consistent with the findings of previous studies. PTA revealed a bilateral sensorineural mild hearing loss for high frequencies (avg 23.40 dB HL; avg > 2 kHz 34.5; avg > 4 kHz 41.66 dBHL). A consistent reduction was found in mid and high range amplitudes when recording baseline TEOAE and DPOAE, compared with controls. Following protocols reported in previous studies,5,7,18 the results revealed a significant increase of TEOAE amplitude at 2.8 and 4.0 kHz 1h after administration of 60 mg pyridostigmine bromide (Mestinon). Amplitudes returned to baseline values after 24 hours. In contrast, no amplitude changes were observed in DPOAEs after drug administration. Measures of single TEOAE frequencies before and after drug administration were as follows: 1.0 kHz = 3.45 dB (before), −1.60 dB (after); 1.4 kHz = 4.65 dB (before), 2.45 dB (after); 2.0 kHz = −2.10 dB (before), −2.35 dB (after); 2.8 kHz = −5.50 dB (before), 2.20 dB (after); 4.0 kHz = −8.40 dB (before), 0.95 dB (after). In addition, the signal to noise ratio was higher than the minimum acceptable value of 5 dB for all frequencies, with an average of 9.45 dB. TEAOE amplitudes recorded before, 1 h and 24 h after drug administration are shown in Figure 1.
Figure 1.
TEOAE amplitudes in a patient with MG before and after 60 mg pyridostigmine bromide (Mestinon) administration. Before drug administration, TEOAE amplitudes were reduced compared with controls. After drug administration, a significant increase of TEOAE amplitudes was recorded, particularly for the 2.8 and 4.0 kHz frequencies; amplitudes returned close to baseline values after 24 hours.
Although CAS did not cause a reduction of TEOAE amplitudes under basal conditions, we observed a clear CAS effect after drug administration, as indicated by a marked reduction of TEOAE amplitude, particularly for 2.8 and 4.0 kHz stimulation.
These findings, particularly the large variation in amplitudes for middle and high frequencies, are in accord with previous reports, confirming that OAEs can play a useful role in the early detection of MG-related effects on the ACh-innervated auditory system. These methods could be utilized for the assessment and monitoring of hearing function in patients with MG.
Conclusion
Increasing evidence suggests that chronic OHC dysfunction in patients with MG is associated with the progressive loss of AChRs on the basolateral portion of OHCs. Taken together, the studies discussed in this review contribute to the understanding of the association between pathophysiological alterations recorded in the inner ear of MG patients and a subclinical or, in some cases, clinically evident hearing disorder Effective diagnosis and treatment thus requires a multidisciplinary approach in clinical management and follow up. Hearing loss in patients with MG often occurs at a subclinical stage, particularly during the first decades of life. However, chronic dysfunction of OHCs can result in a progressive loss of hearing that is rarely noticed by patients. These characteristics suggest that hearing function should always be assessed in patients with MG, particularly via examination of OHCs with otoacoustic emissions, and, to a lesser extent, SR.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Phillips LH., 2nd The epidemiology of myasthenia gravis. Ann N Y Acad Sci 2003; 998: 407–412. [DOI] [PubMed] [Google Scholar]
- 2.Jayam Trouth AJ, Dabi A, Solieman N, et al. Myasthenia gravis: a review. Autoimmune Dis 2012; 2012: 874680–874680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deenen JCW, Horlings CGC, Verschuuren JJGM, et al. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis 2015; 2: 73–85. [PubMed] [Google Scholar]
- 4.Pakzad Z, Aziz T, Oger J. Increasing incidence of myasthenia gravis among elderly in British Columbia, Canada. Neurology 2011; 76: 1526–1528. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda M, Dohi-Iijima N, Nakamura A, et al. Increase in incidence of elderly-onset patients with myasthenia gravis in Nagano Prefecture, Japan. Intern Med 2005; 44: 572–577. [DOI] [PubMed] [Google Scholar]
- 6.Grob D, Arsura EL, Brunner NG, et al. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci 1987; 505: 472–499. [DOI] [PubMed] [Google Scholar]
- 7.Meriggioli MN, Sanders DB. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Rev Clin Immunol 2012; 8: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plinkert PK, Ptok M, Zenner HP. Maturation of postsynaptic acetylcholine receptors in cochlear outer hair cells. HNO 1994; 42: 738–743. [in German, English Abstract]. [PubMed] [Google Scholar]
- 9.Tóth L, Rácz T, Diószeghy P, et al. Otoacoustic emission in myasthenia gravis patients and the role of efferent activation. Hear Res 1998; 126: 123–125. [DOI] [PubMed] [Google Scholar]
- 10.Hamed SA, Elattar AM, Hamed EA. Irreversible cochlear damage in myasthenia gravis – otoacoustic emission analysis. Acta Neurol Scand 2006; 113: 46–54. [DOI] [PubMed] [Google Scholar]
- 11.Fetoni AR, Garzaro M, Ralli M, et al. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: A pilot study. Med Sci Monit 2009; 15: PR1–8. [PubMed]
- 12.Di Girolamo S, d’Ecclesia A, Quaranta N, et al. Effects of contralateral white noise stimulation on distortion product otoacoustic emissions in myasthenic patients. Hear Res 2001; 162: 80–84. [DOI] [PubMed] [Google Scholar]
- 13.Moulin A, Collet L, Duclaux R. Contralateral auditory stimulation alters acoustic distortion products in humans. Hear Res 1993; 65: 193–210. [DOI] [PubMed] [Google Scholar]
- 14.Williams DM, Brown AM. The effect of contralateral broad-band noise on acoustic distortion products from the human ear. Hear Res 1997; 104: 127–146. [DOI] [PubMed] [Google Scholar]
- 15.Collet L, Kemp DT, Veuillet E, et al. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res 1990; 43: 251–261. [DOI] [PubMed] [Google Scholar]
- 16.Blom S, Zakrisson JE. The stapedius reflex in the diagnosis of myasthenia gravis. J Neurol Sci 1974; 21: 71–76. [DOI] [PubMed] [Google Scholar]
- 17.Warren WR, Gutmann L, Cody RC, et al. Stapedius reflex decay in myasthenia gravis. Arch Neurol 1977; 34: 496–497. [DOI] [PubMed] [Google Scholar]
- 18.Kramer LD, Ruth RA, Johns ME, et al. A comparison of stapedial reflex fatigue with repetitive stimulation and single-fiber EMG in myasthenia gravis. Ann Neurol 1981; 9: 531–536. [DOI] [PubMed] [Google Scholar]
- 19.Yamane M, Nomura Y. Analysis of stapedial reflex in neuromuscular diseases. ORL J Otorhinolaryngol Relat Spec 1984; 46: 84–96. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff C, Klingelhöfer J, Conrad B. Decay and recovery of the stapedial reflex by prolonged stimulation in the diagnosis of myasthenia gravis. J Neurol 1989; 236: 343–348. [DOI] [PubMed] [Google Scholar]
- 21.Smith MJ, Brezinova V. Stapedius reflex decay test in diagnosis of myasthenia gravis (MG). Electromyogr Clin Neurophysiol 1991; 31: 317–319. [PubMed] [Google Scholar]
- 22.Tóth L, Lampé I, Diószeghy P, et al. The diagnostic value of stapedius reflex and stapedius reflex exhaustion in myasthenia gravis. Electromyogr Clin Neurophysiol 2000; 40: 17–20. [PubMed] [Google Scholar]
- 23.Paludetti G, Di Nardo W, D’Ecclesia A, et al. The role of cholinergic transmission in outer hair cell functioning evaluated by distortion product otoacoustic emissions in myasthenic patients. Acta Otolaryngol 2001; 121: 119–121. [DOI] [PubMed] [Google Scholar]
- 24.Sheppard A, Hayes SH, Chen GD, et al. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital 2014; 34: 79–93. [PMC free article] [PubMed]
- 25.Cianfrone G, Mazzei F, Salviati M, et al. Tinnitus holistic simplified classification (THoSC): a new assessment for subjective tinnitus, with diagnostic and therapeutic implications. Ann Otol Rhinol Laryngol 2015; 124: 550–560. [DOI] [PubMed] [Google Scholar]