Abstract
Objective
Deep brain stimulation (DBS) for treatment of advanced Parkinson’s disease (PD) has two anatomical targets: the subthalamic nucleus (STN) and the globus pallidus internus (GPI). The clinical effectiveness of these two stimulation targets was compared in the present study.
Methods
A systematic review and meta-analysis was performed to evaluated the postoperative changes in the United Parkinson’s Disease Rating Scale (UPDRS) on- and off-phase, on-stimulation motor scores; activities of daily living score (ADLS); and levodopa equivalent dose (LED) after STN and GPI stimulation. Randomized and nonrandomized controlled trials of PD treated by STN and GPI stimulation were considered for inclusion.
Results
Eight published reports of eligible studies involving 599 patients met the inclusion criteria. No significant differences were observed between the STN and GPI groups in the on-medication, on-stimulation UPDRS motor score [mean difference, 2.15; 95% confidence interval (CI), −0.96–5.27] or ADLS (mean difference, 3.40; 95% CI, 0.95–7.76). Significant differences in favor of STN stimulation were noted in the off-medication, on-stimulation UPDRS motor score (mean difference, 1.67; 95% CI, 0.98–2.37) and LED (mean difference, 130.24; 95% CI, 28.82–231.65).
Conclusion
The STN may be the preferred target for DBS in consideration of medication reduction, economic efficiency, and motor function improvement in the off phase. However, treatment decisions should be made according to the individual patient’s symptoms and expectations.
Keywords: Subthalamic nucleus, globus pallidus internus, deep brain stimulation, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is a severely disabling condition for which current drug therapies do not always achieve satisfactory results. Neurosurgeons worldwide have made many attempts to alleviate the symptoms of PD by performing pallidotomy,1–4 thalamotomy,5 or subthalamotomy6 and high-frequency deep brain stimulation (DBS) of the subthalamic nucleus (STN), globus pallidus internus (GPI), and ventro-intermedial nucleus.7–10
Because the most effective management of advanced PD has not been established, management varies among clinical centers. DBS is widely applied because of its safety and comparative effectiveness.7 Electrical stimulation can produce a functional lesion within a focal area of the brain. In the present study, two targets within the brain were stimulated for treatment of PD: the STN and the GPI. DBS of the STN has gained substantial popularity at most PD specialty centers. Many studies11–14 have revealed that STN stimulation can markedly improve patients’ motor function. However, serious adverse events have also been reported, including infection, depression, mood changes, and psychosis requiring intervention.12,15–18 GPI stimulation can also reportedly enhance patients’ motor function and time spent in the on state.12,19 Serious complications of DBS of the bilateral GPI include hematomas, infection, and equipment issues.21,22
In this comprehensive systematic review and meta-analysis, we compared the clinical advantages of DBS of the STN versus GPI for the treatment of PD. A better understanding of target selection is critical to guide treatment decision-making and patient expectations.
Methodology
Search criteria
All full-text published randomized and nonrandomized controlled trials comparing the United Parkinson’s Disease Rating Scale (UPDRS) on-phase motor score, UPDRS off-phase motor score, activities of daily living score (ADLS), and levodopa equivalent dose (LED) between patients who underwent DBS of the STN versus GPI were included. Case reports containing fewer than five patients, comments, letters, editorials, protocols, guidelines, animal studies, and cadaver articles were excluded.
Search strategy
The Medline, Embase, Cochrane Library, Ovid, and CBM databases were searched for English-language articles published from September 1993 to September 2013. Unpublished studies were excluded. The prespecified search terms were “Parkinson’s disease,” “deep brain stimulation,” “subthalamic nucleus,” “globus pallidum,” “randomized controlled trials,” “random,” “control,” and “trials.” Titles, abstracts, and subject headings were searched. The reference lists of all included articles and review papers were scrutinized for additional publications. In addition, we reviewed the references from all articles identified in the aforementioned search to include any additional papers related to the outcomes of DBS that may have been missed.
Search selection
Two reviewers independently assessed the titles and abstracts of each identified citation. The full texts of potential articles were ordered and evaluated against the eligibility criteria. Any disagreements were resolved by discussion.
Data extraction
Each reviewer independently extracted data from each included paper. All data were tabulated onto a predefined spreadsheet. All articles were anonymized with respect to author name, institution, journal title, and year of publication to blind reviewers during the processes of data extraction, appraisal, and analysis.
Outcome measures
The outcome measures were the changes in the on-phase UPDRS motor score, off-phase UPDRS motor score, ADLS, and LED from preoperatively to >3 months postoperatively. The primary focus of this meta-analysis was the off- and on-medication, on-stimulation UPDRS motor scores (Part III, motor examination section of the UPDRS).23 This is the most commonly reported parameter in DBS studies and reflects the effect of stimulation on patients’ motor function. The motor subscale comprises 14 items with a score ranging from 0 to 104. The UPDRS ADLS is self-reported by patients and focuses on activities such as walking, writing, dressing, and speaking; the total score ranges from 0 to 52. The changes in the ADLS were weighted by the time spent in either the on or off phase.24 Measurement of the LED involves conversion of antiparkinson medication doses into comparable levodopa doses.25
Statistical analysis
The mean difference and 95% confidence interval (CI) of each outcome were assessed by comparing the STN and GPI groups, and the statistical heterogeneity was measured using the I2 statistic. The I2 test for heterogeneity was used to measure the proportion of total variation in the study estimates due to heterogeneity rather than sampling error. If significant heterogeneity was found among the studies based on interpretation of the I2 test, a random-effects model was applied. If no significant heterogeneity among studies was found, a fixed-effects model was applied. The meta-analysis was then carried out using RevMan software (version 5.0 for Windows; Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2008). P values of <0.05 were considered statistically significant. We attempted to contact the original authors to acquire detailed data. For missing standard deviations of changes from baseline, we referred to the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (section 16.1.3.2).
Results
Search strategy
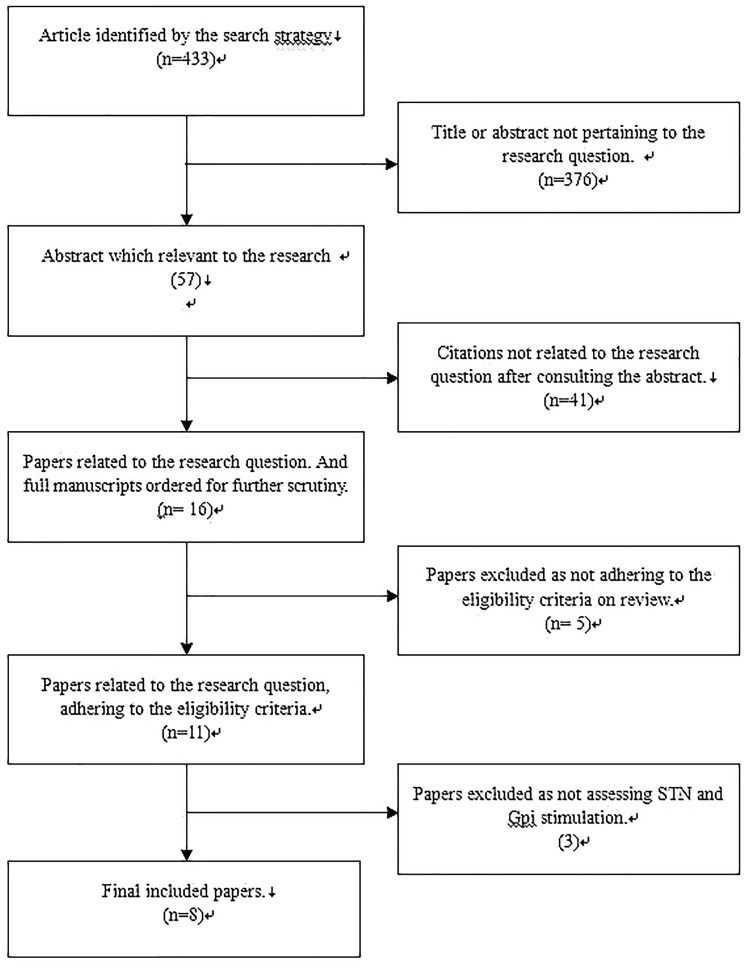
The database search, snowballing, and contact with experts yielded a total of 431 articles. After excluding 423 nonpertinent titles or abstracts, 8 studies22,24,26–32 were retrieved in complete form and assessed according to the selection criteria (Figure 1). In total, 599 patients were included; 300 underwent STN stimulation and 299 underwent GPI stimulation. The mean age of the patients in all eight studies was >40 years, and all follow-up periods were >3 months. The general characteristics of the patients among the eight studies are shown in Table 1.
Figure 1.
A QUORUM Chart.
Table 1.
Characteristics of the patients in the eight studies included in the meta-analysis
| Operation |
Mean age (y) |
Sex (male/female) |
Follow-up | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Paper | STN | GPI | STN | GPI | STN | GPI | ||
| Zahodne et al. 2009 | 20 | 22 | 61.3 (9.0) | 61.3 (5.5) | 14/6 | 16/6 | 6 m | UPDRS3 on/off, ALDS, LEDs |
| Odekerken et al. 2013 | 63 | 65 | 60.9 (7.6) | 59.1 (7.8) | 44/19 | 44/21 | 12 m | UPDRS3 on/off, LEDs, ALDS |
| Evidente et al. 2011 | 12 | 12 | 66.42 (11.13) | 66.92 (10.69) | / | / | 6 m | UPDRS3 |
| Nakamura et al. 2007 | 18 | 15 | 59.9 (2.2) | 59.6 (2.3) | / | / | 3–6 m | UPDRS3 |
| Okun et al. 2009 | 22 | 23 | 59.8 (10.0) | 60.2 (6.2) | 15/7 | 13/10 | 7 m | UPDRS3 on/off, LEDs |
| Krause et al. 2001 | 12 | 6 | 58.7 | 58.5 | / | / | 12 m | UPDRS3 on/off, LEDs |
| Burchiel et al. 1999 | 6 | 4 | 62.5 (12) | 42.5 (11) | / | / | 12 m | ALDs |
| Follett et al. 2010 | 147 | 152 | 61.9 (8.7) | 61.8 (8.7) | 116/31 | 133/19 | 24 m | UPDRS3 |
Outcome measures
Changes in UPDRS motor scores
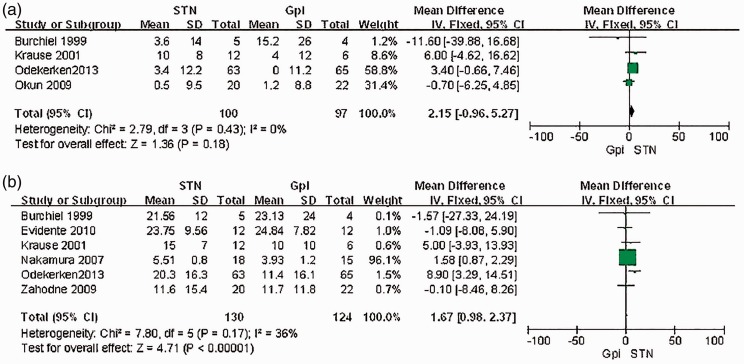
Four studies24,28,30,32 conducted a <1-year follow-up to record the changes in the UPDRS motor score in the on phase. Our meta-analysis showed no significant difference between the STN and GPI groups (mean difference, 2.15; 95% CI, −0.96–5.27) (Figure 2(a)). Six studies22,24,26,28,29,30,32 recorded the change in the UPDRS motor score in the off phase. A meta-analysis of the six studies that recorded the changes in the UPDRS motor score in the off phase showed a significant difference in favor of STN stimulation (mean difference, 1.67; 95% CI, 0.98–2.37; P < 0.01) (Figure 2(b)).
Figure 2.
(a) Meta-analysis of the mean difference of the changes of UPDRS motor scores on phase postoperation in STN stimulation vs.GPI stimulation. There is no significant difference between the two groups, with a mean difference of 2.15, 95%CI (−0.96,5.27); (b) Meta-analysis of the mean difference of the changes of UPDRS motor scores off phase postoperation in STN stimulation vs.GPI stimulation. STN is significantly associated with a decrease in the UPDRS motor scores, with a relative risk of 1.67, 95%CI (0.98,2.37).
Changes in ADLS
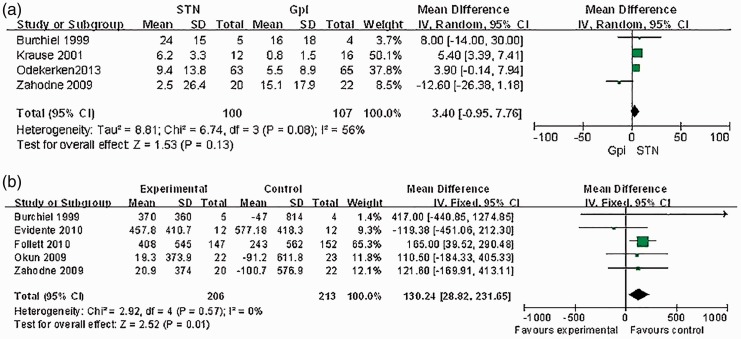
Four studies24,28,30,32 performed a <1-year follow-up to record the ADLS. Our meta-analysis showed no significant difference in the change in the ADLS between STN and GPI stimulation (mean difference, 3.40; 95% CI, −0.95–7.76) (Figure 3(a)).
Figure 3.
(a) Meta-analysis of the mean difference of the changes of ADL scores postoperation in STN stimulation vs.GPI stimulation. There is no significant difference between the two groups, with a mean difference of −1.1, 95%CI [−2.6 to 0.4]; (b) Meta-analysis of the mean difference of the reduction of LEDs postoperation in STN stimulation vs. GPI stimulation. This meta-analysis showed a mean difference across the two groups of 130.24, 95% CI (28.82, 231.65), clearly favouring STN.
Changes in LED
Five studies26,27,28,31,32 recorded the LED reduction. Our meta-analysis showed significant differences in the LED in favor of STN stimulation (mean difference, 130.24; 95% CI, 28.82–231.65; P < 0.05) (Figure 3(b)).
Critical appraisal
The results of the risk of bias assessment are shown in Table 2. Each study appropriately defined its population and eligibility criteria, clearly showing that the study groups were comparable at baseline. Appropriate outcome measures were also selected and defined to answer each study’s research questions.
Table 2.
Risk of bias among the included studies
| Paper | Adequate sequence generation? | Allocation concealment used? | Blinding? | Interventions clearly defined? | Outcome measures clearly defined? | Outcome measures appropriate? | Appropriate follow-up duration? |
|---|---|---|---|---|---|---|---|
| Zahodne et al. 2009 | Yes | No | No | Yes | Yes | Yes | Yes |
| Odekerken et al. 2013 | Yes | No | No | Yes | Yes | Yes | Yes |
| Evidente et al. 2011 | Yes | No | No | Yes | Yes | Yes | Yes |
| Nakamura et al. 2007 | Yes | No | No | Yes | Yes | Yes | Yes |
| Okun et al. 2009 | Yes | No | No | Yes | Yes | Yes | Yes |
| Krause et al. 2001 | Yes | No | No | Yes | Yes | Yes | Yes |
| Burchiel et al. 1999 | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Follett et al. 2010 | Yes | No | No | Yes | Yes | Yes | Yes |
Discussion
The available data from the studies reporting the effects of DBS of the STN and GPI show that both targets have certain benefits. However, many factors must be considered to determine which target is the most appropriate. Among these factors are complications, side effects, motor function, cognition, mood, activities of daily living, medication reduction, and even economic factors.
Major complications of DBS include hemorrhage, infection, and hardware-related failure. Benabid et al. reported that hemorrhage occurred in 8.4% (range, 0.2%–12.5%) of 526 patients who underwent DBS and that only 0.6% had permanent deficits.33,34 Death directly attributable to the surgical procedure is extremely rare in the setting of DBS, but it has been reported.35 In one study, spontaneous intracerebral hemorrhage occurred before insertion of the electrode during a DBS procedure, and the cause may have been related to amyloid angiopathy.36 The infection rate varies widely among studies from 1% to >15%.3,37–39 Skin infections adjacent to the inserted material are mostly superficial at the site of the pulse generator and occurred in 15% of published cases. The stimulator and related hardware should be removed in patients with infection. Hardware-related complications such as lead breakage, extension wire failure, premature battery consumption, or malfunction of the pulse generator40 can lead to discontinuation of treatment and the need for reoperation.41
Because the operation process and implanted device are basically the same between DBS of the STN and GPI, these factors contribute little difference to the postoperative complication rates. Likewise, the side effects caused by stimulation of the two targets appear to be similar. For example, an acute postoperative confusional state may occur in up to 10% of patients,36 and some patients who have undergone DBS (STN or GPI) gain weight.42,43 However, some longer-term studies have also suggested that the adverse effects of DBS are usually transient and reversible.
According to our meta-analysis, DBS of both the STN and GPI can improve the motor function of patients with PD by either pallidal or subthalamic stimulation. However, although no differences were observed in the on phase, significant differences were seen in the off phase; STN was more effective in terms of motor function improvement in the off phase, which is in accordance with many published studies.24,38,29,30,32,44,45 Follett et al. reported that 24 months postoperatively, the results in the on medication state did not differ significantly according to the surgical target; a reduction of 11.8 points was observed in the pallidal-stimulation group, and a reduction of 10.7 points was observed in the subthalamic-stimulation group (difference, −1.1; 95% CI, −4.3–2.1; P = 0.50). However, the UPDRS Part III score in the pallidal-stimulation group decreased by 1.2 points, whereas that in the subthalamic-stimulation group increased by 0.8 points, suggesting that the improvement in long-term motor function after stimulation of the STN is not superior to that after stimulation of the GPI and still needs further exploration. The ADLS is another primary evaluation parameter used in patients undergoing DBS and reflects the general living status of patients with PD. In our meta-analysis, the difference in the ADLS between the two target groups was not significant, and the result remained unchanged in the long term (difference, −1.1; 95% CI, −2.6–0.4).
PD is associated with relatively high rates of mood and cognitive dysfunction, and DBS of the STN and GPI has been proven to mildly ameliorate mood dysfunction and cognitive decline, particularly in verbal fluency tasks.41,46 Some controlled studies have provided evidence that DBS of the STN may lead to cognitive or psychiatric sequelae.15,17,18,26 Okun et al.31 performed a comparative trial focusing on cognition and mood performance in patients with PD who underwent stimulation of the STN versus GPI. They found that stimulation of the STN resulted in worse verbal fluency on the letter task and was associated with a higher number of mood/cognitive/surgical adverse events. Interestingly, in a recent randomized trial of bilateral stimulation of the STN versus GPI with cognition/behavior as the primary outcome, Odekerken et al.24 found no significant differences in the two targets with much larger numbers of patients. Both sites are cognitively safe, and overall target-related differences in cognitive outcomes are small. Nevertheless, the most recent meta-analysis suggested that all declines were found in psychomotor speed, memory, attention, executive functions, and overall cognition in patients who underwent DBS of the STN; DBS of the GPI resulted in fewer neurocognitive declines than DBS of the STN.33
Quality of life shows more improvement when patients with PD are treated with a combination of DBS plus optimal medication than with optimal drug therapy alone.7 However, researchers have divergent opinions regarding the effect of the two stimulation targets on quality of life. Using the 39-item Parkinson’s Disease Questionnaire, some researchers have found no significant difference between the two stimulation targets.27 Other researchers consider that greater improvements in quality-of-life measures are achieved with GPI stimulation.32,47
Although medication reduction is not considered the primary goal of DBS, reductions in medication doses postoperatively may lead to improvements in medication-related side effects, including cognitive slowing, sleepiness, impulse control disorder, mania or hypomania, and dyskinesia. On average, the use of dopaminergic medications decreases more in patients undergoing subthalamic than pallidal stimulation.27,28,31,32 The present meta-analysis also confirmed that STN stimulation has advantages over GPI stimulation in terms of medication reduction.
The cost of DBS is an important factor to consider. The average stimulation amplitudes and pulse widths of STN stimulation are lower than those of GPI stimulation, allowing for potentially longer intervals between pulse generator replacement among patients undergoing subthalamic stimulation, with an attendant reduction in the long-term costs of therapy and a reduction in risks associated with surgical replacement of pulse generators.27 Meanwhile, the cost also decreases due to a dramatic drop in the cost of medication and a probable lower frequency of hospital admissions required for medication adjustments.48,49
Limitations
Several factors may bias the findings of the current study. First, bias can be introduced in retrospective reviews without randomized, prospectively matched groups. Second, the reported infection rate was strongly biased by the duration of follow-up among the studies, and various types of infections continued to occur over time. Therefore, studies with very short follow-up times (3–6 months) showed the lowest infection rates, and studies with longer follow-up times showed higher cumulative infection rates. Third, because the interventionists were surgeons, the interventionists and assessors were poorly blinded to which surgery was being performed. Therefore, this permitted bias. Furthermore, the process of patient randomization was poorly described in the reviewed studies; only one study clearly acknowledged that patient assignment was concealed prior to group allocation. Other potentially confounding factors include the operative decisions and techniques of the different surgeons. Additional well-designed multicenter clinical trials that can minimize bias are needed, especially for evaluation of the long-term outcomes of the two stimulation targets.
Conclusion
Based on our meta-analysis and other available data in the literature, the STN may be the preferred target for DBS in consideration of motor function improvement, medication reduction, and economic efficiency, although GPI stimulation has some advantages in terms of mood and cognition. Treatment decisions should be individualized and based on patients’ specific symptoms and expectations.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Lozano AM, Lang AE. Pallidotomy for Parkinson’s disease. Adv Neurol 2001; 86: 413–420. [PubMed] [Google Scholar]
- 2.Lang AE, Obeso JA. Challenges in Parkinson’s disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol 2004; 3: 309–316. [DOI] [PubMed] [Google Scholar]
- 3.Hariz MI, Bergenheim AT. A 10-year follow-up review of patients who underwent Leksell’s posteroventral pallidotomy for Parkinson disease. J Neurosurg 2001; 94: 552–555. [DOI] [PubMed] [Google Scholar]
- 4.Esselink RA, de Bie M, de Haan RJ, et al. Long-term superiority of subthalamic nucleus stimulation over pallidotomy in Parkinson disease. Neurology 2009; 73: 151–153. [DOI] [PubMed] [Google Scholar]
- 5.Ohye C, Shibazaki T, Sato S. Gamma knife thalamotomy for movement disorders: evaluation of the thalamic lesion and clinical results. J Neurosurg 2005; 102(Suppl): 234–240. [DOI] [PubMed] [Google Scholar]
- 6.Tseng HM, Su PC, Liu HM, et al. Bilateral subthalamotomy for advanced Parkinson disease. Surg Neurol 2007; 68(Suppl 1): S43–S50. discussion S50–S51. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006; 355: 896–908. [DOI] [PubMed] [Google Scholar]
- 8.Wang JW, Zhang YQ, Zhang XH, et al. Cognitive and Psychiatric Effects of STN versus GPi Deep Brain Stimulation in Parkinson's Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS One 2016; 11: e0156721. [DOI] [PMC free article] [PubMed]
- 9.Moro E, Lozano AM, Pollak P, et al. Long-termresults of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord 2010; 25: 578–586. [DOI] [PubMed] [Google Scholar]
- 10.Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 1998; 339: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 11.Obeso JA, Olanow CW, et al. Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars in-terna of the globus pallidus in Parkinson’s disease. N Engl J Med 2001; 345: 956–963. [DOI] [PubMed] [Google Scholar]
- 12.Houeto JL, Damier P, Bejjani PB, et al. Subthalamic stimulation in Parkinson disease: a multidis -ciplinary approach. Arch Neurol 2000; 57: 461–465. [DOI] [PubMed] [Google Scholar]
- 13.Krack P, Benazzouz A, Pollak P, et al. Treatment of tremor in Parkinson’s disease by subthala-mic nucleus stimulation. Mov Disord 1998; 13: 907–914. [DOI] [PubMed] [Google Scholar]
- 14.Ostergaard K, Sunde N, Dupont E. Effects of bilateral stimulation of the subthalamic nucleus in patients with severe Parkinson’s dis-ease and motor fluctuations. Mov Disord 2002; 17: 693–700. [DOI] [PubMed] [Google Scholar]
- 15.Kim YE, Jeon BS, Paek SH, et al. Rapid eye movement sleep behavior disorder after bilateral subthalamic stimulation in Parkinson’s disease. J Clin Neurosci 2015; 22: 315–319. [DOI] [PubMed] [Google Scholar]
- 16.Vesper J, Klostermann F, Stockhammer F, et al. Re-sults of chronic subthalamic nucleus stimulation for Parkinson’s disease: a 1-year follow-up study. Surg Neurol 2002; 57: 306–313. [DOI] [PubMed] [Google Scholar]
- 17.Aono M, Iga J, Ueno S, et al. Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in Parkinson’s disease. J Clin Neurosci 2014; 21: 1595–1598. [DOI] [PubMed] [Google Scholar]
- 18.Thobois S, Mertens P, Guenot M, et al. Subthalamic nucleus stimulation in Parkinson’s disease: clinical evaluation of 18 patients. J Neurol 2002; 249: 529–534. [DOI] [PubMed] [Google Scholar]
- 19.Loher TJ, Burgunder JM, Pohle T, et al. Long-term pallidal deep brain stimulation in patients with advanced Parkinson disease: 1-year follow-up study. J Neu-rosurg 2002; 96: 844–853. [DOI] [PubMed] [Google Scholar]
- 20.Vingerhoets G, Lannoo E, van der Linden C, et al. Changes in quality of life following unilateral pallidal stimulation in Parkinson’s disease. J Psychosom Res 1999; 46: 247–255. [DOI] [PubMed] [Google Scholar]
- 21.Straits-Troster K, Fields JA, Wilkinson SB, et al. Health-related quality of life in Parkinson’s dis-ease after pallidotomy and deep brain stimulation. Brain Cogn 2000; 42: 399–416. [DOI] [PubMed] [Google Scholar]
- 22.Scotto di Luzio AE, Ammannati F, Marini P, et al. Which target for DBS in Parkinson’s disease? Subthalamic nu-cleus versus globus pallidus internus. Neurol Sci 2001; 22: 87–88. [DOI] [PubMed] [Google Scholar]
- 23.Fahn S, Elton RL. UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M. (eds). Recent developments in Parkinson’s disease 1987; Vol. 2, Florham Park, NJ: Macmillan Health Care Information, pp. 153–164. [Google Scholar]
- 24.Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 2013; 12: 37–44. [DOI] [PubMed] [Google Scholar]
- 25.Pahwa R, Wilkinson S, Smith D, et al. High-frequency stimulation of the globus pallidus for the treatment of Parkinson’s disease. Neurology 1997; 49: 249–253. [DOI] [PubMed] [Google Scholar]
- 26.Evidente VG, Premkumar AP, Adler CH, et al. Medication dose reductions after pallidal versus subthalamic stimulation in patients with Parkinson’s disease. Acta Neurol Scand 2011; 124: 211–214. [DOI] [PubMed] [Google Scholar]
- 27.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 2010; 362: 2077–2091. [DOI] [PubMed] [Google Scholar]
- 28.Burchiel KJ, Anderson VC, Favre J, et al. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson’s disease: results of a randomized, blinded pilot study. Neurosurgery 1999; 45: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Christine CW, Starr PA, et al. Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov Disord 2007; 22: 619–626. [DOI] [PubMed] [Google Scholar]
- 30.Krause M, Fogel W, Heck A, et al. Deep brain stimulation for the treatment of Parkinson’s disease: subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry 2001; 70: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brainstimulation: the COMPARE trial. Ann Neurol 2009; 65: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahodne LB, Okun MS, Foote KD, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus ascompared to the subthalamic nucleus. J Neurol 2009; 256: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combs HL, Folley BS, Berry DT, et al. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus palliduspars internus in Parkinson’s disease: a meta-analysis. Neuropsychol Rev 2015; 25: 439–454. [DOI] [PubMed] [Google Scholar]
- 34.Benabid AL, Chabardes S, Mitrofanis J, et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 2009; 8: 67–81. [DOI] [PubMed] [Google Scholar]
- 35.Schoenen J, Di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain 2005; 128(Pt 4): 940–947. [DOI] [PubMed] [Google Scholar]
- 36.Carron R, Fraix V, Maineri C, et al. High frequency deep brain stimulation of the subthalamic nucleus versus continuous subcutaneous apomorphine infusion therapy: a review. J Neural Transm (Vienna) 2011; 118: 915–924. [DOI] [PubMed] [Google Scholar]
- 37.Joint C, Nandi D, Parkin S, et al. Hardware-related problems of deep brain stimulation. Mov Disord 2002; 17(Suppl 3): S175–S180. [DOI] [PubMed] [Google Scholar]
- 38.Lyons KE, Wilkinson SB, Overman J, et al. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology 2004; 63: 612–616. [DOI] [PubMed] [Google Scholar]
- 39.Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery 2008; 62: 360–366. [DOI] [PubMed] [Google Scholar]
- 40.Yágüez L, Costello A, Moriarty J, et al. Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in Parkinson’s disease. J Clin Neurosci 2014; 21: 445–450. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain 2005; 128(Pt 10): 2240–2249. [DOI] [PubMed] [Google Scholar]
- 42.Macia F, Perlemoine C, Coman I, et al. Parkinson’s disease patients with bilateral subthalamic deep brain stimulation gain weight. Mov Disord 2004; 19: 206–212. [DOI] [PubMed] [Google Scholar]
- 43.Strowd RE, Cartwright MS, Passmore LV, et al. Weight change following deep brain stimulation for movement disorders. J Neurol 2010; 257: 1293–1297. [DOI] [PubMed] [Google Scholar]
- 44.Smeding HM, Speelman JD, Koning-Haanstra M, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology 2006; 66: 1830–1836. [DOI] [PubMed] [Google Scholar]
- 45.Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 2005; 62: 554–560. [DOI] [PubMed] [Google Scholar]
- 46.Saint-Cyr JA, Trepanier LL, Kumar R. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain 2000; 123(Pt 10): 2091–2108. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez RL, Miller K, Bowers D, et al. Mood and cognitive changes with deep brain stimulation. What we know and where we should go. Minerva Med 2005; 96: 125–144. [PubMed] [Google Scholar]
- 48.Fraix V, Houeto JL, Lagrange C, et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006; 77: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meissner W, Trottenberg T, Klaffke S, et al. Apomorphine therapy versus deep rain stimulation. Clinical and economic aspects in patients with advanced Parkinson disease. Nervenarzt 2001; 72: 924–927. [in German, English Abstract]. [DOI] [PubMed] [Google Scholar]