Abstract
Objective
To compare the medium-term clinical and radiographic outcomes of Dynesys dynamic stabilization and posterior lumbar interbody fusion (PLIF) for treatment of multisegmental lumbar degenerative disease.
Methods
Fifty-seven patients with multisegmental lumbar degenerative disease underwent Dynesys stabilization (n = 26) or PLIF (n = 31) from December 2008 to February 2010. The mean follow-up period was 50.3 (range, 46–65) months. Clinical outcomes were evaluated using a visual analogue scale (VAS) and the Oswestry disability index (ODI). Radiographic evaluations included disc height and range of motion (ROM) of the operative segments and proximal adjacent segment on lumbar flexion-extension X-rays. The intervertebral disc signal change was defined by magnetic resonance imaging, and disc degeneration was classified by the Pfirrmann grade.
Results
The clinical outcomes including the VAS score and ODI were significantly improved in both groups at 3 months and the final follow-up, but the difference between the two was not significant. At the final follow-up, the disc height of stabilized segments in both groups was significantly increased; the increase was more notable in the Dynesys than PLIF group. The ROM of stabilized segments at the final follow-up decreased from 6.20° to 2.76° and 6.56° to 0.00° in the Dynesys and PLIF groups, respectively. There was no distinct change in the height of the proximal adjacent segment in the two groups. The ROM of the proximal adjacent segment in both groups increased significantly at the final follow-up; the change was significantly greater in the PLIF than Dynesys group. Only one case of adjacent segment degeneration occurred in the PLIF group, and this patient underwent a second operation.
Conclusions
Both Dynesys stabilization and PLIF can improve the clinical and radiographic outcomes of multisegmental lumbar degenerative disease. Compared with PLIF, Dynesys stabilization can maintain the mobility of the stabilized segments with less influence on the proximal adjacent segment and may help to prevent the occurrence of adjacent segment degeneration. Dynesys is reliable for the treatment of multisegmental lumbar degenerative disease at the medium-term follow-up.
Keywords: Dynesys (dynamic neutralization system), posterior lumbar interbody fusion (PLIF), multisegment, lumbar degenerative disease
Introduction
Multisegmental lumbar disc disease is a common problem in spinal surgery and primarily occurs in older patients. Posterior lumbar interbody fusion (PLIF) was historically considered the gold standard treatment for lumbar degenerative disease. With increasing research, more reports have described the deficiencies of fusion surgery, such as adjacent segment degeneration (ASD) and acquired spinal instability after the fusion operation. Non-fusion technology has been developed to solve these problems. As a posterior lumbar non-fusion technique, Dynesys dynamic stabilization has become more widely performed.1,2 The Dynesys system was designed by Dubois et al. and was first applied in the clinical setting in 1994. The Dynesys dynamic stabilization system aims to maintain the range of motion (ROM) of the fixed segment, preserve the lumbar spinal stability, and prevent ASD. Clinical reports of Dynesys stabilization have mainly focused on the short-term clinical efficacy; medium- and long-term clinical follow-up studies, especially those involving PLIF as a control, are lacking.3 In this retrospective study, we analyzed the clinical effects and radiographic results of Dynesys dynamic stabilization versus PLIF in the treatment of multisegmental lumbar degenerative disease.
Materials and methods
General data
A total of 57 patients underwent operations for multisegmental lumbar degenerative disease at The Affiliated Hospital of School of Medicine of Ningbo University from December 2008 to February 2010. Enrolled patients were randomized to either the Dynesys or PLIF group. The Dynesys group comprised 26 patients (14 female, 12 male) with a mean age of 49.6 years (range, 36–73 years). Of these 26 patients, 16 (61.5%) had lumbar intervertebral disc herniation and 10 (38.5%) had degenerative spinal stenosis. Eighteen patients had involvement of two segments, and eight patients had involvement of three segments. The PLIF group comprised 31 patients (18 female, 13 male) with a mean age of 52.5 years (range, 38–77 years). Of these 31 patients, 18 (58.1%) had lumbar intervertebral disc herniation and 13 (41.9%) had degenerative spinal stenosis. Nineteen patients had involvement of 2 segments, 11 patients had involvement of 3 segments, and 1 patient had involvement of 4 segments.
Symptoms in this study included low back pain with or without neurologic claudication or sciatic pain. Three months of nonoperative treatment failed in all patients. This study was approved by the Human Ethics Committee of the Affiliated Hospital of the Medical College of Ningbo University. Each patient enrolled in this study provided written informed consent. All patients were followed up for at least 46 months and underwent X-ray and magnetic resonance imaging (MRI) examinations.
The inclusion criteria were involvement of two or more lumbar segments, neurogenic claudication caused by lumbar spinal stenosis, or low back or leg pain caused by degenerative intervertebral disc disease.4–6 The exclusion criteria were severe osteoporosis; severe spinal deformity; grade ≥2 lumbar degenerative spondylolisthesis; progressive intervertebral disc degeneration; serious instability associated with lumbar spine disease; obesity (body mass index of ≥30 kg/m2); serious heart, brain, or lung disease; trauma; infection; malignant tumors; history of lumbar spine surgery; and pelvic-related disease.
Surgical techniques
All operations were performed by the same surgical team at a single institution. The patients were placed in the prone position, and a standard surgical procedure was used for posterior lumbar spine surgery. Through a midline incision and after subperiosteal dissection of the erector spine muscles, the affected segment and entrance points for the pedicle screws were exposed. Radiographic guidance with a C-arm X-ray machine was used to ensure that the Dynesys pedicle screws were safely inserted. Interlaminar decompression and laminotomy were performed in the Dynesys group depending on each patient’s condition. However, extensive decompression such as semi-laminectomy or total laminectomy was performed in patients with severe stenosis or far lateral stenosis. All procedures were carefully performed to avoid destruction of the facet joints.7 Polycarbonate urethane spacers and polyethylene terephthalate cords were accurately assembled according to the technical suggestions provided by the manufacturer. Finally, the incision was flushed, a drainage tube was placed, and the incision was closed in a step-by-step manner.
Patients in the PLIF group underwent standard semi-laminectomy or total laminectomy according to the severity of their disease. The nucleus pulposus was carefully removed, nerve root was completely released, and an interbody fusion cage filled with artificial bone material was inserted.
Postoperative management
Antibiotics were routinely administered for 1 to 3 days after surgery. The drainage tube was removed 24 to 72 hours postoperatively. The patients were encouraged to cough and produce expectoration as well as move their lower limbs after the operation to prevent deep vein thrombosis. Patients in the Dynesys group wore a soft lumbar orthosis for 12 weeks. In contrast, patients in the PLIF group wore a hard orthosis for 3 months.
Clinical and radiological evaluation
Evaluations were performed preoperatively, 3 months postoperatively, and at the final follow-up. Back and leg pain were evaluated using a visual analogue scale (VAS) and the Oswestry disability index (ODI).8,9 Radiological evaluation included lumbar flexion-extension and lateral standing radiography, computed tomography, and MRI.
Radiological measurements were performed as follows. (1) The average intervertebral space height (AH) was calculated using the ventral intervertebral space height (VH), dorsal intervertebral space height (DH), and central intervertebral space height (CH): AH = (VH + CH + DH)/3. (2) Segmental ROM was calculated as the angle between the inferior surface of the upper vertebrae and the superior surface of the lower vertebrae on the lateral standing lumbar flexion-extension X-ray. (3) All patients underwent lumbar MRI both preoperatively and at the final follow-up to evaluate changes in the height of the adjacent degenerative intervertebral discs and signals of the intervertebral discs. Disc degeneration was graded on T2-weighted sagittal and axial MRI according to the modified method described by Pfirrmann.10
Radiologic ASD was diagnosed by the development of olisthesis of 4 mm, a 10° angular change on flexion-extension lateral radiographs, 10% loss of disc height, or grade ≥2 disc deterioration (University of California at Los Angeles disc degeneration grading scale).3,11,12 On MRI, radiologic ASD was also diagnosed by a modified Pfirrmann grade of IV or V or the presence of spinal stenosis or disc herniation at the adjacent level. Clinical ASD was diagnosed in the presence of (1) symptomatic spinal stenosis, (2) intractable back pain, or (3) subsequent sagittal or coronal balance as suggested by Cheh et al.13 Symptomatic spinal stenosis was defined as MRI-diagnosed stenosis accompanied by clinical neurologic claudication.
Radiographs were evaluated three times independently by two experienced spine surgeons in this study. We used the mean value of each parameter in all analyses and resolved disagreements through discussion or by consultation with a senior surgeon.
Statistical assessment
Statistical analyses were performed using SPSS 18.0 statistics software (SPSS Inc., Chicago, IL, USA). Measurement data are presented as mean ± standard deviation (SD), and enumeration data were evaluated with the chi square test. Categorical data were compared by the Wilcoxon signed rank test. A P-value of <0.05 was considered statistically significant.
Results
Clinical outcomes
All patients were followed up. The Dynesys group was followed up for 50.3 months (range, 46–65 months); the mean operating time was 158.1 minutes (range, 100–255 min), and the mean blood loss volume was 380 ml (range, 200–850 ml). The PLIF group was followed up for 52.8 months (range, 48–68 months); the mean operating time was 182.5 minutes (range, 120–300 min), and the mean blood loss volume was 470 ml (range, 250–1080 ml). The sex, age, follow-up time, and disease distribution were not significantly different between the two groups. However, the operation time and blood loss volume were significantly lower in the Dynesys than PLIF group (P < 0.05).
Clinical symptoms such as back and leg pain were significantly improved at the final follow-up in both groups. The VAS score and ODI were also significantly improved at the 3-month and final follow-ups in both groups. Moreover, the VAS score and ODI were significantly better at the final follow-up than at the 3-month follow-up in the Dynesys group (P < 0.05) (Tables 1, 2, and 3).
Table 1.
Patient characteristics in the two groups.
| Group | n | Sex |
Type of disease |
Follow-up (months) | |
|---|---|---|---|---|---|
| Male/female | Age (years) | LDH/LSS | |||
| Dynesys | 26 | 14/12 | 49.6 ± 8.3 | 16/10 | 50.3 |
| PLIF | 31 | 18/13 | 52.5 ± 6.9 | 18/13 | 52.8 |
Data are presented as n or mean ± standard deviation.
PLIF, posterior lumbar interbody fusion; LDH, lumbar disc herniation; LSS, lumbar spinal stenosis
Table 2.
Visual analogue scale scores.
| Low back pain |
Leg pain |
|||||
|---|---|---|---|---|---|---|
| Preoperative | 3 months | Final follow-up | Preoperative | 3 months | Final follow-up | |
| Dynesys | 5.50 ± 1.61 | 1.69 ± 0.84 | 1.31 ± 0.79 | 5.77 ± 1.37 | 1.81 ± 0.94 | 1.38 ± 0.80 |
| PLIF | 5.48 ± 1.44 | 1.90 ± 1.66 | 1.35 ± 0.80 | 5.74 ± 1.24 | 1.97 ± 1.02 | 1.45 ± 0.77 |
Data are presented as mean ± standard deviation. P values are based on the t test; P > 0.05 compared with Dynesys group. PLIF, posterior lumbar interbody fusion
Table 3.
Oswestry Disability Index.
| Preoperative | 3 months | Final follow-up | |
|---|---|---|---|
| Dynesys | 57.92 ± 7.25 | 29.38 ± 5.95 | 24.38 ± 7.39 |
| PLIF | 57.29 ± 9.83 | 30.26 ± 10.83 | 23.80 ± 7.12 |
Data are presented as mean ± standard deviation. P values are based on the t test; P > 0.05 compared with Dynesys group. PLIF, posterior lumbar interbody fusion
Radiological outcomes
The preoperative and postoperative radiologic parameters in the Dynesys and PLIF groups are shown in Table 4. At the final follow-up, the disc height of the fixed segments was slightly increased in both groups and significantly increased in the PLIF group (P < 0.05). ASD frequently occurs superior to the fixed segment; therefore, we only evaluated the radiological outcomes of the proximal adjacent segment in this study.14,15 The disc height of the proximal adjacent segment was not significantly decreased in the two groups. The ROM of the fixed segments in the Dynesys group decreased from 6.20° preoperatively to 2.76° at the final follow-up; in the PLIF group, the ROM decreased from 6.56° to 0.00°. The height of the proximal adjacent segment was not significantly different between the two groups. The ROM of the proximal adjacent segment was increased at the final follow-up, especially in the PLIF group, with a significant difference between the two groups (P < 0.05). Comparison of proximal adjacent Disc degeneration shows in Table 6. Typical case as shown in Figure 1–2.
Table 4.
Radiographic parameters of surgical and proximal adjacent segments.
| Preoperative | 3 months | Final follow-up | |
|---|---|---|---|
| Intervertebral height of operative segment | |||
| Dynesys | 10.47 ± 1.67 | 11.38 ± 2.06a | 11.05 ± 1.52 |
| PLIF | 10.81 ± 1.43 | 12.93 ± 1.72 | 12.57 ± 1.66a,c |
| Intervertebral height of proximal adjacent segment | |||
| Dynesys | 10.60 ± 1.93 | 10.65 ± 2.02b | 10.54 ± 2.30b |
| PLIF | 10.68 ± 2.08 | 10.71 ± 2.03b | 10.67 ± 2.13b,d |
| ROM of operative segment | |||
| Dynesys | 6.20 ± 1.91 | 2.27 ± 1.73a | 2.76 ± 1.53a |
| PLIF | 6.56 ± 1.61 | 0.00 ± 0.00a | 0.00 ± 0.00a,c |
| ROM of proximal adjacent segment | |||
| Dynesys | 6.85 ± 2.04 | 7.47 ± 2.21a | 8.30 ± 1.65a |
| PLIF | 7.01 ± 1.83 | 7.95 ± 2.35a | 10.86 ± 2.09a,c |
Data are presented as mean ± standard deviation.
Compared with preoperatively, P < 0.05; bCompared with preoperatively, P > 0.05
Compared with Dynesys group, P < 0.05; dCompared with Dynesys group, P > 0.05
ROM, range of motion; PLIF, posterior lumbar interbody fusion
Table 6.
Modified Pfirrmann grade of proximal adjacent segment in the two groups.
| Preoperative grade | Final grade in Dynesys group |
Final grade in PLIF group |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | Total | 3 | 4 | 5 | 6 | 7 | 8 | Total | |
| 3 | 2 | 5 | 1 | 1 | 3 | 2 | ||||||||
| 4 | 1 | 5 | 3 | 3 | 5 | |||||||||
| 5 | 4 | 3 | 2 | 4 | 2 | |||||||||
| 6 | 1 | 1 | 3 | 3 | 1 | |||||||||
| 7 | 1 | 1 | ||||||||||||
| 8 | ||||||||||||||
| Total | 26 | 31 | ||||||||||||
PLIF, postoperative lumbar interbody fusion
Note: The grade of proximal adjacent segment, preoperation/postoperation P < 0.05
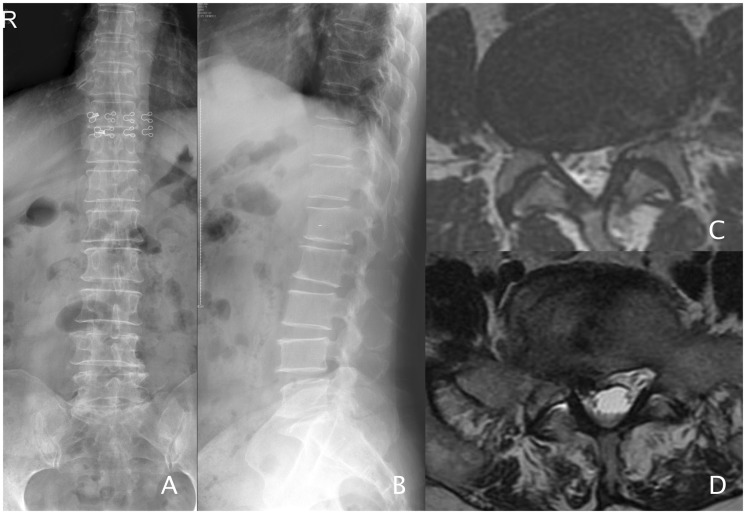
Figure 1.
Femail, 59, Farmer. lumbar spinal stenosis underwent spinal canal decompression and Dynesys fixation in L4/5 and L5/S1. a–b: Preoperative X-rays. c: L4/5(MRI-T2). d: L5/S1(MRI-T2), disc herniation with stenosis.
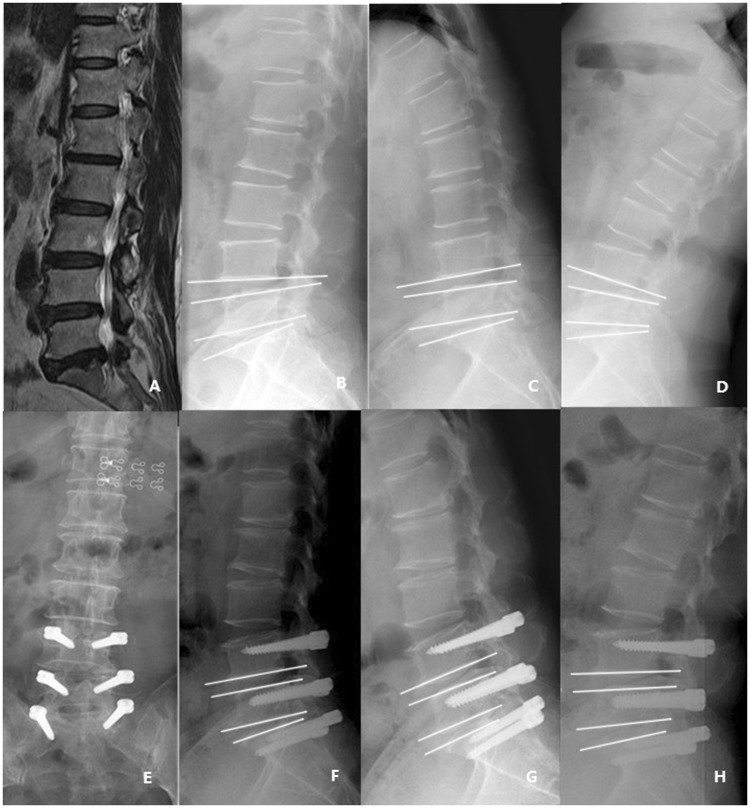
Figure 2.
a: The preoperative lumbar MRI(T2). b–d: The preoperative flexion and extension X-rays. The ROM of L4/5 was 10°, and the ROM of L5/S1 was 7°. e–h: The flexion and extension X-rays 52 months after operation. The ROM of L4/5 was 6°, and the ROM of L5/S1 was 5°.
Complications
Two cases of pedicle screw loosening, both in S1, were found at the final follow-up in two patients in the Dynesys group. No screw breakage occurred in the Dynesys group. One patient in the Dynesys group developed fat liquefaction that progressed to an incision infection. Two patients in the PLIF group developed cerebrospinal fluid leakage (one from an incision infection and one from nerve root injury), and one underwent a second surgery because of symptoms of ASD. No perioperative death occurred in either group (Table 5).
Table 5.
Complications in the two groups.
| Group | Nerve root injury | Dural tear | Screw loosening | Reoperation | Incision infection |
|---|---|---|---|---|---|
| Dynesys | 0 | 0 | 2 | 0 | 1 |
| PLIF | 1 | 2 | 0 | 1 | 1 |
Data are presented as number of patients.
PLIF, posterior lumbar interbody fusion
Discussion
The Dynesys system was developed by Gilles Dubois in 1991 and first applied in the clinical setting in France in 1994.16 The clinical application of the Dynesys system for multilevel lumbar degenerative disease (degenerative scoliosis) was first reported in 2010.17 The Dynesys system is used to preserve the normal function of the fixed segments and maintain spinal stability, thus avoiding ASD.18 Under the condition of keeping certain activity for fixed segments and lumbar lordosis, Dynesys dynamic stabilization not only limits abnormal motion of the unstable segment but also reduces the load on the intervertebral disc and facet joint, achieving the therapeutic goal of the Dynesys system. In contrast to PLIF, Dynesys technology can carry and balance the load between vertebrae while retaining the motor function of the fixed segments and reduce the influences of stress and movement of the adjacent segment. Therefore, the occurrence of ASD can be avoided or delayed. Additionally, after movement recovery and load delivery, the degenerated intervertebral disc has the potential for self-repair.19 Theoretically, Dynesys dynamic stabilization has more advantages than PLIF.
Dynesys stabilization has been performed for more than 20 years, and many studies have verified its clinical effects. However, most of these studies were short-term case reports; comparative studies with PLIF are lacking.20,21 Through its retrospective case-control design, the present study allowed for comparison of the clinical effects and radiographic characteristics of Dynesys stabilization versus PLIF for the treatment of multisegmental lumbar degenerative disease. In addition, the average follow-up duration was more than 46 months. The clinical effects at all follow-up points showed obvious improvement over the condition before treatment (P < 0.05), suggesting that Dynesys stabilization has a definite therapeutic effect in treating multisegmental lumbar degenerative disease. Moreover, the postoperative lumbar disc height of the fixed segment was greater than that preoperatively (P < 0.05), and the ROM decreased from 6.20° ± 1.91° preoperatively to 2.76° ± 1.53° at the final follow-up. This indicates that Dynesys stabilization partially preserves the motion of the operated segment based on stabilization of the lumbar vertebrae. Additionally, there was no significant difference in the lumbar disc height or ROM of the proximal adjacent segment before and after treatment, indicating that Dynesys stabilization can effectively reduce the influence of these parameters on the adjacent segment in the middle-term follow-up. Dynesys stabilization causes a relatively small operative wound and provides a satisfactory effect, and the overall rate of complications such as implant failure and breakage was lower than that of PLIF; therefore, we consider that PLIF can be replaced by Dynesys dynamic stabilization for fixation in patients with lumbar degenerative disease. In total, of the 172 screws used among 26 patients who underwent Dynesys stabilization in this study, only 2 screws had become loose at the medium-term follow-up, both located at S1, within 6 months postoperatively. These patients had no associated symptoms, and screw loosen and breakage were not visible temporarily. Additionally, no delayed infection occurred and no postoperative revision was needed. Stoll et al.22 reported that most cases of screw loosening occurred in the closest proximal or closest distal segment. Consistent with this, both cases of screw loosening in the present study occurred in the closest distal segment. Furthermore, both were located at S1, which can likely be explained by the difficulty in gaining a satisfactory angle during implantation because of the shorter length of the S1 screw and higher tension in the lumbosacral region. The greater stress of the S1 screw after a multisegment operation is probably another cause. Regardless of this complication, we still believe that Dynesys dynamic stabilization is safe and effective in treating multisegmental lumbar degenerative disease.
The clinical features of multisegmental lumbar degenerative disease are complex and atypical, making localization difficult. Extensive decompression and multilevel fusion have been widely adopted. However, ASD after multilevel fusion is attracting increasingly more attention. One study suggested that 24% to 45% of patients with lumbar interbody fusion develop accelerated ASD,23 which leads to spinal stenosis of the adjacent segment, vertebral instability, and facet joint osteoarthritis. Cadaver studies have revealed that the pressure and ROM within the adjacent intervertebral disc after long-level fusion is clearly higher than that after short-level fusion.24 In patients with multisegmental lumbar degenerative disease, the influence of Dynesys stabilization on the development of ASD is a current area of clinical research. When comparing simple lumbar discectomy and lumbar interbody fusion, Dynesys stabilization is capable of removing the herniated nucleus pulposus and releasing nerve compression. Moreover, it can restore the lumbar disc height of the operative segment and maintain the structure of the lumbar spine. Furthermore, it can preserve the ROM of the operative segment, reduce the stress load on the adjacent segment, and compensation of motion range.16 Many studies have also supported the idea that Dynesys stabilization can delay the occurrence of adjacent-level degeneration. However, its specific mechanism is yet unclear. Ciavarro et al.25 determined the glycosaminoglycan content of the intervertebral disc in the fixed segment and adjacent segment after Dynesys stabilization using MRI with delayed enhancement. They found that the glycosaminoglycan content was increased at both 6 and 24 months postoperatively. In addition, the content was higher 24 months than at 6 months postoperatively. The authors concluded that Dynesys stabilization can delay degeneration and promote self-repair of the intervertebral disc. Beastall et al.26 made months MRI follow-up for 24 patients who underwent Dynesys stabilization and found that the fixed segment holds part of motion, but no obvious change occurred at the adjacent segment; additionally, the anterior height of the intervertebral space decreased, while there was no apparent increase of the posterior of intervertebral space. Therefore, Dynesys stabilization could prevent the occurrence of ASD. However, some researchers doubt the role of Dynesys stabilization in preventing and releasing ASD.25–27 Vaga et al.28 performed MRI with delayed enhancement of the cartilage and intervertebral discs preoperatively and 6 months postoperatively in patients who underwent Dynesys stabilization. They discovered that Dynesys stabilization can delay or partly reverse the degeneration of the intervertebral disc, especially for patients with severely degenerative intervertebral discs. However, Dynesys stabilization increased the load on the adjacent segment, which led to the early development of ASD. After the average 50.3-month follow-up in the present study, the differences in the variation of the lumbar space height at the upper end of the adjacent segment between the Dynesys and PLIF groups were not statistically significant. The ROM at the upper end of the adjacent segment was greater at the final follow-up than preoperatively. Additionally, the ROM at the upper end of the adjacent segment was greater in the PLIF than Dynesys group at the final follow-up, indicating that Dynesys stabilization better retains the motion of the adjacent segment than does PLIF. Moreover, temporary development of adjacent lumbar spondylosis was not found during follow-up in the Dynesys group. However, one patient in the PLIF group required a second operation because of symptoms of ASD. The factors that affect the development of ASD remain unclear. Determination of whether Dynesys stabilization can actually prevent the occurrence of ASD requires further high-quality randomized controlled trials with long-term follow-up.
In conclusion, Dynesys dynamic stabilization provides a satisfactory medium-term effect in treating multisegmental lumbar degenerative disease. Dynesys stabilization results in a smaller wound, which benefits for multisegment recovery, and the clinical follow-up data showed that Dynesys stabilization can partially preserve the ROM of the fixed segments and limit abnormal movement of the lumbar spine. It also has little effect on the adjacent segment and is associated with a lower incidence of complications such as screw loosening and breakage. However, the specific mechanism for the prevention of ASD remains unclear. No detailed data are available to verify that Dynesys stabilization can delay ASD. Because of the small number of cases in the present study, determination of the long-term effects of Dynesys dynamic stabilization and its impact on the adjacent segment requires further clinical research with long-term follow-up.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Fay LY, Wu JC, Tsai TY, et al. Dynamic stabilization for degenerative spondylolisthesis: evaluation of radiographic and clinical outcomes. Clin Neurol Neurosurg 2013; 115: 535–541. [DOI] [PubMed] [Google Scholar]
- 2.Wu HT, Jiang GQ, Lu B, et al. Long-term follow-up of Dynesyssystem in clinical application for the treatment of multiple lumbar degenerative disease. Zhong guo Gu Shang 2015; 28: 1000–1005. [in Chinese, English Abstract]. [PubMed] [Google Scholar]
- 3.Min JH, Jang JS, Lee SH. Comparison of anterior- and posterior-approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine 2007; 7: 21–26. [DOI] [PubMed] [Google Scholar]
- 4.Kuo CH, Chang PY, Wu JC, et al. Dynamic stabilization for L4-5 spondylolisthesis: comparison with minimally invasive transforaminal lumbar interbody fusion with more than 2 years of follow-up. Neurosurg Focus 2016; 40: E3–E3. [DOI] [PubMed] [Google Scholar]
- 5.Pham MH, Mehta VA, Patel NN, et al. Complications associated with the Dynesys dynamic stabilization system: a comprehensive review of the literature. Neurosurg Focus 2016; 40: E2–E2. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CH, Chang PY, Tu TH, et al. The effect of lumbar lordosis on screw loosening in dynesys dynamic stabilization: four-year follow-up with computed tomography. Biomed Res Int 2015; 2015: 152435–152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SE, Jahng TA, Kim HJ. Facet joint changes after application of lumbarnonfusion dynamic stabilization. Neurosurg Focus 2016; 40: E6–E6. [DOI] [PubMed] [Google Scholar]
- 8.Turner NM, van de Leemput AJ, Draaisma JM, et al. Validityof the visual analogue scale as an instrument to measure self-efficacy inresuscitation skills. Med Educ 2008; 42: 503–511. [DOI] [PubMed] [Google Scholar]
- 9.Lackey A, Phan K and Mobbs R. A systematic review and meta-analysis of outcomes in hybrid constructs for multi-level lumbar degenerative disc disease. J Clin Neurosci 2016; 34: 23–29. [DOI] [PubMed]
- 10.Griffith JF, Wang YX, Antonio GE, et al. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine(Phila Pa 1976) 2007; 32: E708–E712. [DOI] [PubMed] [Google Scholar]
- 11.Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am 2004; 86–A: 1497–1503. [DOI] [PubMed] [Google Scholar]
- 12.Lee DY, Jung TG, Lee SH. Single-level instrumented mini-open transforaminal lumbar interbody fusion in elderly patients. J Neurosurg Spine 2008; 9: 137–144. [DOI] [PubMed] [Google Scholar]
- 13.Cheh G, Bridwell KH, Lenke LG, et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007; 32: 2253–2257. [DOI] [PubMed] [Google Scholar]
- 14.Hikata T, Kamata M, Furukawa M. Risk factors for adjacent segment disease after posterior lumbar interbody fusion and efficacy of simultaneous decompression surgery for symptomatic adjacent segment disease. J Spinal Disord Tech 2014; 27: 70–75. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Shan JL, Liu XM, et al. Comparison of the Dynesys dynamic stabilization system and posterior lumbar interbody fusion for lumbar degenerative disease. PLoS One 2016; 11: e0148071–e0148071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fay LY, Wu JC, Tsai TY, et al. Dynamic stabilization for degenerative spondylolisthesis: evaluation of radiographic and clinical outcomes. Clin Neurol Neurosurg 2013; 115: 535–541. [DOI] [PubMed] [Google Scholar]
- 17.Di Silvestre M, Lolli F, Bakaloudis G, et al. Dynamic stabilization for degenerative lumbar scoliosis in elderly patients. Spine (Phila Pa 1976) 2010; 35: 227–234. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta DK, Herkowitz HN. Pedicle screw-based posterior dynamic stabilization: literature review. Adv Orthop 2012; 2012: 424268–424268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu SW, Yang SC, Ma CH, et al. Comparison of Dynesys posterior stabilization and posterior lumbar interbody fusion for spinal stenosis L4L5. Acta Orthop Belg 2012; 78: 230–239. [PubMed] [Google Scholar]
- 20.Wang Q, Liu J, Shi Y, et al. Short-term effects of a dynamic neutralization system (Dynesys) for multi-segmental lumbar disc herniation. Eur Spine J 2016; 25: 1409–1416. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Li C, Chen Z, et al. Short term outcome of posterior dynamicstabilization system in degenerative lumbar diseases. Indian J Orthop 2014; 48: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi center study of a novel non fusion system[J]. Eur Spine J 2002; 11(Suppl 2): S170–S178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Untch C, Liu Q, Hart R. Segmental motion adjacent to an instrumented lumbar fusion; the effect of extension of fusion to the secrum[J]. Spine (Phlia Pa 1976) 2004; 29: 2376–2381. [DOI] [PubMed] [Google Scholar]
- 24.Rao RD, David KS, Wang M. Biomechanical changes at adjacent segments following anterior lumbar interbody fusion using tepered cages[J]. Spine (Phlia Pa 1976) 2005; 30: 2772–2776. [DOI] [PubMed] [Google Scholar]
- 25.Ciavarro C, Caiani EG, Brayda Bruno M, et al. Mid-term evaluation of the effects of dynamic neutralization system on lumbar intervertebral discs using quantitative molecular MR imaging. J Magn Reson Imaging 2012; 35: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 26.Beastall J, Karadimas E, Siddiqui M, et al. The Dynesys lumbar spinal stabilization system: a preliminary report onpositional magnetic resonance imaging findings. Spine (Phila Pa 1976) 2007; 32: 685–690. [DOI] [PubMed] [Google Scholar]
- 27.St-Pierre GH, Jack A, Siddiqui MM, et al. Nonfusion does not prevent adjacent segment disease: dynesys long-term outcomes with minimum five-year follow-up. Spine (Phila Pa 1976) 2016; 41: 265–273. [DOI] [PubMed] [Google Scholar]
- 28.Vaga S, Brayda-Bruno M, Perona F, et al. Molecular MR imaging for the evaluation of the effect of dynamic stabilization on lumbar intervertebral discs. Eur Spine J 2009; 18(Suppl 1): 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]