Abstract
Objective
This study aimed to describe treatment of serious complications after primary thoracic endovascular aortic repair (TEVAR) in type B aortic dissection.
Methods
From June 2008 to March 2016, serious complications occurred in 58 patients without Marfan syndrome who received TEVAR for type B aortic dissection.
Results
Complications included endoleak, distal true lumen collapse, retrograde dissection, stroke, stent–graft (SG) migration and mistaken deployment, lower limb ischaemia, and SG fracture. Treatment included endovascular repair, surgical procedures, or conservative medication. Forty-six patients recovered from complications. Twelve patients were not cured. The median follow-up time was 29.5 months (2–61 months). The overall 30-day mortality rate was 1.7% (1/58) and the total mortality rate following secondary complications was 8.6% (5/58). The causes of death were stroke and aortic rupture.
Conclusion
Some treatments need to be performed after TEVAR because of severe complications. A reduction in these complications can be achieved by optimal evaluation of patients, selection of SGs, and specialized endovascular manipulation.
Keywords: Aortic dissection, thoracic endovascular aortic repair, stent–graft, complication
Introduction
Thoracic aortic dissection (TAD) is still one of the most catastrophic vascular events with a mortality rate of up to 80% if left untreated.1,2 Conventional open surgical aortic replacement used to be the only effective treatment for type B aortic dissections, but it results in high mortality and paraplegia.3
Within the last 10 years, minimally invasive thoracic endovascular repair (TEVAR) of type B TAD has become an attractive alternative to conventional open surgery. Thoracotomy, extracorporeal circulation, aortic cross-clamping, high-dose anticoagulation, and single lung ventilation can be avoided. Several studies have shown that the mortality rate following EVAR for type B TAD is acceptably low at 1.5% to 8%.4–6 However, despite short- and mid-term promising clinical results of this new therapy, the long-term durability of EVAR has not been established. Similar to all conventional surgical methods, EVAR for type B TAD is associated with complications.
We present our experience of complications following TAD endovascular procedures, and focus on the occurrence, management, and prevention of complications.
Method
Patient cohort
From June 2008 to March 2016, our department admitted 58 patients without Marfan syndrome who had undergone a thoracic endovascular stent–graft (SG) procedure for acute or chronic type B TAD and developed serious complications.
There were 40 men and 18 women with a mean age of 57.3 years (range, 28–85 years). Twenty-seven patients underwent emergency TEVAR (after onset < 72 hours) for acute TAD, 16 patients received selected TEVAR (after onset > 2 weeks) for acute TAD, and eight patients underwent TEVAR 72 hours to 2 weeks after onset. Endovascular procedures were performed in seven patients with chronic asymptomatic dissection. Thirty-nine patients developed symptomatic complications postoperation (mean time: 3.8 months, range 0–36 months). Nineteen asymptomatic complications were detected at the follow-up after a SG was deployed (mean postoperative time: 5.6 months, range 3–24 months). The patients’ complications are listed in Table 1. The procedural details are listed in Table 2.
Table 1.
Serious complications after stent grafting for type B dissection.
| Complications | Number | Percentage |
|---|---|---|
| Endoleak | 16 | 27.6 |
| Distal true lumen collapse | 16 | 27.6 |
| Retrograde dissection | 6 | 10.3 |
| Paraplegia/paraparesis | 1 | 1.7 |
| Stroke | 6 | 10.3 |
| SG migration and incorrect deployment | 6 | 10.3 |
| Lower limb ischaemia | 5 | 8.6 |
| Stent–graft fracture | 2 | 3.4 |
| Total | 58 | 100 |
Table 2.
Procedural details9.
| Variables | Number (%) | Number (%) |
|---|---|---|
| Landing zone (coverage of the LSA/distal LSA) | 23 (39.7) | 33 (60.3) |
| Length of the SG (> 150 mm/< 150 mm) | 15 (25.9) | 43 (74.1) |
| Calibre of the SG (> 34 mm/< 34 mm) | 24 (41.4) | 34 (58.6) |
| Shape of the SG (conical/tubular) | 8 (13.8) | 50 (86.2) |
| Coated material of the SG (Dacron/PTFE) | 35 (60.3) | 23 (29.7) |
Investigation and imaging studies
Computed tomography (CT)-angiography of the entire aorta with three-dimensional reconstruction was performed in all of the patients to investigate complications after the endovascular procedure. Several variables were carefully determined on contrast-enhanced CT images and angiographic images. These included the location and shape of a previously planted SG, the true lumen, the false lumen, extension, the entry and re-entry sites, and the relationship between the dissection membrane and major aortic branches.
Stent–graft device and grafts
Four types of SG were used in this series, including the Talent and Captivia (Medtronic, USA), TX-1/TX-2 (Cook, USA), and Hercules (Microport, China) straight tube devices. One bare stent, Sinus (OptiMed, Germany), was also used. All of the SGs were available in different lengths and sizes. The bare stents were also sized by individual diameters. PTFE grafts (Gore, USA) were used for surgical bypass.
Classification of complications and treatment
1. Endoleak
Endoleaks were detected in 16 patients, including 12 type 1 endoleaks and four type 2 endoleaks from the left subclavian artery (LSA). We treated five patients with type 1 endoleak with additional SGs proximal to the original SG, and we treated three patients by ballooning the original SG. All of the endoleaks disappeared postoperatively. Four type 1 patients were treated by conservative medical treatment with no enlargement of the false lumen. Four type 2 leaks from the LSA were sealed by coil embolization.
2. Distal true lumen collapse
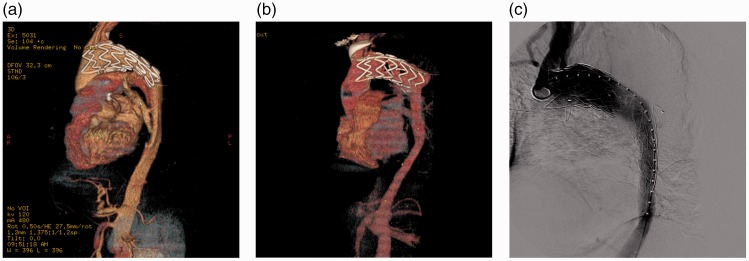
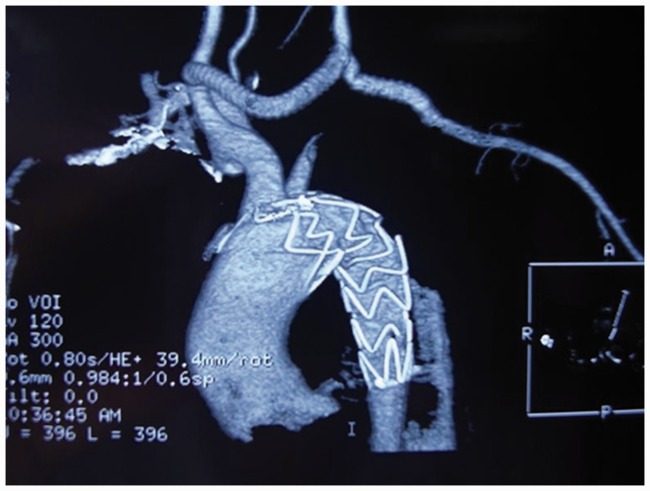
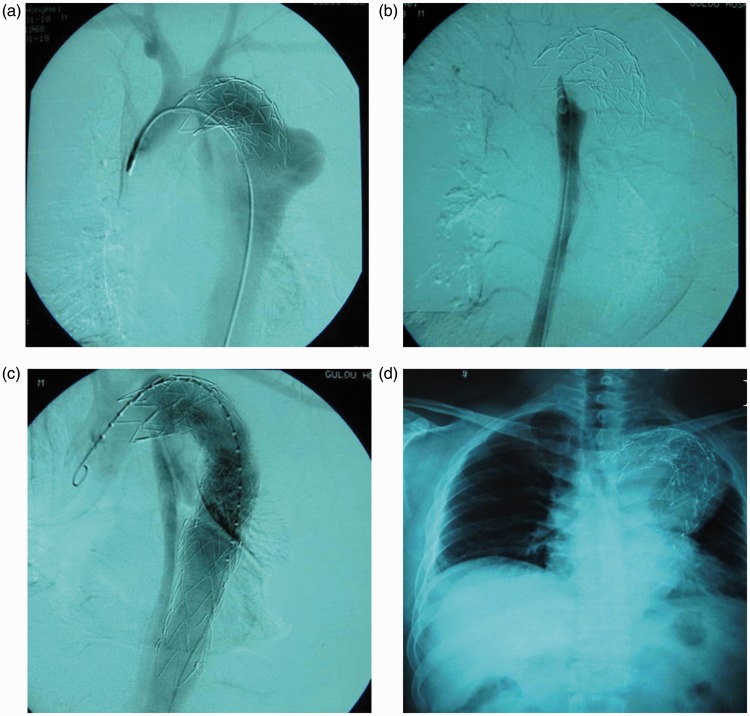
Sixteen patients had distal true lumen collapse following deployment of SGs. Four of them developed a secondary rupture and haemothorax. All of the patients were treated with two additional SGs or one SG with one bare stent. First, we implanted a smaller bare stent or SG extension in the distal aorta. A large SG was then placed in the proximal aorta to block the intimal tear and attach the previous SG and distal stent (Figure 1).
3. Retrograde dissection
Figure 1.
(a) CT shows a type B dissection that was repaired by a SG. (b) Six months after EVAR, a distal true lumen collapse was observed. (c) This collapse was treated using two SG implants. First, a smaller SG fitting the distal lumen was deployed to prevent true lumen collapse, and then a large SG fitted to the previous SG was implanted.
Six retrograde type A dissections were found. One patient had serious chest pain and underwent surgical intervention by ascending aorta replacement. Five patients received conservative medical treatment and were provided follow-up care after discharge because of advanced age and a poor physical condition.
4. Paraplegia/paraparesis
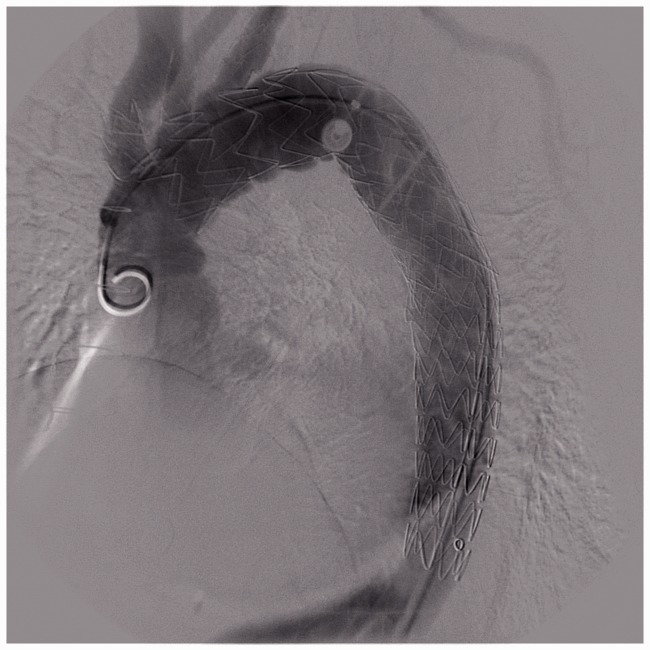
Only one patient with complete paralysis had undergone an aortic arch replacement surgery before EVAR (Figure 2). We treated this condition with thrombolytic therapy using urokinase and anticoagulation therapy with low-molecular-weight heparin.
5. Stroke
Figure 2.
A patient with complete paralysis with previous aortic arch replacement surgery and long descending aortic coverage.
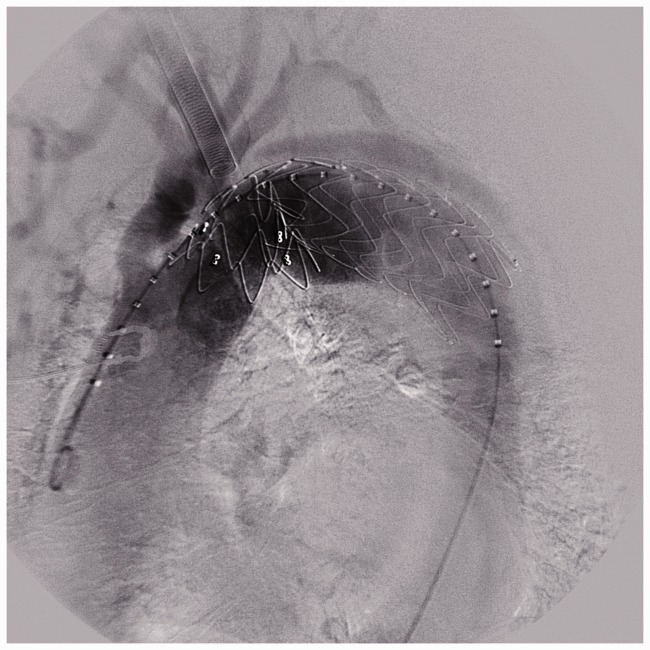
Six patients suffered strokes. Primary strokes occurred in all of the patients because of forward pushing of the SG that led to covering of the left carotid artery. Four patients underwent emergency surgical bypass operations (Figure 3). The other two patients who were transferred from another hospital 2 months after the endovascular procedure only received conservative medical treatment because of a lack of time for emergency surgery.
Figure 3.
Bypass surgery was performed to rescue patients with an SG covering the left carotid artery.
SG migration and mistaken deployment
Four misaligned deployments of the SG occurred, and angiography showed that the proximal bare stents were bent outward and folded over the SG (Figure 4). One patient underwent an open surgical operation involving aortic replacement, and the other three were maintained in follow-up.
Figure 4.
Image showing a proximal bare stent that is bent outward and folded over the SG.
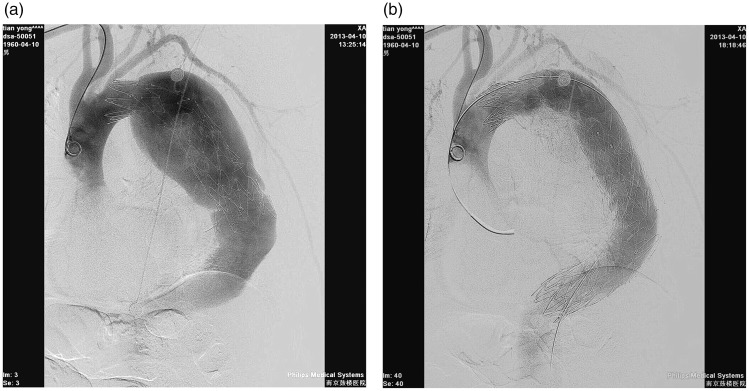
We also experienced two cases involving incorrect recognition of the true lumen, in which SGs were directly placed into the false lumen. These two patients were treated by deploying several long SGs into the aorta such that the proximal lumen and the distal true lumen were connected by the SGs through the false lumen (Figure 5).
6. Lower limb ischaemia
Figure 5.
(a, b) Angiograph showing an SG in the false lumen. (c) Two other long SGs were deployed into the false lumen to connect to the distal true lumen. (d) After 30 months, the patient had severe chest pain and haemothorax. An X-ray shows that the SG is out of shape.
Five patients developed lower limb ischaemia after the procedure. Two of them underwent surgical thrombectomy and artery angioplasty. An emergency femoral–femoral artery bypass was performed in one patient. Delayed lower limb ischaemia occurred in the other two patients. We performed a femoral–femoral bypass in one patient and a stent implant following balloon dilatation in the other patient.
7. Stent–graft fracture
An SG fracture occurred in two patients. These patients developed acute chest pain after the SG implant for several years. CT showed that the SG fractured and the false lumen expanded. We deployed new SGs to repair the broken SG and false lumen (Figure 6).
Figure 6.
(a) Angiograph showing a fractured SG and the false lumen is expanded. (b) A new SG was deployed to repair the broken SG.
Follow-up investigation
The median follow-up time was 29.5 months (range: 2–45 months). Physical examinations and enhanced CT post-discharge were performed at 6 months, 12 months, and annually thereafter.
Results
Endovascular, open surgical, or medical management were performed in the patients. All of the secondary operations succeeded. Forty-six (46/58, 79.3%) patients recovered and 12 (12/58, 20.7%) remained unhealed. The overall 30-day mortality was 1.7% (1/58) for patients undergoing management. Four patients died during follow-up. The survival rate in our group was 91.4% (53/58).
Nine of the 12 type 1 endoleaks were closed by secondary endovascular procedures or conservative medical treatment. Three of the type 1 endoleaks were still present with no evidence of aortic enlargement. One patient with a type 1 endoleak died during follow-up because of aortic rupture resulting from poor blood pressure control. All type 2 endoleaks were closed by coils or occluders.
All of the patients with distal true lumen collapse (16/16) received endovascular management. All of the newly developed distal dissection disappeared and no new complications occurred during follow-up (Figure 1).
One of the six patients with retrograde type A dissection who underwent surgical intervention by ascending aorta replacement survived. Five patients with conservative medical treatment survived, but two showed obvious aortic enlargement, and ultimately died because of aortic rupture. The other three patients died of other causes that did not appear to be related to retrograde type A dissection.
The only patient with paraplegia remained unhealed. Four of the six patients with stroke underwent emergency surgical bypass operations, and three of them recovered with slight sequelae (Figure 3). One patient died 1 week after the operation. The other two patients with stroke remained unhealed with no obvious change.
One of the four patients with SG migration recovered after surgical intervention by ascending aorta replacement. Two patients recovered after endovascular repair by an additional covered SG proximal to the original SG. One patient was treated by conservative medical treatment with no enlargement of the false lumen. One of two patients with mistaken deployment died because of severe SG migration and aortic rupture 30 months after secondary EVAR (Figure 5). Another patient who received a conventional operation was still alive at follow-up.
All of the patients with lower limb ischaemia recovered after surgical intervention and appeared to be in good health. All of the patients with SG fracture recovered after deploying new SGs to repair the broken SG and false lumen.
Discussion
The first endovascular repairs of type B aortic dissection were reported in 1999 by Dake et al.7 and Nienaber et al.8 Since this time, many clinical reports have shown that endovascular repair is technically feasible and has a significantly lower rate of complications compared with conventional surgical repair.9,10,11 Despite the reasonably low early operative morbidity and mortality, later serious complications related to EVAR (e.g., development of aneurysms, aortic rupture, stroke, paraplegia, limb ischaemia, access-related complications, and endoleaks) have been increasingly reported.4,5,10 Therefore, every surgeon should consider methods for reducing the incidence of complications of EVAR for type B dissection.
Detailed preoperative assessment of patients should be carefully performed. In addition to the general condition, observation and measurement of aortic dissection images are required. Some items must be determined, such as the true lumen, false lumen, entry and re-entry sites, location and extension of the lesion, diameter of the aorta, tortuosity of the aorta, access arteries, and the relationship between the dissection membrane and major arterial branches. The effects of EVAR treatment are closely related to preoperative evaluation and selection of cases, configuration and type of SG, as well as the clinical experience of the surgeon.
Endovascular procedures are accompanied by various types of risks, especially for complex aortic lesions. The incidence of complications is closely correlated with the surgeon’s skill and advanced endovascular equipment.12 Therefore, EVAR treatment should be carried out in highly specialized vascular centres where the requirement can be met to routinely perform open and endovascular procedures. This would help to cope with accidents in an effective and timely manner.
The time of endovascular intervention for aortic dissection is still controversial. We believe that EVAR for dissection should not proceed from 3 days to 3 weeks since the onset because of oedema, weakness, and poor flexibility and tensility of the aortic walls. We found that severe complications were more common if EVAR was carried out within 3 weeks. Therefore, if there was no organ or lower limb ischaemia, we still insisted that EVAR should occur 3 weeks after the dissection. To avoid risks, EVAR should be performed in an emergency (<72 hrs) or selectively (>3 weeks). However, for considerable complications, as some authors have indicated,2,13,14 we also believe that asymptomatic chronic patients may benefit more from conservative treatment than from EVAR.
With continuous improvements of SGs and development of endovascular techniques, indications of EVAR for aortic dissections are also expanding. However, because of limits of the SG structure, indications for intervention should be strictly selected to improve the efficacy. There are some relative contraindications to EVAR for aortic dissection. In particular, EVAR should be contraindicated when the anatomy of dissection prevents a safe and effective performance of the SG that often remarkably increases the complication rates. EVAR in patients with an aortic size outside the range of available SG devices should be avoided. Severe tortuosity of the aorta or the access vessels and advanced aortoiliac occlusive disease preclude the safety of EVAR. EVAR should also be avoided in patients with a poor general condition, those with no tolerance of the procedures, and those with serious disorders with an expected survival < 2 years.
Endoleak is the most common complication of endovascular treatment. The incidence of EVAR for a thoracic aorta is approximately 4% to 12%.2,13 Approximately half of the endoleaks may close spontaneously. Short-term follow-up in a previous study showed that there were no significant survival differences between patients with endoleaks closing and non-closing endoleaks.6 However, long-term endoleaks, especially type I endoleaks, may cause an intimal tear to continuously open, and blood flow may persist in the false lumen, thus affecting the long-term effects. The main reasons for this situation include a tortuous aorta that may cause the SG to not completely attach to the aortic wall or SG bending, an anchor zone that is too short, an SG that is too small or too large, and a fragile proximal aorta. Careful preoperative evaluation and selection of the appropriate SG configuration should be carried out to increase the proximal and distal landing zones to prevent the occurrence of endoleaks. If endoleak occurs, balloon dilatation and SG expansion may help to close the endoleak. Small, stable endoleaks can be treated by medication and observation if there is no false lumen growth.15
Neurological complications can be devastating after EVAR for type B dissections. Stroke occurs at similar rates between open and endovascular repairs.10 Deployment of the proximal end of the SG proximal to the left carotid artery can cause a fatal stoke. Emergency revascularization has to be performed as soon as possible for such patients to survive. Moreover, perioperative stroke may occur secondary to manipulation of the arch with catheters, wires, balloons, and SG devices at the origin of the cerebral vessels. Proficiency of an endovascular technique is important and can minimize the risks.
EVAR shows a marked reduction in paraplegia/paraparesis compared with open procedures.16 This may be related to avoidance of thoracic clamping and reduced haemodynamic disturbance at the spinal cord level. However, there is a decrease in cerebrospinal perfusion pressure when overstenting feeding intercostals.5 Surgeons should exercise caution in patients with previous open surgery of the thoracic or abdominal aortas. Overstenting of too long a segment of the total descending thoracic aorta should be avoided. Spinal drainage techniques and steroids can be used to prevent these complications.
Even after successful placement of SGs in type B dissection, the distal descending thoracic aortic segment is still a problem. In the presence of a larger distal SG size and smaller distal true lumen dimension, the descending thoracic aortic segment of the false lumen shows no tendency to thrombose and remodel completely. However, this can lead to late distal true lumen collapse. An extended bare stent or short SG scaffolding of the dissection beyond the main SG and down to the distal aorta results in repositioning and fixation of the distal lamella. This technique can be applied as an adjunctive measure to primary SG insertion to prevent distal true lumen collapse.17 This method also has the capacity to repair such complications in a secondary procedure. A tapered SG adapted to variations in aortic size could solve this problem. However, because this requires customization of the SG individually, it may not be applicable to emergency procedures.
Retrograde type A dissection within hours or even 1 month after EVAR is not a rare complication and can be life-threatening, especially when a type B tear has been over-stented close to or opposite to the left subclavian ostium.18 Fragility of the aortic wall and progression of disease, combined with SG-related causes, contribute to occurrence of retrograde type A dissection. Improvement of the SG system, careful selection of SGs and patients, and specialized endovascular manipulation may prevent such complications. Surgical and medical treatment can be effective management strategies.
Mistaken deployment can occur during deployment of thoracic SGs because of a high flow rate and severe aortic angulation, which are often encountered in the thoracic aorta. This mistake is an unusual phenomenon that tends to occur in the context of certain well-defined anatomical conditions in the thoracic aorta. To date, most of these events have not led to major adverse sequelae. However, careful selection of patients, periprocedural imaging, and case planning can help to identify anatomy in which a misaligned opening is likely to occur, allowing physicians to avoid this complication.19
SG fractures are rare. They occur in irregular homemade products. Products must undergo formal government approval. Strict laws regarding quality should be made, with establishment of criteria for quality inspection and setting up of a regulatory body to ensure that the criteria are strictly adhered to.
With the EVAR technique being widely performed, an increasing number of inexperienced operators have started to perform endovascular repair for type B dissection. A lack of assessment for lesions and an inappropriate choice of SG could cause severe consequences. These consequences include SG migration and incorrect deployment, which are difficult to deal with. Standard specialized treatment will reduce the occurrence of such complications. Therefore, every surgeon should undergo standardized professional training before starting EVAR for aortic diseases.
For lesions involving the aortic arch, important arterial branches, and a short anchor zone (so-called complex dissections), use of a common configuration of SG often leads to various types of severe complications. These complications include endoleak, organ ischaemia, unstable SG implants, and SG migration. LSA overstenting can increase the anchor zone. With preoperative evaluation of transcranial crossflow facilities and the absence of carotid stenosis, it is well tolerated without transposition of the LSA.6,10 According to the range of dissections, a hybrid procedure combines vessel debranching, and endovascular repair can be chosen for complex dissections. There have been no reports of cerebral ischaemia after hybrid procedures.20,21 The chimney technique uses a covered or bare stent that is deployed parallel to the main aortic SG, which can preserve flow to branches by the aortic SG. Standard off-the-shelf SGs can be used to instantly treat lesions with inadequate anchor zones.22,23 However, this may also cause more endoleaks. Specially designed fenestrated or branched SGs can seal the complex dissection and reconstruct important arterial branches. However, currently, this endovascular procedure is still under clinical trial and cannot be widely promoted.24,25
Conclusion
Although long-term data are still unavailable, the advantages of TEVAR over conventional descending aortic surgery are clear. TEVAR is minimally invasive, results in less blood loss, requires a shorter operation time, is easier to operate, leads to faster postoperative recovery, and results in much shorter hospitalizations compared with other techniques. TEVAR also reduces the incidence of common postoperative complications in organs, such as the heart, lungs, and kidney. Older patients or those with severe co-morbidities can be effectively treated with this technique.
Currently, TEVAR is becoming the gold standard for type B TAD. Despite the favourability of TEVAR, notably, complications that are unique to endovascular procedures may occur, and acute or delayed complications may occur. A reduction in these complications could be achieved by optimal evaluation of patients, selection of SGs, and specialized endovascular manipulation. Further studies are still needed to investigate life-long surveillance and long-term clinical effects of TEVAR.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Leurs LJ, Bell R, Degrieck Y, et al. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and Unite d Kingdom Thoracic Endograft registries. J Vasc Surg 2004; 40: 670–679. [discussion 679-80]. [DOI] [PubMed] [Google Scholar]
- 2.Duebener L, Hartmann F, Kurowski V, et al. Surgical interventions after emergency endovascular stent-grafting for acute type B aortic dissections. Interact Cardiovasc Thorac Surg 2007; 6: 288–292. [DOI] [PubMed] [Google Scholar]
- 3.Erbel R, Oelert H, Meyer J, et al. Effect of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiog raphy. Implications for prognosis and therapy. The European Cooperative Study Group on Echocardiography. Circulation 1993; 87: 1604–1615. [DOI] [PubMed] [Google Scholar]
- 4.Piffaretti G, Tozzi M, Lomazzi C, et al. Complications after endovascular stent-grafting of thoracic aortic diseases. J Cardiothorac Surg 2006; 1: 26–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunder-Plassmann L, Orend KH. Stentgrafting of the thoracic aorta-complications. J Cardiovasc Surg (Torino) 2005; 46: 121–130. [PubMed] [Google Scholar]
- 6.Ehrlich MP, Dumfarth J, Schoder M, et al. Midterm results after endovascular treatment of acute, complicated type B aortic dissection. Ann Thorac Surg 2010; 90: 1444–1448. [DOI] [PubMed] [Google Scholar]
- 7.Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340: 1546–1552. [DOI] [PubMed] [Google Scholar]
- 8.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999; 340: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 9.Baril DT, Cho JS, Chaer RA, et al. Thoracic aortic aneurysms and dissections: endovascular treatment. Mt Sinai J Med 2010; 77: 256–269. [DOI] [PubMed] [Google Scholar]
- 10.Neuhauser B, Greiner A, Jaschke W, et al. Serious complications following endovascular thoracic aortic stent-graft repair for type B dissection. Eur J Cardiothorac Surg 2008; 33: 58–63. [DOI] [PubMed] [Google Scholar]
- 11.Xiong J, Jiang B, Guo W, et al. Endovascular stent graft placement in patients with type B aortic dissection: a meta-analysis in China. J Thorac Cardiovasc Surg 2009 Oct; 138: 865–872. Epub 2009 Apr 8. [DOI] [PubMed]
- 12.Hata M, Sezai A, Niino T, et al. Prognosis for patients with type B acute aortic dissection: risk analysis of early death and requirement for elective surgery. Circ J 2007; 71: 1279–1282. [DOI] [PubMed] [Google Scholar]
- 13.Sharif MA, O’Donnell ME, Blair PH, et al. Emergency endovascular repair of acute descending thoracic aortic dissection. Vasc Health Risk Manag 2007; 3: 769–773. [PMC free article] [PubMed] [Google Scholar]
- 14.Nathanson DR, Rodriguez-Lopez JA, Ramaiah VG, et al. Endoluminal stent-graft stabilization for thoracic aortic dissection. J Endovasc Ther 2005; 12: 354–359. [DOI] [PubMed] [Google Scholar]
- 15.Böckler D, Hyhlik-Dürr A, Hakimi M, et al. Type B aortic dissections: treating the many to benefit the few? J Endovasc Ther 2009; 16(Suppl 1): 180–190. [DOI] [PubMed] [Google Scholar]
- 16.Chiesa R, Melissano G, Marrocco Trischitta MM. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 2005; 42: 11–17. [DOI] [PubMed] [Google Scholar]
- 17.Nienaber CA, Kische S, Zeller T, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther 2006; 13: 738–746. [DOI] [PubMed] [Google Scholar]
- 18.Dong ZH, Fu WG, Wang YQ, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009; 119: 735–741. Epub 2009 Jan 26. [DOI] [PubMed] [Google Scholar]
- 19.Kasirajan K, Kwolek CJ, Gupta N, et al. Incidence of and outcomes after misaligned deployment of the talent thoracic stent graft system. J Vasc Surg 2010; 51: 1096–1101. Epub 2010 Mar 29. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Guo W, Liu X, et al. Hybrid endovascular repair in aortic arch pathologies: a retrospective study. Int J Mol Sci 2010; 11: 4687–4696. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Fu WG, Dong ZH, Wang YQ, et al. Strategies for managing the insufficiency of the proximal landing zone during endovascular thoracic aortic repair. Chin Med J 2005; 13: 1066–1071. [PubMed] [Google Scholar]
- 22.Sugiura K, Sonesson B, Akesson M, et al. The applicability of chimney grafts in the aortic arch. J Cardiovasc Surg (Torino) 2009; 50: 475–481. [PubMed] [Google Scholar]
- 23.Shu C, Luo MY, Li QM, et al. Early results of left carotid chimney technique in endovascular repair of acute non-a-non-B aortic dissections. J Endovasc Ther 2011; 18: 477–484. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZG, Li C. Single-branch endograft for treation stanford type B aortic dissectipns with entry tears in proximity to the left subclavian artery. J Endovasc Ther 2005; 12: 588–593. [DOI] [PubMed] [Google Scholar]
- 25.Verhoeven ELG, Zeebregts CJ, Kapma MR, et al. Fenestrated and branched endovascular techniques for thoraco-abdominal aneurysm repair. J Cardiovasc Surg 2005; 46: 131–140. [PubMed] [Google Scholar]