Abstract
Background
Neuropathic pain is one of the most common complaints of neurologic clinics. Neuropathic pain is common and important and has inappropriate complications, and despite their importance, there is no effective treatment for them.
Objective
Because of the importance of neuropathic pain and safe and effective treatment, in this study, we determined the effect of topiramate versus gabapentin in patients with neuropathic pain.
Methods
In this randomized clinical trial, 30 patients with pain attributed to neuropathy who had at least one month of neuropathic pain in one area, were randomized to receive either gabapentin, titrated from 300 mg/day to a maximum of 900 mg/day or topiramate, titrated from 50 mg/day to a maximum of 100 mg/day after a 4-week period in the neurology clinic of Imam Khomeini Hospital of Urmia city, Iran in 2015. Complication, drug tolerance rate and pain were investigated. The pain was measured on visual analog scale (VAS). The data were analyzed by SPSS version 18, and using descriptive statistics, t-test, and ANOVA.
Results
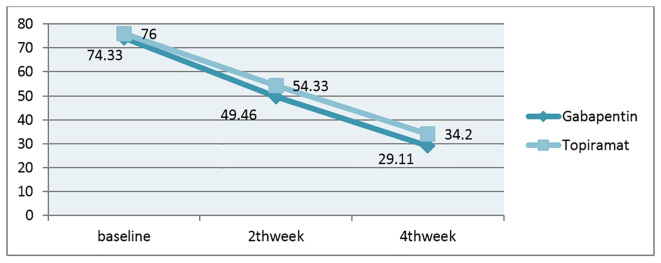
In patients treated by gabapentin, the primary pain score was 74.33±10.29, this score decreased to 49.46±11.41 and 29.93±11.92 in the second and fourth week after intervention with gabapentin. In topiramate treated patients, the primary score was 76.00±9.69. It decreased to 54.33±10.31 and 34.20±6.09 at the same time. There were no significant differences between both groups in terms of average reduction of pain intensity [gabapentin group (59.73%) compared with topiramate (55%) (p=0.48)]. In the present study, the only complication reported in patients treated by gabapentin was drowsiness, but other uncommon side effects were nausea and dizziness.
Conclusion
This study showed that both gabapentin and topiramate reduce pain. Topiramate can also be a good alternative choice, if gabapentin has side effects for patients and it cannot be tolerated, topiramate can be a good replacement.
Trial registration
The trial was registered at the Thai Registry of Clinical Trials (http://www.clinicaltrials.in.th) with the TCTR ID: TCTR20170615001.
Funding
This research has been financially supported by Research Council of Urmia University of Medical Sciences.
Keywords: Neuralgia, Polyneuropathies, Gabapentin, Topiramate
1. Introduction
Peripheral neuropathy can affect sensory, motor or autonomic functions, either alone or in combination, which causes the loss of the sense of heat and burning pains. The nature of neuropathic pain can be sharp and deadly mode or ambiguous and difficult to localization (1), for instance burning and all referral pain, compressive and colic pains are used for paresthesia as well as dysphonia is also related to exaggerated levels of pain (hyperpathy) which is called neuropathy (2). Some studies have reported similar findings in terms of estimated prevalence of neuropathic pain, so that through clinical examination (visit in clinic by specialist) it was reported as 9.8%, using Berger criteria 3% and the S-LANSS (Leeds assessment of neuropathic symptoms and signs) 8.8%, and based on self-reporting, was 12.4% (3–5). Unfortunately, there are no exact statistics of the prevalence of neuropathic pain in Iran. Common drugs used in the treatment of these pains including tricyclic antidepressants, anti-seizure medications, topical capsaicin and opioids were not completely effective or had more side effects limiting their use such as dry mouth, sweating, weakness, drowsiness, dizziness and constipation, which is very problematic, especially in the elderly (6–10). In March 2000, the anti-epileptic drug, gabapentin, had been considered as a first-line therapy in neuropathic pain, based on large clinical trials in two groups of neuropathic pain: Diabetic Neuropathy and Post Herpetic Neuralgia (PHN) (11, 12). Gabapentin is one of the regulators of the calcium channel that binds to the ligand alpha-two-delta, and reduces the passage of calcium into the cells, then decreases the release of neurotransmitter in hyper stimulated neurons (13–15). Topiramate is an antiepileptic drug of which weight loss is the major side effect and it can be used to beneficial effect in the treatment of diabetes complications. Topiramate had been a positive effect in the treatment of neuropathic pains in the presence of failure with other drugs (16, 17). Considering the mentioned subjects which showed notifiable inconsistency among findings, we decided to determine the effect of gabapentin and topiramate in reducing neuropathic pain.
2. Material and Methods
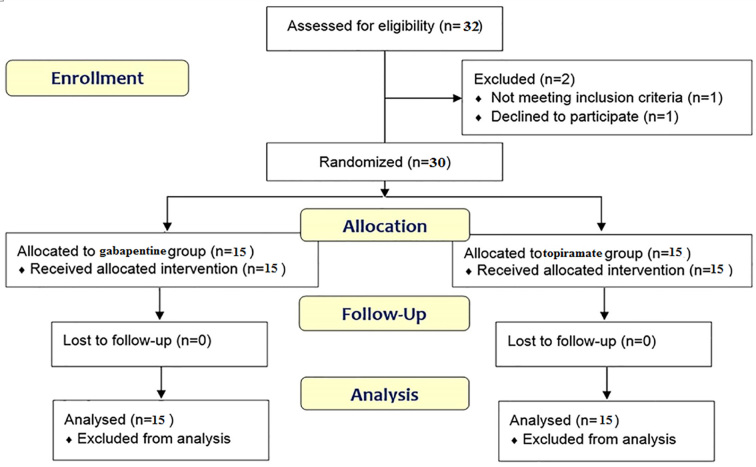
Based on similar conducted studies, 30 patients who referred to the neurology clinic at Imam Khomeini Hospital of Urmia city, Iran in 2015, with pain attributed to neuropathy were blindly randomized to receive either gabapentin, titrated from 300 mg/day to a maximum of 900 mg/day or topiramate, titrated from 50 mg/day to a maximum of 100 mg/day after a 4-week period (Figure 1).
Figure 1.
Follow-up diagram of patients (According to consort statement)
Inclusion criteria were 42–82 years old who had at least one month neuropathic pain in one area. Diagnosis of polyneuropathy was based on neurological examination or electrodiagnostic tests. Patients with cardiovascular and liver lesions, orthostatic hypotension, and history of allergy to gabapentin or topiramate, patients with pain such as neuropathic pain, psychiatric disorders, uncontrolled hypertension, drug abuse and pregnant or lactating women were excluded. Patients who had taken tricyclic antidepressants, mexiletine hydrochloride, carbamazepine, phenytoin, valproate sodium and dextromethorphan drugs in the last 30 days, were also excluded. The pain was measured on visual analog scale (VAS) which is a scale to measure the severity of pain in patients consisting of 0 (No Distress), 1 (2 cm) (Annoying), 2 (4 cm) (Uncomfortable), 3 (6 cm) (Dreadful), 4 (8 cm) (Horrible) and 5 (10 cm) (Agonizing). In order to show the result, it can also be presented as millimeter (mm). All patients were trained to who show themselves pain as score. Patients’ scores were recorded by a person who was unconnected and unaware of the group of patients. The method of study and the possible side effects of both drugs were described for patients. The objectives of the study were explained to all participants and all of them agreed to participate and were assured of the confidentiality of their individual information as well as the voluntary nature of participating in the study. The severity of pain was measured before treatment and after two and four weeks and was recorded in the prepared checklist. The demographic data were collected by checklist. At the end of the study, data were analyzed by SPSS version 18. For the study of descriptive data, frequency, percentage and mean ± SD were used. Also, t-test and analysis of variance (ANOVA) were used to compare pain three times in the two groups. Kolmogorov Smirnov test was used to assess the normality distribution of data.
3. Results
Of 30 patients who participated in the study, 18 patients (60%) were men and 12 (40%) were women. The average age of participants was 63.6±10.36 year and the majority of them were 60–80 years old (Table 1). Fifteen patients (50%) had diabetes type II, and 4 patients (13.3%) had diabetes type I. Patients had breast, ovarian and lung cancer, and 6.7% of patients had chronic renal failure, and possibly in the field of uremia, had developed neuropathic pain. Other causes included one vasculitis and defects of vitamin B 12. In 10% of patients, the specific cause for polyneuropathy was not found. Average years of diabetes development in DM1 and DM2 were 22.2 and 14.5 years, respectively. The mean of recorded pain based on VAS in the group treated by gabapentin at baseline was 74.33±10.29 mm, the second week 49.46±11.41 mm and in the fourth week 29.93±11.92 mm. The mean of recorded pain based on VAS in the group treated by topiramate at baseline was 76.00±9.69 mm, the second week 54.33±10.31 mm and in the fourth week 34.20±6.09 mm. Despite the significant differences in pain scores in each group in different times, there was no statistically significant difference between the two groups (p=0.48). Indeed, the obtained results were close together, so that in the topiramate group, the mean ± SD pain in patients based on the VAS scale in baseline, 2nd week and 4th week were 76.00±9.69, 54.33±10.31 and 54.33±10.31, respectively. As well as in the gabapentin group, the mean ± SD pain in patients based on the VAS scale in baseline, 2nd week and 4th week were 74.33±10.29, 49.46±11.41 and 29.93±11.92, respectively. Distribution of causes of polyneuropathy including uremia, chemotherapy induced, B12 deficiency, vasculitis, DM1 and DM2 in both groups had no significant difference (p>0.05) (Table 2). In both groups, there was a significant difference in pain severity compared to the beginning of treatment (p<0.001). But there was no significant difference in the reduction of pain intensity between the two groups (p=0.27). The average reduction of pain intensity in the gabapentin group (59.73%) compared with topiramate (55%) did not show a significant difference between the two groups (p=0.48) (Figure 2). The most common complication caused by gabapentin was drowsiness (66.7%). The common side effects caused by topiramate were confusion (46.7%)) and anorexia (33.3%). Only two of the patients treated by typical doses of gabapentin were seen in the intolerance list in which the drug dosage had been reduced, there was no significant difference between the two groups in terms of drug tolerance (p=0.5) (Table 3).
Table 1.
Gender and age distribution of patients treated with gabapentin and topiramate
| Drug | Male; n (%) | Female; n (%) | Age (Year) |
|---|---|---|---|
| Gabapentin | 10 (66.7%) | 5 (33.3%) | 61.86±10.24 |
| Topiramate | 8 (53.3%) | 7 (46.7%) | 65.33±10.53 |
| Total | 18 (60%) | 12 (40%) | 63.6±10.36 |
Table 2.
Frequency distribution of causes of polyneuropathy in patients with polyneuropathy
| Diagnosis | Group | Total | |
|---|---|---|---|
| Gabapentin | Topiramate | ||
| DM1 | 1 | 3 | 4 |
| 6.7% | 20.0% | 13.3% | |
| DM2 | 7 | 8 | 15 |
| 46.7% | 53.3% | 50.0% | |
| Uremia | 1 | 1 | 2 |
| 6.7% | 6.7% | 6.7% | |
| Uremia | 1 | 0 | 1 |
| 6.7% | 0.0% | 3.3% | |
| Chemotherapy Induced | 2 | 1 | 3 |
| 13.3% | 6.7% | 10.0% | |
| B12 Deficiency | 1 | 0 | 1 |
| 6.7% | 0.0% | 3.3% | |
| Unknown | 2 | 1 | 3 |
| 13.3% | 6.7% | 10.0% | |
| Vasculitis | 0 | 1 | 1 |
| 0.0% | 6.7% | 3.3% | |
| Total | 15 | 15 | 30 |
| 100.0% | 100.0% | 100.0% | |
Figure 2.
Changes in VAS based on millimeters in treated groups
Table 3.
Frequency of drug complications and tolerance of drug-treated groups in patients with polyneuropathy after treatment
| Variable | Gabapentin | Topiramate | |
|---|---|---|---|
| Complication | Drowsiness | 10 (66.7%) | 0 |
| Confusion | 0 | 7 (46.7%) | |
| Anorexia | 0 | 5 (33.3%) | |
| Tolerance | Yes | 13 (86.66%) | 15 (100%) |
| No | 2 (13.3%) | 0 | |
4. Discussion
In the present study, the primary pain score was 74.33±10.29, this score decreased to 49.46±11.41 and 29.93±11.92 in the second and fourth week after intervention with gabapentin. In topiramate treated patients, the primary score was 76.00±9.69. It decreased to 54.33±10.31 and 34.20±6.09 at the same time. There were significant differences between both groups. In the study of Raskin et al., Treatment with topiramate in patients with polyneuropathy had reduced the VAS from 68 mm to 46.2 mm (32% reduction) (18). The present study, also had reduced the pain by 55% in the topiramate group, so that in the study of Fowler et al., significantly improved neuropathic pain was also reported in patients with polyneuropathy with topiramate, and improved noticeably within one month (16). In the study conducted by Dallocchio et al., 21% (15 mm) had reduction in the average pain of patients in the gabapentin group and 14% (10 mm) had reduction in the average pain of patients in the placebo group (19). In the study of Backonja et al., gabapentin reduced pain in 39% (25 mm) in the gabapentin group and 27.5% (14 mm) in the placebo group (20). In this study of Raskin et al., the most common side effects reported with gabapentin were dizziness (24.2%), nausea and headache (18.4%) and drowsiness (14.4%) and the majority of patients tolerated the drug and only four patients (3.27%) felt the need to reduce the dose of gabapentin (18). In the study of Dallocchio et al., incidence of adverse events in the amitriptyline group compared to gabapentin were higher (92% in the amitriptyline and 31% in the gabapentin group) and the most common side effects reported in the gabapentin group were dizziness (16.7%) and drowsiness (8.3%) (19). In the study of Backonja et al., the most common reported adverse events in the gabapentin group were dizziness (24%) and drowsiness (23%) and feeling of dizziness (8%) and 56 patients out of 84 patients (67%) were tolerant to the doses of gabapentin (20). In the study of Gilron et al., the most common side effects reported in the gabapentin group were drowsiness (10%), dry mouth (8%) and dizziness (6%) (21). In the present study, the only complication reported in patients treated by gabapentin was drowsiness, but other uncommon side effects were nausea and dizziness. In this study of Raskin et al., diarrhea, anorexia and insomnia were the most common adverse reactions in patients treated by topiramate (18). In the present study, confusion and dizziness in 46.7% of patients in the topiramate group and anorexia in 33.3% of patients in the gabapentin group were reported. Gabapentin, pregabalin and lidocaine skin patches are suitable as first-line treatment of neuropathic pain (16). In the present study, the effect of antiepileptic drugs such as gabapentin and topiramate were examined on neuropathic pain in patients with polyneuropathy. Topiramate is an antiepileptic drug that affects through blockage of sodium and calcium channels, inhibition of glutamate receptors, strengthening of the inhibitory effect of gamma-amino butyric acid and carbonic anhydrase, and the incidence of such complications can be used in the treatment of neuropathic pains (13). In this study, gabapentin along with topiramate had significant impact on patients with neuropathic pain, and all patients completed the therapeutic period of study. In statistical analysis, each group separately had significant differences in the scale of VAS in the beginning and the end of the fourth week. The studies of Carroll et al., Dallocchio et al. and Backonja et al. also showed a significant reduction in the average of pain using gabapentin (17, 19, 21). In the study of Raskin et al., also, significant reduction was reported using topiramate on neuropathic pain (18). In a study conducted by Punam et al., the effect of gabapentin, topiramate, levetiracetam and zonisamide were compared for treatment of neuropathic pain induced by anticancer drug (vincristine) (100μg/kg) in albino rats, using thermal method which were divided into five groups, group I was treated with water as control group, group II was treated with oral gabapentin (60 mg/kg), group III received oral topiramate (40 mg/kg), group IV was treated with oral levetiracetam (120 mg/kg) and group V received zonisamide (50 mg/kg). In vincristine induced neuropathic pain gabapentin, topiramate and zonisamide showed a significant increase hot-plate latency (analgesic effect) (22). In terms of gabapentin and topiramate analgesic effect, the obtained results in this study on rats were consistent with our study on patients. Regarding the limitations of the study, the small sample size of included studies was a potential limitation of this study. Another limitation of the current study was the small sample size of this study. There is still need to conduct further studies to access additional information about this subject.
5. Conclusions
This study showed that topiramate is effective on patients with neuropathic pain and there was no clear difference between topiramate and gabapentin, and in cases of intolerance to gabapentin and other drugs such as antidepressants, topiramate can be replaced in the treatment of neuropathic pain. On the other hand, given that none of the aforementioned drugs have never been 100% effective in relieving the pain completely, then it is suggested they be taken with each other. In total, more studies are needed for evaluation of neuropathic pain treated by topiramate.
Acknowledgments
The authors appreciate the research chancellor of Urmia University of Medical Sciences which supported this study financially. This article is obtained from M.D student thesis by Dr. Aynaz Foroughi Eghbal (approval number 92-01-32-1040).
Footnotes
iThenticate screening: August 21, 2017, English editing: October 07, 2017, Quality control: October 15, 2017
Trial registration:
The trial was registered at the Thai Registry of Clinical Trials (http://www.clinicaltrials.in.th) with the TCTR ID: TCTR20170615001.
Funding:
This research has been financially supported by Research Council of Urmia University of Medical Sciences.
Conflict of Interest:
There is no conflict of interest to be declared.
Authors’ contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Longo DL, Kasper DL, Jameson JL, Fauci AS, Houser SL, Loscalzo J, et al. Harrison’s principles of internal medicine. 18th ed. New York: McGraw Hill; 2012. Peripheral Neuropathy; pp. 384–385. [Google Scholar]
- 2.Wolfe GI, Barohn RJ. Painful Peripheral Neuropathy. Curr Treat Options Neurol. 2002;4(3):177–88. doi: 10.1007/s11940-002-0034-0. [DOI] [PubMed] [Google Scholar]
- 3.Yawn BP, Wollan PC, Weingarten TN, Watson JC, Hooten WM, Melton LJ., 3rd The prevalence of neuropathic pain: clinical evaluation compared with screening tools in a community population. Pain Med. 2009;10(3):586–93. doi: 10.1111/j.1526-4637.2009.00588.x. Epub 2009 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7(1):13–7. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- 5.Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5(3):143–9. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Guirguis-Blake J, Kelly C. Are opioids effective in the treatment of neuropathic pain? Am Fam Physician. 2007;75(7):999–1001. [PubMed] [Google Scholar]
- 7.Cayley WE., Jr Antidepressants for the treatment of neuropathic pain. Am Fam Physician. 2006;73(11):1933–4. [PubMed] [Google Scholar]
- 8.Otto M, Bach FW, Jensen TS, Brosen K, Sindrup SH. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139(2):275–83. doi: 10.1016/j.pain.2008.04.012. Epub 2008 Jun 10. [DOI] [PubMed] [Google Scholar]
- 9.Wernicke JF, Pritchett YL, D’Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–20. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 10.Grosskopf J, Mazzola J, Wan Y, Hopwood M. A randomized, placebo-controlled study of oxcarbazepine in painful diabetic neuropathy. Acta Neurol Scand. 2006;114(3):177–80. doi: 10.1111/j.1600-0404.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 11.McCleane GJ. Comment on: Serpell et al., gabapentin in neuropathic pain syndromes: a randomised double-blind, placebo controlled trial (Pain 2002; 99: 557–66) Pain. 2003;103(1–2):227. doi: 10.1016/S0304-3959(03)00072-1. author reply 8. [DOI] [PubMed] [Google Scholar]
- 12.Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One. 2013;8(9):e74195. doi: 10.1371/journal.pone.0074195. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong MS, Libretto SE. The rationale and use of topiramate for treating neuropathic pain. Clin J Pain. 2003;19(1):59–68. doi: 10.1097/00002508-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Boyd AL, Barlow PM, Pittenger GL, Simmons KF, Vinik AI. Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2010;3:431–7. doi: 10.2147/DMSOTT.S13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dib JG. Focus on topiramate in neuropathic pain. Curr Med Res Opin. 2004 Dec;20(12):1857–61. doi: 10.1185/030079904X11358. [DOI] [PubMed] [Google Scholar]
- 16.Fowler JA, Shen JY, Bettinger TL. Successful Use of Topiramate in a Patient with Severe Postherpetic Neuralgia. Ann Pharmacother. 2009;43:139–42. doi: 10.1345/aph.1L470. Epub 2008 Dec 23. [DOI] [PubMed] [Google Scholar]
- 17.Carroll DG, Kline KM, Malnar KF. Role of topiramate for the treatment of painful diabetic peripheral neuropathy. Pharmacotherapy: Pharmacotherapy. 2004;24(9):1186–93. doi: 10.1592/phco.24.13.1186.38096. [DOI] [PubMed] [Google Scholar]
- 18.Raskin P, Donofrio PD, Rosenthal NR, Hewitt DJ, Jordan DM, Xiang J, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neourology. 2004 Sep 14;63(5):865–73. doi: 10.1212/01.WNL.0000137341.89781.14. [DOI] [PubMed] [Google Scholar]
- 19.Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. J Pain Symptom Manage. 2000 Oct 31;20(4):280–5. doi: 10.1016/S0885-3924(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 20.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus. JAMA. 1998;280(21):1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 21.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, Gabapentin, or Their Combination for Neuropathic Pain. N Engl J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 22.Punam J, Omi C, Anusuya G, RKR, Raghuveer C, Rajesh C. A Comparative Study of the Effect of Gabapentin, Topiramate, Levetiracetam and Zonisamide for Neuropathic Pain Induced by Anticancer Drug (Vincristine) in Rats. JPRCP. 2014;4(1):1–6. [Google Scholar]