Abstract
Triclosan and triclocarban (TCs) are broad-spectrum microbicides found in household and personal wash products. We sought to determine whether TC exposure from wash products or urinary triclosan level modified thyroid function during pregnancy or anthropometric measurements at birth. A randomized intervention of wash products with or without TCs, including toothpaste, enrolled pregnant women from 20 weeks’ gestation. Urinary triclosan, TSH, T4 and T3 were assessed at enrollment, 36 weeks’ gestation and/or post-delivery; anthropometric measures at birth were ascertained from medical records. 78 and 76 mothers were assigned to the TC-containing and no-TC-containing product arms, respectively. No differences were observed in any thyroid function measure at any time point or in any anthropometric measurement at birth between either exposure arms or lowest and highest urinary triclosan quartile groups. TCs from wash products, primarily liquid and bar soaps, did not affect thyroid function measures during pregnancy or babies’ anthropometric measures at delivery.
Keywords: Triclosan, triclocarban, thyroid function, anthropometry, pregnancy, randomized intervention
INTRODUCTION
Triclosan and triclocarban (together, TCs) are synthetic, broad-spectrum biocides first licensed for use in the 1960s. Found worldwide in a vast array of household and personal products including toys and kitchenware, TCs are present most notably in toothpaste and liquid soaps (triclosan) and bar soaps (triclocarban) [1]. They are frequent environmental contaminants [2] and are prevalent in human populations. For example, in 2003 a US national population survey detected triclosan in 74% of urine samples [3].
TCs have been reported to disrupt thyroid function in a variety of laboratory and cell culture systems. In rats, triclosan has been shown to reduce levels of both triiodothyronine (T3) and thyroxine (T4) [4–7]; in minnows, triclosan exposure delayed hatching [8]. In vitro studies have demonstrated changes in TH-responsive gene transcripts at high triclocarban concentrations [9], thyroid hormone receptor activity by irradiated triclosan solutions [10], and inhibition of thyroid hormone synthesis genes by both triclosan and triclocarban [11].
The thyroid hormones T3 and T4 are key metabolic hormones, essential for development, growth, neural differentiation and metabolic regulation [12]. Thyroid dysfunction has well documented adverse effects on human gestation including premature birth, low birth weight, miscarriage, fetal loss and impaired fetal neurocognitive development [13, 14]. Few studies, though, have explored the effects of TCs on thyroid function in humans. In one small study, short-term use of triclosan-containing toothpaste did not modify thyroid function measures [15]. A small cross-over trial of TC-containing wash products including toothpaste showed no change in either thyrotropin (TSH) or T4 levels after four months [16]. Finally, a cohort study of heart disease patients randomized to triclosan-containing or placebo toothpaste identified no effect on thyroid function measures over a four-year period [17]. Although triclosan has commonly been found in urine samples from pregnant women [18–24] (triclocarban has been less studied but is also prevalent [23]), only one study has explored its impact on thyroid function during pregnancy and no changes in any thyroid function measure across time were observed [25]. Anthropometric measurements at birth, affected by thyroid dysfunction during pregnancy, have for the most part not been shown to be related to urinary triclosan levels in cohort studies [26–29].
In 2011, we initiated a birth cohort study, STanford’s Outcomes Research in Kids (STORK), to better understand the role of early childhood infectious diseases on growth and development [30]. Within STORK, we nested a randomized trial of TC-containing wash products and toothpaste. In a post-hoc analysis, we explored whether TC exposure modified either maternal thyroid function measures during pregnancy or babies’ anthropometric measurements at delivery.
METHODS
STORK study design
Detailed methods for the STORK cohort have been described previously [30]. In brief, participants were recruited through flyers or attendance at public obstetric clinics (the Lucile Packard Children’s Hospital (LPCH; Stanford, CA) and the Valley Health Center at Tully (Santa Clara Valley Medical Center [SCVMC], San Jose, CA). Enrollment criteria for mothers included: Spanish or English fluency, a low-risk healthy pregnancy with a single fetus, and self-reported absence of non-gestational diabetes, thyroid dysfunction or other endocrine conditions. At enrollment, cohort subjects were invited to participate in a nested, randomized trial of the effect of TC-containing wash products on infectious disease incidence in the index baby. Participants were randomly allocated to the TC arm or the control (nTC) arm and were provided commercially available wash products (liquid and bar soap, toothpaste, dishwashing liquid), all of which either did or did not contain TCs; supplies were replenished every four months as needed. Triclosan-containing toothpaste was provided to households assigned to the TC arm only after the baby was born.
Household visits were performed every four months after enrollment to obtain demographic and household information. Chart review from the hospital record at delivery was performed to determine the baby’s sex, gestational age at delivery and anthropometric measurements as available; abstractors were blinded as to each household’s intervention arm assignment.
Urine samples were collected at each visit and stored at −80°C until processed. A blood sample was collected at enrollment. An additional sample was drawn at a second household visit before delivery if this occurred, at the routine 36 week clinic visit if this occurred, or at the first household visit after delivery.
All questionnaire information was collected on tablet computers and uploaded using SAS version 9.4 (Cary, NC) for storage and management in REDCap [31] hosted at Stanford University. Information from chart abstraction was entered into Medrio (San Francisco, CA). The study was approved by the institutional review boards of Stanford University and the SCVMC (ClinicalTrials.gov identifier: NCT01442701).
Measurements of thyroid function
All maternal serum specimens with an available volume equal to or greater than 0.9 ml were tested for TSH, total T3, and both total and free T4 (TT4, FT4). Testing used immunoassays (TSH and FT4: Siemens Dimension Vista homogeneous chemiluminescent immunoassay on LOCI technology; total T3: Abbott Architect chemiluminescent microparticle immunoassay (CMIA); TT4: Siemens Dimension Vista homogeneous enzyme immunoassay) and was performed at the Stanford Anatomic Pathology & Clinical Laboratories at Hillview (Stanford, CA). Reference ranges were not adjusted for pregnancy (TSH: 0.4–4.00μl/mL; T3: 70–180μg/dL; TT4: 6.1–11.8μg/dL; FT4: 0.6–1.6ng/dL).
Urinary triclosan measurements
Urinary triclosan levels were measured using liquid chromatography-mass spectrometry with liquid-liquid extraction using ethyl acetate (see Supplemental Material). Testing was performed at the Vincent Coates Foundation Stanford University Mass Spectrometry Laboratory (http://mass-spec.stanford.edu) (Stanford, CA). (Urinary triclocarban levels were not assessed.)
Statistical methods
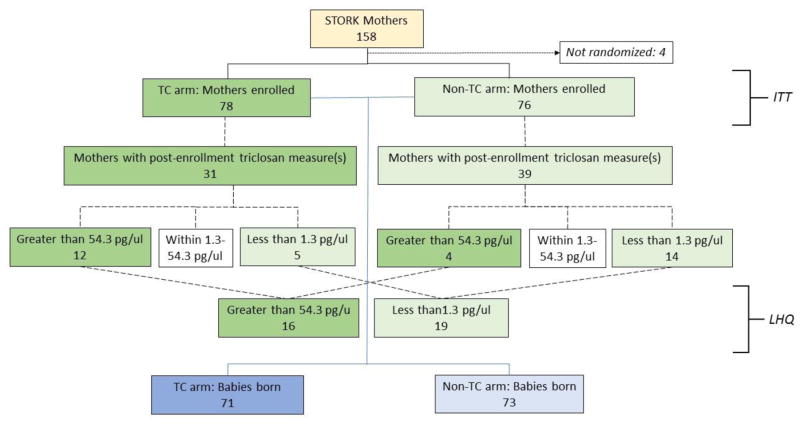
TC exposure was defined in two ways: 1) by intervention arm assignment (intention to treat [ITT]), or 2) by categorizing lowest (<1.3 pg/μl) and highest (>54.8 pg/μl) quartiles of urinary triclosan using the distribution of all available urinary triclosan results from throughout the analysis time frame (baseline visit, late pregnancy visit and/or first post-delivery visit). If more than one post-baseline level was available, we calculated the mean of the two results prior to categorization by quartile. Results from the mothers with levels in the lowest and highest quartiles were compared for the lowest-highest quartile (LHQ) analysis (Figure 1).
Figure 1.
Participant disposition by randomization arm, showing intention-to-treat (ITT) and lowest vs. highest quartiles of urinary triclosan (LHQ) cohorts.
We tested differences in both thyroid function measures and anthropometric measurements between the exposure groups (either ITT or LHQ) using Wilcoxon rank-sum tests or t-tests for continuous variables and chi-square or Fisher’s Exact tests for categorical variables, as appropriate. Generalized linear modeling (GLM) was used to determine whether TC exposure was a predictor of thyroid function measures during pregnancy. Potential confounders considered in the analyses included both gestational age in weeks when samples were drawn (or weeks post-partum in the case of post-delivery samples) and time since enrollment in weeks (as a marker of cumulative TC exposure from the intervention).
We defined the 5th percentile of head circumference (less than two standard deviations below the mean for sex at birth) for term girls and boys when head circumference was less than 32.251 cm for girls and 32.148 cm for boys [32]. Additional GLM models considered the effect of TC exposure on baby anthropometric measures at birth.
All analyses were performed in SAS Version 9.4 (Cary, NC).
RESULTS
Of the 158 mothers enrolled in the STORK cohort, four declined participation in the intervention; of the remainder, 78 (50.7%) were assigned to the TC arm and 76 (49.4%) were assigned to the nTC arm (Figure 1). Baseline characteristics of mothers overall have been described previously [30]. Intervention arms appeared balanced with regard to demographic and household factors (data not shown). Both gestational age at enrollment and weeks from enrollment in the study to delivery were similar between intervention arms (median [Q1–Q3]: gestational age at enrollment: 23.0 [16.9–31.6] weeks in the TC arm and 23.3 [14.3–30.9] weeks in the nTC arm, p=0.65; weeks enrolled in the study to delivery: 17.3 [7.4–22.6] in the TC arm and 15.7 [9.0–24.4] in the nTC arm, p=0.68).
Triclosan levels
At least one urinary triclosan level was available from 117 mothers, with 50, 54 and 13 providing one, two or three results, respectively, across time. The overall distribution of triclosan from all time points was skewed: 14.2% of samples were below the level of quantification and 8.1% were above 200 pg/μl (range: 388–7356 pg/μl). At enrollment, triclosan levels were low and similar across arms (median: 6.8 pg/μl in both arms, p=0.71) (Figure 2; Table 1); 20 (13.0%) and 27 (17.5%) mothers had urinary triclosan levels ≤ 1.3 pg/μl and ≥ 54.3 pg/μl, respectively. Considering only post-TC randomization urine samples, median triclosan levels were seven-fold higher in the TC arm than in the nTC arm (median: 19.0 pg/μl in the TC arm vs. 2.7 pg/μl in the nTC arm, p=0.002). A total of four mothers had post enrollment triclosan levels greater than 200 pg/μl (three in the TC arm; one in the nTC arm).
Figure 2.
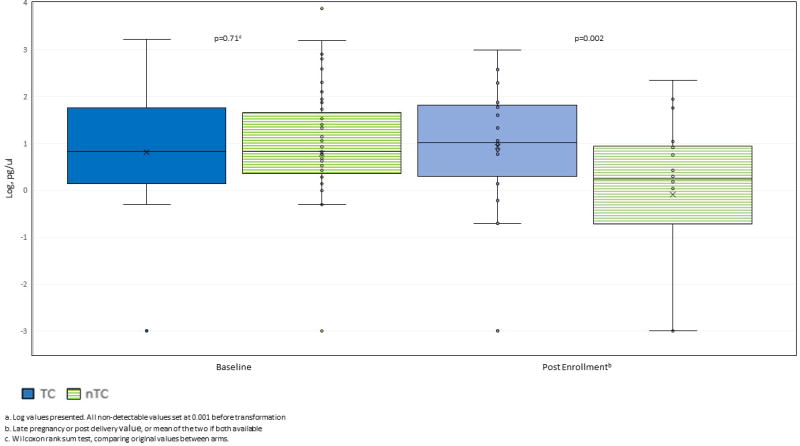
Distributiona of urinary triclosan at baseline and post enrollment, by intervention arm (TC, nTC)
Table 1.
Urinary triclosan levels (pg/μl) for all samples by time point and intervention arm
| Measure | TC arm (median [Q1–Q3]) |
nTC arm (median [Q1–Q3]) |
P c |
|---|---|---|---|
| Baselinea | 6.8 [1.4 – 56.4] | 6.8 [2.7–37.5] | 0.71 |
| Late pregnancy | 39.4 [7.2–118.3] | 3.9 [1.4–11.0] | 0.002 |
| Post delivery | 1.4 [0.6–2.0] | 0 [0–0.2] | 0.003 |
| Post enrollment b | 19.0 [3.1–80.6] | 2.7 [0.3–10.9] | 0.002 |
Baseline: approximately 23 weeks gestation (51 women in the TC arm and 61 in the nTC arm); late pregnancy: approximately 36 weeks gestation (27 in the TC arm and 32 in the nTC arm); post-delivery: approximately 6 weeks (10 in the TC arm and 16 in the nTC arm)
Late pregnancy or post-delivery value, or mean of the two if both available (31 women in the TC arm and 39 in the nTC arm)
Wilcoxon rank-sum test
Measures of thyroid function
All mothers in the intervention provided at least one blood sample that potentially could have been used for this study; however, 42 women did not provide a quantity of serum sufficient for testing (≥0.9 ml). These 42 did not differ from the 106 women who were ultimately tested for thyroid function (45 with one sample only [35 baseline, 9 late pregnancy, 1 post-delivery] and 61 with two samples [49 baseline/late pregnancy, 12 baseline/post-delivery]).
As expected from previous physiological studies during pregnancy [33], mean TT4 and T3 tended to be higher than non-pregnant levels (Table 2). At medical chart review, we identified one woman taking thyroxine and three women with a history of thyroid dysfunction (one with hyperthyroidism, one hypothyroidism and one unspecified). In our study, all four of these women had post-enrollment thyroid function measures within normal ranges. A total of 29 other women had at least one value of their post enrollment thyroid function measures outside of the references ranges. One had low TSH levels with normal FT4 and T3 levels, and two had high T3 levels with normal TSH and FT4 levels. The largest group of women with values outside the normal ranges were 26 with high TT4 levels, a common finding in pregnancy due to increased protein binding. For 23 of these women, the remaining thyroid function tests were normal. For three, however, other abnormalities were observed---low TSH levels in one, and high T3 levels in two. No woman had a post enrollment FT4 value outside of the normal range. No difference in distribution between intervention arms was observed at baseline or at any other time point for any thyroid function measure; TSH, TT4 and T3 decreased post-delivery but these changes were not different between arms (Figure 3). A total of 49 mothers had results from two pre-delivery samples. No difference in change in levels between the two time points was observed across intervention arms for any thyroid function measure (Figure 4). These findings did not change after adjustment for either gestational age in months or time since enrollment (GLM; data not shown). Comparison of the highest and lowest quartiles of post enrollment urinary triclosan showed no difference in any thyroid function measure at any time point or in any difference in measures between time points (Supplemental Material, Table S1). Among the women with post enrollment urinary triclosan values greater than 200 pg/μl, all thyroid function measures were within the reference ranges.
Table 2.
Thyroid function measures by time point and difference over time, by intervention arm
| Measure | TC arm (median [Q1–Q3]) |
nTC arm (median [Q1–Q3]) |
P c |
|---|---|---|---|
| TSH (μl/mL) | |||
| Baseline a | 1.19 [0.82–1.38] | 1.23 [0.77–1.56] | 0.65 |
| Late pregnancy | 1.45 [1.07–2.33] | 1.44 [1.11–2.00] | 0.82 |
| Post delivery | 0.89 [0.81–1.08] | 0.95 [0.73–1.58] | 0.94 |
| Difference b from baseline | 0.34 [−0.02 – 0.56] | 0.51 [0.1 – 0.91] | 0.26 d |
| T3 (ng/dL) | |||
| Baseline | 144 [117–167] | 144 [113–156] | 0.57 |
| Late pregnancy | 131 [123–154] | 141 [134–157] | 0.14 |
| Post delivery | 109 [94–110] | 100 [95.5–108] | 0.94 |
| Difference from baseline | −14 [−27 –8] | −0.5 [−14–28] | 0.24 d |
| TT4 (μg/dL) | |||
| Baseline | 12.3 [10.7–14.8] | 12.1 [10.9–13.5] | 0.54 |
| Late pregnancy | 11.8 [10.7–13.2] | 11.3 [10.2–12.6] | 0.34 |
| Post delivery | 7.5 [7.3–7.9] | 8.0 [6.7–8.9] | 0.71 |
| Difference from baseline | −0.4 [−1.6 – 0.4] | −0.9 [−2.5 – 0.4] | 0.32 d |
| FT4 (ng/dL) | |||
| Baseline | 0.9 [0.9–1.0] | 0.9 [0.9–1.0] | 0.48 |
| Late pregnancy | 0.9 [0.9–1.0] | 0.9 [0.8–1.0] | 0.11 |
| Post delivery | 0.9 [0.9–1.0] | 0.95 [0.85–1.05] | 1.00 |
| Difference from baseline | 0 [−0.1 – 0] | −0.1 [−0.1 – 0] | 0.12 d |
TSH: thyrotropin, T3: total triiodothyronine; TT4 and FT4: total and free thyroxine
Baseline: approximately 23 weeks gestation (51 women in the TC arm and 45 in the nTC arm); late pregnancy: approximately 36 weeks gestation (29 in each arm); post-delivery: approximately 8 weeks (5 in the TC arm and 8 in the nTC arm)
Among women with two pre-delivery measures only (25 women in the TC arm and 24 in the nTC arm)
Wilcoxon rank-sum test unless otherwise indicated
T-test
Figure 3.
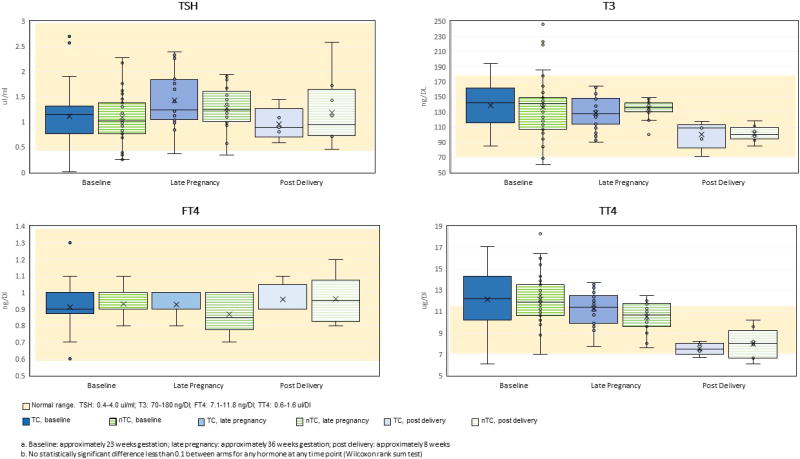
Distribution of thyroid function measures at baselinea, late pregnancy and post-delivery, comparing intervention armsb (TC, nTC)
Figure 4.
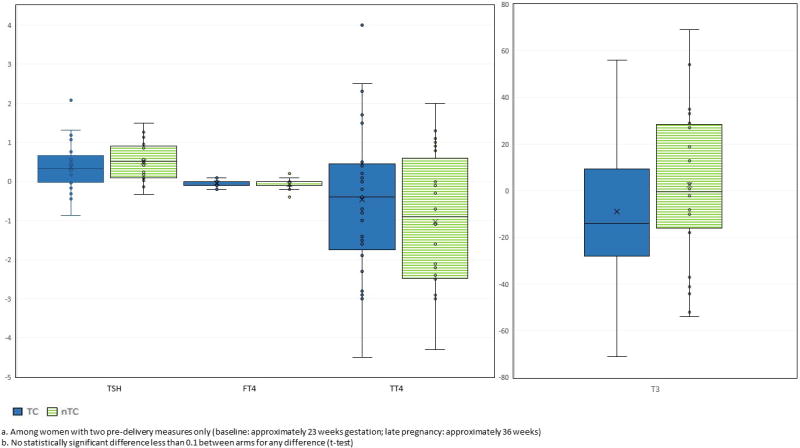
Change in thyroid function measures over timea, comparing intervention armsb (TC, nTC)
Babies’ anthropometric measurements
Medical records were available for review of delivery details for 70 girls and 74 boys, equally distributed between intervention arms (Table 3). Distributions of birth weight, length, body mass index (BMI) and head circumference overall were similar between intervention arms (Figure 5). The proportion of head circumferences in the 5th percentile was not different between arms. No differences were observed between highest and lowest urinary triclosan quartile groups for any baby anthropometric measurement (Supplemental Material, Table S2).
Table 3.
Baby anthropometric measurements a by intervention arm
| Measure | TC arm | nTC arm | P d |
|---|---|---|---|
|
| |||
| Sex | |||
| Girls [N(%)] | 34 (48%) | 36 (49%) | 0.86 e |
| Boys | 37 (52%) | 37 (51%) | |
|
| |||
| Weight (kg) | |||
| Mean (SD) | 3.41 (0.49) | 3.49 (0.43) | 0.32 |
| Range | [1.97–4.72] | [2.63–4.58] | |
|
| |||
| Length (cm) a | |||
| Mean (SD) | 50.13 (2.69) | 50.81 (2.26) | 0.12 |
| Range | [43.2–58.5] | [45.1–55.0] | |
|
| |||
| BMI b | |||
| Mean (SD) | 13.56 (1.50) | 13.48 (1.33) | 0.75 |
| Range | [9.7–17.1] | [11.3–16.7] | |
|
| |||
| Head circumference (cm) a | |||
| Mean (SD) | 34.22 (1.39) | 34.60 (1.35) | 0.17 |
| Range | [31.0–36.5] | [31.5–37.5] | |
| 5th percentile c [N(%)] | 5 (12%) | 2 (3.3%) | 0.12 f |
A total of 9 babies were missing birth length (6 in the TC arm and 3 in the nTC arm) and 41 babies were missing head circumference (30 in the TC arm and 11 in the nTC arm).
Body mass index: weight in kg/(length in cm) 2
<32.25 cm for girls and <32.15 cm for boys
T-test unless otherwise indicated
Chi square test
Fisher’s Exact test
Figure 5.
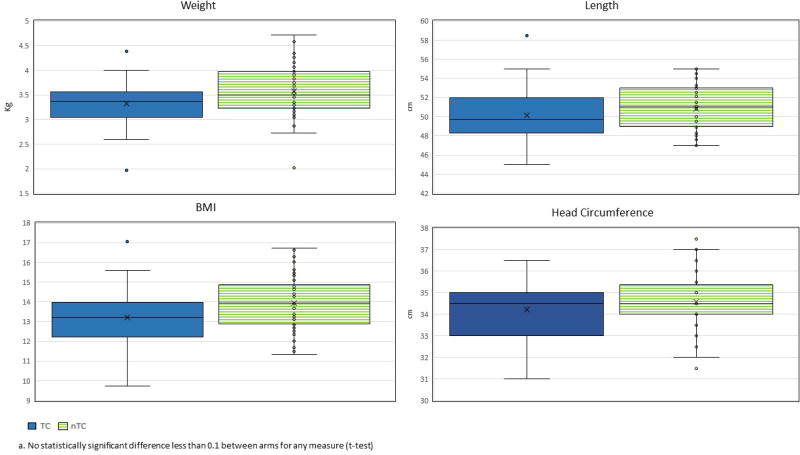
Distribution of anthropometric measures at delivery, comparing intervention armsa (TC, nTC)
DISCUSSION
Our randomized intervention compared the use of TC-containing and non-TC-containing wash products in 154 healthy women undergoing a low-risk pregnancy with a single fetus. Levels of thyroid hormones in 106 of these mothers during pregnancy were comparable to those in the literature: normal changes in thyroid function during pregnancy include a steady increase in TT4 and T3 to approximately 1.5 times the non-pregnant levels by mid second trimester, and a gradual decrease in FT4 and FT3 throughout the second and third trimesters, still within the normal reference range [33]. In our analysis, exposure to TCs had no effect on thyroid function measures. These findings support those of a previous investigation that tested thyroid function earlier during pregnancy (16–20 and 24–28 weeks) [25]. Our study, with sampling time points at approximately 23 and 36 weeks, expands the exposure timeframe throughout pregnancy, still with no observed effect of TCs on thyroid function measures.
Our analysis also did not identify any association between intervention arm and anthropologic measurements of the baby at delivery, including weight, length, BMI and head circumference either overall or as the lowest fifth percentile. Anthropometric measurements in this study are compatible with those of a geographically similar sample [34]. Few studies have examined TC exposure and birth outcomes, mostly identifying no association but with some exceptions. An inverse association of birth weight and length with TC exposure was shown in boys [29]; decreased head circumference with TC exposure was suggested, again in boys [27, 28]; finally, early gestational age was associated with triclocarban exposure [26]. We could confirm none of these findings.
Our intervention was successful, in that mothers in the TC arm evinced more TC exposure than mothers in the nTC arm, with a 7–10 fold difference in median urinary triclosan level between arms; in fact, the median urinary triclosan level in the TC arm (19 pg/μl) was 2.5 times higher than the US adult female 50th percentile for triclosan (7.4 ug/L) [3]. Levels overall though, even in the TC arm, were low. Much of the TC exposure attributable to consumer products is likely to be from ingestion of toothpaste, as dermal adsorption from soaps is not high [15, 35]. In our cohort, however, it is impossible to know for certain the primary sources of urinary triclosan since: a) triclosan-containing toothpaste, unlike other wash products, was not provided until the first visit after delivery (until this time, mothers in these households used their own toothpaste); b) mothers may have had TC exposure outside the home; and c) dosage of TC from wash products on skin or residual on dishes is impossible to quantify. A recent ruling from the FDA has banned TCs from liquid and bar soaps but not from toothpaste [36]; it will be intriguing to see whether future TC prevalence estimates are affected by this change.
There were several limitations to our study. First, very few mothers had high urinary triclosan levels. We therefore cannot prove that differences in thyroid function measures would not have been observed had triclosan levels been considerably higher. Levels identified in this study, however, fit well within other population estimates [3, 21, 28, 29]. Secondly, exposure to intervention arm was not a particularly good marker of TC exposure. This randomized intervention was not blinded and participants could have used wash products other than those assigned. Additionally, TC exposure could have occurred outside of the household. We attempted to correct for potential misclassification by performing a comparison of high vs. low urinary triclosan quartile groups (LHQ analysis) but did not identify any association with any thyroid function measure, although sample size was small. Finally, ascertainment of anthropometric measures at delivery was incomplete, particularly for head circumference. Medical records were not available for all births, and within a record, some measures may have been lacking; chart review, however, was performed blinded to intervention arm assignment. Despite these limitations, our analysis suggests that thyroid function measures during pregnancy are not grossly affected by “real-world” use of TCs from wash products.
CONCLUSION
In this randomized intervention, TCs from wash products, primarily liquid and bar soaps, did not have an effect on either thyroid function measures during pregnancy or anthropometric measures at delivery. The effect of TC-containing toothpaste and other TC-containing household products on thyroid function remains uncertain.
Supplementary Material
HIGHLIGHTS.
Triclosan and triclocarban (TCs) disrupt thyroid function in animals.
We randomized pregnant women to receive TC- or no-TC-containing wash products.
We assessed urinary triclosan and thyroid hormones over time and birth anthropometry.
Neither thyroid function measures nor babies’ anthropometry were affected by intervention arm.
Acknowledgments
The authors thank TD Haggerty for assistance with sample coordination. This project was supported in part by the National Institutes of Health (grant numbers 5R01HD063142 and 5R21ES023371). Colgate-Palmolive provided funding for running some triclosan urinary assays and all of the thyroid function tests. Neither Dr. Parsonnet, Dr. Pischel nor Dr. Ley received salary support or honoraria from Colgate-Palmolive and have no financial interests in the company. Colgate-Palmolive received the data and reviewed the manuscript but did not participate directly in the analyses, their interpretation or the study’s conclusions. The authors declare they have no known or potential competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halden RU. On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol. 2014;48:3603–3611. doi: 10.1021/es500495p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalew TE, Halden RU. Environmental Exposure of Aquatic and Terrestrial Biota to Triclosan and Triclocarban. J Am Water Works Assoc. 2009;45:4–13. doi: 10.1111/j.1752-1688.2008.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calafat AM, Ye X, Wong L-YY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–7. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelstad M, Boberg J, Vinggaard A, Christiansen S, Hass U. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem Toxicol. 2013;59:534–540. doi: 10.1016/j.fct.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol Sci. 2010;117:45–53. doi: 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- 6.Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicol Sci. 2010;113:367–79. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler JG, Frédérich B, Dussenne M, Klaren PH, Silvestre F, Das K. Triclosan exposure results in alterations of thyroid hormone status and retarded early development and metamorphosis in Cyprinodon variegatus. Aquat Toxicol. 2016;181:1–10. doi: 10.1016/j.aquatox.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Hinther A, Bromba CM, Wulff JE, Helbing CC. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol. 2011;45:5395–402. doi: 10.1021/es1041942. [DOI] [PubMed] [Google Scholar]
- 10.Sankoda K, Matsuo H, Ito M, Nomiyama K, Arizono K, Shinohara R. Identification of triclosan intermediates produced by oxidative degradation using TiO2 in pure water and their endocrine disrupting activities. Bull Environ Contam Toxicol. 2011;86:470–5. doi: 10.1007/s00128-011-0249-4. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Beland FA, Fang JL. Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol In Vitro. 2016;32:310–9. doi: 10.1016/j.tiv.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weetman AP. Thyroid disease in pregnancy in 2011: Thyroid function--effects on mother and baby unraveled. Nat Rev Endocrinol. 2011;8:69–70. doi: 10.1038/nrendo.2011.217. [DOI] [PubMed] [Google Scholar]
- 14.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 15.Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic Clin Pharmacol Toxicol. 2009;105:339–44. doi: 10.1111/j.1742-7843.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 16.Poole AC, Pischel L, Ley C, Suh G, Goodrich JK, Haggerty TD, et al. Crossover Control Study of the Effect of Personal Care Products Containing Triclosan on the Microbiome. mSphere. 2016;1:e00056–15. doi: 10.1128/mSphere.00056-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullinan M, Palmer J, Carle A, West M, Seymour G. Long term use of triclosan toothpaste and thyroid function. Sci Total Environ. 2012;416:75–79. doi: 10.1016/j.scitotenv.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 18.Arbuckle TE, Marro L, Davis K, Fisher M, Ayotte P, Bélanger P, et al. Exposure to free and conjugated forms of bisphenol A and triclosan among pregnant women in the MIREC cohort. Environ Health Perspect. 2015;123:277–84. doi: 10.1289/ehp.1408187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertelsen RJ, Engel SM, Jusko TA, Calafat AM, Hoppin JA, London SJ, et al. Reliability of triclosan measures in repeated urine samples from Norwegian pregnant women. J Expo Sci Environ Epidemiol. 2014;24:517–21. doi: 10.1038/jes.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol. 2013;47:3439–47. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen ME, Calafat AM, Ye X, Wong LY, Wright DJ, Pirkle JL, et al. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children’s Study. Environ Res. 2014;129:32–8. doi: 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, et al. Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ Health Perspect. 2013;121:1225–31. doi: 10.1289/ehp.1206335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pycke BF, Geer LA, Dalloul M, Abulafia O, Jenck AM, Halden RU. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol. 2014;48:8831–8. doi: 10.1021/es501100w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss L, Arbuckle T, Fisher M, Ramsay T, Mallick R, Hauser R, et al. Temporal variability and sources of triclosan exposure in pregnancy. Int J Hyg Environ Health. 2015;218:507–513. doi: 10.1016/j.ijheh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30–37. doi: 10.1016/j.envres.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geer LA, Pycke BF, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater. 2017;323:177–183. doi: 10.1016/j.jhazmat.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lassen TH, Frederiksen H, Kyhl HB, Swan SH, Main KM, Andersson AM, et al. Prenatal Triclosan Exposure and Anthropometric Measures Including Anogenital Distance in Danish Infants. Environ Health Perspect. 2016;124:1261–8. doi: 10.1289/ehp.1409637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R, et al. Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25:625–35. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley C, de Sanchez ML, Mathur A, Yang S, Sundaram V, Parsonnet J. Stanford’s Outcomes Research in Kids (STORK): a prospective study of healthy pregnant women and their babies in Northern California. BMJ Open. 2016;6:e010810. doi: 10.1136/bmjopen-2015-010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. [accessed 2/13/2017]; https://www.cdc.gov/growthcharts/html_charts/hcageinf.htm.
- 33.Lazarus JH. Thyroid function in pregnancy. Br Med Bull. 2010;97:137–148. doi: 10.1093/bmb/ldq039. [DOI] [PubMed] [Google Scholar]
- 34.Madan A, Holland S, Humbert JE, Benitz WE. Racial differences in birth weight of term infants in a northern California population. J Perinatol. 2002;22:230–5. doi: 10.1038/sj.jp.7210703. [DOI] [PubMed] [Google Scholar]
- 35.Queckenberg C, Meins J, Wachall B, Doroshyenko O, Tomalik-Scharte D, Bastian B, et al. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother. 2010;54:570–2. doi: 10.1128/AAC.00615-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FDA. Safety and Effectiveness of Consumer Antiseptics. Topical Antimicrobial Drug Products for Over-the-Counter Human Use. 2016 https://www.federalregister.gov/documents/2016/09/06/2016-21337/safety-and-effectiveness-of-consumer-antiseptics-topical-antimicrobial-drug-products-for. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.