Abstract
Objective
Vitamin D and probiotics are nutrients of interest in the context of type 1 diabetes (T1D). We assessed the prevalence of and factors associated with vitamin D and probiotic supplementations among young children with genetic risk of T1D.
Subjects/Methods
Use of supplements during the first two years of life was collected prospectively from 8 674 children in the Environmental Determinants of Diabetes in the Young (TEDDY) study.
Results
Single and/or multivitamin/mineral (MVM) supplements were reported by 81% of the children. The majority of participants in Finland, Germany, and Sweden (97-99%) and 50% in the US received vitamin D supplements that were mostly MVMs. Probiotics use varied from 6% in the US to 60% in Finland and was primarily from probiotics-only preparations. More than 80% of the vitamin D and probiotics supplementation was initiated during infancy, and more than half of the uses lasted longer than a year. Being the first child, longer duration of breastfeeding, born in a later year, older maternal age, and higher maternal education level were associated with both vitamin D and probiotics use. Shorter gestational age and mother not smoking during pregnancy were associated with a higher likelihood of probiotics supplementation only.
Conclusion
Vitamin D and probiotics supplementations are popular in children 0–2 years old and are associated with common factors. Data documented here will allow evaluation of the relationship between early childhood dietary intake and the development of islet autoimmunity and progression to T1D.
Keywords: childhood, dietary supplement, vitamin D, probiotics, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is an autoimmune disease that usually manifests during childhood and is hypothesized to be triggered by both genetic and environmental factors1. The major genetic determinants of this disease are polymorphisms of class II Human Leukocyte Antigen (HLA) genes encoding the human leukocyte antigen D related (HLA-DR) antigens and human leukocyte antigen DQ subregion (HLA-DQ). The major haplotypes of concern include DRB1*03, DQB1*0201, DRB1*04, DQB1*0302, and DRB1*0301, DQB1*0201/ DRB1*04, DQB1*0302. In efforts to identify plausible environmental triggers of the disease, such as dietary exposures, the association between the risk of T1D and supplemental intake of vitamin D and/or probiotics has been studied in different populations with inconclusive findings.2–5 Vitamin D has an active role in immunological regulation and in metabolic pathways pertinent to diabetes.6 Gut microbiota modifications via ingestion may prevent autoimmune diseases such as T1D through intestinal immune regulation although most of the evidence so far comes from animal models.7 However, there is limited data collected in a prospective observational manner that profile the voluntary supplementation during early childhood (0–2 years). This is a critical time period when dietary intake can make a profound and lasting impact on growth and health. Dietary supplements are concentrated sources of nutrients and can be used to achieve nutritional adequacy. National recommendations on vitamin D supplementation at this age are established in some countries with variations. In 2003, the American Academy of Pediatrics (AAP) recommended 5 mcg daily supplementation to infants (0–12 months of age), children, and adolescents, starting within the first two months after birth.8 This recommendation was changed in 2008 to 10 mcg everyday beginning within days after birth and continue throughout childhood.8 Infants in Germany are recommended to supplement with 10 mcg vitamin D every day.9, 10 In Sweden, daily supplementation of 10 mcg vitamin D is recommended for children under two years of age.11 For children in the same age group in Finland, the recommendations changed from 5–10 mcg daily depending on the use of infant formulas and milk products in 200412 to 10 mcg daily regardless of formula and milk consumption in 2011.13 The research on the health benefits of probiotics has grown remarkably in recent years; however, there are no current recommendations on probiotics supplementation for the pediatric population.14
The objective of this study is to assess the prevalence of vitamin D and probiotics supplementation in children with genetic predisposition of T1D during the first two years of life and to identify demographic and behavioral factors associated with this voluntary behavior.
Subjects and Methods
The Environmental Determinants of Diabetes in the Young (TEDDY) is a prospective study that examines the roles of dietary and other environmental exposures in the development of islet autoimmunity (IA) and T1D among children carrying high-risk HLA-DR-DQ genotypes.15
A total of 424 788 newborns were screened for high-risk HLA genotypes between September 2004 and February 2010. The screening identified 21 589 infants with eligible genotypes. Among them, 8 676 infants were enrolled at six clinical centers (three in the US: Colorado, Washington, and Georgia/Florida, and three in Europe: Finland, Sweden, and Germany). The participants are followed from birth until clinical diagnosis of T1D or until the child turns 15 years old. Data on their dietary supplement use was collected from parents or caretakers during the first two years of life.15, 16 Detailed description about study design have been previously published.15, 16 Separate consent letters for genetic screening and participation in prospective follow-up were obtained for all study participants from a parent or primary caregiver. The study was approved by local Ethics or Institutional Review Boards and is monitored by an External Evaluation Committee formed by the National Institutes of Health (clinical trial registration number NCT00279318).
The caretakers of the participants reported supplement name and duration of usage on a self-administered questionnaire every three months between ages 0 and 2. The staff reviewed the reports shortly after they were returned in-person or by mail to the study clinics and contacted the caretakers for missing or ambiguous data. At each visit, intake dates were confirmed against those reported at previous visits. When needed, the staff would reach out to the manufacturer or distributor to verify the ingredient composition. Products that contain one or any combination of the following nutrients or constituents are recorded in the study: vitamin, mineral, fatty acid, probiotics, and antioxidant compounds.17 Fiber, herbal, homeopathic, amino acid, and protein supplements were excluded. Any nutrient administered intramuscularly or subcutaneously was deemed a medication and excluded. Recorded supplements were categorized into one of the 37 mutually exclusive subgroups based on nutrient profile (27 single and 10 multivitamin/mineral (MVM) subgroups, see Supplementary Information). A supplement user was defined as an individual who used any supplement for at least one week during ages 0 and 2. The duration of supplementation was calculated based on self-reported starting and ending dates. If two or more supplements were reported in a subgroup (e.g. two vitamin D products reported), the maximum overall duration was used in the analysis. This present study included data from 8 674 participants, after two participants were excluded for missing supplement information.
Maternal and Child Characteristics
Maternal age referred to the woman’s age at time of delivery, and was treated as both a continuous and a categorical variable. Maternal education was recorded on a ten-category scale designed to account for different education systems across countries. The variable was then aggregated into two categories, basic primary education (primary school through trade school) and higher education (completed trade school or higher), to achieve comparability. Pre-pregnancy body mass index (BMI) was calculated by using self-reported weight before pregnancy (in kilograms) divided by the square of height (in meters) and was categorized based on the WHO classification.18 Smoking and alcohol consumption during pregnancy were defined as ‘yes’ if reported, regardless of frequency and duration. Self-reported maternal diabetes status during pregnancy was self-reported and was grouped into ‘no diabetes’, ‘type 1 diabetes’, ‘type 2 diabetes’, ‘gestational diabetes’, and ‘unknown’. Child-related variables included location of study center, year of birth, mode of delivery (vaginal v. caesarean), sex, gestational age (in weeks), birth order (first-born v. others), birth weight (in grams), and whether the child has a first-degree relative (FDR) (i.e. mother, father, and/or sibling) diagnosed with T1D. TEDDY enrollment started in late 2004 and ended in early 2010, so the “year of birth” variable has five values (2004–2005, 2006, 2007, 2008, and 2009–2010). The duration of breastfeeding (in months), irrespective of the use of infant formula, was calculated based on self-reported starting and ending dates. This variable and gestational age were dichotomized based on median values in the analysis.
Statistical Analysis
The 37 supplement subgroups were collapsed into vitamin D-containing and probiotics-containing categories. Logistic regression was used to model the odds of supplementation. The study centre was always included as an independent variable in the model because preliminary examination of the data suggested that supplement use differed across centres. The remaining independent variables were chosen via a backward selection algorithm and a 0.05 level cut-off. Analyses were performed using the Statistical Analysis Software (Version 9.4, SAS Institute, Cary NC, USA).
Results
The maternal and child characteristics of the study cohort are presented in Table 1. Overall, 81% (n=7 008) of the participants took at least one dietary supplement for at least one week during the first two years of life. A total of 11 141 supplement products were reported. Table 2 shows the use of single and MVM supplements that contained vitamin D and probiotics.
Table 1.
Maternal and child characteristics of the TEDDY participants by country.
| United States | Finland | Germany | Sweden | |
|---|---|---|---|---|
| n=3725 | n=1832 | n=594 | n=2525 | |
| Maternal characteristics | ||||
| Maternal age (years) | 30.1 (5.8) | 29.9 (5.0) | 31.4 (5.0) | 30.8 (4.7) |
| Pre-pregnancy BMIa | 25.7 (6.1) | 24.2 (4.6) | 24.3 (5.0) | 24.4 (4.6) |
| Maternal diabetes status | ||||
| Gestational | 191 (5.1) | 184 (10.0) | 28 (4.7) | 74 (2.9) |
| Type 1 | 121 (3.2) | 66 (3.6) | 106 (17.8) | 44 (1.7) |
| Type 2 | 16 (0.4) | 0 | 0 | 7 (0.3) |
| None | 3246 (87.1) | 1482 (80.9) | 394 (66.3) | 2336 (92.5) |
| Missing | 151 (4.1) | 100 (5.5) | 66 (11.1) | 64 (2.5) |
| Maternal education | ||||
| Basic | 567 (15.2) | 181 (9.9) | 66 (11.1) | 776 (30.7) |
| Higher | 2723 (73.1) | 1521 (83.0) | 449 (75.6) | 1510 (59.8) |
| Missing | 435 (11.7) | 130 (7.1) | 79 (13.3) | 239 (9.5) |
| Mother smoking during pregnancy | ||||
| No | 3211 (86.2) | 1516 (82.8) | 470 (79.1) | 2150 (85.1) |
| Yes | 420 (11.3) | 275 (15.0) | 123 (20.7) | 350 (13.9) |
| Missing | 94 (2.5) | 41 (2.2) | 1 (0.2) | 25 (1.0) |
| Mother drinking during pregnancy | ||||
| No | 2266 (60.8) | 1242 (67.8) | 319 (53.7) | 1803 (71.4) |
| Yes | 1372 (36.8) | 549 (30.0) | 274 (46.1) | 697 (27.6) |
| Missing | 87 (2.3) | 41 (2.2) | 1 (0.2) | 25 (1.0) |
| Child characteristics | ||||
| Birth weight (g)b | 3408 (538) | 3540 (554) | 3455 (550) | 3598 (537) |
| Gestational age (weeks)c | 39.2 (1.7) | 39.7 (1.6) | 39.3 (1.6) | 39.8 (1.6) |
| Premature birth (< 37 weeks) | ||||
| Yes | 245 (6.6) | 84 (4.6) | 32 (5.4) | 122 (4.8) |
| No | 3472 (93.4) | 1748 (95.4) | 562 (94.6) | 2402 (95.2) |
| Sex | ||||
| Female | 1838 (49.3) | 900 (49.1) | 298 (50.2) | 1249 (49.5) |
| Male | 1887 (50.7) | 932 (50.9) | 296 (49.8) | 1276 (50.5) |
| Mode of delivery | ||||
| Vaginal | 2363 (63.4) | 1511 (82.5) | 386 (65.0) | 2150 (85.2) |
| Caesarean | 1359 (36.5) | 317 (17.3) | 208 (35.0) | 375 (14.9) |
| Missing | 3 (<0.01) | 4 (0.2) | 0 | 0 |
| Being the first child | ||||
| No | 1949 (52.3) | 958 (52.3) | 264 (44.4) | 1229 (48.7) |
| Yes | 1373 (36.9) | 772 (42.1) | 267 (44.9) | 1096 (43.4) |
| Missing | 403 (10.8) | 102 (5.6) | 63 (10.6) | 200 (7.9) |
| Having FDR with T1D | ||||
| No | 3333 (89.5) | 1663 (90.8) | 375 (63.1) | 2354 (93.2) |
| Yes | 392 (10.5) | 169 (9.2) | 219 (36.9) | 171 (6.8) |
| Breastfed during the first year of year | ||||
| No | 269 (7.2) | 0 | 26 (4.4) | 27 (1.1) |
| Yes | 3455 (92.8) | 1831 (99.9) | 568 (95.6) | 2498 (98.9) |
| Missing | 1 (<0.1) | 1 (0.1) | 0 | 0 |
| Duration of breastfeeding (months)d | 7.5 (7.4) | 8.5 (5.6) | 7.2 (6.0) | 7.0 (5.1) |
| Year of Birth | ||||
| 2004-05 | 367 (9.9) | 402 (21.9) | 80 (13.5) | 588 (23.3) |
| 2006 | 609 (16.3) | 335 (18.3) | 114 (19.2) | 483 (19.1) |
| 2007 | 871 (23.4) | 373 (20.4) | 107 (18.0) | 500 (19.8) |
| 2008 | 867 (23.3) | 308 (16.8) | 136 (22.9) | 439 (17.4) |
| 2009-10 | 1011 (27.1) | 414 (22.6) | 157 (26.4) | 515 (20.4) |
TEDDY, The Environmental Determinants of Diabetes in the Young; BMI, body mass index; FDR, first-degree relative; T1D, type 1 diabetes.
Data expressed as n (%) or mean (SD).
Data was available in 3571 US, 1790 Finnish, 592 German, and 2472 Swedish mothers.
Data was available in 3491 US, 1828 Finnish, 594 German, and 2524 Swedish children.
Data was available in 3717 US, 1828 Finnish, 592 German, and 2524 Swedish children.
Data was available in 3687 US, 1817 Finnish, 571 German, and 2510 Swedish children.
Table 2.
Use of vitamin D and probiotics supplements by TEDDY participants during the first two years of life.
| Single Supplements,
n(%)a
|
MVMs, n(%)a,b
|
MVMs, n(%)a,c
|
No Supplement Use |
||||
|---|---|---|---|---|---|---|---|
| Vitamin D | Probiotics | Vitamin D+ | Vitamin D− | Probiotics+ | Probiotics− | ||
| United States (n=3725) | 291 (7.8) | 208 (5.6) | 1824 (49.0) | 394 (10.6) | 26 (0.7) | 2192 (58.9) | 1533 (41.1) |
| Finland (n=1832) | 1822 (99.4) | 1081 (59.0) | 326 (17.8) | 96 (5.2) | 64 (3.5) | 358 (19.6) | 7 (0.4) |
| Germany (n=594) | 296 (49.8) | 39 (6.6) | 485 (81.7) | 12 (2.0) | 4 (0.7) | 493 (82.7) | 11 (1.9) |
| Sweden (n=2525) | 903 (35.8) | 369 (14.6) | 2149 (85.1) | 40 (1.6) | 1 (<0.1) | 2188 (86.7) | 8 (0.3) |
| Total (n=8674) | 3312 (38.1) | 1697 (19.6) | 4784 (55.2) | 542 (6.2) | 95 (1.1) | 5231 (60.3) | 1557 (18.0) |
TEDDY, The Environmental Determinants of Diabetes in the Young; MVMs, multivitamin and mineral supplements
n= number of reported products. % = user percentage within a country.
MVMs are separated into vitamin D-containing products (Vitamin D+) and vitamin D-free products (Vitamin D−).
MVMs are separated into probiotics-containing products (Probiotics +) and probiotics-free products (Probiotics−).
A total of 6 776 (78%) children obtained vitamin D from either single or MVM formulations, with the latter being the more popular source (4784/8096 products, 59%). Vitamin D supplementation lasted an average of 81±32 weeks across all countries during the first two years. In comparison, 1 735 (20%) participants reported taking probiotics, and the main source was single supplements that do not contain vitamin or mineral (1697/1792 products, 95%). The mean total duration of probiotics use over the first two years was 61±36 weeks. Among children taking either type of supplements, more than half started the use during infancy. Country-specific information on the timing of supplement initiation and duration of use is shown in Table 3.
Table 3.
Age of initiation and duration of vitamin D and probiotics supplements in the TEDDY study.
| Vitamin D |
Probiotics |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| United States | Finland | Germany | Sweden | United States | Finland | Germany | Sweden | ||
| % of participants taking supplements during the first two years of life | 49.8 | 99.6 | 97.5 | 99.7 | 6.0 | 59.9 | 7.2 | 14.7 | |
| % of supplement users started in the first year of life | 67.5 | 100 | 99.5 | 100 | 61.2 | 84.5 | 53.5 | 88.7 | |
| % of supplement users started in the second year of life | 32.5 | 0 | 0.5 | 0 | 38.8 | 15.5 | 46.5 | 11.3 | |
| Duration of use within the first two years of life (weeks) | |||||||||
| Minimum | 1 | 2 | 2 | 5 | 1 | 1 | 3 | 1 | |
| 25th Percentile | 26 | 101 | 87 | 96 | 30 | 38 | 36 | 7 | |
| Mean(SD) | 57(34) | 93(23) | 87(28) | 89(25) | 54(29) | 65(35) | 51(23) | 53(42) | |
| Median (IQR) | 52 (68) | 102 (1) | 102 (16) | 100 (4) | 55 (48) | 76 (62) | 54 (29) | 61 (89) | |
| 75th Percentile | 94 | 102 | 103 | 100 | 78 | 100 | 65 | 96 | |
| Maximum | 104 | 104 | 104 | 104 | 104 | 104 | 94 | 104 | |
| % of users lasted > 12 months | 50.0 | 90.3 | 84.8 | 88.6 | 50.5 | 64.1 | 51.2 | 51.4 | |
TEDDY, The Environmental Determinants of Diabetes in the Young
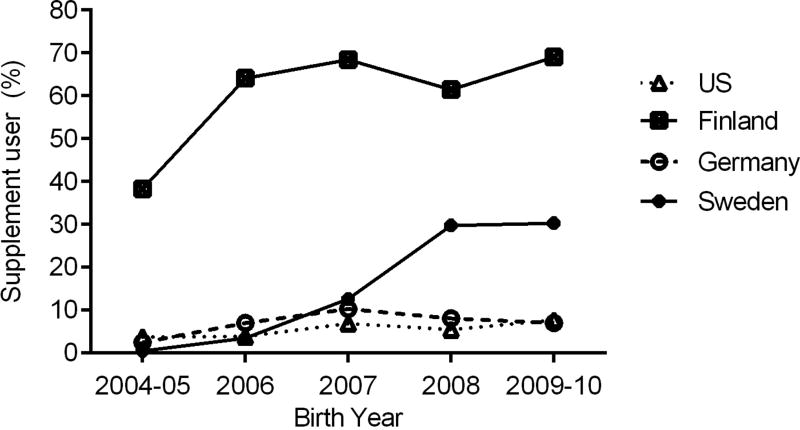
The investigation of significant predictors of vitamin D supplement use was done solely for the US participants because the prevalence in Finland, Germany, and Sweden was 97% ~ >99%. Across the three study centers in the US, 57% of the Washington participants (787/1 377), 45% of the Colorado participants (626/1 377), and 45% of the Georgia/Florida participants (442/973) took vitamin D supplements, respectively. Higher maternal education level (p<0.001), older maternal age at delivery (p<0.001), being the first child (p=0.002), longer duration of breast feeding (p<0.001), and being born in 2009–2010 (p<0.001) were associated with vitamin D use in the US (Table 4). The analysis of probiotic supplementation included all four countries because user proportions ranged from 6% in the US (6% in Washington, 6% in Colorado, and 7% in Georgia/Florida) to 60% in Finland. The same factors associated with vitamin D use, plus no smoking during pregnancy (p=0.004) and shorter gestational age (p=0.008), were associated with higher likelihood of probiotics use (Table 4). The year of birth exhibited a stronger independent association with probiotics use than with vitamin D use (Table 4). The incidence of probiotics use by year of birth and by country is shown in Figure 1.
Table 4.
Factors associated with vitamin D and probiotics supplementation during the first two years of life in the TEDDY study
| Vitamin D (US only)a | Probioticsb | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Maternal age (years) | ||||||
| ≤30 | reference | reference | ||||
| >30 | 1.03 | 1.01, 1.04 | 0.001 | 1.03 | 1.01, 1.04 | <0.001 |
| Maternal educationc | ||||||
| Basic | reference | reference | ||||
| Higher | 1.81 | 1.47, 2.24 | <0.001 | 1.46 | 1.20, 1.78 | <0.001 |
| Being the first child | ||||||
| No | reference | reference | ||||
| Yes | 1.28 | 1.09, 1.49 | 0.002 | 1.64 | 1.43, 1.89 | <0.001 |
| Duration of breastfeeding (months)d | ||||||
| ≤7.5 | reference | reference | ||||
| >7.5 | 1.06 | 1.05, 1.07 | <0.001 | 1.02 | 1.01, 1.03 | <0.001 |
| Year of Birth | ||||||
| 2004-05 | reference | reference | ||||
| 2006 | 0.98 | 0.73, 1.31 | 0.872 | 2.10 | 1.64, 2.71 | <0.001 |
| 2007 | 1.17 | 0.88, 1.55 | 0.278 | 3.77 | 2.96, 4.79 | <0.001 |
| 2008 | 1.31 | 0.99, 1.73 | 0.062 | 4.63 | 3.62, 5.91 | <0.001 |
| 2009-10 | 1.48 | 1.12, 1.94 | 0.005 | 5.49 | 4.34, 6.93 | <0.001 |
| Gestational age (weeks)e | ||||||
| >39.5 | - | - | - | reference | ||
| ≤39.5 | - | - | - | 0.95 | 0.91, 0.99 | 0.008 |
| Mother smoking during pregnancy | ||||||
| No | - | - | - | reference | ||
| Yes | - | - | - | 0.72 | 0.57, 0.890 | 0.004 |
TEDDY, The Environmental Determinants of Diabetes in the Young; OR, odds ratio; CI, confidence interval.
ORs and CIs are for the US population only due to highly prevalent use in Finland, Germany, and Sweden and are adjusted for study centers.
ORs and CIs are for the entire study population and are adjusted for country.
Basic education = primary school through trader school; higher education = completed trader school or higher.
Duration of breastfeeding is dichotomized based on the median value (7.5 months).
Gestational age is dichotomized based on the median value (39.5 weeks).
Figure 1.
Incidence of probiotics use during the first two years of life by country and by year of birth in the TEDDY study.
Discussion
Data from this multi-national prospective cohort indicated that dietary supplement use under 2 years of age was popular among children with known genetic risk of T1D. More than half of the US participants and almost everyone in Finland, Germany, and Sweden took vitamin D supplements, whereas the prevalence of probiotics use varied notably among the countries. The MVMs were the main source of vitamin D in this age group, while probiotics mainly came from single supplements. Supplementation was initiated in the first year of life and continued for longer than one year by the majority of the users. A common set of demographic and behavioral factors were associated with taking vitamin D and probiotics supplements across all TEDDY countries.
Our findings can be compared to national recommendations and results from similar studies. The overwhelming majority of German children receiving supplemental vitamin D for longer than one year indicated a good implementation of the national recommendation. Our data echoed the BABYDIET Study in which 96.5% of children with genetic risk of T1D received vitamin D supplements during infancy.19 The vitamin D recommendation in Sweden was also well followed. Almost all Swedish participants used vitamin D supplements within the first year of life and continued the intake for an average of 89 weeks. Seventy percent of the products reported in Sweden were a liquid preparation that contained vitamin A and D (AD-drops), which reflected the market availability during the TEDDY enrollment period (2004–2010), as the AD-drops was discontinued in 2009. The popular use of vitamin D was also noted in another study in southeastern Sweden where 99% of children were given AD-drops during infancy and 87.8% at 2.5 years of age.20 Similarly, in northern Sweden 76% of 7-month-old infants and 74% of 10-month-old infants were given supplemental vitamin D.21 The high prevalence and long duration of vitamin D supplementation in Finland aligned with the national recommendations. The Type I Diabetes Prediction and Prevention (DIPP) study in Finland found 91% of the children took vitamin D at 3 and 6 months of age, and 81% were still taking the vitamin at one year old.22 Another Finnish study reported 50% of children took vitamin D supplements at age 2 and the most commonly used variety was vitamin D or a combination of vitamin A and D.23 In the US, the proportion of supplement users (56%) was higher than the data (17%) from young children (< 2 years old) surveyed in the National Health and Nutrition Examination Survey (NHANES) around the same time (2007–2010 cycles).24 Similar to the NHANES results, more TEDDY participants used MVM supplements than single supplements. The higher prevalence in TEDDY might be related to the parental knowledge of the child’s genetic risk and/or the belief that supplementation may be a preventive approach as reported by 1.3% of the TEDDY families at 6 months of age and 2.2% at 15 months of age.25 Children in NHANES were more likely to receive supplements for “tooth health and cavity prevention” than for disease treatment or prevention.24
The research on the health benefits of probiotics has boomed in recent years. Despite lack of national recommendations, giving probiotics to neonates is on the rise.26 Among TEDDY participants in the US, Germany, and Sweden, less than 5% born at the beginning of the study enrollment (2004–2005) took probiotics. This number doubled in those born five years later (2009–2010) in Germany and the US. In Sweden, the use increased drastically from 0.5% to 30%. Given that TEDDY is an observational study, it is reasonable to believe the difference by birth year reflected the growing popularity of probiotics over the years. In Germany, infants were exposed to probiotics mostly through formulas that are fortified with probiotics (90.3%),5 which might explain the relatively lower use of a separate probiotics supplement. The drastic increase in probiotics use in Sweden, particularly between 2007 and 2008, may have resulted from various marketing efforts of probiotics supplements around that time. For example, Semper® Mag Droppar, a Lactobacillus reuteri Protectis supplement introduced in Sweden around 2006/2007, has been advertised for various health problems (e.g. acute diarrhea, colic) to “keep the stomach in balance.” The favorable effect of Lactobacillus reuteri in treating infantile colic, as seen in a clinical trial in Italy,27 might also have boosted the use of probiotics in young children. In Finland, taking probiotics during antibiotic treatment is a common practice, which might have explained the higher proportion of probiotics users seen in the 2004–2005 birth cohort.5 The continued higher prevalence and longer use in that country might be attributed to the uptake of media coverage of an intervention study on probiotics and health outcomes among Finnish infants.28 Probiotics exposure between 0–27 days of age from supplements and infant formulas was found to lower the risk of islet autoimmunity.5 Such discovery may stimulate more interest in giving probiotics to children.
Our analysis captured the initiation time and duration of supplement use, which is less frequently reported in the literature. Vitamin D supplementation was seen in more participants in the second year of life, but the majority of the use was initiated in the first year and more than half of the use lasted longer than a year. That further supported the notion that national recommendations on vitamin D supplementation were adhered to by many TEDDY families. Vitamin D supplements were not only taken more widely in the TEDDY European countries, but also lasted longer than in the US. One possible explanation of the shorter use in the US was that all US participants received infant formulas fortified with vitamin D by 12 months of age,29 which might have made the parents less likely to consider a dietary supplement. It needs to be pointed out, however, that one liter of formula supplies 10 mcg vitamin D and it is unlikely that an infant would consume that much formula per day.8 This indicates a need to better communicate the AAP recommendations to the public to ensure all children, regardless of the consumption of formula, have adequate vitamin D intake.
The predictors of supplementation identified in this study were similar for vitamin D and probiotics, which was a good confirmation given the analysis of vitamin D included the US participants only. Some of the predictors were consistent with those reported in other studies. The effect of maternal age and parental education attainment were seen in two Finnish studies.22, 23 The NHANES 2007–2010 revealed similar associations with supplement use in children in the US.30 A recent analysis of the TEDDY data suggested being the first child, later birth year, and mother not smoking during pregnancy were associated with probiotics exposure during infancy (0–1 year).5 Being pregnant with the first child and higher maternal education level were also found to predict mother’s supplement use during pregnancy in TEDDY.31 It is worth noting that some of these significant factors, namely older maternal age at the child’s birth, higher maternal education, no smoking during pregnancy, and born in the later years of study screening period, were found to be associated with lower likelihood of withdrawal from TEDDY during the first three years.32, 33 It is possible that families with these characteristics engage more actively in a variety of behaviors, such as remaining in TEDDY and using supplements. This engagement may be related to other factors, such as higher socioeconomic status, as suggested by some of the associated variables, such as older maternal age and higher education. The parental awareness of T1D risk and the decision to enroll in the study itself may have influenced the decision of giving vitamin D and probiotics supplements to their children.
To conclude, findings from this prospective study add to the knowledge of voluntary supplementation behavior in early childhood and suggested many parents of children with T1D risk paid attention to the nutrient intake recommendations. Using age-appropriate dietary supplements could be a convenient and effective way to reach the intake goal. Data from this prospective cohort will enable future evaluation of relationships between early childhood dietary intake (from foods and supplements) and the development of islet autoimmunity and progression to T1D in the at-risk children. It would also be valuable to profile the temporal trend of supplementation among the TEDDY participants as they grow older, and to evaluate whether the development of islet autoantibody, if detected during study participation, affects the supplementation behavior in these children.
Supplementary Material
Acknowledgments
The authors thank the participation of all families and the work of the TEDDY Study Group.
Financial Support:
The Environmental Determinants of Diabetes in the Young (TEDDY) study is funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC). This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
The NIDDK, NIAID, NICHD, NIEHS, JDRF, and CDC had no role in the design, analysis or writing of this article.
Footnotes
Conflicts of Interest:
The authors declare no conflict of interest.
Authorship:
JY formulated research questions, coordinated and supervised data collection, and drafted and revised the manuscript. RNT carried out statistical analyses, reviewed, and revised the manuscript. UMU, CAA, KS, AR, NF, GJ, and CW supervised data collection and critically reviewed the manuscript. JMN and SMV conceptualized the study, designed the data collection instruments and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. JY has full access to the data in the study and final responsibility for the decision to submit for publication.
Ethical Standards Disclosure
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by local Institutional Review Board at each TEDDY clinical center and were monitored by an External Evaluation Committee formed by the National Institutes of Health. Written informed consents were obtained from a parent or primary caregiver, separately, for genetic screening and participation in the prospective follow-up.
References
- 1.Virtanen SM. Dietary factors in the development of type 1 diabetes. Pediatr. Diabetes. 2016;17:49–55. doi: 10.1111/pedi.12341. [DOI] [PubMed] [Google Scholar]
- 2.Hyppönen E, Läärä E, Reunanen A, Järvelin M-R, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. The Lancet. 2001;358(9292):1500–1503. doi: 10.1016/s0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 3.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch. Dis. Child. 2008;93(6):512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 4.Simpson M, Brady H, Yin X, Seifert J, Barriga K, Hoffman M, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 2011;54(11):2779–2788. doi: 10.1007/s00125-011-2278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uusitalo U, Liu X, Yang J, Aronsson CA, Hummel S, Butterworth M, et al. Association of early exposure of probiotics and islet autoimmunity in the teddy study. JAMA Pediatrics. 2016;170(1):20–28. doi: 10.1001/jamapediatrics.2015.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. The Lancet. 2016;387(10035):2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorini C, Falcone M. Shaping the (auto)immune response in the gut: the role of intestinal immune regulation in the prevention of type 1 diabetes. American journal of clinical and experimental immunology. 2013;2(2):156–171. [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner CL, Greer FR the Section on Breastfeeding Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 9.Koletzko B, Bauer C, Brönstrup A, Cremer M, Flothkötter M, Hellmers C, et al. Key Statements for Infant Nutrition During the First Year of Life. Monatsschr. Kinderheilkd. 2013;161:237–246. [Google Scholar]
- 10.German Nutrition Society. [Accessed 23 March 2017];The DACH Reference Values. https://www.dge.de/wissenschaft/referenzwerte/
- 11.Nordic Council of Ministers. Nordic Nutrition Recommendations. Copenhagen, Denmark: 2012. Report no.: 978-92-983-2629-2. [Google Scholar]
- 12.Hasunen K, Kalavainen M, Keinonen H, editors. Helsinki: Publications of the ministry of social affairs and health. 2004. The child, family, and food. Nutrition recommendations for infants and young children as well as pregnant and breastfeeding mothers. [Google Scholar]
- 13.Virtanen S, Sarlio-Lahteenkorva S. [Accessed 23 March 2017];D-vitamiinivalmisteiden käyttösuositukseen muutoksia. http://www.mynewsdesk.com/fi/pressreleases/d-vitamiinivalmisteiden-kaeyttoesuositukseen-muutoksia-562100.
- 14.Thomas DW, Greer FR Nutrition Co, Section on Gastroenterology H, Nutrition. Probiotics and Prebiotics in Pediatrics. Pediatrics. 2010;126(6):1217–1231. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 15.The TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr. Diabetes. 2007;8(5):286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 16.Hagopian WA, Lernmark A, Rewers MJ, Simell OG, She JX, Ziegler AG, et al. TEDDY--The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Ann. N. Y. Acad. Sci. 2006;1079:320–326. doi: 10.1196/annals.1375.049. [DOI] [PubMed] [Google Scholar]
- 17.Moyers S, Richesson R, Krischer J. Trans-atlantic data harmonization in the classification of medicines and dietary supplements: a challenge for epidemiologic study and clinical research. Int. J. Med. Inform. 2008;77(1):58–67. doi: 10.1016/j.ijmedinf.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO; Geneva: 2000. [PubMed] [Google Scholar]
- 19.Pflüger M, Winkler C, Hummel S, Ziegler AG. Early Infant Diet in Children at High Risk for Type 1 Diabetes. Horm. Metab. Res. 2010;42(02):143–148. doi: 10.1055/s-0029-1241830. [DOI] [PubMed] [Google Scholar]
- 20.Brekke HK, Ludvigsson J. Vitamin D supplementation and diabetes-related autoimmunity in the ABIS study. Pediatr. Diabetes. 2007;8(1):11–14. doi: 10.1111/j.1399-5448.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 21.Blomquist HK, Frangsmyr A, Hernell O, Stenberg B, Back O. Dietary intake of vitamin D during the second half of infancy in Swedish infants. Scandinavian Journal of Nutrition. 2004;48(4):173–177. [Google Scholar]
- 22.Rasanen M, Kronberg-Kippila C, Ahonen S, Uusitalo L, Kautiainen S, Erkkola M, et al. Intake of vitamin D by Finnish children aged 3 months to 3 years in relation to sociodemographic factors. Eur. J. Clin. Nutr. 2006;60(11):1317–1322. doi: 10.1038/sj.ejcn.1602459. [DOI] [PubMed] [Google Scholar]
- 23.Marjamäki Räsänen, Uusitalo Ahonen, Veijola Knip, et al. Use of Vitamin D and other Dietary Supplements by Finnish Children at the Age of 2 and 3 Years. Int. J. Vitam. Nutr. Res. 2004;74(1):27–34. doi: 10.1024/0300-9831.74.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Bailey RL, Gahche JJ, Thomas PR, Dwyer JT. Why US children use dietary supplements. Pediatr. Res. 2013;74(6):737–741. doi: 10.1038/pr.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LB, Lynch KF, Baxter J, Lernmark B, Roth R, Simell T, et al. Factors Associated With Maternal-Reported Actions to Prevent Type 1 Diabetes in the First Year of the TEDDY Study. Diabetes Care. 2014;37(2):325–331. doi: 10.2337/dc13-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderhoof JA, Young R. Probiotics in the United States. Clin. Infect. Dis. 2008;46(Supplement 2):S67–S72. doi: 10.1086/523339. [DOI] [PubMed] [Google Scholar]
- 27.Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) Versus Simethicone in the Treatment of Infantile Colic: A Prospective Randomized Study. Pediatrics. 2007;119(1):e124–130. doi: 10.1542/peds.2006-1222. [DOI] [PubMed] [Google Scholar]
- 28.Salminen MK, Tynkkynen S, Rautelin H, Saxelin M, Vaara M, Ruutu P, et al. Lactobacillus Bacteremia during a Rapid Increase in Probiotic Use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002;35(10):1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 29.Hummel S, Vehik K, Uusitalo U, McLeod W, Aronsson CA, Frank N, et al. Infant feeding patterns in families with a diabetes history – observations from The Environmental Determinants of Diabetes in the Young (TEDDY) birth cohort study. Public Health Nutr. 2014;17(12):2853–2862. doi: 10.1017/S1368980013003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picciano MF, Dwyer JT, Radimer KL, Wilson DH, Fisher KD, Thomas PR, et al. Dietary supplement use among infants, children, and adolescents in the United States, 1999–2002. Arch. Pediatr. Adolesc. Med. 2007;161(10):978–985. doi: 10.1001/archpedi.161.10.978. [DOI] [PubMed] [Google Scholar]
- 31.Aronsson CA, Vehik K, Yang J, Uusitalo U, Hay K, Joslowski G, et al. Use of dietary supplements in pregnant women in relation to sociodemographic factors - a report from The Environmental Determinants of Diabetes in the Young (TEDDY) study. Public Health Nutr. 2013;16(8):1390–1402. doi: 10.1017/S1368980013000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SB, Lee HS, Baxter J, Lernmark B, Roth R, Simell T. The Environmental Determinants of Diabetes in the Young (TEDDY) study: predictors of early study withdrawal among participants with no family history of type 1 diabetes. Pediatr. Diabetes. 2011;12(3 Pt 1):165–171. doi: 10.1111/j.1399-5448.2010.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson SB, Lynch KF, Baxter J, Lernmark B, Roth R, Simell T, et al. Predicting Later Study Withdrawal in Participants Active in a Longitudinal Birth Cohort Study for 1 Year: The TEDDY Study. J. Pediatr. Psychol. 2015 doi: 10.1093/jpepsy/jsv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.