Abstract
Objective
Although BLT-humanized mice provide a robust model for HIV-1 infection and enable evaluation of cure strategies dependent on endogenous immune responses, most mice develop graft versus host disease (GVHD), limiting their utility for extended HIV cure studies. This study aimed to: 1) Evaluate the GVHD-resistant C57BL/6 Rag2−/−γc−/−CD47−/− triple knockout (TKO)-BLT mouse as a model to establish HIV-1 latency. 2) Determine whether TKO-BLT mice could be maintained on ART for extended periods of time. 3) Assess the rapidity of viral rebound following therapy interruption.
Design
TKO-BLT mice were HIV-1 infected, treated with various ART regimens over extended periods of time and assayed for viral rebound following therapy interruption.
Methods
Daily subcutaneous injection and oral ART-mediated suppression of HIV-1 infection was tested at various doses in TKO-BLT mice. Mice were monitored for suppression of viremia and cellular HIV-1 RNA and DNA prior to and following therapy interruption.
Results
Mice remained healthy for 45 weeks post-humanization and could be treated with ART for up to 18 weeks. Viremia was suppressed to < 200 copies/ml in the majority of mice with significant reductions in cellular HIV-1 RNA and DNA. Treatment interruption resulted in rapid viral recrudescence.
Conclusions
HIV-1 latency can be maintained in TKO-BLT mice over extended periods on ART and rapid viral rebound occurs following therapy removal. The additional 15–18 weeks of healthy longevity compared to other BLT models provides sufficient time to examine the decay kinetics of the latent reservoir as well as observe delays in recrudescence in HIV-1 cure studies.
Keywords: HIV-1, latency, antiretroviral therapy, cure, humanized mouse
INTRODUCTION
A major barrier to the development of a cure for HIV-1 is the persistence of a reservoir of latently infected cells that is not eliminated by antiretroviral therapy (ART). Efforts are underway to test latency reversal agents (LRAs) that reactivate HIV-1 to expose latently infected cells to pharmaceutical and/or immunological elimination, the so-called shock and kill strategy. Many kill strategies incorporate aspects of the host immune response to destroy these cells. Cure studies would be greatly facilitated by a small animal model that could be infected with HIV-1, establish a reservoir of latently infected cells, undergo ART for sufficient time to suppress viremia, and then survive in a healthy state long enough to evaluate shock and kill approaches. Additionally, animals provide significantly greater sensitivity over in vitro assays in the ability to detect remaining latent infection. In cases where the latent reservoir has been reduced but not eliminated, it could take significant time following treatment interruption for residual virus to emerge from latency, so long-lived models are essential.
BLT-humanized mice can be treated with ART to establish latent HIV-1 infections[1, 2] and, importantly for cure studies, they also develop HIV-1-specific immune responses[3, 4]. Another advantage of BLT-humanized mice is that up to 50 mice can be reconstituted with tissue from a single human donor, providing sufficient numbers of animals for statistically powerful studies. Although BLT-humanized mice are the best model for HIV-1 studies in most respects, they have a major drawback. Coincident with human reconstitution, BLT mice often develop graft versus host disease (GVHD) and begin to die around 22 weeks post-humanization[5]. This time limitation represents a major obstacle in the use of BLT mice for cure studies[6]. In order to avoid GVHD issues, humanized mice such as T cell only-reconstituted mice, have been developed to study latency[7]. However, such mice lack B cell responses and human macrophages, which may be an important reservoir for HIV-1[8].
Here we investigate the utility of C57BL/6 Rag2−/−γc−/−CD47−/− triple knockout BLT (TKO-BLT) mice, which are resistant to the development of GVHD[4], to study long-term ART-suppression of HIV-1. TKO-BLT mice develop high-levels of multilineage hematopoiesis with organized lymphoid tissues and have an intact complement system. They exhibit hallmarks of human HIV-1 infection including CD4+ T-cell depletion and immune activation. Infection is characterized by transient high-level viremia that is resolved to low levels, broad HIV-1 specific T cell responses and low but detectable virus-specific IgG responses[4]. Previously, TKO-BLT mice showed no signs of GVHD out to 29 weeks after humanization[4]. The current study extends this to 45 weeks in mice generated from numerous human donors and includes 6 weeks of infection before initiating an 18 week regimen of oral ART to suppress HIV-1 followed by 4 weeks of therapy interruption. The results indicate that the TKO-BLT mouse is a viable model in which to study HIV-1 cure strategies. Following the time to establish an HIV-1 reservoir and achieve virus suppression by ART, the mice survive at least another five months in a healthy state, sufficient time to rigorously test cure strategies. Given that the mice remained well reconstituted and healthy at the final time-point, experiments may be extended even further.
MATERIALS AND METHODS
Ethics statement
Anonymous tissue donations were obtained with informed written consent via Advanced Bioscience Resources. Research was conducted under the NIH Office of Human Subjects Research Exemption #4980. Animal study proposals RML2014-032, RML2015-022, RML2015-081, and RML2016-030 were approved by the Rocky Mountain Laboratories ACUC. Experiments were done in accordance with Public Health Service Office of Laboratory Animal Welfare regulations. Rocky Mountain Laboratories is fully accredited by AAALAC.
Humanized TKO-BLT mice
C57BL/6 Rag2−/−γc−/−CD47−/− (TKO) mice were humanized using the BLT method as previously described[4, 9]. Briefly, 6–10 week old mice were irradiated and transplanted with 17–22 week gestation human thymus and liver followed by injection of autologous liver-derived CD34+ hematopoietic progenitor cells. Animals were housed under specific pathogen-free conditions.
HIV-1 challenge and antiretroviral therapy
HIV-1JR-CSF stocks were prepared as previously described[4]. Intraperitoneal infections were one-time, 100μl injections. Liquid formulations of tenofovir disoproxil fumarate (TDF), emtricitabine (FTC) and dolutegravir (DTG) were provided by Gilead Sciences (Foster City, CA) and administered daily by subcutaneous injection. Tablets of TDF, FTC and raltegravir (RAL; Merck & Co., Kenilworth, NJ) were crushed and formulated with TestDiet 5B1Q feed as previously described[10]. Briefly, powdered TDF, FTC and RAL were incorporated into TestDiet 5B1Q irradiated feed pellets at a final concentration of 720 mg/kg TFV, 520 mg/kg FTC, and 4800 mg/kg RAL.
Isolation of plasma and human leukocytes
Blood was collected in EDTA and centrifuged to obtain plasma. Blood leukocytes were purified using RBC Lysis Buffer (BioLegend, San Diego, CA). Splenocytes and thymic organoid cells were obtained by passage through 100μm filters and bone marrow was flushed from femurs and tibias prior to lysis with ACK (NH4Cl 0.15M, KHCO3 10mM, EDTA 0.1M). CD4+ splenocytes used for proviral quantification were pre-enriched using positive selection (Miltenyi, San Diego, CA).
Flow cytometry
Leukocytes were stained using CD3-V450, CD4-APC, CD45-V500, CD123-PE and lin-1 (CD3, CD14, CD16, CD19, CD20, CD56)-FITC (BD Biosciences, San Jose, CA); CD11c-APC and HLA-DR Alexa Fluor 700 (Biolegend); CD8-APC-eFluor 780, CD19 PerCP-Cy5.5 and CD45 eFluor450 (eBioscience, San Diego, CA). Samples were run on an LSRII (BD Biosciences) and analyzed using FlowJo 9.9 (TreeStar Inc., Ashland, OR).
Quantification of HIV-1 antigenemia, viral RNA and proviral DNA
Plasma HIV-1 p24 levels were determined by ELISA (Advanced Bioscience Laboratories, Rockville MD). Plasma and cell-associated vRNA was measured by using either the COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0 or Abbott RealTime HIV-1 assay on the m2000 system. Genomic DNA was isolated with the QIAamp DNA Mini Kit (Qiagen). Determination of proviral DNA was performed by quantitative real-time PCR as detailed previously[11] using lysates from 5×106 CD4-enriched splenocytes.
Statistics
Statistical calculations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
RESULTS
TKO-BLT mice sustain long-term human engraftment and HIV-1 infection
To assess the durability of human engraftment in TKO-BLT mice, blood was analyzed at 12, 35 and 45 weeks post-transplant (wpt). Increasing frequencies of human leukocytes (hCD45+) were observed over the 45 week period (Fig. 1A top) but the absolute number of human leukocytes remained stable between 12 and 45wpt peaking at 35wpt (1.5×106/ml) (Fig. 1A bottom). This suggested that the human compartment remained stable and the residual mouse myeloid compartment was gradually lost. Over time, total T cells (CD3+) increased with slightly higher proportions and numbers of CD4+ cells and lower CD8+ cells (Fig. 1A top). The loss of B cells (Fig. 1A top) between 12 and 35wpt is characteristic of TKO-BLT mice[4] and is likely due to the earlier release of B cells into the the periphery compared to T cells, which first migrate and mature within the human thymic organoid. However, the overall reduction in B cell numbers (Fig. 1A bottom) suggests that the murine mileu is insufficient for the maintenance of a robust B cell compartment, which is a concern in BLT-humanized mice[12] [13]. It is interesting but unclear why B cell numbers slightly rebounded between 35 and 45wpt. At study termination (45wpt), spleens were harvested and analyzed. Cell frequencies (Fig. 1B, top) and absolute numbers (Fig. 1B, bottom) for individual mice were variable, but all spleens contained at least 10% human leukocytes and ~4×106 human cells per spleen. CD4+ to CD8+ T cells ratio averaged 2:1, typical of this model[4]. Spleens at 45wpt contained an average of 6×106 CD4+ T cells, providing ample cell numbers to support HIV-1 infection and to detect cell-associated viral RNA (ca-RNA) or HIV-1 DNA (vDNA) with reasonable sensitivity in individual mice. In addition, lineage negative, HLA-DRhi CD11c+ myeloid (mDC) and CD123+ plasmacytoid (pDC) dendritic cells remained detectable at 45wpt. Overall, TKO-BLT mice maintained high-levels of human engraftment over 45 weeks with no clinical signs of GVHD.
Figure 1. Long-term human engraftment of blood and spleen and sustained stable HIV-1 plasma antigenemia in TKO-BLT mice.

(A) Frequencies (top row) and numbers (bottom row) of human cell subsets determined by flow cytometric analysis of blood samples obtained from a representative uninfected TKO-BLT mice generated from a single human donor at 12 (n=35), 35 (n=32) and 45 (n=31) weeks post-transplant (wpt) or (B) spleen samples at 45wpt (n=15). (C) Plasma HIV-1 antigenemia in TKO-BLT mice as determined by p24 ELISA at 7, 12, 15, 18 and 22 weeks post infection (wpi). Splenic data is shown represent individual mice with horizontal bar at the median. Data points on longitudinal graphs denote mean values ±SEM at each time point. Statistical analyses were done by one way ANOVA with a Tukey multiple comparsions post test to compare all groups. Differences with a P value below 0.05 are shown. *P<0.05, **P<0.01***P<0.001, ****P<0.0001.
TKO-BLT mice were next assessed for their ability to support HIV-1 infection over an extended period. Groups of TKO-BLT mice were infected with HIV-1JR-CSF at 12wpt and all sustained p24 antigen in plasma over 22 weeks of infection (Fig. 1C). Starting at 7 weeks post infection (wpi), antigenemia remained relatively stable with no significant decline over 15 weeks, suggesting that peak viremia had resolved and the proviral reservoir was established by 7wpi. In summary, TKO-BLT mice remained healthy, maintained high-levels of human engraftment and supported HIV-1 infection over the extended periods required to test cure strategies.
Daily-injected ART regimens suppress viremia and cell-associated viral RNA
Cure studies require viremia suppression below a stringent limit of detection (LOD) for a period of time sufficient to establish latency. Previously, small plasma volumes from mice restricted the LOD to 800 copies of viral RNA/ml. Now more sensitive kits permit a LOD of 140 copies/ml. Reduction of viral RNA (vRNA) to levels below this LOD indicate that latent HIV-1 provirus remains as the primary source of virus upon therapy removal. As depicted in Figure 2A, mice were infected with HIV-1JR-CSF for 6 weeks. At 6wpi mice were distributed into treatment groups with relatively equivalent levels of plasma vRNA. Half of the mice received a daily subcutaneous (s.q.) regimen of ART and half received control injections. Viremia was monitored for up to 6 weeks of therapy and in some groups therapy was removed to assess for recrudescence as diagrammed (Fig. 2A). Four ART regimens were selected based on preliminary pharmacokinetic data from C57BL/6 and TKO-BLT mice to determine dose ranges that would provide efficacious plasma drug levels (data not shown). The lowest dose regimen, Regimen 1, achieved significant suppression of viremia compared to mock controls after 14 days of administration but did not suppress vRNA below the LOD that would be required for cure studies. Continued treatment for an additional 2 weeks did not further reduce viremia so the group was terminated at day 28 (Fig. 2B). Mice receiving Regimen 2 achieved plasma vRNA levels below the level of 800 copies/ml reported in other humanized mouse studies[1, 10, 14–18] by day 14 and became undetectable by the more sensitive assay employed in our study by day 28 (Fig. 2C). Suppression was maintained until day 42 when the mice were removed from therapy to test for recrudescence. Viremia recrudesced to untreated levels 2 weeks later (day 56, Fig. 2C). We expected higher dose regimens to drop viremia below the 140 copies/ml LOD even more quickly than Regimen 2 but this was not the case. The original Regimen 3, (Regimen 3a), produced rapid but no better suppression of viremia than Regimen 2 by day 14 (Fig. 2D) and mice receiving this dosage showed minor signs of toxicity. Thus, the dosages of FTC and DTG were cut in half on day 21, which alleviated the toxicity and reduced viremia below 140 copies/ml after an additional 2 weeks of administration (Fig. 2D). Therapy interruption at day 36 in all the treated animals resulted in recrudescence to levels similar to untreated by day 56 (Fig. 2D). Surpisingly, the highest dose regimen, Regimen 4, did not prove to be more effective so therapy was discontinued at day 21 and the mice were euthanized at day 36, after virus recrudescence (Fig. 2E). Thus, the daily subcutaneous ART regimens varied in their ability to suppress viremia below stringent LODs in TKO-BLT mice, with Regimen 2 appearing optimal among the doses tested. Removal of ART-therapy resulted in rapid viral recrudescence in TKO-BLT mice even when plasma vRNA levels were below the LOD.
Figure 2. Dose-dependent suppression of plasma viral load using subcutaneously injected daily ART regimens.
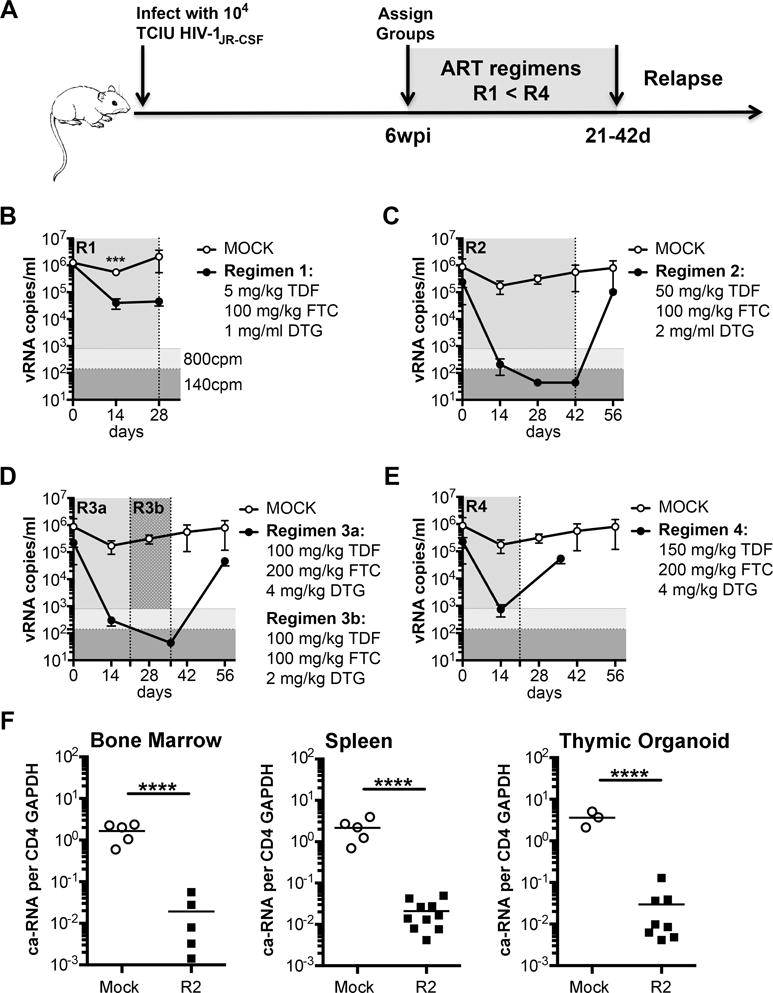
(A) Schematic of the experimental timeline(s) used to test the ART regimens. (B) Plasma viral loads (vRNA copies/ml (cpm)) of mock-treated (open circles) or treated (closed circles) after 14 and 28 days of Regimen 1; (C) days 14, 28 and 42 of Regimen 2 plus an additional bleed 2 weeks after treatment withdrawal at day 56; (D) after 14 days of treatment with the original Regimen 3 formulation (3a), and after switching to the amended Regimen 3b dose for 2 weeks at day 36 and after treatment interruption at day 56 (20 days after treatment withdrawal); (E) after 14 days of treatment with Regimen 4 followed by a day 36 sample 2 weeks after treatment withdrawal at day 21. (B–E) The same mock treated mice are shown in each panel for comparison to the treated group. (F) Levels of cell-associated (ca-RNA) detected in cells harvested from the bone marrow, spleen and human thymic organoid of mice treated for 4 weeks with Regimen 2. CD4+ T cells from some mice were combined to obtain sufficient material for the ca-RNA assay. Vertical shading denotes periods of ART administration in the treatment group, vertical dotted lines indicate points where there were changes in the treatment protocol. Horizontal shading highlights the previously reporting limit of detection (LOD) of 800 copies/ml (light grey) and the more stringent LOD of 140 copies/ml used in this study (dark grey). Horizontal bars denote the mean and single points represent the mean ±SEM at each time point. ****P<0.0001, ***P<0.001 unpaired T-test at the indicated time point.
Humanized mouse models have the advantage of access to organs to assess the effect of treatments on tissue vRNA levels. To evaluate cell-associated vRNA (ca-RNA) in the tissues, TKO-BLT were infected for 8 weeks prior to receiving daily s.q. doses of the most efficacious regimen, Regimen 2, for 4 weeks. After treatment tissues were assessed for ca-RNA levels. HIV-1 ca-RNA was detectable but significantly decreased in treated mice compared to untreated mice (Fig. 2F). Thus, daily s.q. ART decreased but did not eliminate tissue vRNA despite suppression of viremia below detectable levels. This result is similar to what is seen in humans[19] and other humanized mouse models[16].
HIV-1 suppression in TKO-BLT mice using ART-supplemented mouse chow
Since daily subcutaneous injections are labor intensive over the extended periods required to perform cure studies and sharp use is contraindicated when working with HIV-1, we next sought to induce HIV-1 latency using a previously reported free-fed, ART-supplemented mouse chow formulation[10] As illustrated in Figure 3A, mice were infected and infection was allowed to progress for 6 weeks. At 6wpi, mice were assigned to either ART-treated (ART FOOD) or untreated (MOCK) groups based on equivalent levels of plasma p24 (Fig. 3B). After 4 weeks of treatment (10wpi) the mice were tested for viremia and half the mice in each group were euthanized to assess splenic vDNA (Fig. 3A). Viremia was significantly suppressed in the treated animals compared to controls with 7 of 10 mice below the LOD of 800 copies/ml and 5 below 300 copies/ml (Fig. 3C left). The mice receiving ART also had significantly less vDNA in CD4+ T cells harvested from spleen compared to untreated animals after 4 weeks of therapy (Fig. 3C right). To assess recrudescence following treatment interruption, the remaining mice were changed back to regular mouse chow for 2 weeks (Fig. 3A). Mice that had received ART all recrudesced and had vRNA and vDNA levels similar to the untreated group (Fig. 3D). Therefore, oral ART suppressed viremia in TKO-BLT mice while maintaining a detectable vDNA reservoir capable of re-establishing viremia upon treatment withdrawal.
Figure 3. Significant suppression of HIV-1 RNA and DNA using free-fed ART chow and rapid recrudescence after treatment withdrawal.
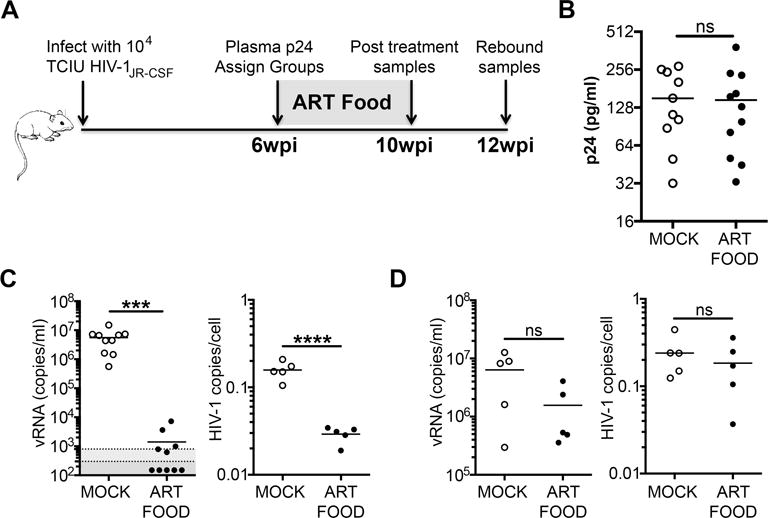
(A) Schematic of the experimental timeline. (B) Levels of HIV-1 p24 in plasma of untreated mice 6wpi were used to assign groups with equal plasma antigenemia prior to treatment commencement. (C) Levels of vRNA in plasma (left panel) and HIV-1 DNA in CD4+ enriched cells from spleen (right panel) in mice at 10wpi that had either received 4 weeks of ART-supplemented chow (ART FOOD) or normal mouse chow (MOCK). (D) Comparison of vRNA in plasma (left panel) or HIV-1 DNA in CD4+ enriched spleen cells (right panel) from mice at 12wpi that had either never received ART (MOCK) or that had received ART for 4 weeks and then had it withdrawn for 2 weeks to assess viral recrudescence (ART FOOD). Horizontal bars denote mean. Horizontal shading demarks LODs of 800 copies/ml (light grey) or 300 copies/ml (dark grey). ns=not significant, ***P<0.0001, ****P<0.0001 unpaired T-tests.
Oral ART in HIV-1 infected TKO-BLT mice prevents T cell depletion and inversion of the CD4:CD8 ratio
Ideally a model for HIV-1 cure studies would replicate the immunological benefits of ART, primarily the preservation of CD4+ T cell counts and a high CD4:CD8 ratio. To assess these parameters in TKO-BLT mice receiving oral ART we examined the blood cell frequencies (Fig. 4A top) and counts (Fig. 4A bottom) of ART-treated compared to untreated animals. Blood was tested prior to ART initiation at 6wpi, at the end of ART at 10wpi, and 2 weeks after treatment interruption (12wpi). Cell frequencies and counts of human leukocytes (hCD45+), CD3+ T cells, CD4+ and CD8+ T cells and CD19+ B cells were all similar between groups at the initiation of ART (Fig. 4A). Overall human cell frequencies were significantly higher in ART-treated animals at the end of 4 weeks of therapy (10wpi), as were CD4+ T cell frequencies (Fig. 4A top). Notably, within the CD3+ T cell compartment the CD4+ frequency remained high compared to the CD8+ T cell frequency i.e. there was a higher CD4:CD8 ratio of 2.52±0.28 in the ART group compared to the untreated group (ratio of 1.52±0.62) (Fig. 4A). Similarly, at the completion of ART at 10wpi, cell counts had significantly increased for all cell types in treated mice compared to untreated mice, including B cells, which was surprising as this cell type is not a target for HIV-1 and typically declines over time in TKO-BLT mice even in the absence of infection (Fig. 1A bottom). These increases appeared related to viral suppression as within 2 weeks of ART withdrawal cell numbers had declined and were no longer significantly different from untreated HIV-1-infected animals (Fig. 4B).
Figure 4. Preservation of cell subsets, including CD4+ T cells in HIV-1 infected TKO-BLT mice receiving ART via free-fed mouse chow.
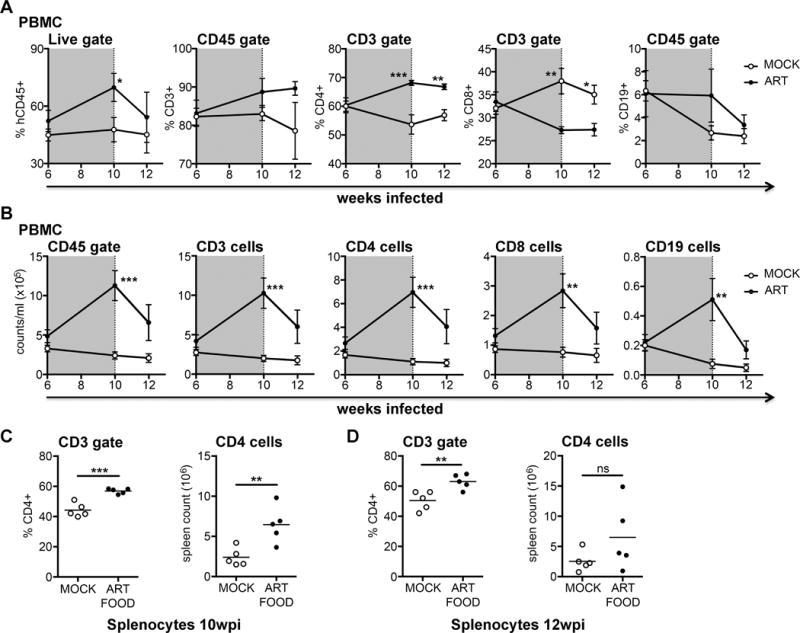
Cellular frequencies (A) and numbers (B) in blood of HIV-1+ TKO-BLT mice at 6, 10 and 12wpi that had either received ART-supplemented chow (filled circles) or normal chow (open circles) between 6 and 10wpi. Data points denote mean ±SEM at each time point. Vertical shading indicates the period where animals in the treatment group received ART-chow. Frequency (left panel) or number (right panel) of CD4+ T cells in spleens of HIV-1+ animals at 10wpi, immediately after 4 weeks of ART food had been administered to the ART FOOD treatment group (C) or at 12wpi after withdrawing ART food for 2 weeks from the previously 4-week treated group (ART FOOD) compared to untreated controls (MOCK) (D). Each data point represents an individual mouse. Horizontal bars represent the mean. *P<0.05, **P<0.01, ***P<0.001 unpaired T-tests.
Next we examined the effect of oral ART on the frequency and number of CD4+ T cells in spleen. Mice after 4 weeks of ART (at 10wpi) contained significantly higher frequencies and numbers of CD4+ T cells in spleen compared to untreated mice (Fig. 4C), indicating that ART had penetrated the tissues and suppressed HIV-1. Evaluation of spleens 2 weeks after ART interruption (12wpi) showed that while frequencies of CD4+ T cells remained higher than untreated mice, the cell counts had declined and were no longer different from controls (Fig. 4D). Overall, the results demonstrated that oral ART in the TKO-BLT model afforded immunological benefits in both blood and tissues analogous to those seen in humans.
The human hematopoietic graft and latent HIV-1 reservoir is maintained over extended periods of ART-mediated viral load suppression in TKO-BLT mice
Previous humanized mouse studies reported administration of ART for periods of 14 weeks with no assessment of virus recrudescence[15] or for 9 weeks followed by virus recrudescence for 3 weeks[18]. To determine if we could significantly extend these periods in our model we administered ART to mice at 4wpi, and continued therapy for 18 weeks, followed by treatment interruption for 4 weeks. Viremia was monitored as shown in Fig. 5A. HIV-1 viremia declined to below 200 copies/ml in 29 of 47 mice and below 800 copies/ml in 43 of the 47 mice after 4 weeks of treatment (Fig. 5A, 8wpi). After 18 weeks (22wpi) only two of 22 mice had viremia above 800 copies/ml and 16 of 22 had levels below the 200 copies/ml LOD. The two mice above 800 copies/ml at 22wpi had both been undetectable at previous time points and may have been undergoing viral load “blips” similar to those seen in ART-treated human patients[20]. Further optimization of the ART dose in chow will be required to determine if the percentage of mice below the more stringent LOD can be increased or if viral suppression can be achieved more quickly. Note: The reduced number of mice at 22wpi was not a result of mortality but assignment of half mice to a separate study. The reassigned mice had equivalent virus suppression levels (data not shown).
Figure 5. Suppression of viral replication, the latent reservoir and the human immune compartment are maintained over 18 weeks of ART.
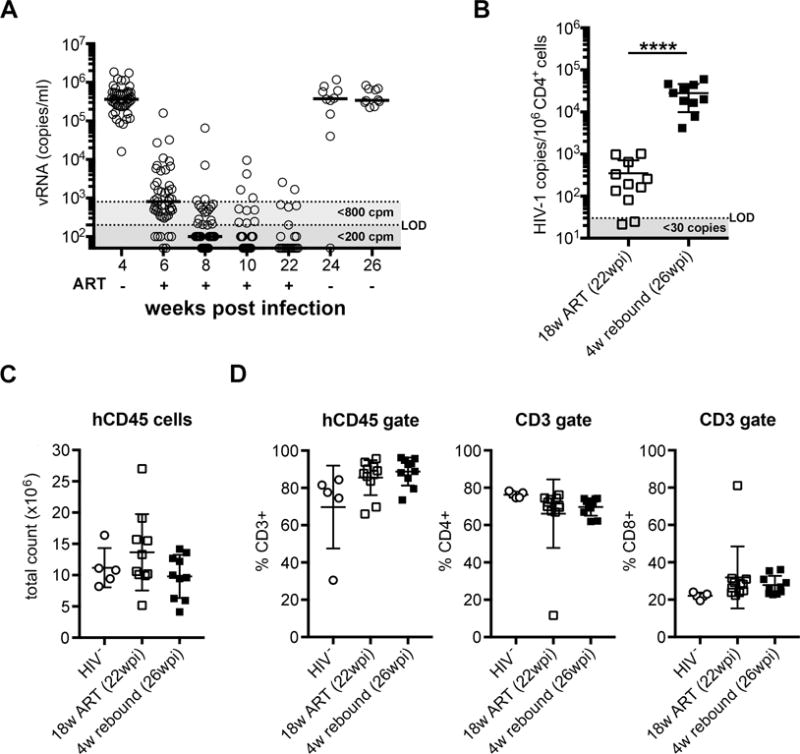
(A) HIV-1 viral RNA (vRNA, copies/ml (cpm)) in plasma of mice before (4wpi, n=47) during (6, 8 and 10wpi, n=47 and 22wpi, n=22) and after discontinuation of ART-medicated mouse chow (24 and 26wpi, n=10). Horizontal bars indicate median. Horizontal shading demarks LODs of 800 copies/ml (light grey) or 140 copies/ml (dark grey). (B) Total HIV-1 viral DNA in CD4+ enriched splenocytes after 18 weeks of ART (22wpi) and after 4 weeks of discontinued therapy (26wpi). Mean±SD. Unpaired t-test. ***P<0.0001. (C) Total human leukocyte count and (D) total CD3+ T cell and CD4+/CD8+ T cell subset frequencies in spleens from HIV-1 negative mice (n=5), ART-medicated mice after 18 weeks of treatment (22wpi, n=11) and after an additional 4 weeks of discontinued ART (26wpi, n=10). Mean±SD. Each symbol represents an individual mouse. Horizontal shading demarks limits of detection (LOD).
After 18 weeks on ART (22wpi), half of the mice were euthanized for analysis and half underwent treatment interruption for 4 weeks to assess viral recrudescence. Viremia increased to pre-therapy levels after 2 weeks (24wpi) and remained high after 4 weeks (26wpi) without ART. At that point the remaining mice were euthanized for tissue analysis (Fig. 5A, 26wpi). HIV-1 DNA in the CD4+ splenocytes of mice was significantly reduced immediately after 18 weeks of ART (22wpi) compared to mice that underwent ART interruption for 4 weeks (Fig. 5B, 26wpi). Two mice at 22wpi, immediately post-therapy, had vDNA levels below the assay’s LOD but all mice undergoing ART interruption had detectable cellular vDNA and plasma vRNA. These results indicated that, as in humans, ART did not purge the latent HIV-1 reservoir from the mice (Fig. 5A, B). Assessment of the number of human leukocytes in the spleens at 22 and 26wpi (Fig. 5C) and the frequency of total CD3+, CD4+ and CD8+ T cell splenocytes (Fig. 5D) revealed no significant differences compared to HIV-1 negative controls. At the time of euthanasia, all mice appeared healthy.
These data demonstrated that TKO-BLT mice administered oral ART over an extended period of 18 weeks followed by treatment interruption and viral recrudescence to a total of 26 weeks of infection (39–43 weeks post-humanization) not only remain healthy and well engrafted with a high CD4+:CD8+ ratio but are able to achieve long-term viral suppression and significant contraction, but not loss, of the HIV-1 proviral reservoir that rapidly reactivates upon therapy removal.
DISCUSSION
HIV-1 cure strategies require a small animal model that recapitulates latent HIV-1 infections in humans being treated with ART. Humanized mice have become well-established for the study of HIV-1 infection and testing of therapeutics[21]. They are particularly valuable for studies that rely on both an authentic HIV-1 infection and/or a genuine human immune system[6]. The BLT method of humanization produces robust multi-lineage reconstitution in multiple lymphoid tissues complete with innate and adaptive immune responses. HIV-1 infection in the humanized mouse simulates human infection, including high virus titers, tissue dissemination, proviral reservoir seeding, immune dysfunction and CD4+ T cell loss[22].
The utility of BLT-humanized mice has been shown for in vivo studies evaluating the cell types harboring latent virus[7, 8, 23], the role of interferon in reservoir size and persistence[18], the ability of a histone deacetylase inhibitor to reactivate latent HIV-1[17] as well as short-term evaluations of reservoir purging strategies[10, 16]. However, the failure of these mice to thrive in long-term experiments has restricted their utility for cure studies. Here we demonstrate that using TKO-BLT mice allows the extension of studies for more than 4 months longer than previously reported in other BLT models. This longevity makes it feasible to test cure therapies in immunologically competent and healthy mice that survive and remain highly reconstituted with human cells for at least 45 weeks post-transplantation. As the mice remained healthy at study’s end suggests that experiments may be extended even further.
In summary, we demonstrate that upon HIV-1 infection of the TKO-BLT mouse, subcutaneous or oral ART can be administered to suppress viremia below stringent LODs and that viral suppression results in CD4+ T cell count recovery similar to that seen in humans. Additionally, the mice contain abundant cells for ex vivo analysis such as assessing LRA-induced ca-RNA increases or therapeutic reductions in provirus. Importantly, HIV-1 recrudesces rapidly after ART interruption facilitating observations of delays in recrudescence due to interventions that reduce viable provirus and serves as the most stringent test for functional cure. Finally, the immune responses in this model[4] make it suitable to test strategies that utilize endogenous immune-modalities to clear the HIV-1 reservoir, particularly when such experiments require extended timelines.
Acknowledgments
We thank Drs. Stefano Boi, Alyssa B. Evans and Ron Swanstrom for critical reading of the manuscript. KJL designed and performed experiments and analyzed and interpreted data; CP designed experiments and analyzed data; KS, RJM, DLP, NWC, SN, JZ, JG, MW and ESVD performed experiments; KJL, RJM, KP and BR humanized the mice; UD, GK and KJH designed experiments and interpreted data. KJH and KJL wrote the paper.
Funding: Support for this work was provided by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institute of Health, USA, by the Mayo Clinic, by Gilead Sciences and the Deutsche Forschungsgemeinschaft, project TRR60.
Footnotes
The authors have no conflicts of interest.
References
- 1.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, et al. Generation of HIV Latency in Humanized BLT Mice. Journal of virology. 2012;86(1):630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, et al. HIV Latency in the Humanized BLT Mouse. Journal of virology. 2012;86(1):339–347. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. Journal of virology. 2009;83(14):7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavender KJ, Pang WW, Messer RJ, Duley AK, Race B, Phillips K, et al. BLT-humanized C57BL/6 Rag2−/−gammac−/−CD47−/− mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013;122(25):4013–4020. doi: 10.1182/blood-2013-06-506949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenblatt MB, Vbranac V, Tivey T, Tsang K, Tager AM, Aliprantis AO. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PloS one. 2012;7(9):e44664. doi: 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins NW, Sainski AM, Dai H, Natesampillai S, Pang YP, Bren GD, et al. Prime, Shock, and Kill: Priming CD4 T Cells from HIV Patients with a BCL-2 Antagonist before HIV Reactivation Reduces HIV Reservoir Size. Journal of virology. 2016;90(8):4032–4048. doi: 10.1128/JVI.03179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D, Garcia JV. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology. 2013;10 doi: 10.1186/1742-4690-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arainga M, Edagwa B, Mosley RL, Poluektova LY, Gorantla S, Gendelman HE. A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy. Retrovirology. 2017;14(1):17. doi: 10.1186/s12977-017-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavender KJ, Messer RJ, Race B, Hasenkrug KJ. Production of bone marrow, liver, thymus (BLT) humanized mice on the C57BL/6 Rag2(−/−)gammac(−/−)CD47(−/−) background. Journal of immunological methods. 2014;407:127–134. doi: 10.1016/j.jim.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malnati MS, Scarlatti G, Gatto F, Salvatori F, Cassina G, Rutigliano T, et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nature protocols. 2008;3(7):1240–1248. doi: 10.1038/nprot.2008.108. [DOI] [PubMed] [Google Scholar]
- 12.Seung E, Tager AM. Humoral immunity in humanized mice: a work in progress. The Journal of infectious diseases. 2013;208(Suppl 2):S155–159. doi: 10.1093/infdis/jit448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jangalwe S, Shultz LD, Mathew A, Brehm MA. Improved B cell development in humanized NOD-scid IL2Rgammanull mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun Inflamm Dis. 2016;4(4):427–440. doi: 10.1002/iid3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. Latent HIV-1 Infection of Resting CD4(+) T Cells in the Humanized Rag2(−/−) gamma(−/−)(c) Mouse. Journal of virology. 2012;86(1):114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nischang M, Sutmuller R, Gers-Huber G, Audige A, Li D, Rochat MA, et al. Humanized Mice Recapitulate Key Features of HIV-1 Infection: A Novel Concept Using Long-Acting AntiRetroviral Drugs for Treating HIV-1. PloS one. 2012;7(6) doi: 10.1371/journal.pone.0038853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS pathogens. 2014;10(1):e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai P, Wu G, Baker CE, Thayer WO, Spagnuolo RA, Sanchez R, et al. In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection. Retrovirology. 2016;13(1):36. doi: 10.1186/s12977-016-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Ma J, Li J, Li D, Li G, Li F, et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. The Journal of clinical investigation. 2017;127(1):269–279. doi: 10.1172/JCI90745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. The Journal of infectious diseases. 2013;208(8):1212–1220. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC, et al. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA: the journal of the American Medical Association. 2001;286(2):171–179. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 21.Garcia VJ. Humanized mice for HIV and AIDS research. Curr Opin Virol. 2016;19:56–64. doi: 10.1016/j.coviro.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpel ME, Boutwell CL, Allen TM. BLT humanized mice as a small animal model of HIV infection. Current opinion in virology. 2015;13:75–80. doi: 10.1016/j.coviro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, et al. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nature medicine. 2017;23(5):638–643. doi: 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]


