Abstract
Bisphenol A (BPA) is a commonly used plasticizer. Previous studies show that in utero exposure to BPA affects reproductive outcomes in the F1–F3 generations of mice. However, its multigenerational effects on ovarian histology and steroidogenesis over the reproductive lifespan are unknown. Thus, we tested the hypothesis that BPA has multigenerational effects on follicle numbers and steroidogenesis. Mice were exposed in utero to vehicle control or BPA (0.5, 20, and 50 µg/kg/day). Ovaries were collected for histological and gene expression analyses and sera were collected for hormone assays. In utero BPA exposure decreased preantral follicle numbers, cytochrome P450 aromatase mRNA levels, and estradiol levels in the F1 generation, whereas it decreased testosterone levels and altered steroidogenic acute regulatory protein, cytochrome P450 cholesterol side-chain cleavage, 3β-hydroxysteroid dehydrogenase 1, and cytochrome P450 aromatase mRNA levels in the F2 generation. These data suggest that BPA has multigenerational effects on the ovary in mice.
Keywords: Bisphenol A (BPA), In utero exposure, Multigenerational, Ovary, Sex steroid hormones, Steroidogenesis, Diethylstilbestrol (DES)
Introduction
Exposure to endocrine disrupting chemicals in the environment has been implicated as a factor in reproductive disorders including infertility [1]. Bisphenol A (BPA) is a widely studied endocrine disrupting chemical with estrogenic properties that is used as a plasticizer in epoxy resins and polycarbonate plastics. Several epidemiological studies in humans and experimental studies in animal models suggest that BPA is a reproductive toxicant [2–11]. BPA is found in various products including plastic reusable food and beverage containers, baby bottles, toys, food and beverage can liners, thermal receipt paper, and dental sealants. BPA can leach out of these products when subjected to heat (e.g., microwaving), acids and bases (e.g. detergents), and ultraviolet light. Thus, daily human exposure to BPA is a potential hazard given its endocrine disrupting properties. Exposure is most commonly through the oral route, and BPA has been detected in human tissues including blood, urine, saliva, breast milk, amniotic fluid, placental tissues, and ovarian follicular fluid [12–14].
The presence of BPA in maternal reproductive tissues, placenta, amniotic fluid, and umbilical cord blood is of concern as this implies possible BPA exposure to the developing embryo. Thus, BPA may be capable of altering the germline, leading to undesirable effects in future generations. Previous studies in mice show that in utero exposure to BPA can affect several developmental and reproductive parameters in the F1, F2, and F3 generations [15–17]. Specifically, in utero BPA exposure inhibited ovarian germ cell nest breakdown, and decreased fertility and litter size in the F1 generation [15], whereas it reduced the ability of mice to maintain pregnancies to term in the F1 and F2 generations [16]. Further, in utero BPA exposure delayed puberty, and decreased fertility and conception rates in the F3 generation [16]. Additionally, in utero BPA exposure altered ovarian follicle numbers on postnatal day (PND) 21 in the F1 and F2 generations, and altered the gene expression of certain steroidogenic enzymes on PND 21 in the F1, F2, and F3 generations of mice [17]. Overall, these findings suggest that BPA may have multigenerational (i.e., effects in more than one generation) and transgenerational (i.e., effects in the F3 generation) effects on some reproductive outcomes in mice.
The aim of the present study was to investigate further the multigenerational effects of BPA on the F1 and F2 generations of mice, with a focus on ovarian follicle numbers, atresia, serum sex steroid hormone levels, and ovarian mRNA levels of steroidogenic enzymes in aging mice. Specifically, this study tested the hypothesis that in utero BPA exposure affects ovarian follicle numbers, atresia, and steroidogenic capacity of the F1 and F2 generations of mice at three, 10, and 12 months of age.
Materials and methods
Chemicals
BPA (99% purity; National Institutes of Environmental Health Sciences, USA) and diethylstilbestrol (DES; Sigma Chemical Co., USA) were dissolved in ethanol and then diluted to selected doses in tocopherol-stripped corn oil (vehicle). The final ethanol concentration in the tocopherol-stripped corn oil for the oral dosing solutions was 0.1%.
Animals
Inbred FVB mice (Charles River, USA) were housed in polysulfone cages in a controlled environment (25°C, 12-hour light-dark cycles). The mice were fed Teklad Rodent Diet 8604 (Harlan) and provided with reverse osmosis filtered water in glass bottles ad libitum. All animal procedures were approved by the Institutional Animal Use and Care Committee at the University of Illinois at Urbana–Champaign, USA.
Study design
Female mice (F0 generation) at 12 weeks of age were mated with fertility-confirmed unexposed control males. The day of detection of a vaginal sperm plug after mating was considered as gestational day (GD) 1. On GD 1, the female mice with vaginal sperm plugs were individually housed and thereafter, daily weights were recorded for further confirmation of successful pregnancy. On GD 9, confirmed pregnant females were randomly assigned to five treatment groups: vehicle control (tocopherol-stripped corn oil), DES (0.05 µg/kg/day), or BPA (0.5, 20, and 50 µg/kg/day). DES at 0.05 µg/kg/day was used in the study as a control to confirm that the mice were responsive to estrogenic compounds. BPA at 0.5 µg/kg/day was used to mimic estimated human exposure from bottle feeding [18], and this dose was based on a previous study that showed reproductive abnormalities in female CD-1 mice exposed to a similar dose of BPA in utero [19]. BPA at 20 µg/kg/day was used because this dose was shown to disrupt oocyte meiosis and cause aneuploidy in mice late in their reproductive lifespan [20]. BPA at 50 µg/kg/day was used because it is the United States Environmental Protection Agency (USEPA) referenced safe dose [21]. Daily oral dosing of the dams in the respective treatment groups was started on GD 11 and continued until the birth of pups. The exposure window of GD 11 to birth was selected because this is a critical period of ovarian development in the mouse. Oral dosing was achieved by placing a pipette tip containing the dosing solution into the mouth, and the doses were administered in volumes of 28–32 µl based on daily body weight. The F0 dams were allowed to deliver naturally and the birth date of the F1 progeny was considered as PND 0. Litter size was standardized to 10 pups per litter on PND 4, as described in a previous study [15]. At least one F1 female from each litter was euthanized by CO2 asphyxiation followed by decapitation at three months and 10 months of age. Blood was collected using cardiac puncture, centrifuged to obtain serum, and then serum samples were used for analysis of sex steroid hormone levels. Ovaries were collected and used for assessment of histological analysis of follicle numbers and health as well as mRNA levels of steroidogenic enzymes. Some of the adult F1 females were used to generate the F2 progeny, and at least one F2 female from each litter was euthanized at three months and 12 months of age. Serum samples and ovaries were collected from these F2 mice as described for the F1 generation.
Histological Analysis
Ovaries from the F1 and F2 mice at three months of age were collected and fixed in 10% neutral buffered formalin, followed by transfer to 70% ethanol after 24 hours until further processing for histological analysis. The fixed ovaries were subjected to paraffin embedding, serial sectioning (8 µm thick sections), and mounting on glass slides, which were then stained using hematoxylin and eosin. Every tenth section of each ovary was used to count healthy primordial, primary, preantral, and antral follicles by experimenters blind to treatment group as previously described [17,22]. Every tenth section of each ovary was also used to count unhealthy follicles; specifically follicles showing abnormal morphology (e.g., follicles with multiple/disintegrated oocytes) and atresia (i.e., follicles with apoptotic bodies encompassing more than 5% of the total area of the follicles) [17,22]. The raw follicle numbers as well as the percentage of each healthy follicle type (healthy follicles divided by the total number of follicles) per sample were used for statistical analysis.
Hormone Assays
Serum samples were collected from the F1 mice at three months of age and from the F2 mice at 12 months of age for analysis of estradiol, testosterone, and progesterone levels. The selected sex steroid hormones are essential for normal female reproduction. Blood was drawn from the mice immediately after euthanasia. All samples from the F1 and F2 generations at three months were collected during estrus. For the F2 generation at 12 months, some samples from the control, DES, 0.5 µg/kg BPA, and 20 µg/kg BPA treatment groups were collected during diestrus and some samples from the control and 50 µg/kg BPA treatment groups were collected during estrus. Thus, hormone data from samples collected during each of these cycle stages were analyzed and presented separately because hormone levels differ depending on the stage of the cycle. The sera were subjected to enzyme-linked immunosorbent assays (ELISAs) and the levels of each hormone were measured in one run using ELISA kits purchased from Diagnostics Research Group (DRG, Springfield, NJ) according to manufacturer’s instructions. Samples were run in duplicate, and had coefficients of variation less than 10%. The analytical sensitivity of each kit was 0.1 ng/ml for progesterone, 0.083 ng/ml for testosterone, 9.71 pg/ml for estradiol. The mean values for each sample were used for statistical analysis.
Quantitative real-time polymerase chain reaction
Ovaries from the F1 mice at three and 10 months of age and from the F2 mice at three and 12 months of age were collected and snap frozen for quantitative real-time polymerase chain reaction (qPCR) analysis of steroidogenic enzymes. We chose to measure the mRNA levels of the transport protein, steroidogenic acute regulatory protein (Star), as well as cytochrome P450 cholesterol side-chain cleavage (Cyp11a1), 3β-hydroxysteroid dehydrogenase 1 (Hsd3b1), 17β-hydroxysteroid dehydrogenase 1 (Hsd17b1), and cytochrome P450 aromatase (Cyp19a1) because they are known regulators of ovarian steroidogenesis [23]. Total RNA was extracted from the ovaries using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) and quantified with the Nanodrop® spectrophotometer following manufacturer's instructions. Total RNA (100 ng per sample) was then reverse transcribed to complementary DNA (cDNA) using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, CA) and subsequently subjected to qPCR using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) and accompanying CFX Manager Software as per manufacturer's recommendations. The gene-specific primers are listed in Table 1. The qPCR program protocol was based on previously published methods [24],[25], with minor modifications: initial incubation at 95°C for five minutes (instead of 10 minutes) and final extension at 72°C for two minutes (instead of 10 minutes). Standard curves, amplification, melt curves and temperatures were generated for each run. All samples were run in triplicate, except the samples analyzed for steroidogenic enzymes at 10 months in the F1 generation and at 12 months in the F2 generation, which were run in duplicate due to limited amount of sample. Beta-actin (Actb) was used as a reference gene because its mRNA levels did not differ among the five treatment groups (data not shown). Thus, data from each generation at each time-point were normalized to the corresponding value of Actb for that generation/time-point, and the Pfaffl method for relative quantification [26] was used to calculate relative fold changes, which were used for statistical analysis.
Table 1.
Sequence of Primers used for Gene Expression Analysis
| Gene Name | Gene symbol |
Accession No. |
Forward primer | Reverse primer |
|---|---|---|---|---|
| Beta-actin | Actb | NM_007393 | GGGCACAGTGTGGGTGAC | CTGGCACCACACCTTCTAC |
| Steroidogenic acute regulatory protein | Star | NM_007810 | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
| Cytochrome P450 cholesterol side-chain cleavage | Cyp11a1 | NM_008293 | AGATCCCTTCCCCTGGCGACAATG | CGCATGAGAAGAGTATCGACGCATC |
| 3β-hydroxysteroid dehydrogenase 1 | Hsd3b1 | NM_008293 | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC |
| 17β-hydroxysteroid dehydrogenase 1 | Hsd17b1 | NM_010475 | ACTGTGCCAGCAAGTTTGCG | AAGCGGTTCGTGGAGAAGTAG |
| Cytochrome P450 aromatase | Cyp19a1 | NM_007810 | CATGGTCCCGGAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
Statistical Analysis
Data were expressed as means ± standard error of the means (SEM), and analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Multiple comparisons between normally distributed experimental groups (vehicle control group and BPA treated groups) were made using one-way analysis of variance, followed by the two-sided Dunnett’s test when variances were equal, or the Welch test followed by the Games-Howell test for post-hoc comparison when variances were unequal. Multiple comparisons between non-normally distributed experimental groups (vehicle control group and BPA treated groups) were made using the Kruskal–Wallis test and the Mann-Whitney test [27]. Comparisons between the vehicle control group and the DES treated group were made using the independent samples t-test when data showed normal distribution, and using the Mann-Whitney test when data were not normally distributed. Statistical significance was assigned at p ≤ 0.05. In the F2 generation, we only had an n of 2 for serum samples in the DES treatment group at 12 months of age and for mRNA levels in the BPA 50 µg/kg/day group at three months of age. Thus, values from those groups were not used in the statistical analysis and the data are not shown in the graphs.
Results
Effect of BPA and DES on ovarian histology in the F1 and F2 generations
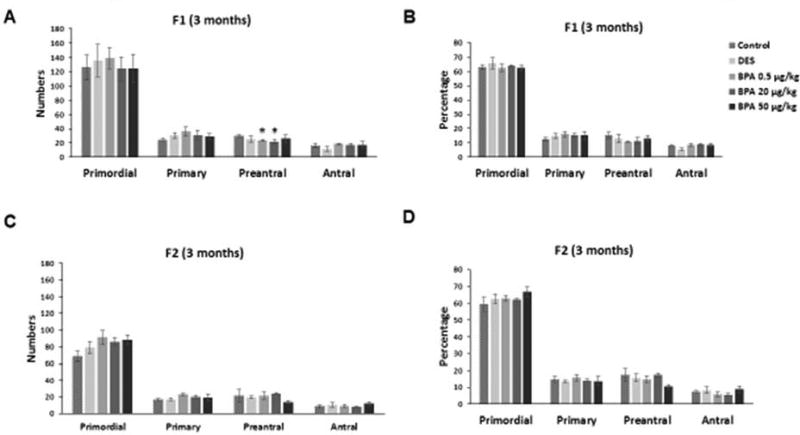
We investigated whether in utero exposure to BPA affected ovarian histology, specifically ovarian follicle numbers and atresia in the F1 and F2 generations of mice at three months of age. BPA at 0.5 µg/kg/day and at 20 µg/kg/day significantly decreased the number of preantral follicles when compared to control in the F1 generation (Figure 1A; n=3, p≤0.05). However, BPA did not affect the numbers of primordial, primary, or antral follicles in the F1 generation (Figure 1A). Further, it did not affect the percentage of primordial, primary, preantral, or antral follicles in the F1 generation (Figure 1B). In the F2 generation, BPA did not affect ovarian follicle numbers or follicle percentage when compared to controls (Figures 1C–D). Further, BPA exposure did not affect the percentage of abnormal or atretic follicles in the F1 and F2 generations at three months when compared to controls (data not shown). Similarly, DES exposure at 0.05 µg/kg/day did not affect ovarian follicle numbers or follicle percentage in the F1 and F2 generations of mice when compared to control (Figures 1A–D).
Figure 1. Effect of BPA and DES on ovarian follicle numbers and percentage.
Ovaries were collected from F1 and F2 generations of mice at three months of age and then subjected to histological evaluation of primordial, primary, preantral, and antral follicles. The graphs represent the means ± SEM (n = 3 ovaries/treatment) of follicle numbers (panel A) and percentage of follicle types from the total (panel B) counted in the F1 generation. Similarly, the graph represents the follicle numbers (panel C) and percentage (panel D) in the F2 generation as means ± SEM (n = 3 ovaries/treatment). Asterisks (*) represent statistically significant differences from the vehicle control (p ≤ 0.05).
Effect of BPA and DES on serum levels of sex steroid hormones in the F1 generation
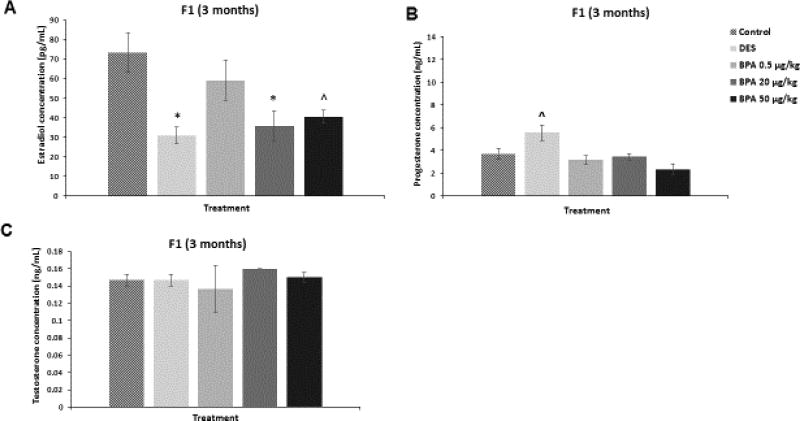
We assessed whether in utero exposure to BPA affected the serum levels of estradiol, testosterone, and progesterone in the F1 generation of mice at three months of age. BPA at 20 µg/kg/day significantly decreased estradiol levels when compared to control (Figure 2A; n=3, p≤0.05). BPA at 50 µg/kg/day also decreased estradiol levels in the three month old F1 mice compared to control, but this was borderline significant (Figure 2A; n=3, p=0.06). BPA did not significantly affect the levels of progesterone (Figure 2B) and testosterone (Figure 2C) compared to controls. DES at 0.05 µg/kg/day significantly reduced estradiol levels (Figure 2A; n=3, p≤0.05), increased progesterone in a borderline manner (Figure 2B; n=3, p=0.09), and did not affect testosterone levels (Figure 2C) at three months of age when compared to controls.
Figure 2. Effect of BPA and DES on Serum Sex Steroid Hormone Levels in the F1 generation.
Serum was collected from the F1 generation of mice at three months of age, and then subjected to enzyme-linked immunosorbent assays for estradiol, progesterone, and testosterone. The graph represents the means ± SEM (n = 3) of serum levels of estradiol (panel A), progesterone (panel B), and testosterone (panel C). Asterisks (*) represent statistically significant differences from the vehicle control (p ≤ 0.05). ^ indicates p = 0.06 (panel A) and p = 0.09 (panel B).
Effect of BPA and DES on serum levels of sex steroid hormones in the F2 generation
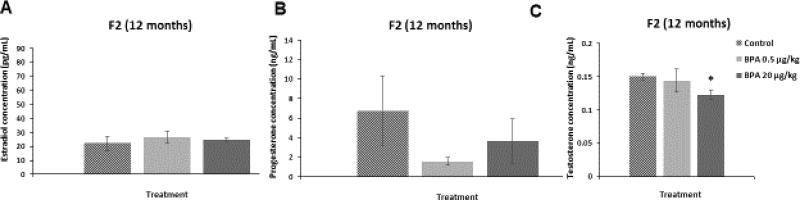
We assessed whether in utero exposure to BPA affected the serum levels of estradiol, testosterone, and progesterone in the F2 generation of mice at 12 months of age. BPA at 20 µg/kg/day significantly reduced testosterone levels during diestrus when compared to control (Figure 3C; n=4–5, p≤0.05). In contrast, BPA did not affect the levels of estradiol (Figure 3A) or progesterone (Figure 3B) during diestrus when compared to controls. In addition, BPA at 50 µg/kg/day did not significantly alter sex hormone levels during estrus in the F2 mice at 12 months of age (estradiol: control = 24.47±0.5 pg/mL, BPA 50 µg/kg/day = 23.15±11.72 pg/mL; progesterone: control = 1.76±0.65 ng/mL, BPA 50 µg/kg/day = 1.99±0.79 ng/mL, testosterone: control = 0.26±0.08 ng/mL, BPA 50 µg/kg/day = 0.23±0.09 ng/mL).
Figure 3. Effect of BPA on Serum Sex Steroid Hormone Levels in the F2 generation.
Serum was collected from the F2 generation of mice at 12 months of age, and then subjected to enzyme-linked immunosorbent assays for estradiol, progesterone, and testosterone. The graph represents the means ± SEM (n = 3–5) of serum levels of estradiol, progesterone, and testosterone in the F2 mice at 12 months (panels A–C). Asterisk (*) represents statistically significant differences from the vehicle control (p ≤ 0.05).
Effect of BPA and DES on the mRNA levels of transport protein and steroidogenic enzymes in the F1 generation
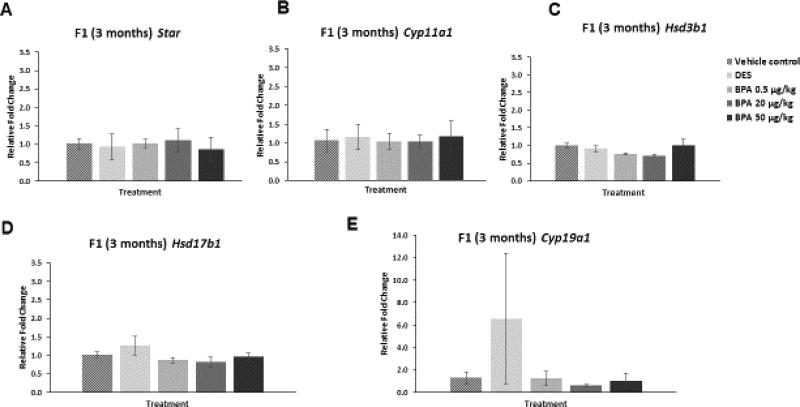
We examined whether in utero exposure to BPA affected the ovarian mRNA levels of Star, Cyp11a1, Hsd3b1, Hsd17b1, and Cyp19a1 in the F1 generation of mice at three and 10 months of age. In the F1 generation at three months of age, BPA did not significantly affect the mRNA levels of Star or any of the selected steroidogenic enzymes when compared to control (Figure 4A–E). Similarly, in the F1 generation at three months of age, DES did not affect the mRNA levels of Star or any of the selected steroidogenic enzymes when compared to control (Figure 4A–E).
Figure 4. Effect of BPA and DES on Steroidogenic Enzyme mRNA Levels in the F1 generation (3 months).
Ovaries were collected from the F1 generation of mice at three months of age, and then subjected to quantitative polymerase chain reaction for Star (panel A), Cyp11a1 (panel B), Hsd3b1 (panel C), Hsd17b1 (panel D), and Cyp19a1 (panel E). The graph represents the means ± SEM (n = 3) of relative fold changes normalized to Actb.
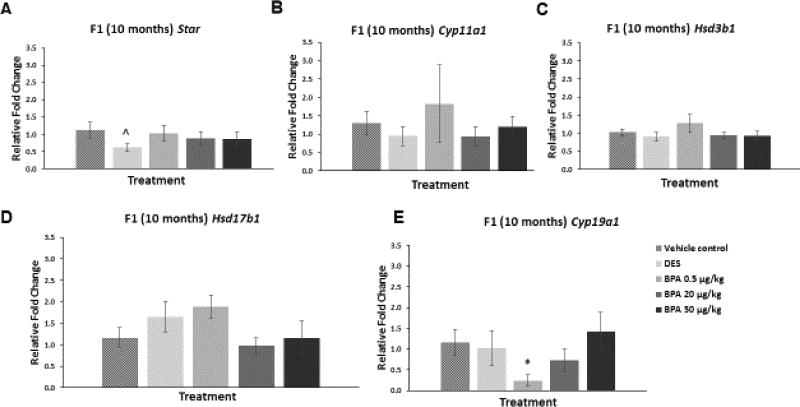
However, in the F1 generation at 10 months of age, BPA at 0.5 µg/kg/day significantly decreased the mRNA levels of Cyp19a1 compared to control (Figure 5E; n=3–7, p≤0.05). BPA did not alter mRNA levels of the other steroidogenic enzymes in the 10 month old F1 mice when compared to control (Figure 5A–D). DES at 0.05 µg/kg/day reduced the mRNA levels of Star in the F1 generation of mice at 10 months of age when compared to control, but this reduction was not statistically significant (Figure 5A; n=6–7, p=0.09).
Figure 5. Effect of BPA and DES on Steroidogenic Enzyme mRNA Levels in the F1 generation (10 months).
Ovaries were collected from the F1 generation of mice at 10 months of age, and then subjected to quantitative polymerase chain reaction for Star (panel A), Cyp11a1 (panel B), Hsd3b1 (panel C), Hsd17b1 (panel D), and Cyp19a1 (panel E). The graph represents the means ± SEM (n = 3–7 ovaries/treatment) of relative fold changes normalized to Actb. Asterisk (*) represents statistically significant difference from the vehicle control (p ≤ 0.05). ^ indicates p = 0.09.
Effect of BPA and DES on the mRNA levels of transport protein and steroidogenic enzymes in the F2 generation
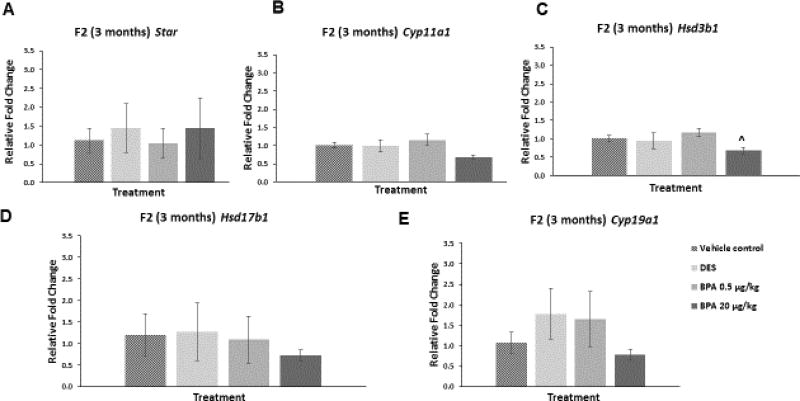
We examined whether in utero exposure to BPA affected the ovarian mRNA levels of Star, Cyp11a1, Hsd3b1, Hsd17b1, and Cyp19a1 in the F2 generation of mice at three and 12 months of age. At three months of age, BPA at 20 µg/kg/day decreased the mRNA levels of Hsd3b1 compared to control, though this decrease was borderline significant (Figure 6C; n=3, p=0.07). BPA did not affect the mRNA levels of the other selected enzymes and Star at three months when compared to control (Figure 6). Further, DES did not alter the mRNA levels of the selected genes at three months when compared to control (Figure 6A–E).
Figure 6. Effect of BPA and DES on Steroidogenic Enzyme mRNA Levels in the F2 generation (3 months).
Ovaries were collected from the F2 generation of mice at three months of age, and then subjected to quantitative polymerase chain reaction for Star (panel A), Cyp11a1 (panel B), Hsd3b1 (panel C), Hsd17b1 (panel D), and Cyp19a1 (panel E). The graph represents the means ± SEM (n = 3) of relative fold changes normalized to Actb. ^ indicates p = 0.07.
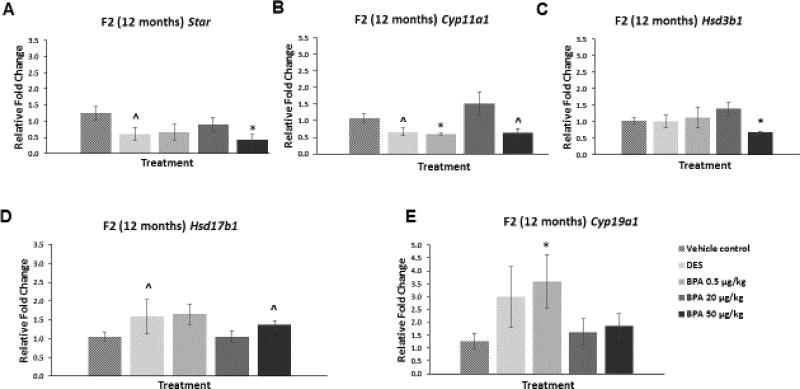
At 12 months of age, BPA at 50 µg/kg/day significantly decreased the mRNA levels of Star (Figure 7A; n=4–9, p≤0.05) and Hsd3b1 (Figure 7C; n=4–9, p≤0.05) when compared to control. Further, BPA at 50 µg/kg/day reduced the mRNA levels of Cyp11a1 and increased the levels of Hsd17b1 when compared to control, but these alterations were not statistically significant (Figure 7B and D; n=3–9). Additionally, BPA treatment at 0.5 µg/kg/day significantly decreased the mRNA levels of Cyp11a1 (Figure 7B; n=3–9, p≤0.05), whereas it significantly increased the mRNA levels of Cyp19a1 (Figure 7E; n=4–9, p≤0.05) when compared to control. DES at 0.05 µg/kg/day decreased the mRNA levels of Star when compared to control, although this was not statistically significant (Figure 7A; n=4–9, p=0.09). DES also decreased Cyp11a1 mRNA levels when compared to control in a borderline significant manner (Figure 7B; n=4–9, p=0.07). Further, DES at 0.05 µg/kg/day increased mRNA of Hsd17b1 compared to control, although this was again of borderline significance (Figure 7D; n=4–9, p=0.06).
Figure 7. Effect of BPA and DES on Steroidogenic Enzyme mRNA Levels in the F2 generation (12 months).
Ovaries were collected from the F2 generation of mice at 12 months of age, and then subjected to quantitative polymerase chain reaction for Star (panel A), Cyp11a1 (panel B), Hsd3b1 (panel C), Hsd17b1 (panel D), and Cyp19a1 (panel E). The graph represents the means ± SEM (n = 3–9 ovaries/treatment) of relative fold changes normalized to Actb. Asterisks (*) represent statistically significant differences from the vehicle control (p ≤ 0.05). ^ indicates p = 0.09 for DES versus control (panel A), p = 0.07 for DES versus control (panel B), p = 0.11 for BPA 50 µg/kg versus control (panel B), p = 0.06 for DES versus control (panel D), and p = 0.07 for BPA 50 µg/kg versus control (panel D).
Discussion
We investigated the multigenerational effects of BPA on ovarian follicle numbers and steroidogenesis over the reproductive lifespan of mice. Our study design allowed us to determine whether in utero exposure to environmentally relevant levels of BPA during a critical period of embryonic ovarian development has adverse effects on ovarian structure and function in the later reproductive life of the F1 and F2 generations. In our dosing paradigm, the F1 mice were exposed to vehicle or BPA as fetuses, whereas the F2 mice were exposed to vehicle or BPA as germ cells. The study design included a DES treatment group as positive control to verify that the mice in our study were responsive to estrogenic compounds, and to examine differential responses to BPA and DES.
Our data indicate that in the F1 generation at three months, BPA (0.5 µg/kg/day and 20 µg/kg/day) decreased the absolute number of preantral follicles, without a shift in the percentage of total follicles or an increase in the percentage of abnormal or atretic follicles. Collectively, these data suggest that in utero BPA exposure decreases preantral follicle numbers, potentially by causing death of these follicles at time-points earlier than three months. Previous studies using different experimental models show that BPA is capable of inducing cytotoxicity in ovarian follicular cells by various mechanisms [28–33]. For example, one study found that BPA exposure reduced the health of granulosa cells in cultured mouse preantral follicles [33]. Other studies showed that BPA induced death of murine ovarian granulosa cells [28] and antral follicles [32] via cell cycle arrest and dysregulation of the apoptotic pathway. Thus, the decreased numbers of preantral follicles in the F1 generation at three months of age in the present study could be due to similar mechanisms. Further, a study conducted in rats as part of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) found that prenatal exposure to BPA decreased preantral as well as primordial and primary follicle numbers at PND 21, but not at three months [34]. Although the decrease in the number of preantral follicles in the F1 generation observed in the CLARITY-BPA study is consistent with the present study, the age at which this effect is seen is different. This age-related difference in the effect of BPA on preantral follicles could be due to species differences in rats versus mice. It also could be due to differences in dosing paradigm. In the current study, mice were dosed with BPA during gestation using an oral route of exposure (gentle pipetting vehicle or BPA into the mouth). In CLARITY-BPA, rats were continuously dosed with vehicle or BPA from gestation using oral gavage [34].
In the F2 generation at three months of age, in utero BPA exposure did not affect ovarian follicle numbers. The differing results on preantral follicle numbers at three months between the two generations is likely because the F1 and F2 generations were exposed to BPA during different developmental windows. The F1 generation was exposed to BPA as fetuses and the F2 generation was exposed to BPA as germ cells. This likely resulted in differential effects of BPA on follicle numbers in the F1 and F2 generations.
Sex steroid hormones are essential for normal reproductive function. Thus, we measured the effects of BPA on serum levels of estradiol and two of its main precursors, testosterone and progesterone. Our data indicate that BPA at 20 µg/kg/day decreased serum levels of estradiol in the F1 mice at three months of age. It is possible that the reduced estradiol levels in the BPA 20 µg/kg/day treatment group at three months may be due to the observed decrease in the number of preantral follicles at this same time-point. Preantral follicles are a major source of sex steroid hormones; thus, reduced numbers of these follicles could lead to decreased hormone production, resulting in low serum estradiol levels. It is also possible that BPA reduces estradiol levels by altering the levels or activity of the steroidogenic factors involved in the conversion of cholesterol to estradiol in the ovary. However, our data indicate that in utero BPA exposure did not affect the mRNA levels of Star, Cyp11a1, Hsd3b1, Hsd17b1 or Cyp19a1 in the F1 generation at three months of age. It is possible that BPA is affecting the protein levels or activity of the enzymes, leading to abnormal sex steroid hormone levels. Hence, further studies are needed to determine whether the reduction in estradiol levels observed in the F1 mice at three months is due to disruption of upstream steroidogenic enzymes.
Some of our data on the effects of BPA on ovarian steroidogenesis in the F1 and F2 generations of mice are consistent with previous studies that investigated whether and how BPA affects steroidogenesis. For example, an in vitro study found that BPA reduced the levels of estradiol, testosterone, progesterone, DHEA, androstenedione, and estrone produced by isolated CD-1 mouse antral follicles [25]. The same study found that BPA decreased the mRNA levels of Star, Cyp11a1, and Hsd3b1 in isolated follicles [25]. This is consistent with our findings of BPA-induced reductions in estradiol and testosterone levels, as well as decreased mRNA levels of Star, Cyp11a1, and Hsd3b1 in some of our BPA-treated groups. Additionally, our findings are consistent with a study conducted as part of CLARITY-BPA that found that prenatal and continuous dosing with BPA decreased serum estradiol levels at 12 months of age in the F1 generation of rats [34]. Taken together, these results indicate that some of the effects of BPA on ovarian steroidogenesis are consistent among different strains (FVB and CD-1 mice) and species (mice and rats).
Some of our data on the effects of BPA on ovarian steroidogenesis in the F1 and F2 generations also differ from previous studies. In the current study, we did not observe any effects of BPA on mRNA levels of Star and Hsd17b1 at three months of age. However, a previous study found that in utero BPA exposure increased Hsd17b1 mRNA levels at PND 21 in both the F1 and F2 generations and that it increased Star mRNA levels in the F2 generation of FVB mice [17]. Collectively, these findings indicate that in utero BPA exposure has different effects on the gene expression of steroidogenic enzymes and that these effects depend on the dose of BPA, the age of the mice, and the generation of the mice. This finding is typical of endocrine disrupting chemicals that can often have different effects depending on the dose [35] and the generation [36].
The present study used DES mainly as a control to ensure that the mice were responding to estrogenic compounds. However, our data indicate some similarities between the effects of DES and BPA on the ovary. For example, our data indicate that neither chemical affected primordial, primary, and antral follicle numbers or percentage in the F1 or F2 generations or steroidogenic enzyme mRNA levels in the F1 and F2 generations at three months, and both chemicals reduced estradiol levels in the F1 generation at three months. In contrast, our data indicate differences between the effects of DES and BPA on the ovary. Specifically, BPA, but not DES, significantly decreased preantral follicle numbers in the F1 generation at three months, decreased mRNA levels of Cyp19a1 in the F1 generation at 10 months, decreased Hsd3b1 in the F2 generation at 12 months, and increased Cyp19a1 in the F2 at 12 months compared to controls.
In conclusion, our results indicate that in utero exposure to BPA has multigenerational effects on ovarian follicle numbers and steroidogenesis. These effects are dependent on the dose of BPA as well as the age of the mice. The results of the present study are important because they imply that in utero exposure to BPA at environmentally relevant levels can affect ovarian function over the reproductive life of future generations. Hence, it is essential to conduct further studies to understand both the multigenerational and transgenerational effects of BPA. Such studies should address the actions of BPA on the estradiol biosynthesis pathway in more detail and characterize the mechanisms underlying differences between the effects of BPA and DES.
Highlights.
In utero BPA exposure decreases preantral follicle numbers in the F1 generation
In utero BPA exposure decreases cytochrome P450 aromatase mRNA and estradiol levels in the F1 generation
In utero BPA exposure decreases testosterone levels and alters mRNA levels of several steroidogenic factors in the F2 generation
In utero BPA exposure has multigenerational effects on the ovary and steroidogenesis in mice
Acknowledgments
The authors thank all members of Dr. Flaws’ laboratory for their assistance with dosing and animal handling. They also thank Hannah Beers for her help with the hormone assays. This work was supported by NIH P01 ES022848 and EPA RD-83459301.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36:E1–e150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007–2016) Fertil Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich S, Williams PL, Hauser R, Missmer SA, Peretz J, Calafat AM, Flaws JA. Urinary bisphenol A concentrations and cytochrome P450 19 A1 (Cyp19) gene expression in ovarian granulosa cells: an in vivo human study. Reprod Toxicol. 2013;42:18–23. doi: 10.1016/j.reprotox.2013.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27:3583–3592. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 7.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziv-Gal A, Craig ZR, Wang W, Flaws JA. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod Toxicol. 2013;42:58–67. doi: 10.1016/j.reprotox.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, Soto AM. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi K, Watanabe S. Measurement of bisphenol A in human urine using liquid chromatography with multichannel coulometric electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780:365–370. doi: 10.1016/s1570-0232(02)00547-0. [DOI] [PubMed] [Google Scholar]
- 14.Mendonca K, Hauser R, Calafat AM, Arbuckle TE, Duty SM. Bisphenol A concentrations in maternal breast milk and infant urine. Int Arch Occup Environ Health. 2014;87:13–20. doi: 10.1007/s00420-012-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284:354–362. doi: 10.1016/j.taap.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol. 2016;60:39–52. doi: 10.1016/j.reprotox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.USFDA. Exposure to bisphenol A (BPA) for infants, toddlers, and adults fromthe consumption of infant formula, toddler food and adult canned food. United States Department of Health and Human Services, Public Health Service, Food and Drug Administration (memorandum dated 22 October 2009) 2009 [Google Scholar]
- 19.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 20.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EPA. Health assessment information on bisphenol A (CASRN80-05-7) 1998 [Google Scholar]
- 22.Flaws JA, Hirshfield AN, Hewitt JA, Babus JK, Furth PA. Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol Reprod. 2001;64:1153–1159. doi: 10.1095/biolreprod64.4.1153. [DOI] [PubMed] [Google Scholar]
- 23.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 24.Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2015;284:42–53. doi: 10.1016/j.taap.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci. 2001;61:201–210. doi: 10.1093/toxsci/61.2.201. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun. 2002;292:456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- 29.Lee SG, Kim JY, Chung JY, Kim YJ, Park JE, Oh S, Yoon YD, Yoo KS, Yoo YH, Kim JM. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17beta-estradiol synthesis via downregulation of aromatase in rat ovary. Environ Health Perspect. 2013;121:663–669. doi: 10.1289/ehp.1205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganesan S, Keating AF. Bisphenol A-Induced Ovotoxicity Involves DNA Damage Induction to Which the Ovary Mounts a Protective Response Indicated by Increased Expression of Proteins Involved in DNA Repair and Xenobiotic Biotransformation. Toxicol Sci. 2016;152:169–180. doi: 10.1093/toxsci/kfw076. [DOI] [PubMed] [Google Scholar]
- 31.Peretz J, Neese SL, Flaws JA. Mouse strain does not influence the overall effects of bisphenol a-induced toxicity in adult antral follicles. Biol Reprod. 2013;89:108. doi: 10.1095/biolreprod.113.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012;87:63. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Brehm E, Gao L, Rattan S, Ziv-Gal A, Flaws JA. Bisphenol A Exposure, Ovarian Follicle Numbers, and Female Sex Steroid Hormone Levels: Results from a CLARITY-BPA Study. Endocrinology. 2017 doi: 10.1210/en.2016-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology. 2017 doi: 10.1210/en.2017-00100. [DOI] [PMC free article] [PubMed] [Google Scholar]