Summary
The oncometabolite 2-hydroxyglutarate (2-HG) is a signature biomarker in various cancers where it accumulates as a result of mutations in isocitrate dehydrogenase (IDH). The metabolic source of 2-HG, in a wide variety of cancers, dictates both its generation and also potential therapeutic strategies, but this remains difficult to access in vivo. Here, utilizing patient-derived chondrosarcoma cells harboring endogenous mutations in IDH1 and 2, we report that 2-HG can be rapidly generated from glutamine in vitro. Then, using hyperpolarized magnetic resonance imaging (HP-MRI), we demonstrate that in vivo HP [1-13C] glutamine can be used to non-invasively measure glutamine-derived HP 2-HG production. This can be readily modulated utilizing a selective IDH1 inhibitor, opening the door to targeting glutamine-derived 2-HG therapeutically. Rapid rates of HP 2-HG generation in vivo further demonstrate that, in a context dependent manner, glutamine can be a primary carbon source for 2-HG production in mutant IDH tumors.
Keywords: 2-hydroxyglutarate, hyperpolarized imaging, glutamine, mutant isocitrate dehydrogenase
eTOC blurb

Salamanca-Cardona et al. show that glutamine is a primary carbon-source for the biosynthesis of the oncometabolite 2-hydroxyglutarate in mutant IDH tumors. They develop a novel hyperpolarized MRI method using glutamine as a probe to detect 2-hydroxyglutarate formation in vivo in real-time, non-invasively, and with high specificity.
Introduction
Metabolic reprogramming has been established as an important hallmark of cancer cells since the discovery of the ‘Warburg effect’ and more recently with the annotation of glutamine addiction in various cancer types (DeBerardinis et al., 2007; Ward and Thompson, 2012). Additionally, the abnormal accumulation of certain metabolites (oncometabolites) as a result of genetic mutations in metabolic genes further supports the importance of a dysregulated metabolism for carcinogenesis and cancer cell survival (Nowicki and Gottlieb, 2015). However, it remains unclear to what extent these factors interact to promote tumorigenesis (Yang et al., 2013). Nevertheless, this reprogrammed metabolism has been leveraged for cancer diagnosis, staging and grading, as well as for response to therapy in tumors (Galluzzi et al., 2013; Ward and Thompson, 2012). For example, the glucose analogue 18F-flurorodeoxyglucose (18F-FDG) is routinely used in the clinic for evaluating increased glucose uptake as a result of the Warburg effect using positron emission tomography (PET) imaging (Weber, 2005).
The importance of understanding metabolic reprogramming in vivo for diagnostics and therapeutics is further highlighted with the recent development of 18F-fluoroglutamine (18F-FGln) PET imaging which has been exploited to assess nutrient-uptake alterations in glioma tumors in vivo in order to overcome the limitations of 18F-FDG imaging in glutamine-addicted tumors with reduced glucose uptake (Lieberman et al., 2011; Venneti et al., 2015). Nevertheless, while useful in the context of glutamine uptake, 18F-FGln cannot be used to resolve the metabolic fates of glutamine thus limiting its utility in cancers where glutamine anaplerosis has been linked to tumor cell metabolism such as the formation of the oncometabolite 2-hydroxyglutarate (2-HG) in the setting of IDH mutations (Dang et al., 2009; Ohka et al., 2014).
2-HG is the metabolic product of a gain-of-function mutation in the isocitrate dehydrogenase (mIDH1/2) enzyme (Losman and Kaelin, 2013). Wild type IDH1 and IDH2, located in the cytoplasm and the mitochondria respectively, catalyze the conversion of isocitrate and NADP+ into alpha-ketoglutarate (α-KG) and CO2 with the concomitant generation of NADPH. Mutant IDH1/2 enzymes instead convert α-KG into 2-HG with the oxidation of NADPH into NADP+. As a result, 2-HG is abnormally accumulated in cells that harbor IDH1/2 mutations. The role of 2-HG as an oncometabolite has been linked to epigenetic changes through the inhibition of α-KG dependent dioxygenases and demethylases, which has been hypothesized to be a driver for carcinogenesis (Lu et al., 2012; Xu et al., 2011b; Yen et al., 2010). In addition, the redirection of α-KG flux from normal reductive metabolism in the TCA cycle towards 2-HG generation has been suggested to dysregulate other metabolic fluxes and disrupt redox balance (Ohka et al., 2014; Parker and Metallo, 2015; Tateishi et al., 2015). Multiple cancers have been identified to carry an IDH1 or IDH2 mutation including low-grade gliomas, secondary glioblastomas (GBM), chondrosarcomas, and acute myelogeneous leukemia (AML) (de Botton et al., 2016). Despite these recent advances, the primary metabolic source of 2-HG in vivo has remained unresolved and difficult to annotate. 2-HG has great potential as a biomarker for the detection, diagnosis and therapeutic monitoring, but targets beyond direct inhibition of IDH are required to address its metabolism (Dang et al., 2010; Dubbink et al., 2009; Marcucci et al., 2010).
Current methods for detecting IDH1/2 mutations include DNA sequencing, immunochemistry, mass spectrometry (MS), and proton magnetic resonance spectroscopy (1H-MRS) (Andronesi et al., 2013; Capper et al., 2009; Fathi et al., 2012). Of these, 1H-MRS is the only non-invasive and non-destructive method but clinical application of this approach is limited because 2-HG cannot be detected below mM levels and the presence of other abundant metabolites with similar chemical structures, such as glutamine (Gln) and glutathione, which are challenging to deconvolve in 1H-MRS (Andronesi et al., 2012; Pope et al., 2012). Additionally, none of these methods can assess the carbon sources for 2-HG nor can they give information regarding the flux kinetics of 2-HG generation (Andronesi et al., 2013; Choi et al., 2012). Consequently, there is still a need for safe, non-invasive methods for interrogating the metabolism of 2-HG in the context of cancers carrying IDH mutations in vivo. Hyperpolarized magnetic resonance imaging (HP-MRI) can be a key player in meeting this need being an imaging technique that relies on the spectral resolution of 13C magnetic resonance spectroscopy with enhanced sensitivity of >10,000 fold via the dynamic nuclear polarization technique (Keshari and Wilson, 2014; Salamanca-Cardona and Keshari, 2015). HP-MRI allows real-time imaging of metabolism in vivo and has been translated to the clinic with [1-13C] pyruvate used successfully as an imaging probe in prostate cancer (Nelson et al., 2013).
In this study we demonstrate production of 2-HG from glutamine occurs rapidly in patient-derived chondrosarcoma cell lines harboring endogenous IDH mutations and highlight some of the fundamental metabolic differences between cells harboring IDH1 and IDH2 mutations. We then exploit this rapid metabolism of glutamine to 2-HG and present in vivo imaging of 2-HG via HP-MRI using hyperpolarized [1-13C] Gln as the imaging probe. With this approach we demonstrate that glutamine can be a primary source for 2-HG production in vivo and also develop a novel non-invasive imaging method that detects 2-HG unambiguously, with high sensitivity, and in real time thus addressing major limitations of other diagnostic methods (1H-MRS and 18F-FGln).
Results
Glutamine flux is directed towards production of 2-hydroxyglutarate in IDH1/2 mutants in vitro
The carbon source for 2-HG formation has been suggested to be glutamine in experiments done using ectopically expressed IDH1 mutants in glioma cells (Dang et al., 2009; Grassian et al., 2014) consequently we sought to confirm that glutamine is converted to 2-HG in our cell models. Both JJ012 and CS1 are patient-derived chondrosarcoma cell lines with IDH1 and IDH2 mutations respectively, and have been previously shown to accumulate high levels of 2-HG (Ward et al., 2013). We grew each cell line in glutamine-free media supplemented with [13C5] Gln to isotopically follow the fate of glutamine (Fig. 1). After 24 h of incubation nearly all of the total glutamate and 2-HG pools are fully labeled (m+5) in both IDH mutants (Fig. 1A and S1). Additionally, both cell lines showed minimal (~5%) incorporation of glucose carbons into 2-HG and glutamate after 24 h incubation with [13C6] Glucose (Gluc) (Fig 1B and 1C, S2A and S2B) despite high levels of labeling in TCA cycle precursors for α-ketoglutarate (Fig. S2C and S2D). These results corroborate that the main source for 2-HG under normal growth conditions is glutamine. Our results also demonstrate that the cytosolic IDH1 mutant fails to accumulate substantial 2-HG when incubated in glutamine-depleted media (Fig. 1D), while mitochondrial IDH2 mutant cells remain capable of accumulating 2-HG under these conditions, though to a lesser degree. The 2-HG derived from subtrates other than glutamine or glucose are slightly higher in CS1 than in JJ012 cells (Fig. 1B and 1C), highlighting a potential fundamental difference in metabolism resulting from the localization of the IDH mutation.
Fig. 1. Glutamine flux is directed towards production of 2-hydroxyglutarate in IDH1/2 mutants in vitro.
A Representative metabolic map of TCA cycle metabolism in mIDH1 chondrosarcoma cells (JJ012) grown with 5 mM [13C5] Gln. The time-course analysis shows nearly all of the glutamate and 2-HG pool is labeled in all carbons after 24 h. The map also shows evidence of reductive carboxylation taking place as observed in the m+5 labeling of isocitrate and citrate, and it extends to aspartate metabolism as well as malate and fumarate as evidenced by labeling m+3 labeling of these metabolites. The LC-MS method was sensitive enough to detect the second pass of the oxidative reduction of TCA cycle as shown by the m+3 labeling of citrate and isocitrate. The representative metabolic map for mIDH2 cells (CS1) is shown in Fig. S1. B and C show labeling of 2-HG from JJ012 and CS1 after 24 h incubation with various combinations of unlabeled and [13C6 ] Gluc [1-13C] Gln. Cells incubated with [13C6] Gluc show ~5% m+2 fractional enrichment. D JJ012 and CS1 cells were grown in media supplemented with 5 mM glutamine (Gln +) and in glutamine-depleted media (Gln -). JJ012 cells do not produce 2-HG in the absence of glutamine while CS1 cells are capable of limited 2-HG production in these conditions. Data are representative of mean ± S.D. of 3 replicates from 3 experiments carried independently.
Using the m+5 labeled metabolite measurements in time, we determined the fractional enrichment of glutamate and 2-HG production in vitro from glutamine (Fig. 2A-2D). Apparent rates of production were approximated using the total pools from unlabeled samples (t= 0 h) determined by 1H-NMR and the fractional enrichment when cells reached an isotopic steady-state (t= 8 h). Glutamate was labeled from glutamine at a rate of 17.9 ± 1.3 nmol and 16.0 ± 1.4 nmol per million cells in JJ012 and CS1, respectively. 2-HG labeling from glutamine in JJ012 and CS1 reached 1.90 ± 0.08 nmol and 1.27 ± 0.07 nmol per million cells thus supporting the notion that 2-HG production occurs at a high enough rate to account for the levels of 2-HG accumulation found in tumors (Dang et al., 2009), and is potentially fast enough for its detection in vivo using HP glutamine as an imaging probe.
Fig. 2. Kinetics of glutamine-derived glutamate and 2-HG in vitro provide evidence for the possible translation to in vivo HP-MRI.
Fractional enrichment of glutamate and 2-HG from glutamine in vitro was determined by LC-MS analysis following the time-course m+5 labeling of these metabolites from [13C5] Gln. A and B show absolute glutamate pools (blue) and m+5 labeling obtained from LC-MS over time (red) for JJ012 and CS1 cells respectively. Glutamate enrichment reached approximately 80% by 3 h on JJ012 and CS1. C and D show total 2-HG fractional enrichment with absolute glutamate pools (blue) and m+5 labeling from LC-MS over time (red) for JJ012 and CS1 cells respectively. At 8 h approximately 80% of 2-HG has been labeled in both cell lines. Data are representative of mean ± S.D. of 3 replicates from 3 experiments carried independently.
A difference in overall glutamine metabolism is observed between the IDH1 (JJ012) and IDH2 (CS1) mutant cells. The latter (CS1) label glutamate at a faster rate than JJ012, and consequently 2-HG is also labeled faster. This is not unexpected based on previous reports showing higher 2-HG accumulation in mIDH2 cells compared to mIDH1 (Ward et al., 2013). Another difference is the pattern of citrate labeling (Fig. 1 and S1) with cytosolic IDH1 mutant cells showing an isotopic labeling pattern in citrate and isocitrate characteristic of the presence of both reductive (m+5) and oxidative (m+4) metabolism of glutamine-derived α-KG. In contrast, mitochondrial IDH2 mutant cells direct glutamine-derived-carbons almost entirely in the oxidative direction.
Kinetics of glutamine-derived 2-hydroxyglutarate can be modulated in vitro with IDH inhibition
One of the desired traits in a diagnostic imaging modality for cancer is the potential for detecting therapy response of a given tumor to a selected drug. In our proposed method for imaging metabolism of glutamine to 2-HG in mIDH1/2 tumors in vivo, we rely on the production of 2-HG as a biomarker and driver for carcinogenesis and consequently seek to detect changes in 2-HG production resulting from a given treatment. For this purpose, we assessed if production of 2-HG could be modulated in vitro in our mIDH1 model. We first determined the specificity of the mIDH1 inhibitor AGI-5198, which has been previously validated in ectopic mIDH1 mutants (Rohle et al., 2013), by growing both JJ012 and CS1 cells, exposing them to increasing concentrations of AGI-5198 and analyzing 2-HG levels after 96 h of exposure to the drug. As expected the JJ012 (mIDH1) cells showed a dose-dependent response to the inhibitor with highest inhibition of 2-HG formation (approximately 90% compared to untreated) at 3 and 5 µM (Fig. 3A) and an IC50 of 0.21 µM. We observed a minor inhibitory effect of the drug to CS1 (mIDH2) cells, (IC50 = 2.9 µM) with an inhibition of 2-HG production at this concentration of 24% (Fig. 3B).
Fig. 3. The rate of 2-HG production in mIDH1 cells can be modulated in vitro using an mIDH1 inhibitor drug.
A and B IC50 plot of JJ012 and CS1 cells respectively, grown in media supplemented with 5 mM glutamine and increasing concentrations of IDH1 inhibitor (IDHi) AGI-5198. JJ012 cells show sensitivity to the drug, with a maximum inhibition of 2-HG production of approximately 90%. CS1 cells show limited sensitivity to AGI-5198. C and D Isotopic enrichment in JJ012 cells incubated with [13C5] Gln without (control) or with (IDHi) 2 µM of IDHi demonstrates the glutamate pool is unchanged by inhibition of mutant IDH1 while the apparent rate of 2-HG production is reduced as a function of total 2-HG pools. Data are representative of mean ± S.D. of 3 replicates from 3 experiments carried independently.
We next sought to determine how inhibition of the mutant IDH1 affects the kinetics of glutamine-derived 2-HG formation from [13C5] Gln in JJ012 cells (Fig. S3) following 96 h incubation with 2 µM of AGI-5198. Inhibition did not affect the flux of glutamine to glutamate (Fig. 3C). As expected, generation of 2-HG is reduced by approximately 70% to 0.33 ± 0.02 nmol per million cells at 8 h (Fig. 3D), an appreciable difference that is potentially large enough to be quantified by HP-MRI in vivo. No differences in the other TCA cycle metabolites were observed (Fig. S3).
Hyperpolarization of [1-13C] glutamine with minimal pyroglutamate formation
The usefulness of glutamine as an imaging probe for 2-HG production in vivo is dependent on the polarization levels that can be achieved and the T1 relaxation time (Salamanca-Cardona and Keshari, 2015). High levels of polarization are necessary because the signal needs to be carried from dissolution, injection, delivery, transport into the cell, and the conversion of glutamine to 2-HG via glutamate and α-KG (Fig. 4A)(Keshari and Wilson, 2014; Salamanca-Cardona and Keshari, 2015). To determine these two parameters [1-13C] Gln was dissolved in an equimolar amount of NaOH and mixed with a solution of OX063 radical in glycerol. The sample was hyperpolarized for >1h and polarization levels immediately after dissolution reached 34.7 ± 7 % and the T1 was determined to be 31 ± 3 s (Fig. 4B and 4C). In solution, glutamine is spontaneously converted to pyroglutamate (PG). Their 13C NMR chemical shifts at physiological pH are 174.7 ppm and 180 ppm respectively, and a small amount of HP pyroglutamate can be observed immediately after dissolution (Fig. 4D and 4C).
Fig. 4. [1-13C] glutamine can be hyperpolarized with minimal pyroglutamate formation. A.
Schematic representation of metabolism of HP [1-13C] Gln into [1-13C] 2-HG in vivo: (i) a sample of [1-13C] Gln dissolved in NaOH, combined with OX063 radical and Gd-Dota is hyperpolarized at 3.35 T for >1 h, rapidly dissolved in Tris buffer and intravenously injected into mice. (ii) HP [1-13C] Gln is converted to HP [1-13C] Glu by glutaminase (GLS). (iii) HP [1-13C] Glu is metabolized to HP [1-13C] 2-oxoglutarate (2-OG) by glutamate dehydrogenase (GDH). (iv) HP [1-13C] 2-OG is converted to HP [1-13C] 2-HG by mutant isocitrate dehydrogenase 1/2 (mIDH1/2). B Degradation of glutamine in solution leads to the formation of pyroglutamate (PG), which we minimized during preparation. HP [1-13C] Gln shows resonance at 175 ppm and PG at 180 ppm following dissolution. C The T1 relaxation [1-13C] Gln is 31±3 s at 1 Tesla. D Chemical shifts at physiological pH for [1-13C] 2-HG (180.8 ppm), [5-13C] PG (180 ppm), [1-13C] Glu (175.5 ppm), [1-13C] Gln (174.8 ppm) and 13C Urea (163 ppm) measured at 14 Tesla.
During the course of several dissolutions, we observed that the chemical shift of C1 glutamine drifts by several ppm depending on pH (Fig. S4), which could result in misleading data interpretation when comparing in vitro measurements to determine chemical shifts and polarization levels with results obtained in vivo. Therefore, upon dissolution of the HP [1-13C] Gln sample in 100 mM Tris, the sample was neutralized with HCl to obtain a polarized solution at physiological pH to avoid any possible unambiguity in our data resulting from the chemical shift variation of the C1 in glutamine at different pHs. Moreover, pH was confirmed for each of the injections conducted in animal studies.
HP [1-13C] 2-hydroxyglutarate formation can be imaged in vivo using HP [1-13C] glutamine
Based on the kinetics of glutamine conversion to 2-HG our in vitro models, we hypothesized that 2-HG formation could be detected in real-time using hyperpolarized [1-13C] Gln as an imaging probe. We determined the dynamics of glutamine delivery into JJ012 xenograft tumors and 2-HG appearance by acquiring 13C dynamic spectra in a 20 mm slab covering the entire tumor. Data was acquired with a 10 degree excitation every 3 s starting at the time of injection (t=0). The [1-13C] Gln resonance is readily detected at 3 s and it reaches a maximum at 15 s (Fig. S5A). The observed glutamine signal is most likely a composite signal from [1-13C] Gln and [1-13C] Glu (referred to as [1-13C] Gln+Glu) since their respective chemical shifts are only 0.8 ppm apart (Fig. 4D) and with a large influx of HP glutamine they are difficult to resolve at a 1T magnetic field strength. A small pyroglutamate signal is detected, which reaches a maximum at approximately 12 s and disappears at 24 s. The [1-13C] 2-HG signal appears between 24 and 27 s reaching a maximum between 30 and 33 s and it disappears at 42 s (Fig. S5A).
Next, using the times derived from dynamic acquistions, 2D chemical shift imaging was performed on both tumor models. Acquisition was started 26 s after the start of injection for approximately 10 s in order to optimally sample the [1-13C] 2-HG signal. The results are summarized in Fig. 5. Chemical shifts were assigned based on the [1-13C] urea phantom resonance at 163 ppm. The acquired spectra show the distribution of the [1-13C] Gln+Glu signal (peak at 175 ppm) over the mouse body as illustrated by the heatmap and representative spectra shown in Fig. 5A and 5B. In the tumor region an additional peak can be observed at 180.8 ppm (Fig. 5A, second panel). The second peak was not observed in any surrounding normal tissue voxels, demonstrating that the second peak could not come from pyroglutamate that is spontaneously formed during the preparation of the glutamine sample. This is further supported by the disappearance of the pyroglutamate signal before the appearance of the 2-HG signal in the dynamic data (Fig. S5A).
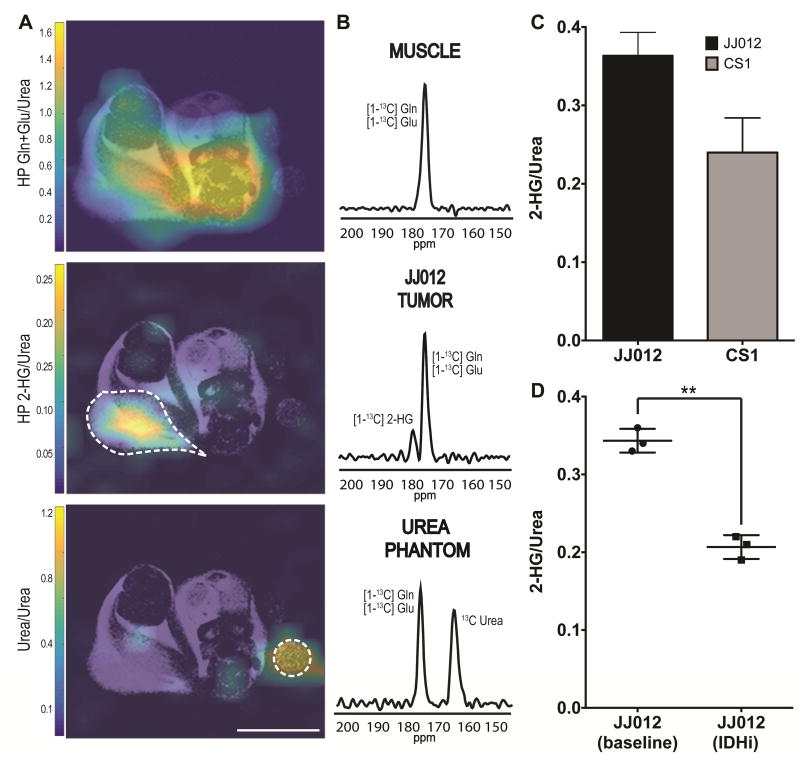
Fig. 5. The real-time formation of 2-HG can be detected in vivo using HP [1-13C] Gln as an imaging probe.
A Representative heatmap of spectral data from a mouse with a mIDH1 tumor xenograft following injection of [1-13C] Gln. The top picture shows distribution of glutamine and glutamate over the whole body. The middle picture shows accumulation of 2-HG in the tumor region only. The bottom picture shows the 13C urea phantom used for resonance reference. The dotted lines highlight tumor and urea phantom. White line at the bottom is 10 mm for scaling. B Selected voxels showing the presence of a second peak approximately 5.5 ppm away from [1-13C] Gln+Glu in tumor regions only. C Bar plots representing relative [1-13C] 2-HG signal integration from animals with JJ012 (n=11) and CS1 (n=8) tumors. Signal integrations were normalized to 13C urea signals from the same acquisition experiment. D Relative [1-13C] 2-HG signal integration from longitudinal study of animals with JJ012 tumors (n=3*) at baseline (without treatment) and treated with IDH1 inhibitor.
We assigned the 180.8 ppm resonance to hyperpolarized [1-13C] 2-HG. The chemical shift difference between the signals is approximately 6 ppm, which correlates to the chemical shifts determined via 13C NMR for C1 of Gln+Glu and 2-HG in solution at physiological pH (Fig. 4D, Fig. S3B). This spectral profile, where a second peak appears only in tumor regions, was observed in all JJ012 animals (n=11) and all CS1 animals (n=8) studied (Fig. 5, Fig. S6), but not in other glutamine avid tumor models (HPAC and MiaPaCa-2, Fig. S7) where no evidence of 2-HG accumulation has been reported. In these glutamine avid models, distribution of the [1-13C] Gln+Glu signal is observed in the whole mouse body, including the tumors, at the 175 ppm resonance, but no additional peak is observed in the normal or tumor tissue (Fig. S7). All these results combined, strongly demonstrate that the second peak observed in the JJ012 and CS1 tumors is indeed [1-13C] 2-HG derived from the HP [1-13C] Gln probe.
To assess if our method reflected the difference in kinetics observed in vitro between the IDH1 mutants and the IDH2 mutants, we normalized the area of the HP 2-HG signal over the signal of the urea phantom for both tumor types. The results show the signal from CS1 (IDH2) tumors is 34% (p<0.005, unpaired t-test) lower than the signal from JJ012 (IDH1) tumors (Fig. 5C). Quantitative assessment was done using the resonance integration from the 2 M 13C urea phantom (Xu et al., 2011a). Fitting of the experimental intensities resulted in approximately 2.15 ± 0.17 nmol/g and 1.42 ± 0.26 nmol/g of 2-HG in JJ012 and CSI tumors, respectively, in 40 s of metabolism from injection to end of acquisition. For both qualitative and quantitative measurements, voxels that contained at least 80% of tumor across the slab were selected. The quantitative measurements for JJ012 roughly correlate to the expected values using the quantitative kinetic data from our in vitro experiments determined by LC-MS.
Inhibition of IDH1 mutant can be assessed in vivo using HP [1-13C] glutamine
Next, we evaluated the potential of using HP [1-13C] Gln to assess inhibition of mutant IDH1 with AGI-5198 in vivo. We collected images in JJ012 animals without treatment to determine the baseline spectra and baseline 2-HG levels. After 2 days following the first HP [1-13C] Gln injection, the animals were treated intraperitoneally with the mIDH1 inhibitor and reimaged following a second injection with HP [1-13C] Gln. This longitudinal approach allowed us to eliminate any ambiguity that could be introduced from differences in tumor microenvironment. Treatment with the mIDH1 inhibitor was done 3 hours prior to the second injection based on the pharmacokinetics of the compound to ensure the drug was present in the tumor (Rohle et al., 2013). Following treatment, a significant decrease in HP 2-HG signal of approximately 40% could be observed compared to the baseline (p<0.005, paired t-test, Fig. 5D). These results are in accordance with the inhibition levels observed in vitro (Fig. 3) and support the ability of our method to assess glutamine-derived 2-HG modulation in vivo and response to inhibitory therapies in real-time shortly after single dose administration of the drug.
Kinetics of glutamine conversion to 2-hydroxyglutarate in vivo facilitate HP imaging
The results obtained from in vitro experiments support our hypothesis that glutamine is a primary carbon source for 2-HG under conditions used for HP imaging, and this conversion happens fast enough to detect it with HP [1-13C] Gln in vivo. In order to eliminate any ambiguity from translating in vitro results to in vivo imaging, we confirmed the rates of 2-HG production in the tumor by infusing tumor-bearing mice with a bolus of [13C5] Gln, extracting and flash-freezing the tumor 30 mins after the start of injection. We determined total pools of 2-HG and glutamate via 1H-NMR analysis of tumor tissue (Fig. 6A). We observed total levels of 2-HG reaching 6.2 ± 0.6 mM in JJ012 tumors and 4.7 ± 0.6 mM in CS1 tumors, while the total glutamate pool was 16.6 ± 1.5 mM and 13.5 ± 2.6 mM for each model, respectively (Fig. 6B). In addition, LC-MS metabolic tracing analysis shows that in 30 min, [13C5] 2-HG accumulates to 38.7 ± 0.9 nmol/g and 37.9 ± 6.7 nmol/g of tumor in JJ012 and CS1 models, respectively (Fig. 6C). These data further confirm the fast kinetics of glutamine metabolism into 2-HG in IDH mutants in vivo, and validate the results obtained from imaging experiments.
Fig. 6. Kinetics of glutamine conversion to 2-hydroxyglutarate in vivo are fast enough for HP imaging.
Tumors were extracted from mice, xenografted with JJ012 and CS1 cells, following a 12 s intravenous infusion of [13C5] Gln and metabolite levels (glutamate and 2-HG) were quantified. A Representative 1H-NMR spectra of tissue-extracts from JJ012 (top) and CS1 (bottom) tumors used for quantification of total glutamate (Glu) and 2-HG pools. For glutamate quantification the area of gamma protons signal was used (dark pink area) and for 2-HG the area of one beta proton was used (light blue area). B Bar plots showed concentration of glutamate and 2-HG in tumors as resolved from the 1H-NMR spectra. For both metabolites, JJ012 shows higher levels than CS1. C Concentration of [13C5] 2-HG in JJ012 and CS1 tumors 30 min after the end of infusion was determined via LC-MS. Both JJ012 (black dot) and CS1 (gray square) show similar levels of labeling after 30 min. Additionally, we quantified concentrations of [13C5] 2-HG at 1, 5 and 10 min to assess initial rates of labeling in JJ012 tumors. Quantitative data are representative of mean ± S.D. of 3 replicates from 3 experiments carried independently.
Next, we sought to translate in vitro results obtained from [13C6] Gluc labeling to in vivo and validate to the observed metabolic phenotype of glutamine as a primary source for formation of 2-HG under the quick-infusion scheme necessary for HP imaging. For this purpose we infused additional tumor-bearing animals with [13C6] Gluc or with [13C5] Gln and analyzed the relative labeling at 30 min post infusion. The metabolism of [13C6] Gluc in both tumor types (JJ012 and CS1) is confirmed by the increase in m+3 of glyceraldehyde-3-phosphate as well as entry to the TCA cycle as shown by m+2 labeling of isocitrate (Fig. 7A–7D, second set). Similarly, metabolism of [13C5] Gln carbons is evidenced in the m+3 and m+4 labeling of glyceraldehyde-3-phosphate and isocitrate respectively (Fig. 7A–7D, third set). Despite the entry of [13C6] Gluc carbons into the TCA cycle, m+2 labeling of 2-HG is not observed (Fig. 7E and 7F), while relative labeling 2-HG m+5 from [13C5] Gln reaches levels expected from the quantitative experiments (Fig. 6C). Finally, these ex vivo studies corroborate the results obtained from inhibitory studies in imaging experiments and support the feasibility of our approach to test for efficacy of potential therapies (Fig. 7G).
Fig. 7. Glucose carbons are not incorporated into 2-HG in the time frame of an HP injection.
Tumors were extracted from mice, xenografted with JJ012 and CS1 cells, following a 12 s intravenous infusion of [13C6] Gluc or [13C5] Gln and fractional enrichment (for each tracer) of selected metabolites (glyceraldehyde-3-phosphate, isocitrate, and 2-HG) was obtained. A and B show fractional enrichment of glyceraldehyde-3-phosphate confirming the metabolism of glucose in the tumor (m+3, middle set). C and D show enrichment of isocitrate to confirm entry of glucose and glutamine carbons into the TCA cycle (m+2 and m+4 respectively). E and F show fractional enrichment 2-HG m+5 in tumors infused with [13C5] Gln while no fractional enrichment of m+2 is detected in tumors infused with [13C6] Gluc. G shows fractional enrichment of 2-HG m+5 from [13C5] Gln with or without mIDH inhibitor. These results show similar inhibition levels detected in imaging experiments. Qualitative data are representative of mean ± S.D. of 3 replicates from 3 experiments carried independently.
Discussion
Accumulation of 2-hydroxyglutarate as a result of mutations in IDH1/2 enzymes has been identified in a wide variety of cancer types and has been used as a diagnostic biomarker (de Botton et al., 2016; Losman and Kaelin, 2013). It has been previously reported that in vitro 2-HG is primarily derived from glutamine via glutaminolysis in mIDH1/2 cell lines, however there is no conclusive evidence regarding the primary source for 2-HG production in vivo restricting the scope of potential therapies for mIDH1/2-driven cancers ((Dang et al., 2009; Grassian et al., 2014). Moreover, there has been recent reports which argue that 2-HG could be derived from other nutrient sources (Izquierdo-Garcia et al., 2014; Seltzer et al., 2010). The lack of evidence could be due to limitations in current methods to assess mIDH1/2 tumors in vivo, which cannot assess real-time metabolism (Babakoohi et al., 2016). In this work we provide evidence to support that glutamine can be a primary source of 2-HG and have developed a non-invasive imaging method to detect the active formation 2-HG from glutamine in real-time using HP-MRI.
Using isotopic tracing, we confirmed that in tumor cell lines expressing IDH1/2 mutations (JJ012 and CS1) 2-HG is primarily derived from glutamine via glutaminolysis. While this reaction might not be the sole source for 2-HG production, based on isotopic enrichment using labeled glucose and glutamine, it is likely to be a primary source that is dependent on the availability of glutamine to the cells. Additionally, the results show that in contrast to the cytosolic IDH1 mutants, which do not accumulate 2-HG in glutamine-depleted conditions, mIDH2 cells can still accumulate a small amount of 2-HG. These findings suggest a fundamental difference in the metabolic rewiring of each type of mutant cell, which has also been suggested in previous reports using isogenic mutants (Reitman et al., 2011; Wen et al., 2015). It is possible that in mIDH2 cells, glycolytic flux is redirected towards the TCA cycle in the absence of endogenous glutamine. This could mean that mIDH2 cells use glucose, as well as glutamine, to maintain TCA cycle homeostasis (Wen et al., 2015). Nevertheless, our experiments confirm the biology observed in other mIDH1/2 cell models in vitro since under glutamine-replete conditions over 90% and 80% of 2-HG comes directly from glutamine in both JJ012 and CS1 models, respectively (Fig. 1, 2C, 2D), thus validating a commonality in mIDH1/2 models from various types of cancer.
We also found the kinetics of 2-HG formation to be within the same order of magnitude as glutamate formation via glutaminolysis (DeBerardinis et al., 2007; Wise et al., 2008). This is expected based on the enzyme kinetics of α-KG conversion to 2-HG found in mIDH1, and that many cancer cells depend on glutamine as an anaplerotic substrate (Dang et al., 2009; Daye and Wellen, 2012; DeBerardinis et al., 2007). It is not unreasonable that cells already under the burden of glutamine addiction to maintain anaplerosis, increase glutaminolysis rates when a large portion of glutaminolytic α-KG is quickly converted to a dead-end metabolite like 2-HG. Our kinetic results show that kinetics of glutamine-derived 2-HG has the potential to be fast enough for detection in vivo with HP-MRI.
In order to use HP-MRI to image the conversion of glutamine to 2-HG in vivo, two physicochemical properties of glutamine need to be considered: (i) T1 relaxation time and (ii) signature spectral resonances. T1 governs how long the signal will last and in general, for any given molecule, quaternary and carbonyl carbons will have the longest T1 (Salamanca-Cardona and Keshari, 2015). For glutamine the T1 values of its two carbonyl carbons (C1, carboxyl, C5, amide) have been reported with the amine carbon (C5) having a longer T1 in the deuterated form (Keshari and Wilson, 2014; Qu et al., 2011). However, when also considering spectral resoances at which each of the carbonyls of glutamine and its metabolic products resonate, the C5 of glutamine is inadequate due to the overlap of the C5 of glutamate and the C5 of 2-HG, especially at lower magnetic fields, thus we selected [1-13C] Gln for the HP-MRI experiments. In previous studies that tested HP glutamine as an imaging probe, the spontaneous formation of pyroglutamate during sample preparation was presented as a limitation to the potential translation to a clinical setting (Cabella et al., 2013; Gallagher et al., 2008). With our preparation method, we minimized the amount of PG formed and the [5-13C] PG present in the spectra immediately after dissolution was not present at the start of spectra acquisition (Fig. 5).
The combination of our results from in vitro experiments and HP-MRI imaging in vivo make a strong case for the unambigious detection of real-time metabolic conversion of glutamine to 2-HG in vivo. In order to further confirm that 2-HG was indeed forming fast enough for detection in the time frame of the HP experiment in the tumor following injection of glutamine, we devised a quick-infusion scheme that, to our knowledge, has not been reported before for assessing metabolic rates of a potential HP-MRI probe. This allowed us to confirm that the rates of 2-HG production in mIDH1 and IDH2 tumors were fast enough for in vivo detection via HP-MRI. The method was also sensitive enough to detect the difference in 2-HG formation rates in mIDH1 and mIDH2 models. In theory, this quick-infusion scheme can be extended to any probe and metabolites with high rates of accumulation within the first 30 min and validate potential novel probes for metabolic imaging using HP-MRI.
Knowing the limitations to elucidate the main metabolic source for 2-HG in vivo, we sought to develop a method that could unambigiously show glutamine is the metabolite fulfilling this role. In achieving this, we developed an HP-MRI method with great potential for translation to the clinic because of the safety of glutamine when injected intravenously. Intravenous injection of glutamine has been used as support therapy for patients in other contexts with doses of 320 mg/kg without any adverse effects (Berg et al., 2006; Berg et al., 2007). Our animal subjects received injections of 148 mg/kg, which translates to a human equivalent dose of 12 mg/kg (Nair and Jacob, 2016). The safety of glutamine in cancer human patients has also been evidenced in studies using fluorinated glutamine for PET imaging in gliomas (Venneti et al., 2015). Additionally, no adverse effects were observed in any of our animal subjects following injection of glutamine, making this methodology as well as the potential biology interrogated ideal for translation to patients. On the other hand the method presented here as proof-of-concept, has limitations before it can be used in the clinic. We recognize slightly longer injection times may be required in humans than the HP experiments in mice. In this case, this probe would benefit from having a longer T1. Strategies to increase T1 include exchange of neighboring 14N for 15N and/or exchange of 1H for 2H and have been used to extend T1s of glucose and [5-13C] Gln (Keshari and Wilson, 2014). This approach can be applied to [1-13C] Gln and it should result in extended relaxation times not only for glutamine, but also glutamate thus effectively extending the life-time of the signal throughout the entire experiment.
The imaging method presented here addresses the sensitivity and specificity limitations of other methods dedicated to identify mIDH1/2 tumors. Current imaging techniques for identifying IDH1/2 mutations used in the clinic are 1H-MRS based and typically do not require the infusion of an exogenous agent, taking advantage of tailored MR pulse sequences to quantify 2-HG. These methods though are limited by overlapping resonances from other metabolites that are not trivial to resolve (Andronesi et al., 2012; Andronesi et al., 2013; Turkalp et al., 2014). Furthermore, 1H-MRS detects steady-state, total 2-HG levels that include intracellular and extracellular pools. This makes it difficult to differentiate between metabolically active and necrotic tumors that have potentially accumulated 2-HG or other metabolites. More importantly, with 1H-MRS the specific nutrient source of mIDH1/2-derived 2-HG in vivo cannot be resolved. These are limitations shared by HP α-KG which has been evaluated for its potential to identify mIDH1 mutations. While [1-13C] α-KG benefits from a longer T1 than [1-13C] Gln, the presence of [5-13C] α-KG 0.1 ppm apart from the [1-13C] 2-HG signal limits the unambiguity of the method (Chaumeil et al., 2013). Additionally, the transport of α-KG is likely to be limiting in many cell systems which is why historically metabolic studies of the TCA cycle have taken advantage of dimethyl-α-KG as a cell permeable α-KG derivative (MacKenzie et al., 2007).
The work presented here provides strong evidence from imaging data as well as ex vivo tumor analysis, that in vivo the specific nutrient source for 2-HG in mIDH1/2 mutants is glutamine, which to our knowledge has not been demonstrated before. We acknowledge that our data is constrained to the context of a quick-infusion set up and further experiments are necessary to obtain conclusive evidence that this phenotype extends to normal physiological conditions in which case these findings could have wide implications with regards to potential therapies. As evidenced by our results, this approach has the specificity to detect the real-time formation of 2-HG without the need to de-convolute overlapping resonances, while also being sensitive enough to detect differences in rates of 2-HG generation and assess therapy response. Though continued work is needed to take this endogenous substrate forward into the clinic to explore its clinical utility, we propose that our method has great potential to diagnose and monitor therapy response of mIDH1/2 tumors. While this approach was tested in chondrosarcoma xenograft models, we expect it to be extremely useful in the setting of brain tumors due to their high glutamine uptake, which has already been exploited for diagnosis using 18F-Gln PET imaging (Venneti et al., 2015). Combining 18F-Gln PET with HP glutamine MRI provides a multimodal means to interrogate both its quantitative uptake and utilization in vivo and specifically differentiate between PTEN-driven and mIDH1/2-driven biology, a great need in the glioma community given the prevalence of IDH1/2 mutations (Dang et al., 2009). In summary, these studies suggest that HP glutamine MRI can play an important role in the annotation of IDH mutations in vivo and could aid in the development of novel therapeutics geared toward addressing IDH.
STAR Methods
Contact for Reagents and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kayvan R. Keshari (rahimikk@mskcc.org)
Experimental Models and Subject Details
Cell lines and culture conditions
JJ012 (male) and CS1 (male) human chondrosarcoma cells and HPAC and MiaPaCa-2 pancreatic cells were cultured and maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (FBS), 100 µg/mL streptomycin prepared at Memorial Sloan Kettering Media Preparation core and grown as monolayers at 37 °C with 5% CO2. Cell lines were validated at the Integrated Genomics Operation core at Memorial Sloan Kettering Cancer Center.
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee at Memorial Sloan Kettering Cancer Center. SCID female mice (4–6 weeks old, average weight 25 g, Jackson Laboratories) were xenografted on the right flank via subcutaneous injection of 5.0×106 cells for CS1, HPAC, MiaPaCa-2 and 10.0×106 cells for JJ012 tumors. Cells were injected in a 150 µL suspension of 1:1 v/v DMEM with Matrigel. All mice were housed under standard conditions (group housing up to five per cage) with ad libitum access to food and water and 12hr light/dark cycle. Tumors were allowed to develop until a size of 0.8–1.0 cc for HP-MRI experiments, and until a size of 0.25–0.35 cc for LC-MS analysis.
Method Details
Culture conditions for metabolite profiling
For kinetics of 2-HG formation, approximately 2.0×105 cells/well (JJ012 and CS1) were seeded in 6 well plates and left overnight to attach. Media in each plate was exchanged with isotope tracing media consisting of glutamine-free or glucose-free DMEM supplemented with streptomycin and FBS and 5 mM [13C5] Gln or 25 mM [13C6] Gluc or 5 mM [1-13C] Gln. Cells were harvested at 0.5, 1, 3, 8, 24 h after media exchange, and after 24 h for glucose and [1-13C] Gln. An additional set of cells used as a control was incubated with media supplemented with 5 mM unlabeled glutamine (no isotopic enrichment) and harvested at 24 h after media exchange.
For inhibitor studies, the small molecule IDH1 inhibitor AGI-5198 (Agios) was used with DMSO as vehicle. Media was exchanged after seeding to DMEM supplemented with FBS, streptomycin and AGI-5198 with concentration ranging from 0 to 5 µM. Fresh media with inhibitor was exchanged at 24 h and 72 h and cells were harvested at 96 h following initial exposure to inhibitor. Kinetics of 2-HG formation with IDH1 inhibition (2 µM AGI-5198) was carried in JJ012 cells following the treatment scheme outlined above, but cells were harvested at 0.5, 1, 3, 8 h after isotope-containing media was exchanged.
LC-MS isotope tracing analysis
Following incubation all media was aspirated and a 20:80 ice-cold solution of water and methanol was added to the cells for metabolite extraction and incubated at −80 °C. For in vitro quantitation experiments a calibration curve of 2-HG included in the LC-MS analysis and 2 µM d5-2-HG was added to the extraction solvent as an internal standard. After overnight incubation, cells were scraped, transferred to Eppendorf tubes and clarified by centrifugation at 21,000 g for 20 min at 4°C. Supernatant was collected in clean tubes and dried in a vacuum evaporator (Genevac). Metabolite extracts were resuspended in 160 µL of 60/40 acetonitrile/water containing 5 mM ammonium formate. Samples were vortexed well and incubated on ice for 20 min and clarified by centrifugation at 21,000 g for 15 min at 4°C. 130 µL of supernatant was transferred to autosampler vial for LC-MS analysis. LC separation was using an Agilent 1290 UPLC system and BEH Amide column (150 mm × 2.1 mm, 3.5 µm; Waters). Mobile phase A was 50:50 methanol:water with 10 mM Ammonium formate, 5 µM EDTA, pH 3. Mobile phase B was 90:10 acetonitrile:water with 10 mM ammonium formate, 5 µM EDTA, pH 3. The injection volume was 5 µL and LC gradient conditions were: 0 min: 95% B; 9 min: 70% B; 9.75 min: 40% B; 12 min: 40% B; 13 min: 30% B; 13 min: 30% B; 14.5 min 95% B with 8 min of re-equilibration time, flow rate was 0.4 mL/min and column temperature was 45°C. MS detection was using an Agilent 6545 Q-TOF mass spectrometer with Dual JetStream source operating in negative ionization mode. MS parameters were: gas temp: 200°C; gas flow: 10 l/min; nebulizer pressure: 40 psig; sheath gas temp: 300°C; sheath gas flow: 12 l/min; VCap: 3,000 V; Fragmentor: 125 V; Skimmer: 45 V; Octopole RF: 750 V. Active reference mass correction was through a second nebulizer using masses with m/z: 119.03632 and 966.000725. Data was acquired from m/z 50 – 1700 at 1 Hz. Data analysis and isotopic natural abundance correction was performed within MassHunter VistaFlux and MassHunter Quant software (Agilent).
Polarization of [1-13C] glutamine
[1-13C] Gln (Sigma-Aldrich, US) was completely dissolved in 8 N NaOH for a final concentration of 4M. This solution was combined 1:1 with a solution of glycerol, 35 mM of OX063 radical (Oxford Chemicals, UK) and 0.1 mM of Gd-DOTA (Biocyclics). The sample was rapidly frozen in liquid nitrogen and kept frozen as much as possible throughout all experimental steps to minimize the formation of pyroglutamate. The sample was polarized in a SpinLab DNP polarizer (General Electric) for more than 1 hour and rapidly dissolved to a final concentration of 50 mM in 100 Tris buffer (100 mM, pH 7.4) supplemented with 0.1 mM EDTA.
Polarization quantification was carried immediately after dissolution on a bench-top NMR spectrometer equipped with 1 Tesla permanent magnet and a dual tuned 1H-13C coil (Magritek Ltd.). Apparent relaxation time (T1) was estimated from sequential spectra collected every 5 s after a 5° excitation fitted to a mono-exponential curve and corrected for flip angle. Thermal polarization was determined from the average spectrum of 256 scans acquired with 90° flip angle every 10 s. Final polarization values are reported after correcting for flip angles and the apparent T1.
In vivo hyperpolarized studies
All in vivo hyperpolarized experiments were carried on a small animal imaging system (Nanoscan Mediso Inc.) equipped with a 1 Tesla permanent magnet using a dual tuned 1H-13C coil. For anatomical imaging animals were maintained under continuous isoflurane flow (1 – 2%) throughout the experiments. 1H-T2 anatomical images to determine tumor location were obtained using a 2D Fast Spin Echo (FSE) sequence, axial, TE/TR = 12.4/671 ms, 32×32 mm field of view (FOV), 10 slices at 2 mm/slice. For each experiment a13C-Urea sample (5M) was placed next to the animal for quantitative measurements of 13C compounds.
Intravenous injection of HP [1-13C] Gln in Tris buffer was started 15 s post dissolution. A total of 300 µL of HP solution was injected over 12 seconds via a 23-gauge catheter secured in the tail. 13C dynamic spectra was acquired at the start of injection with 10° excitations ever 3 seconds in a 20 mm thick slab. 2D CSI was acquired 26 s after the start of injection for 8 s with 20° constant flip angle, 6×6 in-plane resolution, 32×32 mm FOV, and 20 mm slab thickness. For inhibition studies, baseline spectra were acquired without treatment on animals inoculated with JJ012 cells. The animals were left to recover for 2–3 days and a second injection on selected animals was done following treatment (IP injection, 150 mg/kg in MC 0.5% and tween-80 0.02%) with the IDH1 inhibitor AGI-5198. All treatment was administered within 3 h prior to HP [1-13C] Gln injection. For all HP experiments, isoflurane flow was stopped 5 s before start of injection and re-started 20 s after start of injection.
Post mortem analysis
Tumor-bearing animals were restrained and kept under isoflurane flow. A 300 µL bolus of buffered [13C5] Gln or [13C6] Gluc (50 mM in Tris) was intravenously injected over 12 s. JJ012 animals were euthanized 1 min, 5 min, 15 min and 30 min after start of injection for Gln infusions and 30 min for Gluc infusions. For CS1 tumor-bearing animal studies, tumors were extracted 30 min after the start of injection. Tumors were quickly resected and snap frozen in liquid nitrogen.
LC-MS analisys of [13C5] 2-HG in tumor tissue
Tumor tissues were ground in a mortar and pestle with liquid nitrogen and ~40 mg of powdered tissue was weighed into a cold tube. Powdered tissue was extracted using 4:4:2 acetonitrole:methanol:water containing 2.1 µM [13C5,15N] Glu internal standard to a final tissue concentration of 50 mg/mL. Samples were sonicated, vortexed and subjected to 2 freeze-thaw cycles then centrifuged at 21,000 g for 20 min at 4°C and an equal volume (600 µL, 30 mg) was dried in a Genevac concentrator and re-suspended in 100 µL 60:40 acetonitrile:water containing 5 mM ammonium formate as for the cell samples.
LC-MS quantitation was using an 2.1 × 100 mm, 1.7 µm BEH amide column (Waters Corporation) and Agilent 1260 pump coupled to Thermo scientific TSQ vantage triple quadruple mass spectrometer operating in SRM and negative ionization modes. Mobile phases and LC parameters were as described for cell samples except that the injection volume was 10 µL and LC gradient conditions were: 0 min: 95% B; 0.5 min: 95% B; 6.0 min: 60% B; 6.5 min: 40% B; 8.0 min: 40% B; 8.5 min: 30% B; 10.5 min 95% B with 8 min of re-equilibration time. MS source parameters were spray voltage: 2500 V; capillary temperature: 300°C; vaporizer temperature: 400°C; sheath gas pressure: 50 psi; aux gas pressure: 40 psi. S-lens was 40 V and individual reactions monitored and collision energies (CE) were: 2-HG m+5 m/z 152.1 → 60.0 (CE: 20 V)*, 134.1 (CE: 12 V); [13C5,15N] glutamate m/z 152.1 → 134.1 (CE: 12 V)*, 107.1 (CE: 14 V); with * indicating the primary transition used to quantify each metabolite. Quantitation was performed using calibration curve prepared using deuterated-2-HG (d5-2-HG) and 2.1 µM [13C515N] Glu in tumor tissue extract from untreated animals. The use of a d5-2-HG curve to quantify [13C5] 2-HG was validated as an approach that corrected for ion suppression due to the high concentration of unlabeled 2-HG present in the tumor tissue extracts. Chromatograms were acquired and processed with Xcalibur and TraceFinder software (ThermoFisher).
Isotopic enrichment following Gln and Gluc infusions were carried on tissue extracts using the same method as outlined for isotopic tracing in cells.
NMR analysis of cell extract and tumor tissue
Metabolites from dried cells were extracted as previously outlined for LC-MS isotope tracing analysis. Dried tumor extract from JJ012 and CS1 tumors dissected 30 min after [13C5] Gln infusion and dried cell extract were re-suspended in 550 µL of D2O. For 1H-NMR determination of total levels of 2-HG and glutamate in the tumor and cell extracts, an internal standard of 20 µM of 4,4-dimethyl-4-silapentane-1-sulfonic acid was used. 1H, and 13C-NMR spectroscopy was carried on a 14.1 T NMR spectrometer (Bruker Biospin) equipped with a cryoprobe (1H and 13C) and automatic sample changer. 1H spectra were acquired using a one-pulse sequence (30 degree pulse), repetition time of 4s, selective presaturation of the water signal, without composite adiabatic 13C decoupling with a total of 526 scans per sample. Identification and quantification of resonance signals were done with Chenomx NMR Suite 8.0 professional (Chenomx Inc). Experimental spectra were adjusted for T1 saturation effects by comparison to a sample fully relaxed spectra (180s recycle delay) for each metabolite (Di Gialleonardo et al., 2016). For 13C analysis, were acquired using a one-pulse sequence (30 degree pulse), repetition time of 2s, with a total of 35000 scans per sample. The area of C1 and C5 of 2-HG and glutamate were used for quantification normalized to a 13C urea internal standard.
Quantification and Statistical analysis
All animal experiments were performed following approval from the MSKCC Institutional Animal Care and Use Committee and were conducted following NIH guidelines for animal welfare. Individuals handling the animals and conducting all animal experiments were blinded to the experimental design. In the evaluation of the potential of HP-MRI to assess response to the IDHi, imaging was performed in a non-blinded fashion. For animal studies, n=3 to n=11 was selected to validate the methodology. For ex vivo LC-MS analysis n=3 was selected, as for imaging with IDHi. For cell culture studies, all experiments were conducted using three independent experiments (n=3). No samples or animals were excluded from data analyses. Quantitative data are representative of mean ± S.D. and as applicable, determined to be statistically significant when p < 0.05 by two-tailed Student’s t test. In figures, asterisks denote statistical significance as calculated by Student’s t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
Supplementary Material
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals | ||
| [1-13C] Glutamine | Sigma | Cat # 605018 |
| OX063 | Oxford Chemicals | N/A |
| [13C5] glutamine | Sigma | Cat # 605166 |
| [d5]-2-hydroxuglutarate | Sigma | Cat # 16059 |
| [13C6] Glucose | Sigma | Cat # 389374 |
| [5-13C, 15N] glutamine | Sigma | Cat # 607983 |
| AGI-5198 | Agios | N/A |
| 4,4-dimethyl-4-silapentane-1-sulfonic acid | Sigma | Cat # 613150 |
| Gd-DOTA | Biocyclics | Cat # M-147 |
| Experimental Models: Cell Lines | ||
| JJ012 | MSKCC | N/A |
| CS1 | MSKCC | N/A |
| MiaPaCa-2 | ATCC | Cat # CRL-1420 |
| HPAC | ATCC | Cat # CRL-2119 |
| Experimental Models: Organisms/Strains | ||
| Mice strain NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ | Jackson Laboratory | Cat # 005557 |
| Software and Algorithms | ||
| Prism | Graphpad | https://www.graphpad.com/scientific-software/prism/ |
| Osirix | Osirix | http://www.osirix-viewer.com/ |
| SIVIC | Sourceforge | https://sourceforge.net/projects/sivic/ |
| MATLAB | Mathworks | https://www.mathworks.com/ |
| Chenomx | ChenomX | http://www.chenomx.com/ |
| Xcalibur | Thermo Scientific | https://www.thermofisher.com/order/catalog/product/OPTON-30487 |
| TraceFinder | Thermo Scientific | https://www.thermofisher.com/order/catalog/product/OPTON-30491 |
| Nanoscan | Mediso | N/A |
| SpinSolve | Magritek | http://www.magritek.com |
| MassHunter VistaFlux | Agilent | N/A |
| MassHunter Quant | Agilent | N/A |
Highlights.
Glutamine is a primary carbon source for the oncometabolite 2-hydroxyglutarate.
2-HG formation from glutamine occurs with fast kinetics in vitro and in vivo
With HP MRI, 2-HG formation from glutamine can be imaged in real time in vivo.
Specific inhibition of mIDH in vivo was detected non-invasively using HP 2-HG.
Acknowledgments
The authors wish to thank Christian Felt for help with MATLAB scripts for data reconstruction as well as the Agilent MassHunter software development team for expert support in analysis of stable isotope mass spectrometry tracing data. We also acknowledge Sangmoo Jeong for input in discussions of the experimental work. We thank George Sukenik of the NMR analytical Core Facility and members of the Donald B. and Catherine C. Marron Cancer Metabolism Center at MSKCC. Grant support: This work was supported by NIH R00 EB014328; NIH/NCI Cancer Center Support Grant P30 CA008748; Geoffrey Beene Cancer Research Center, Center for Molecular Imaging and Nanotechnology at MSKCC, Starr Cancer Consortium grant I6-A616, The Center for Experimental Therapeutics at MSKCC, Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and the American Italian Cancer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: Conception and design: L. Salamanca-Cardona, J. R. Cross, C. B. Thompson, K. R. Keshari. Development of methodology: L Salamanca-Cardona, H. Shah, A. J. Poot, F. M. Correa, V. Di Gialleonardo, K. L. Granlund, V. Z. Miloushev, J. R. Cross, K. R. Keshari. Data Acquisition: L. Salamanca-Cardona (HP-MRI, tumor tissue NMR, in vitro and ex vivo LC-MS, animal experiments), F. M. Correa (HP-MRI, tumor extractions, animal experiments), H. Shah (in vitro and ex vivo LC-MS, tumor extractions) H. Lui (in vitro and ex vivo LC-MS, tumor extractions). Analysis and interpretation of data: L. Salamanca-Cardona, H. Shah, J. R. Cross, K. R. Keshari. Writing of manuscript: L. Salamanca-Cardona, J. R. Cross, C. B. Thompson, K. R. Keshari. Review and revision of manuscript: L. Salamanca-Cardona, S. T. Tee, J. R. Cross, C. B. Thompson, K. R. Keshari.
Disclosures: C.B. Thompson is a co-founder and chairman of scientific advisory board, of Agios Pharmaceuticals, Inc.
References
- Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-Hydroxyglutarate in IDH-Mutated Glioma Patients by In Vivo Spectral-Editing and 2D Correlation Magnetic Resonance Spectroscopy. Sci Transl Med. 2012;4:116ra114–116ra114. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronesi OC, Rapalino O, Gerstner E, Chi A, Batchelor TT, Cahill DP, Sorensen AG, Rosen BR. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013;123:3659–3663. doi: 10.1172/JCI67229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babakoohi S, Lapidus RG, Faramand R, Sausville EA, Emadi A. Comparative Analysis of Methods for Detecting Isocitrate Dehydrogenase 1 and 2 Mutations and Their Metabolic Consequence, 2-Hydroxyglutarate, in Different Neoplasms. Appl Immunohistochem Mol Morphol. 2016 doi: 10.1097/PAI.0000000000000342. [DOI] [PubMed] [Google Scholar]
- Berg A, Bellander BM, Wanecek M, Gamrin L, Elving A, Rooyackers O, Ungerstedt U, Wernerman J. Intravenous glutamine supplementation to head trauma patients leaves cerebral glutamate concentration unaffected. Intensive Care Med. 2006;32:1741–1746. doi: 10.1007/s00134-006-0375-3. [DOI] [PubMed] [Google Scholar]
- Berg A, Norberg A, Martling CR, Gamrin L, Rooyackers O, Wernerman J. Glutamine kinetics during intravenous glutamine supplementation in ICU patients on continuous renal replacement therapy. Intensive Care Med. 2007;33:660–666. doi: 10.1007/s00134-007-0547-9. [DOI] [PubMed] [Google Scholar]
- Cabella C, Karlsson M, Canape C, Catanzaro G, Colombo Serra S, Miragoli L, Poggi L, Uggeri F, Venturi L, Jensen PR, et al. In vivo and in vitro liver cancer metabolism observed with hyperpolarized [5-13C]glutamine. J Mag Res. 2013;232:45–52. doi: 10.1016/j.jmr.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- Chaumeil MM, Larson PE, Yoshihara HA, Danforth OM, Vigneron DB, Nelson SJ, Pieper RO, Phillips JJ, Ronen SM. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nature Commun. 2013;4:2429. doi: 10.1038/ncomms3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang X-L, Mashimo T, Raisanen JM, Marin-Valencia I, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16:387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daye D, Wellen KE. Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Sem Cell Dev Biol. 2012;23:362–369. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- de Botton S, Mondesir J, Willekens C, Touat M. IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. J Blood Med. 2016;7:171–180. doi: 10.2147/JBM.S70716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Prod Natl Acad Sci U.S.A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gialleonardo V, Tee SS, Aldeborgh HN, Miloushev VZ, Cunha LS, Sukenick GD, Keshari KR. High-Throughput Indirect Quantitation of 13C Enriched Metabolites Using 1H NMR. Anal Chem. 2016;88:11147–11153. doi: 10.1021/acs.analchem.6b03307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbink HJ, Taal W, van Marion R, Kros JM, van Heuvel I, Bromberg JE, Zonnenberg BA, Zonnenberg CB, Postma TJ, Gijtenbeek JM, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73:1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- Fathi AT, Sadrzadeh H, Borger DR, Ballen KK, Amrein PC, Attar EC, Foster J, Burke M, Lopez HU, Matulis CR, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood. 2012;120:4649–4652. doi: 10.1182/blood-2012-06-438267. [DOI] [PubMed] [Google Scholar]
- Gallagher FA, Kettunen MI, Day SE, Hu D-E, Ardenkjær-Larsen JH, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453:940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- Grassian AR, Parker SJ, Davidson SM, Divakaruni AS, Green CR, Zhang X, Slocum KL, Pu M, Lin F, Vickers C, et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014;74:3317–3331. doi: 10.1158/0008-5472.CAN-14-0772-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Garcia JL, Cai LM, Chaumeil MM, Eriksson P, Robinson AE, Pieper RO, Phillips JJ, Ronen SM. Glioma cells with the IDH1 mutation modulate metabolic fractional flux through pyruvate carboxylase. PLoS One. 2014;9:e108289. doi: 10.1371/journal.pone.0108289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari KR, Wilson DM. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem Soc Rev. 2014;43:1627–1659. doi: 10.1039/c3cs60124b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman BP, Ploessl K, Wang L, Qu W, Zha Z, Wise DR, Chodosh LA, Belka G, Thompson CB, Kung HF. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med. 2011;52:1947–1955. doi: 10.2967/jnumed.111.093815. [DOI] [PubMed] [Google Scholar]
- Losman JA, Kaelin WG., Jr What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 2013;27:836–852. doi: 10.1101/gad.217406.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci G, Maharry K, Wu Y-Z, Radmacher MD, Mrózek K, Margeson D, Holland KB, Whitman SP, Becker H, Schwind S, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki S, Gottlieb E. Oncometabolites: tailoring our genes. The FEBS Journal. 2015;282:2796–2805. doi: 10.1111/febs.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohka F, Ito M, Ranjit M, Senga T, Motomura A, Motomura K, Saito K, Kato K, Kato Y, Wakabayashi T, et al. Quantitative metabolome analysis profiles activation of glutaminolysis in glioma with IDH1 mutation. Tumor Biology. 2014;35:5911–5920. doi: 10.1007/s13277-014-1784-5. [DOI] [PubMed] [Google Scholar]
- Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther. 2015;152:54–62. doi: 10.1016/j.pharmthera.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107:197–205. doi: 10.1007/s11060-011-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Zha Z, Lieberman BP, Mancuso A, Stetz M, Rizzi R, Ploessl K, Wise D, Thompson C, Kung HF. Facile synthesis [5-(13)C-4-(2)H(2)]-L-glutamine for hyperpolarized MRS imaging of cancer cell metabolism. Acad Radiol. 2011;18:932–939. doi: 10.1016/j.acra.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A. 2011;108:3270–3275. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca-Cardona L, Keshari KR. (13)C-labeled biochemical probes for the study of cancer metabolism with dynamic nuclear polarization-enhanced magnetic resonance imaging. Cancer Metab. 2015;3:9. doi: 10.1186/s40170-015-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, Tsukamoto T, Rojas CJ, Slusher BS, Rabinowitz JD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N, Wiederschain D, Bedel O, Deng G, Zhang B, et al. Extreme Vulnerability of IDH1 Mutant Cancers to NAD+ Depletion. Cancer Cell. 2015;28:773–784. doi: 10.1016/j.ccell.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkalp Z, Karamchandani J, Das S. IDH mutation in glioma: new insights and promises for the future. JAMA Neurol. 2014;71:1319–1325. doi: 10.1001/jamaneurol.2014.1205. [DOI] [PubMed] [Google Scholar]
- Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7:274ra217–274ra217. doi: 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Lu C, Cross JR, Abdel-Wahab O, Levine RL, Schwartz GK, Thompson CB. The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization. J Biol Chem. 2013;288:3804–3815. doi: 10.1074/jbc.M112.435495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber WA. Use of PET for Monitoring Cancer Therapy and for Predicting Outcome. J Nucl Med. 2005;46:983–995. [PubMed] [Google Scholar]
- Wen H, Cho HR, Yun T, Kim H, Park CK, Lee SH, Choi SH, Park S. Metabolomic comparison between cells over-expressing isocitrate dehydrogenase 1 and 2 mutants and the effects of an inhibitor on the metabolism. J Neurochem. 2015;132:183–193. doi: 10.1111/jnc.12950. [DOI] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Mayer D, Gu M, Yen YF, Josan S, Tropp J, Pfefferbaum A, Hurd R, Spielman D. Quantification of in vivo metabolic kinetics of hyperpolarized pyruvate in rat kidneys using dynamic 13C MRSI. NMR Biomed. 2011a;24:997–1005. doi: 10.1002/nbm.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim S-H, Ito S, Yang C, Wang P, Xiao M-T, et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer cell. 2011b;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652–3658. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen KE, Bittinger MA, Su SM, Fantin VR. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene. 2010;29:6409–6417. doi: 10.1038/onc.2010.444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.